Abstract
To identify novel coding association signals and facilitate characterization of mechanisms influencing glycemic traits and type 2 diabetes risk, we analyzed 109,215 variants derived from exome array genotyping together with an additional 390,225 variants from exome sequence in up to 39,339 normoglycemic individuals from five ancestry groups. We identified a novel association between the coding variant (p.Pro50Thr) in AKT2 and fasting plasma insulin (FI), a gene in which rare fully penetrant mutations are causal for monogenic glycemic disorders. The low-frequency allele is associated with a 12% increase in FI levels. This variant is present at 1.1% frequency in Finns but virtually absent in individuals from other ancestries. Carriers of the FI-increasing allele had increased 2-h insulin values, decreased insulin sensitivity, and increased risk of type 2 diabetes (odds ratio 1.05). In cellular studies, the AKT2-Thr50 protein exhibited a partial loss of function. We extend the allelic spectrum for coding variants in AKT2 associated with disorders of glucose homeostasis and demonstrate bidirectional effects of variants within the pleckstrin homology domain of AKT2.
Introduction
The increasing prevalence of type 2 diabetes is a global health crisis, making it critical to promote the development of more efficient strategies for prevention and treatment (1). Individuals with type 2 diabetes display both pancreatic β-cell dysfunction and insulin resistance. Genetic studies of surrogate measures of these glycemic traits can identify variants that influence these central features of type 2 diabetes (2), highlighting potential pathways for therapeutic manipulation. Comprehensive surveys of the influence of common genetic variants on fasting plasma glucose (FG) and fasting plasma insulin (FI) have highlighted defects in pathways involved in glucose metabolism and insulin processing, secretion, and action (3). Recent studies have identified type 2 diabetes–associated alleles that are common in one population but rare or absent in others (4–6). These associations were observed either due to an increase in frequency of older alleles based on population dynamics and demography (5) or the emergence of population-specific alleles (4,6).
We set out to identify and characterize low-frequency allele (minor allele frequency [MAF] <5%) glycemic trait associations by meta-analysis of exome sequence and exome array genotype data in a multiancestry sample. We also performed in vitro functional studies of protein expression, localization, and activity to understand the consequences of our novel findings.
Research Design and Methods
Genetic Association Studies
Study Samples
The Genetics of Type 2 Diabetes (GoT2D) and Type 2 Diabetes Genetic Exploration by Next-generation sequencing in multi-Ethnic Samples (T2D-GENES) Consortia were initially designed to evaluate the contribution of coding variants to type 2 diabetes risk (7). We performed a discovery association analysis to find novel coding variants associated with fasting glycemic traits in 14 studies from GoT2D that contributed exome array information on 33,231 individuals without diabetes of European ancestry. Further discovery analysis was performed with GoT2D and T2D-GENES with exome sequence data (average 80× coverage) in five ancestral groups comprised of 12,940 individuals (6,504 with type 2 diabetes, 6,436 without) with measured FG or FI levels available in 2,144 European, 508 South Asian, 1,104 East Asian, 844 Hispanic, and 508 African American individuals without diabetes. We performed a replication analysis and an assessment of allele frequency distributions in 5,747 individuals from four Finnish cohorts: The Cardiovascular Risk in Young Finns Study (YFS) (8), Helsinki Birth Cohort Study (HBCS) (9), Health 2000 GenMets Study (GenMets) (10), and National FINRISK Study 1997 and 2002 (11). We also assessed the allele frequencies of novel findings in 46,658 individuals from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium with available exome array data (12), although none of the studies passed our quality control filter of a minor allele count (MAC) greater than 5 for inclusion in our replication analysis. See Supplementary Table 1 for study details, sample characteristics, ascertainment criteria, detailed genotype calling, and quality control procedures for each cohort. The relevant institutional review boards, conducted according to the Declaration of Helsinki, approved all human research, and all participants provided written informed consent. A detailed description of ethics permissions is provided in the Supplementary Data.
Phenotypes
For the discovery and replication analysis, we excluded individuals from the analysis if they had a diagnosis of type 2 diabetes, were currently receiving oral or injected diabetes treatment, had FG measures ≥7 mmol/L, had 2-h postload glucose measures ≥11.1mmol/L, or had HbA1c measures ≥6.5% (48 mmol/mol). Additional exclusions occurring at the study level included pregnancy, nonfasting at time of exam, type 1 diabetes, or impaired glucose tolerance. See Supplementary Table 1A for details. Within each study, we adjusted FG and log-transformed FI levels for age, sex, BMI, and additional study-specific covariates. We applied rank-based inverse-normal transformations to study- or ancestry-specific residuals to obtain satisfactory asymptotic properties of the exome-wide association tests.
We tested for genetic associations with type 2 diabetes, hypertension (HTN), and other related quantitative traits in the Finnish discovery and replication cohorts. We analyzed lipid levels (total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides), blood pressure (systolic [SBP] and diastolic [DBP] blood pressure and HTN), height, BMI, central adiposity measures (waist-to-hip ratio, waist circumference, hip circumference), adiponectin level, 2-h insulin level, and Matsuda index, which is known to correlate with whole-body insulin sensitivity as measured by the hyperinsulinemic-euglycemic clamp (r = 0.7, P < 1.0 × 10−4) (13). For quantitative traits and HTN, we adjusted for age, sex, BMI (for glycemic, blood pressure and central adiposity measures), stratified by type 2 diabetes status and sex (for central adiposity measures) within study. We adjusted LDL and total cholesterol for use of lipid-lowering medication, by dividing total cholesterol by 0.8 if on lipid-lowering medication, prior to calculating LDL cholesterol using the Friedewald equation (14). SBP and DBP were adjusted for use of blood pressure–lowering medication by adding 15 mmHg to SBP and 10 mmHg to DBP measurements if an individual reported taking blood pressure–lowering medication (15). The Matsuda index was log transformed and analyzed in individuals without diabetes only. After adjusting for covariates, traits were inverse-normalized within strata. In addition to studying these metabolic outcomes, we used ICD codes to query electronic medical records in the METSIM (METabolic Syndrome In Men) study and FINRISK 1997 and 2002 cohorts (in all individuals regardless of type 2 diabetes status) and categorized affection status for lipodystrophy, polycystic ovary disease, and ovarian or breast cancer.
Statistical Analysis
Discovery Analysis.
We performed association analyses within each study for the exome array data sets and within ancestry for the exome sequence data sets. We used linear mixed models implemented in EMMAX (16) to account for relatedness. Within each study/ancestry, we required variants to have a MAC greater than or equal to five alleles for single variant association tests. We meta-analyzed the single variant results from the (European ancestry) exome array studies using the inverse-variance meta-analysis approach implemented in METAL (17) and combined these with the European ancestry exome sequence results. Then, we meta-analyzed summary statistics across ancestries. We used P < 5 × 10−7 as exome-wide statistical significance thresholds for the single variant tests (18). We used the binomial distribution to assess enrichment of previously reported associations with FG or FI by calculating a P value for the number of nonsignificant variants with consistent direction of effects.
Gene-Based Association Analysis.
We performed gene-based association tests using variants with MAF <1% (including rare variants with MAC ≤5), annotating and aggregating variants based on predicted deleteriousness using previously described methods (7). Briefly, we defined four different variant groupings: “PTV-only,” containing only variants predicted to severely impair protein function; “PTV+missense,” containing protein-truncating variants (PTV) and nonsynonymous (NS) variants with MAF <1%; “PTV+NSstrict,” composed of PTV and NS variants predicted damaging by five algorithms (SIFT, LRT, MutationTaster, PolyPhen-2 HDIV, and PolyPhen-2 HVAR); and “PTV+NSbroad,” composed of PTV and NS variants with MAF <1% and predicted damaging by at least one prediction algorithm above. We used the sequence kernel association test (SKAT) (19) and a frequency-weighted burden test to conduct exome array meta-analyses in an unrelated subset of individuals using RAREMETAL (20). We conducted exome sequence gene-based analyses within ancestry using a linear mixed model to account for relatedness and combined results across ancestries with MetaSKAT (21), which accounts for heterogeneous effects. We further combined gene-based results from exome array and exome sequences using the Stouffer method with equal weights. For gene-based tests, we considered P < 2.5 × 10−6 as exome-wide significant, corresponding to Bonferroni correction for 20,000 genes in the genome (18).
Replication Analysis.
The AKT2 p.Pro50Thr variant was observed at sufficient frequency in the independent Finnish cohorts to perform single variant association test of association with FI. We tested association in SNPTEST (22) (v.2.4.0) in each study with the same additive linear model used in the discovery analysis. Covariate adjustments for FI levels were sex, age, and 10 principal components, and models were run with and without adjustment for BMI.
Estimate of Effect on Raw FI Level and Variance Explained.
To characterize the association between AKT2 p.Pro50Thr and FI, we examined full regression models with raw FI in three studies (Finland-United States Investigation of NIDDM Genetics [FUSION], METSIM, and YFS). We estimated the raw effect on log-transformed FI levels with a fixed-effects meta-analysis. The variance in log-transformed FI explained by AKT2 p.Pro50Thr was estimated by a weighted average of the narrow-sense heritability of AKT2 p.Pro50Thr seen in these three studies.
Population Genetics and Constraint.
We used the Exome Aggregation Consortium (ExAC) for constraint metrics and allele frequencies (23). We obtained sequence alignments for AKT proteins and mRNAs in 100 vertebrates from the University of California, Santa Cruz Genome Browser (24), used Shannon entropy (normalized K = 21) as a conservation score (25), and plotted the sequence logos in R using the RWebLogo library (26).
Associations With Other Traits.
We conducted association tests for traits other than FI and FG within studies for both discovery studies as well as the independent Finnish studies used for replication. P values for type 2 diabetes and HTN came from EMMAX (16) or the Wald test from logistic regression (Finnish replication data sets) and meta-analyzed using an N-weighted meta-analysis (17). Odds ratios (ORs) were obtained from logistic regression adjusting for age, sex, with and without BMI, and principal components and meta-analyzed using an inverse-variance meta-analysis.
Trait Distributions and Phenotype Clustering.
We examined distributions of traits among AKT2 missense allele carriers (p.Pro50Thr, p.Arg208Lys, and p.Arg467Trp) in the T2D-GENES exome sequencing data set. We used nonparametric rank–based methods (kruskal.wallis and permKS functions in R) on both the inverse-normalized covariate-adjusted traits used in the genetic association studies and normalized raw trait values (scale function in R). We clustered AKT2 missense allele carriers on scaled trait values (pheatmap function in R).
In Vitro Functional Studies
Plasmids and Cell Lines
The generation of the AKT2 allelic series was initiated by the production of pDONR223-AKT2 through PCR of the human AKT2 open reading frame with the integration of terminal attR sites using primers (see below). HeLa, HuH7, and 293T cells were obtained at The Broad Institute and maintained in 10% FBS DMEM, 100 units/mL penicillin and 100 μg/mL streptomycin, and documented mycoplasma-free. HeLa and HuH7 cells were starved for 18 h and stimulated for 15 min with 100 nmol/L insulin for activation analyses.
Primers for Functional Work
The generation of the AKT2 allelic series was initiated by the production of pDONR223-AKT2 through PCR of the human AKT2 open reading frame with the integration of terminal attR sites using primers FWD: 5′-GGGGACAAGTTTGTACAAAAAAGTTGGCACCATGAATGAGGTGTCTGTCATC−3′, REV: 5′-GGGGACCACTTTGTACAAGAAAGTTGGCAACTCGCGGATGCTG−3′ and subsequent Gateway BP reaction into pDONR223 obtained from The Broad Institute Genetic Perturbation Platform. Site-directed mutagenesis was then performed to generate AKT2.E17K (AKT2.Lys17), AKT2.P50T (AKT2.Thr50), AKT2.R208K (AKT2.Lys208), AKT2.R274H (AKT2.His274), AKT2.R467W (AKT2.Trp467) with the following primers: AKT2.E17K: FWD: 5′-GGCTCCACAAGCGTGGTAAATACATCAAGACCTGG−3′, REV: 5′-CCAGGTCTTGATGTATTTACCACGCTTGTGGAGCC−3′; AKT2.P50T: FWD: 5′-AGGCCCCTGATCAGACTCTAACCCCCTTAAAC−3′, REV: 5′-GTTTAAGGGGGTTAGAGTCTGATCAGGGGCCT−3′; AKT2.R208K: FWD: 5′-GTCCTCCAGAACACCAAGCACCCGTTCC−3′, REV: 5′-GGAACGGGTGCTTGGTGTTCTGGAGGAC−3′; AKT2.R274H: FWD: 5′-GGGACGTGGTATACCACGACATCAAGCTGGA−3′, REV3′REV: 5′-TCCAGCTTGATGTCGTGGTATACCACGTCCC−3′; and AKT2.R467W: FWD: 5′-GGAGCTGGACCAGTGGACCCACTTCCC−3′, REV: 5′-GGGAAGTGGGTCCACTGGTCCAGCTCC−3′. COOH-terminal, V5-tagged lentiviral pLX304-AKT2.E17K, pLX304-AKT2.P50T, pLX304-AKT2.R208K, pLX304-AKT2.R274H, and pLX304-AKT2.R467W were each generated by subsequent Gateway LR reactions with pDONR223-AKT2.E17K, pDONR223-AKT2.P50T, pDONR223-AKT2.R208K, pDONR223-AKT2.R274H, and pDONR223-AKT2.R467W, respectively, and pLX304 obtained from The Broad Institute Genetic Perturbation Platform. Control plasmid pLX304-empty vector was additionally acquired from The Broad Institute Genetic Perturbation Platform.
Antibodies
Anti-Akt (#4685), anti–phospho-Akt S473 (#4060), anti–phospho-Akt T308 (#9275), anti–β-actin (#4970), anti-GSK3β (#9315), anti–phospho-GSK3β (#9336), anti-GST (#2625), and anti-V5 (#13202) were purchased from Cell Signaling Technologies. Horseradish peroxidase–conjugated anti-rabbit and anti-mouse IgG antibodies were purchased from Millipore.
3-D Modeling
The 3-D structure of AKT2 with the full allelic series was predicted using IntFOLD (27) and visualized in PyMOL (28).
In Vitro Kinase Assays
Following lentiviral infection and subsequent 5μg/mL blasticidin selection, V5-AKT2, V5-AKT2.Lys17, V5-AKT2.Thr50, V5-AKT2.Lys208, V5-AKT2.His274, and V5-AKT2.Trp467 variants were each isolated from HeLa cell lysate with V5 agarose beads (Sigma-Aldrich) and incubated with 150 ng GST-GSK3β substrate peptide (Cell Signaling Technologies) and 250 mmol/L cold ATP in kinase assay buffer (Cell Signaling Technologies) for 35 min at 30°C.
Proliferation Assay
Lentiviral pLX304 control or V5-AKT2 variant infected HuH7 cells were cultured in 24-well plates for 72 h in 10% FBS /phenol red-free DMEM for 72 h. We added WST-1 (Takara Clontech) to each well at the manufacturer-recommended 1:10 ratio and incubated for 4 h at 37°C prior to absorbance measurement at 450 nm with BioTek Synergy H4 plate reader.
Immunoblots
We washed cells with PBS and lysed in EBC buffer (120 mmol/L NaCl, 50 mmol/L Tris-HCl [pH 7.4], 50 nmol/L calyculin, cOmplete protease inhibitor cocktail [Roche], 20 mmol/L sodium fluoride, 1 mmol/L sodium pyrophosphate, 2 mmol/L ethylene glycol tetraacetic acid, 2 mmol/L ethylenediaminetetraacetic acid, and 0.5% NP-40) for 20 min on ice. To preclear cell lysates, we centrifuged at 12,700 rpm at 4°C for 15 min. We measured protein concentration with Pierce BCA protein assay kit using a BioTek Synergy H4 plate reader. We resolved lysates on Bio-Rad Any kD Mini-PROTEAN TGX polyacrylamide gels by SDS-PAGE and transferred by electrophoresis to nitrocellulose membrane (Life Technologies) at 100 V for 70 min. We blocked membranes in 5% nonfat dry milk/TBST (10 mmol/L Tris-HCl, 150 mmol/L NaCl, 0.2% Tween 20) buffer pH 7.6 for 30 min. We incubated blots with indicated antibody overnight at 4°C. The membrane was then washed in TBST, three times at 15-min intervals, before a 1-h secondary horseradish peroxidase–conjugated antibody incubation at room temperature. We again washed nitrocellulose membranes in TBST, three times for 15 min, prior to enhanced chemiluminescent substrate detection (Pierce).
Statistical Analysis
The quantified results of the in vitro kinase and proliferation assays were normalized to internal control values for each replicate. We used generalized linear models of the quantified assay results to assess effects of variants within and across replicate rounds, allowing for interaction by replicate. The graphical representation was produced using functions in the effects (v 3.0-3) package in R.
Gene Expression Studies
Study Samples
We compared the expression pattern of AKT2 to the two other members of the AKT gene family, AKT1 and AKT3, using multitissue RNA sequencing (RNA-seq) data from the pilot phase of the Genotype-Tissue Expression (GTEx) project (dbGaP accession number: phs000424.v3.p1) in 44 tissues with data from more than one individual. Detailed procedures for sample collection, RNA extraction, RNA-seq, and gene and transcript quantifications have been previously described (29). Using data from the Identifying Biomarkers of Ageing using whole Transcriptome Sequencing (EuroBATS) project, samples from photo-protected subcutaneous adipose tissue from 766 twins were extracted (130 unrelated individuals, 131 monozygotic and 187 dizygotic twin pairs) and processed as previously described (30,31). Using data from METSIM, subcutaneous fat biopsy samples were obtained from a sample of 770 participants and processed as previously described (32).
Phenotypes
We studied the association of age, BMI, and FI levels with gene expression levels and with expression-associated SNPs (expression quantitative trait loci [eQTL]) in the AKT2 region. Age and sex were available for the GTEx study samples. In additional to age and BMI, FI level was measured at the same time point as the fat biopsies in the EuroBATS sample data, following a previously described protocol (33). Baseline age, BMI, and FI levels were used for the METSIM participants (34).
Statistical Analysis
The comparison of expression levels of AKT2 versus AKT1 and AKT2 versus AKT3 was performed using log2-transformed reads per kilobase per million mapped reads (RPKMs). We studied BMI, age, and FI (not available in GTEx data) associations with AKT2 expression using linear mixed models as implemented in the lme4 package in R. The gene expression RPKM values were inverse-variance rank normalized for these analyses. Covariates included study-specific fixed- and random-effects (see Supplementary Data for additional details on each cohort), using sex, BMI, and age as additional fixed-effects as appropriate. The eQTL analysis was performed on single nucleotide polymorphisms (SNPs) within a 1 Mb of AKT2 using linear mixed models to assess the association of the SNPs with the inverse-normalized RPKM expression values.
Results
Genetic Association Studies
We tested the association of FI and FG with 390,225 variants from exome sequence data (GoT2D and T2D-GENES) and 109,215 variants derived from exome array genotyping (GoT2D) (7) (individual study genomic inflation factor [λGC] <1.06; Supplementary Fig. 1). We examined variants that had been previously associated with FG and FI (3,18). Of 28 FG and 14 FI loci with the reported SNPs or close proxies in our data set, 13 FG and 4 FI showed directionally consistent significant associations. Among the remaining genome-wide association study loci not significant in our data, we observed directionally consistent associations in 14 of 15 FG and 9 of 10 FI loci (Penrichment = 5 × 10−4 for FG and 0.01 for FI) (Supplementary Data, Supplementary Table 2).
In addition, we identified a novel significant single variant association between rs184042322 and FI (MAF 1.2%, P = 1.2 × 10−7), a coding variant in AKT2 (V-AKT Murine Thymoma Viral Oncogene Homolog 2) where amino acid Pro50 is substituted with a threonine (NP_001617.1:p.Pro50Thr) (Fig. 1, Supplementary Fig. 1). The same allele drove a significant FI signal for AKT2 in gene-based analysis (P = 6.1 × 10−7), in which we discovered two additional significant gene-based associations between GIMAP8 and FG (PPTV = 2.3 × 10−6) and between NDUFAF1 and FI (PPTV+NSBroad = 9.2 × 10−7) (Supplementary Fig. 2, Supplementary Table 2D).
Figure 1.
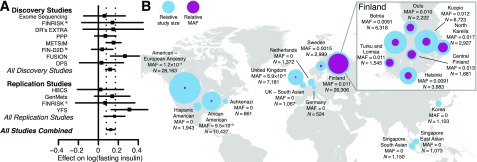
AKT2 Pro50Thr association with FI levels. A: For each study, the square represents the estimate of the additive genetic effect for the association of the AKT2 Pro50Thr allele with log-transformed FI levels and the horizontal line gives the corresponding 95% CI of the estimate. Inverse-variance meta-analyses were performed for all discovery studies, all replication studies, and all studies combined. The vertical dashed lines indicate the 95% CI for the estimate obtained in the meta-analysis of all studies combined. DPS, The Finnish Diabetes Prevention Study; DR’s EXTRA, Dose-Responses to Exercise Training study; FIN-D2D, National Diabetes Prevention Programme in Finland; PPP, Prevalence, Prediction and Prevention of Diabetes (PPP)-Botnia study. B: MAF for each available region and ancestry. Across countries of the world, the MAF ranges from 0 to 1.1%. The relative sample sizes (N) for each region/ancestry are displayed with the blue circles and the relative MAFs of AKT2 Pro50Thr are displayed with the purple circles, with the size of the circles showing comparative differences. Within Finland (inset), where the MAF ranges from 0.9 to 1.7%, birthplace and study center data were used to show the allele distribution across the country. aFINRISK 2007, bFIN-D2D 2007, cFINRISK 1997 and 2002.
In an effort to replicate the single variant association of AKT2 Pro50Thr with FI, we aggregated the allele frequency estimates of AKT2 Pro50Thr in our data with data from the CHARGE Consortium and the four Finnish studies. In ExAC, rs184042322 is multiallelic (p.Pro50Thr and p.Pro50Ala) but Pro50Ala is observed only twice in the Latino population sample and not seen in our exome sequencing data, which includes 1,021 individuals of Hispanic ancestry. AKT2 Pro50Thr was observed at a much higher frequency in Finnish individuals (MAF 1.1%) than other non-Finnish pooled European (MAF 0.02%), African American (MAF 0.01%), Asian (MAF <0.01%), or Hispanic (MAF <0.01%) individuals (Fig. 1). We replicated the association between FI and AKT2 Pro50Thr by meta-analysis of the association in the four Finnish studies (P = 5.4 × 10−4, N = 5,747) with the discovery studies (Pcombined = 9.98 × 10−10, N = 25,316). We observed no evidence of effect-size heterogeneity between studies (Pheterogeneity = 0.76). The minor T allele was associated with a 12% (95% CI 7–18) increase in FI levels in the discovery and replication studies, a per allele effect of 10.4 pmol/L (95% CI 6.6–14.3).
The serine/threonine protein kinases AKT1, AKT2, and AKT3 are conserved across all vertebrates (Fig. 2). Pro50 and the seven preceding residues in the pleckstrin homology (PH) domain appear to be specific for the AKT2 isoform. Population genetic studies show a strong intolerance to missense and loss-of-function (LoF) variation in AKT2 (Supplementary Data, Supplementary Fig. 3, Supplementary Fig. 4, Supplementary Table 3). Notably, in ExAC data, AKT2 contains fewer missense variants than expected (the missense constraint metric, Z = 3.5, is in the 94th percentile of all genes) and extreme constraint against LoF variation (estimated probability of being LoF intolerant = 1).
Figure 2.
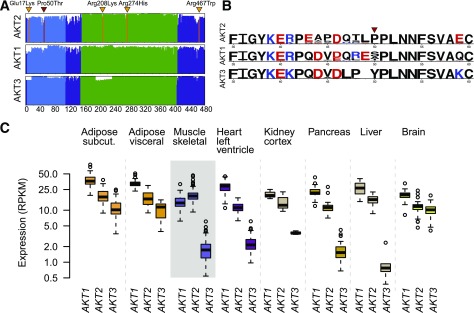
Expression and conservation properties. A: Amino acid alignment and conservation of the three AKT proteins in vertebrates. The x-axis gives the amino acid position and the height of the lines shows the conservation score across 100 vertebrate genome alignments. The functional domains are the PH domain (blue) and the kinase domain (green). The position of AKT2 Pro50Thr is shown in red and the locations of the other AKT2 disease-causing mutations (37–40) are shown in orange: Glu17Lys, Arg208Lys, Arg274His, and Arg467Trp. B: WebLogo plots of amino acids 35–60 are shown for AKT2, AKT1, and AKT3, contrasting the homology of the three isoforms. The height of letters gives the relative frequency of different amino acids across the 100 vertebrate species, with the colors showing amino acids with similar charge. C: Expression of AKT1, AKT2, and AKT3 in eight insulin-sensitive tissues using RNA sequencing data from the GTEx Consortium. subcut., subcutaneous.
AKT2 is a primary transducer of PI3K signaling downstream of the insulin receptor and is responsible for mediating the physiological effects of insulin in tissues including liver, skeletal muscle, and adipose. Akt2 null mice are characterized by hyperglycemia and hyperinsulinemia, and some develop diabetes (35,36). In humans, highly penetrant rare alleles in AKT2 cause familial partial lipodystrophy and hypoinsulinemic hypoglycemia with hemihypertrophy (Glu17Lys) (37,38) and a syndrome featuring severe insulin resistance, hyperinsulinemia, and diabetes (Arg274His) (39). Additional rare alleles have been observed in individuals with severe insulin resistance (Arg208Lys and Arg467Trp), but no variant has been associated with glycemic traits at the population level (40).
Given the spectrum of diseases and traits associated with AKT2 (41), we hypothesized that AKT2 Pro50Thr would be associated with features of metabolic syndrome or lipodystrophy. In quantitative trait analysis in the initial discovery and replication cohorts, we did observe a constellation of features indicative of a milder lipodystrophy-like phenotype associated with the rare allele: associations with increased 2-h insulin values (effect = 0.2 SD of log-transformed 2-h insulin, 95% CI 0.1–0.4, P = 7.9 × 10−8, N = 14,150), lower insulin sensitivity (effect = −0.3 SD of the log-transformed Matsuda index, 95% CI −0.5 to −0.2, P = 1.2 × 10−6, N = 8,566), and increased risk of type 2 diabetes (OR 1.05, 95% CI 1.0–1.1, P = 8.1 × 10−5, 9,783 individuals with type 2 diabetes and 22,662 without diabetes), with no effects on FG, postprandial glucose, or fasting lipid levels (P ≥ 0.01) (Supplementary Table 4). In the T2D-GENES exome sequencing data where FG and FI levels were available in individuals with diabetes, we observed one individual who was homozygous for the P50T allele with FI and FG levels in the 99.8th and 98.8th percentiles, respectively. There was a significant difference in trait distributions by P50T genotype (FI, P = 0.002; FG, P = 0.02) (Supplementary Fig. 5, Supplementary Table 4). Next, we used electronic health records available in the Finnish METSIM and FINRISK cohorts to characterize the impact of AKT2 Pro50Thr on disease risk. We found no evidence for association with any cancer, polycystic ovary disease, or acanthosis nigricans (Supplementary Table 5); however, these tests are underpowered due to the low number of cases and potential for misclassification. Nor did we find evidence for enrichment of low-frequency associations in any AKT2-related pathways or genes implicated in monogenic forms of glycemic disease (Supplementary Data, Supplementary Table 6, Supplementary Table 7, Supplementary Fig. 6, Supplementary Fig. 7).
In Vitro Functional Studies
To understand the functional consequences of the AKT2 Pro50Thr variant on the protein, we investigated protein expression, activation, kinase activity, and downstream effector phosphorylation.
First, we used in silico classifiers that predict potential functional consequences of alleles on protein function. Two of the five classifiers predicted AKT2 Pro50Thr to be deleterious (Supplementary Table 3). Second, we used 3-D models of AKT2 viewed in the PyMol software, which predicted that the Pro50Thr variant causes a change in the conformations of the lipid binding pleckstrin homology (PH) domain (Fig. 3, Supplementary Fig. 8). We hypothesized that the variant protein is inefficiently recruited to the plasma membrane thereby impacting AKT2 phosphorylation and downstream activity.
Figure 3.
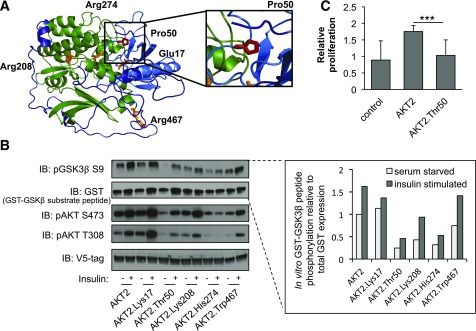
Functional properties of AKT2-Thr50. A: Predicted protein structure of AKT2. Domain and variants are highlighted as in Fig. 2A. The relative spatial positioning of the AKT2-Pro50 residue is magnified within the inset. B: HeLa cells were infected with lentiviral V5-AKT2, V5-AKT2-Lys17, V5-AKT2-Thr50, V5-AKT2-Lys208, V5-AKT2-His274, or V5-AKT2-Trp467; starved for 18 h (white bar); and stimulated for 20 min with 100 nmol/L insulin (gray bar). V5-tagged AKT2 was isolated from cell lysates with anti-V5 agarose beads and incubated with GSK3β-GST peptide in an in vitro kinase assay. Quantification of phosphorylated substrate peptide (pGSK3β) relative to total peptide (GST-GSK3β) is shown at the inset. Immunoblots and quantification shown are representative of three independent replicates. Linear model statistical analyses across all three independent replicates are available in Supplementary Fig. 9. The in vitro kinase was immunoblotted (IB) with the indicated antibodies. C: HuH7 cells were infected with lentiviral V5-AKT2, V5-AKT2-Thr50, or control pLX304. At 72 h, relative cellular proliferation was determined with WST-1 assay of HuH7 cells. Error bars represent SD. ***P = 4.5 × 10−5.
To assess the molecular and cellular consequence of the AKT2 Thr50 variant on protein function, we performed a comparative analysis of AKT2-Thr50 with inactivating and activating alleles implicated in monogenic disorders of insulin signaling. Analysis of AKT2-Thr50 expression showed that while AKT2 protein levels remained unchanged, there was a partial loss of AKT2-Thr50 phosphorylation at its activation sites (Thr308 and Ser473) in HeLa cells, suggesting impaired AKT2 signaling (Fig. 3, Supplementary Fig. 9). Similar effects were observed in human liver–derived HuH7 cells (Supplementary Fig. 10). AKT2-Thr50 also showed a reduced ability to phosphorylate its downstream target GSK3β. These defects in AKT2-Thr50 activity were confirmed through an in vitro kinase assay (P < 0.01) (Fig. 3). AKT2-Thr50 showed a similar decrease in kinase function to the lipodystrophy-causing AKT2-His274 variant. Using a 4-h time course analysis of AKT2 activity, we verified a reduction in both maximally phosphorylated Thr308 and Ser473 in AKT2-Thr50 (Supplementary Fig. 11). To understand how this loss of activity could manifest as a defect in a known cellular function of AKT2 (42), we determined the impact of AKT2-Thr50 on cell proliferation in HuH7 cells. While the addition of AKT2 stimulated hepatocyte proliferation, the response to AKT2-Thr50 was reduced (effect = −1.2, P < 1.0 × 10−3) (Fig. 3C, Supplementary Fig. 12).
Gene Expression Studies
We queried RNA sequencing data from the GTEx Project and found that, in agreement with previous studies (43), AKT2 is highly and ubiquitously expressed across all tissues (44 tissue types, 3–156 individuals/tissue). Notably the AKT2 Pro50Thr containing exon is expressed in all tissues and individuals (Supplementary Fig. 13), suggesting that the PH domain is important to AKT2 function (44). Of the three AKT homologs, AKT2 had 1.4-fold higher expression in skeletal muscle than AKT1 (P = 1.5 × 10−19) and 11-fold higher expression than AKT3 (P = 7.8 × 10−91). Skeletal muscle was the only tested tissue displaying such pronounced AKT2 enrichment (Fig. 2, Supplementary Data, Supplementary Fig. 14, Supplementary Table 8).
Motivated by the age-related loss of adipose tissue in Akt2 null mice (35,36) and the growth and lipodystrophy phenotypes in carriers of fully penetrant alleles (37–40), we examined associations of expression levels of AKT2 with BMI, FI, and age in the three adipose tissue data sets (Supplementary Table 9). We found an association between lower BMI levels and higher AKT2 expression in two cohorts (EuroBATS: effect = −0.07 SD, P = 6.1 × 10−28; METSIM: effect = −0.06 SD, P = 8.1 × 10−8) and also observed that higher AKT2 expression was associated with lower log-transformed FI (EuroBATS: effect = −0.04 SD, P = 1.1 × 10−3; METSIM: effect = −0.4 SD, P = 3.3 × 10−11). We next tested for gene eQTL and found an eQTL in the 5′UTR of AKT2 (rs11880261, MAF 35%, r2 = 0.002, D’ = 0.47 in the Finnish 1000 Genomes samples) with the common allele associated with lower AKT2 expression levels (METSIM: P = 6.9 × 10−14; EuroBATS: P = 2.3 × 10−8; GTEx: P = 0.08) (Supplementary Fig. 15). No association was detected between rs11880261 and FI levels, suggesting that the common variant eQTL does not drive the initial FI association (Supplementary Data, Supplementary Table 10).
Discussion
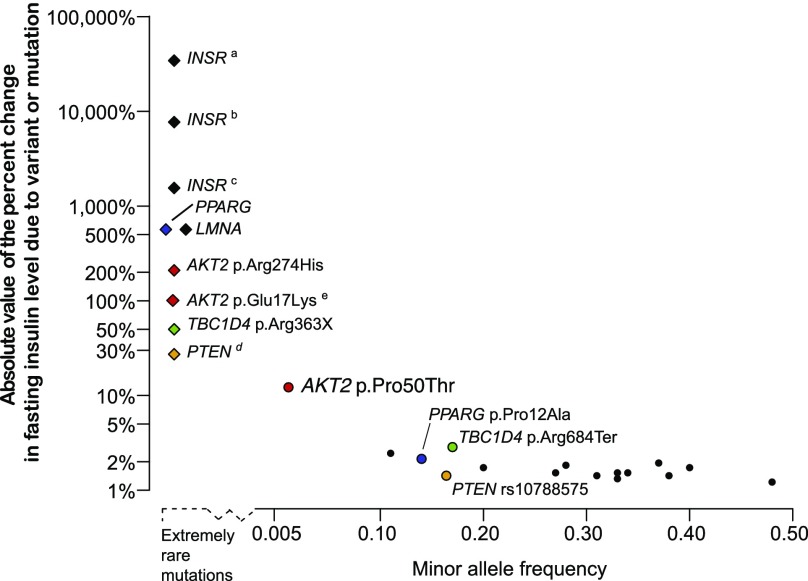
Meta-analyses of exome sequence and array genotyping data in up to 38,339 normoglycemic individuals enabled the discovery, characterization, and functional validation of a FI association with a low-frequency AKT2 coding variant. Rare, penetrant variants in genes encoding components of the insulin-signaling pathway, including AKT2, cause monogenic but heterogeneous glycemic disorders (45). In parallel, common alleles in or near many of these genes impact FI levels—the AKT2 Pro50Thr association shows an effect 5–10 times larger than those of these previous published associations (3). This discovery expands both the known genetic architecture of glucose homeostasis and the allelic spectrum for AKT2 coding variants associated with glucose homeostasis into the low-frequency range and highlights the effects of both locus and allelic heterogeneity (Fig. 4).
Figure 4.
Genetic architecture of rare, low-frequency, and common variants associated with FI levels. In this plot, the absolute values of the percent change in FI level due to rare monogenic mutations (diamonds) and common genetic variants (circles) are plotted against the MAF of the variant. The extremely rare monogenic mutations (above the dashed line to the left of the x-axis) were observed in 2–18 individuals (3,37–40,48,53,54), with the height of the point indicating the percent change in FI levels of mutation carriers from 40 pmol/L, an estimate of population mean FI level. Mutations in INSR and AKT2 p.Arg274His cause compensatory hyperinsulinemia, individuals with TBC1D4 p.Arg363Ter show normal FI levels but postprandial hyperinsulinemia, and mutations in PTEN cause enhanced insulin sensitivity providing protection against type 2 diabetes. For common variants, the percent change in FI levels per insulin-increasing allele is plotted above the solid horizontal axis. These observations are from sequencing (6) and array-based genome-wide association studies (3). For several genes, the effects from rare mutations can be compared with the effects of common variants in or near the gene: PPARG (blue), TBC1D4 (green), PTEN (orange), and AKT2 (red). aDonohue syndrome: biallelic LoF mutations in INSR (54). bRabson-Mendenhall syndrome: biallelic LoF mutations in INSR (54). cPostpubertal severe insulin resistance: heterozygous or homozygous LoF mutations in INSR (54). dLoF PTEN mutations cause Cowden syndrome in which carriers exhibit a lowered FI level (mean 29 pmol/L) compared with matched control subjects (3). eCarriers with the AKT2 p.Glu17Lys mutation were described with hypoinsulinemic hypoketotic hypoglycemia and hemihypertrophy with undetectable serum insulin (37,38).
Individuals of Finnish ancestry drove the AKT2 Pro50Thr association signal. This demonstrates the value of association studies in different ancestries where frequencies of rare alleles may increase due to selective pressure or stochastic changes from population bottlenecks and genetic drift. The allele associated with increased FI most likely rose to a higher frequency due to genetic drift and exists within the spectrum of rare and low-frequency variation observed in Finland, the excess of which facilitates the study of complex trait associations (46).
Although the AKT2 Pro50Thr allele shows a strong effect on all of the insulin measures and modest increased type 2 diabetes risk (OR 1.05), we see no effect on any of the glucose measures in individuals without diabetes. Due to the effects of both type 2 diabetes and its treatment on glucose homeostasis, we have not tested genetic associations of FG and FI in individuals with type 2 diabetes, although we observed an individual with diabetes homozygous for P50T with extreme FI and FG levels. The mechanism for such heterogeneous effects is unclear and detailed in vivo physiological studies are needed.
We leveraged similar findings to generate hypotheses for future work on AKT2 and downstream targets to further illuminate tissue-specific mechanisms. All reported carriers of the lipodystrophy-causing AKT2 Arg274His allele are hyperinsulinemic, and three of the four carriers have diabetes (39). These observations are similar to the ones made for TBC1D4 (which encodes a protein that acts as a substrate immediately downstream of AKT2 in the PI3K pathway). In TBC1D4, a population-specific, protein-truncating variant (Arg684Ter) is associated with increased type 2 diabetes risk (OR 10.3), increased postprandial glucose and insulin levels, and a modest decrease in FI and FG levels (6) (Fig. 4). Arg363Ter, another stop codon allele in TBC1D4, is rare (not observed in ExAC) and has been reported with a modest elevation in FI levels but extreme postprandial hyperinsulinemia and acanthosis nigricans (47). Small interfering RNA–mediated gene knockdown of AKT2 in human primary myotubes completely abolishes insulin action on glucose uptake and glycogen synthesis (48), which highlights the importance of an intact AKT2-TBC1D4 signaling pathway in the regulation of insulin sensitivity in humans. TBC1D4 is ubiquitously expressed with adipose and skeletal muscle tissue ranking among the tissues with highest expression in GTEx. TBC1D4 Arg363Ter seems to have an effect in adipocytes (47), whereas Arg684Ter falls in an exon that is exclusively expressed in skeletal and heart muscle (6,49). This is a likely cause of the TBC1D4 Arg684Ter tissue specificity, which appears to differ from the other TBC1D4 Arg363Ter variant as well as the AKT2 variants.
The phenotypes exhibited by carriers of rare, penetrant AKT2 alleles reflect differential AKT2 activation with kinetically inactivating variants, resulting in hyperinsulinemia and lipodystrophy, whereas kinetically activating variants lead to hypoglycemia (37–39). The decrease of cellular proliferation we observe demonstrates that the downstream signaling changes caused by AKT2-Thr50 are sufficient in hepatocytes to impair AKT2 function at the cellular level while maintaining varying portions of regulatory capacity. Along with the observed association with increased FI levels in human populations, these results support AKT2 Pro50Thr as a partial LoF variant. The inactivating AKT2 Pro50Thr variant contrasts with the known activating AKT2 Glu17Lys mutation and showcases bidirectional effects within the PH domain of AKT2. Although the Pro50 residue is conserved in AKT2 throughout all vertebrates, the variant lies within the PH domain that is not conserved between AKT isoforms (Fig. 2). These residues, harboring the Pro50 variant, may functionally distinguish AKT2 from AKT1 and AKT3. Although AKT isoforms are activated in the same mechanism within the PI3K pathway downstream of insulin, the Akt2−/− mouse is the only knockout of the gene family to be characterized by insulin resistance and diabetes (35,50–52). A deeper understanding of what makes the AKT2 isoform distinct could offer potential sites for therapeutic intervention and enable more targeted approaches to disease prevention.
Supplementary Material
Article Information
Acknowledgments. The authors thank the more than 44,412 volunteers who participated in this study.
Funding. The authors acknowledge the following funding sources: Academy of Finland (102318, 129293, 128315, 129330, 131593, 139635, 121584, 126925, 124282, 129378, 258753, 123885, 124243); Action on Hearing Loss (G51); Jalmari and Rauha Ahokas Foundation; American Diabetes Association (7-12-MN-02); Archimedes Foundation (3.2.1001.11-0033); Atlantic Canada Opportunities Agency; Augustinus Foundation; Becket Foundation; Alfred Benzon Foundation; Biomedical Research Council; British Heart Foundation (SP/04/002); Canada Foundation for Innovation; City of Kuopio and Social Insurance Institution of Finland (4/26/2010); Commission of the European Communities; Directorate C–Public Health (2004310); Copenhagen County; Danish Centre for Evaluation and Health Technology Assessment; Danish Council for Independent Research; Danish Heart Foundation (07-10-R61-A1754-B838-22392F); Danish Medical Research Council; Danish Pharmaceutical Association (Dansk Apotekerforening); Development Fund of the University of Tartu (SP1GVARENG); Emil Aaltonen Foundation; Estonian Research Council (IUT20-60); European Research Council Advanced Research Grant; European Commission (HEALTH-F2-2007-201681, HEALTH-F4-2007-201413, LSHM-CT-2004-005272, Marie Curie Fellowship PIEF-GA-2012-329156); European Commission FP6 (EXGENESIS); European Commission FP7 (EpiMigrant, 279143, FP7/2007-2013, 259749, 278913); European Commission Horizon 2020 (633589, 654248, 676550); European Research Council Advanced Research Grant; Finland's Slot Machine Association; Finnish Cultural Foundation; Finnish Diabetes Association; Finnish Diabetes Research Foundation; Finnish Foundation for Cardiovascular Research; Finnish Foundation for Diabetes Research; Finnish Heart Association; Finnish Medical Society; Finnish National Public Health Institute; Finska Läkaresällskapet; Folkhälsan Research Foundation; Foundation for Life and Health in Finland; German Center for Diabetes Research (DZD); German Federal Ministry of Education and Research and the State of Bavaria; German Research Center for Environmental Health; Guy's and St Thomas' NHS Foundation Trust; German Federal Ministry of Education and Research; health care centers in Vaasa, Närpes, and Korsholm; Health Insurance Foundation (Helsefonden) (2012B233); Helmholtz Zentrum München - German Research Center for Environmental Health; Helsinki University Central Hospital Research Foundation; hospital districts of Pirkanmaa, Southern Ostrobothnia, Northern Ostrobothnia, Central Finland, and Northern Savonia; Ib Henriksen Foundation; Juho Vainio Foundation; Korea Centers for Disease Control and Prevention (4845-301, 4851-302, 4851-307, KBP-2013-11, KBP-2014-68); Korea National Research Institute of Health (2012-N73002-00); Kuopio University Hospital; Li Ka Shing Foundation; Liv och Hälsa; Ludwig-Maximilians-Universität, as part of LMUinnovativ; Lundbeck Foundation; Medical Research Council (G0601261, G0900747-91070, G0601966, G0700931); Ministry of Education and Culture of Finland (627;2004-2011); Ministry of Social Affairs and Health in Finland; MRC-PHE Centre for Environment and Health; Munich Center of Health Sciences; municipal health care center and hospital in Jakobstad; Närpes Health Care Foundation; National Institute for Health Research (RP-PG-0407-10371); National Cancer Institute/SAIC-Frederick, Inc. (10XS170, 10XS171, X10S172); National Cancer Institute (K12 CA1391602); National Heart, Lung, and Blood Institute (R01 HL102830, T32 HL007055); National Human Genome Research Institute (Z01 HG000024); National Institute on Aging (PO1 AG027734, R01 AG046949, 1R01 AG042188, P30 AG038072); National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK066358, U01 DK062370, P30 DK020595, R01 DK073541, R01 DK078616, R01 DK098032, U01 DK085501, U01 DK085524, U01 DK085545, U01 DK085584, K24 DK080140, RC2 DK088389, U01 DK085526, DK072193); National Institute on Drug Abuse (R01 DA006227, R01 DA033684); National Institute of General Medical Sciences (T32 GM007753); National Institutes of Health (HHSN 268201300046C, HHSN 268201300047C, HHSN 268201300048C, HHSN 268201300049C, HHSN 268201300050C, HHSN 268201000029C, HHSN 261200800001E); National Institutes of Health Office of the Director (R01 MH101820, R01 MH090937, R01 MH090941, R01 MH101814, R01 MH090951, R01 MH101825, R01 MH090936, R01 MH101819, R01 MH090948, R01 MH101782, R01 MH101810, R01 MH101822); National Institute of Mental Health (R01 MH107666, N01 MH000028); Singapore National Medical Research Council; National Research Foundation of Korea (NRF-2012R1A2A1A03006155); Nordic Centre of Excellence in Disease Genetics; Novo Nordisk Foundation; Nuffield Department of Medicine (NDM) Prize Studentship; Ollqvist Foundation; Orion-Farmos Research Foundation; Oxford Biomedical Research Centre; Paavo Nurmi Foundation; Påhlssons Foundation; Päivikki and Sakari Sohlberg Foundation; Perklén Foundation; Samfundet Folkhälsan; Signe and Ane Gyllenberg Foundation; Sigrid Juselius Foundation; Singapore National Research Foundation; Social Insurance Institution of Finland; South-Eastern Norway Regional Health Authority (2011060); Swedish Cultural Foundation in Finland; Swedish Heart-Lung Foundation; Swedish Research Council (Linné and Strategic Research Grant); American Federation for Aging Research; The Einstein Glenn Center; The Påhlssons Foundation; the Canadian provinces of Newfoundland and Labrador, Nova Scotia, and New Brunswick; Skåne Regional Health Authority; Tekes Finnish Funding Agency for Innovation (1510/31/06); Timber Merchant Vilhelm Bang’s Foundation; Turku University Foundation; UK National Institute for Health Research (RP-PG-0407-10371); Uppsala University; Uppsala University Hospital; and Wellcome Trust (064890, 083948, 085475, 086596, 090367, 090532/Z/09/Z, 092447, 095101/Z/10/Z, 200837/Z/16/Z, 095552, 098017, 098381, 098051, 084723, 072960/2/ 03/2, 086113/Z/08/Z). Detailed acknowledgment of funding sources is provided in the Supplementary Data.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. N.G., A.Mah., N.P.B., C.Lad., J.B.-J., N.R.R., N.W.R., R.A.S., A.P.G., A.U.J., C.J.G., C.B., D.Bu., G.B., G.J., H.M.S., J.R.H., J.Mu., J.M.J., J.Tr., K.S.S., M.M., M.N., M.H., R.O., S.G., A.R.W., A.T.H., H.E.A., A.C., R.A.D., A.St., A.H.R., A.Me., A.J.F., A.-C.S., A.Kä., Y.A.K., R.A., A.Sw., T.A., B.L., B.G., B.I.F., B.-G.H., C.Me., C.G., C.Lan., D.Pa., D.Ag., D.W.B., D.H., ES.T., E.C., C.-Y.C., W.Y.L., E.M., S.P.F., F.B.H., G.At., G.W., D.E.H., H.G., H.A.K., H.O., H.A.T., T.I., J.S.K., J.Se., J.Li., J.Kra., J.E.C., C.P.J., J.E.B., J.Kri., J.H., J. Liu, J.Fa., J.C.C., J.C.L., K.R.O., K.S.C., C.-C.K., L.L.B., J.-Y.L., L.K., D.M.L., L.H., L.Mi., L.Lia., M. Loh, M.O-.M., M.W., M.M-.N., T.M., M.G., M.R., M.C.Y.N., N.D.P., N.N., L.Q., N.J.W., N.B., O.M., O.R., P.J.H., P.W.F., P.N., A.Pe., Q.Q., R.M., S.-T.T., S.Ku., S.K.M., S.P.O., S.Pup., K.St., T.M.F., T.K., T.E., F.T., B.T., T.V.V., T.Y.W., T.A.L., T.La., T.I.P., U.A., V.S.F., W.R.S., Y.S.C., A.D.M., A.S.F.D., A.L., B.I., C.N.A.P., F.S.C., C.C., E.I., F.K., G.L.S., I.Br., J.Tu., J.Ku., L.La., L.Lin., L.G., M.E.J., M.U., O.P., R.R., R.N.B., T.Tuo., T.D.S., T.H., T.J., V.S., G.I.B., J.G.W., J.B., N.J.C., R.D., K.L.M., M.La., C.L.H., A.P.M., M.B., D.Al., and M.I.M. contributed to sample collection and phenotyping. T.Tuk., A.V., A.A.B., Y.W., A.Pa., A.J., J.G.E., O.T.R., S.Ko., T.Le., J.W., A.Y.C., R.A.S., M.O.G., V.S., J.D., S.R., J.C.F., J.B.M., M.La., and K.L.M. contributed to replication and expression studies. X.S., N.G., A.Mah., C.F., N.P.B., C.Ha., C.Lad., J.B-.J., N.R.R., N.W.R., A.P.G., A.U.J., C.J.G., C.B., D.Bu., G.B., G.J., H.M.S., J.R.H., J.Mu., J.M.J., J.Tr., K.E.S., K.S.S., M.M., M.N., M.H., R.O., P.S.C., S.G., M.O.C., M.D., E.B., Y.F., M.H.d.A., K.Sh., R.P., T.Fe., T.S., T.W., T.M.S., K.St., T.M., P.D., M.B., and M.I.M. contributed to data production (sequencing and genotyping). M.A.R., K.J.G., H.M.K., G.J., B.M.N., G.G., J.Ma., J.Ca., J.D.S., J.I.G., and S.Pur. contributed to variant calling and panel generation. A.Man., H.M.H., J.G., X.S., T.Tuk., P.Fo., N.G., M.A.R., A.Mah., A.E.L., P.C., T.H.P., J.Fl., C.F., E.R.G., K.J.G., H.K.I., T.M.T., A.Ku., N.P.B., C.Ha., C.Lad., H.M.K., J.B-.J., Y.C., J.R.B.P., L.J.S., C. Ma, M.v.d.B., L.Mo., N.R.R., R.D.P., T.W.B., T.G., N.W.R., A.P.G., A.U.J., C.J.G., C.B., D.Bu., G.B., G.J., H.M.S., J.R.H., J.Mu., J.M.J., J.Tr., K.S.S., M.M., M.N., M.H., R.O., S.G., J.B.M., A.P.M., M.B., M.I.M., and C.M.L. contributed to statistical analysis. J.G., S.B.R.J., and A.L.G. contributed to functional studies. A.Man., H.M.H., J.G., X.S., T.Tuk., P.Fo., J.C.F., M.B., M.I.M., A.L.G., and C.M.L. wrote the manuscript. X.S., L.J.S., A.T.H., H.E.A., R.A.D., B.G., ES.T., G.At., J.S.K., C.P.J., J.C.C., K.S.C., J.-Y.L., D.M.L., T.M., T.M.F., T.I.P., Y.S.C., C. Hu, G.R.C., D.Bh., P.J.D., D.Pr., E.Z., I.Ba., J.Sc., J.Ch., G.M., M.J.D., M.Sa., N.T., P.E., P.Fr., R.C.W.M., R.S., S.B.E., Y.Y.T., T.P., T.Fi., W.J., R.M.W., J.Tu., L.G., G.I.B., G.Ab., J.G.W., J.B., M.Se., N.J.C., R.D., J.D., I.P., J.C.F., K.L.M., M.La., J.B.M., C.L.H., A.P.M., M.B., D.Al., M.I.M., A.L.G., and C.M.L. contributed to study design. G.I.B., G.Ab., J.G.W., J.B., M.Se., N.J.C., R.D., J.C.F., K.L.M., J.B.M., C.L.H., A.P.M., M.B., D.Al., M.I.M., A.L.G., and C.M.L. contributed to study supervision. C.M.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-1329/-/DC1.
References
- 1.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–846 [DOI] [PubMed] [Google Scholar]
- 2.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med 1994;11:286–292 [DOI] [PubMed] [Google Scholar]
- 3.Manning AK, Hivert MF, Scott RA, et al.; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium; Multiple Tissue Human Expression Resource (MUTHER) Consortium . A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet 2012;44:659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estrada K, Aukrust I, Bjørkhaug L, et al.; SIGMA Type 2 Diabetes Consortium . Association of a low-frequency variant in HNF1A with type 2 diabetes in a Latino population [published correction appears in JAMA 2014;312:1932]. JAMA 2014;311:2305–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams AL, Jacobs SB, Moreno-Macías H, et al.; SIGMA Type 2 Diabetes Consortium . Sequence variants in SLC16A11 are a common risk factor for type 2 diabetes in Mexico. Nature 2014;506:97–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moltke I, Grarup N, Jørgensen ME, et al. A common Greenlandic TBC1D4 variant confers muscle insulin resistance and type 2 diabetes. Nature 2014;512:190–193 [DOI] [PubMed] [Google Scholar]
- 7.Fuchsberger C, Flannick J, Teslovich TM, et al. The genetic architecture of type 2 diabetes. Nature 2016;536:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raitakari OT, Juonala M, Rönnemaa T, et al. Cohort profile: the cardiovascular risk in Young Finns Study. Int J Epidemiol 2008;37:1220–1226 [DOI] [PubMed] [Google Scholar]
- 9.Eriksson JG. Epidemiology, genes and the environment: lessons learned from the Helsinki Birth Cohort Study. J Intern Med 2007;261:418–425 [DOI] [PubMed] [Google Scholar]
- 10.Perttilä J, Merikanto K, Naukkarinen J, et al. OSBPL10, a novel candidate gene for high triglyceride trait in dyslipidemic Finnish subjects, regulates cellular lipid metabolism. J Mol Med (Berl) 2009;87:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vartiainen E, Laatikainen T, Peltonen M, et al. Thirty-five-year trends in cardiovascular risk factors in Finland. Int J Epidemiol 2010;39:504–518 [DOI] [PubMed] [Google Scholar]
- 12.Grove ML, Yu B, Cochran BJ, et al. Best practices and joint calling of the HumanExome BeadChip: the CHARGE Consortium. PLoS One 2013;8:e68095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 14.Peloso GM, Auer PL, Bis JC, et al.; NHLBI GO Exome Sequencing Project . Association of low-frequency and rare coding-sequence variants with blood lipids and coronary heart disease in 56,000 whites and blacks. Am J Hum Genet 2014;94:223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med 2005;24:2911–2935 [DOI] [PubMed] [Google Scholar]
- 16.Kang HM, Sul JH, Service SK, et al. Variance component model to account for sample structure in genome-wide association studies. Nat Genet 2010;42:348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 2010;26:2190–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahajan A, Sim X, Ng HJ, et al.; T2D-GENES Consortium, GoT2D Consortium . Identification and functional characterization of G6PC2 coding variants influencing glycemic traits define an effector transcript at the G6PC2-ABCB11 locus. PLoS Genet 2015;11:e1004876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu MC, Lee S, Cai T, Li Y, Boehnke M, Lin X. Rare-variant association testing for sequencing data with the sequence kernel association test. Am J Hum Genet 2011;89:82–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu DJ, Peloso GM, Zhan X, et al. Meta-analysis of gene-level tests for rare variant association. Nat Genet 2014;46:200–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S, Teslovich TM, Boehnke M, Lin X. General framework for meta-analysis of rare variants in sequencing association studies. Am J Hum Genet 2013;93:42–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet 2007;39:906–913 [DOI] [PubMed] [Google Scholar]
- 23.Exome Aggregation Consortium. ExAC Browser. Available from http://exac.broadinstitute.org/. Accessed 29 January 2016
- 24.Karolchik D, Barber GP, Casper J, et al. The UCSC Genome Browser database: 2014 update. Nucleic Acids Res 2014;42:D764–D770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valdar WS. Scoring residue conservation. Proteins 2002;48:227–241 [DOI] [PubMed] [Google Scholar]
- 26.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res 2004;14:1188–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roche DB, Buenavista MT, Tetchner SJ, McGuffin LJ. The IntFOLD server: an integrated web resource for protein fold recognition, 3D model quality assessment, intrinsic disorder prediction, domain prediction and ligand binding site prediction. Nucleic Acids Res 2011;39:W171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The PyMOL Molecular Graphics System, version 1.3r1. Cambridge, MA, Schrödinger, LLC, 2010
- 29.GTEx Consortium Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 2015;348:648–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buil A, Brown AA, Lappalainen T, et al. Gene-gene and gene-environment interactions detected by transcriptome sequence analysis in twins. Nat Genet 2015;47:88–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown AA, Buil A, Viñuela A, et al. Genetic interactions affecting human gene expression identified by variance association mapping. eLife 2014;3:e01381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Civelek M, Wu Y, Pan C, et al. Genetic regulation of adipose gene expression and cardio-metabolic traits. Am J Hum Genet 2017;100:428–443 [DOI] [PMC free article] [PubMed]
- 33.Falchi M, Wilson SG, Paximadas D, Swaminathan R, Spector TD. Quantitative linkage analysis for pancreatic B-cell function and insulin resistance in a large twin cohort. Diabetes 2008;57:1120–1124 [DOI] [PubMed] [Google Scholar]
- 34.Stancáková A, Javorský M, Kuulasmaa T, Haffner SM, Kuusisto J, Laakso M. Changes in insulin sensitivity and insulin release in relation to glycemia and glucose tolerance in 6,414 Finnish men. Diabetes 2009;58:1212–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho H, Mu J, Kim JK, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 2001;292:1728–1731 [DOI] [PubMed] [Google Scholar]
- 36.Garofalo RS, Orena SJ, Rafidi K, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest 2003;112:197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hussain K, Challis B, Rocha N, et al. An activating mutation of AKT2 and human hypoglycemia. Science 2011;334:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arya VB, Flanagan SE, Schober E, Rami-Merhar B, Ellard S, Hussain K. Activating AKT2 mutation: hypoinsulinemic hypoketotic hypoglycemia. J Clin Endocrinol Metab 2014;99:391–394 [DOI] [PubMed] [Google Scholar]
- 39.George S, Rochford JJ, Wolfrum C, et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science 2004;304:1325–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan K, Kimber WA, Luan J, et al. Analysis of genetic variation in Akt2/PKB-beta in severe insulin resistance, lipodystrophy, type 2 diabetes, and related metabolic phenotypes. Diabetes 2007;56:714–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parikh C, Janakiraman V, Wu WI, et al. Disruption of PH-kinase domain interactions leads to oncogenic activation of AKT in human cancers. Proc Natl Acad Sci U S A 2012;109:19368–19373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci 2001;114:2903–2910 [DOI] [PubMed] [Google Scholar]
- 43.Zinda MJ, Johnson MA, Paul JD, et al. AKT-1, -2, and -3 are expressed in both normal and tumor tissues of the lung, breast, prostate, and colon. Clin Cancer Res 2001;7:2475–2479 [PubMed] [Google Scholar]
- 44.Peng XD, Xu PZ, Chen ML, et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev 2003;17:1352–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Rahilly S. Human genetics illuminates the paths to metabolic disease. Nature 2009;462:307–314 [DOI] [PubMed] [Google Scholar]
- 46.Lim ET, Würtz P, Havulinna AS, et al.; Sequencing Initiative Suomi (SISu) Project . Distribution and medical impact of loss-of-function variants in the Finnish founder population. PLoS Genet 2014;10:e1004494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dash S, Sano H, Rochford JJ, et al. A truncation mutation in TBC1D4 in a family with acanthosis nigricans and postprandial hyperinsulinemia. Proc Natl Acad Sci U S A 2009;106:9350–9355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bouzakri K, Zachrisson A, Al-Khalili L, et al. siRNA-based gene silencing reveals specialized roles of IRS-1/Akt2 and IRS-2/Akt1 in glucose and lipid metabolism in human skeletal muscle. Cell Metab 2006;4:89–96 [DOI] [PubMed] [Google Scholar]
- 49.Baus D, Heermeier K, De Hoop M, et al. Identification of a novel AS160 splice variant that regulates GLUT4 translocation and glucose-uptake in rat muscle cells. Cell Signal 2008;20:2237–2246 [DOI] [PubMed] [Google Scholar]
- 50.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem 2001;276:38349–38352 [DOI] [PubMed] [Google Scholar]
- 51.Toker A, Marmiroli S. Signaling specificity in the Akt pathway in biology and disease. Adv Biol Regul 2014;55:28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tschopp O, Yang ZZ, Brodbeck D, et al. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development 2005;132:2943–2954 [DOI] [PubMed] [Google Scholar]
- 53.Savage DB, Tan GD, Acerini CL, et al. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma. Diabetes 2003;52:910–917 [DOI] [PubMed] [Google Scholar]
- 54.Semple RK, Sleigh A, Murgatroyd PR, et al. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest 2009;119:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.