Abstract
Chrysanthemum indicum Linné extract (CIL) is used in herbal medicine in East Asia. In the present study, gerbils were orally pre-treated with CIL, and changes of antioxidant enzymes including superoxide dismutase (SOD) 1 and SOD2, catalase (CAT) and glutathione peroxidase (GPX) in the hippocampal CA1 region following 5 min of transient cerebral ischemia were investigated and the neuroprotective effect of CIL in the ischemic CA1 region was examined. SOD1, SOD2, CAT and GPX immunoreactivities were observed in the pyramidal cells of the CA1 region and their immunoreactivities were gradually decreased following ischemia-reperfusion and barely detectable at 5 days post-ischemia. CIL pre-treatment significantly increased immunoreactivities of SOD1, CAT and GPX, but not SOD2, in the CA1 pyramidal cells of the sham-operated animals. In addition, SOD1, SOD2, CAT and GPX immunoreactivities in the CA1 pyramidal cells were significantly higher compared with the ischemia-operated animals. Furthermore, it was identified that pre-treatment with CIL protected the CA1 pyramidal cells in the CA1 region using neuronal nuclei immunohistochemistry and Fluoro-Jade B histofluorescence staining; the protected CA1 pyramidal cells were 67.5% compared with the sham-operated animals. In conclusion, oral CIL pre-treatment increased endogenous antioxidant enzymes in CA1 pyramidal cells in the gerbil hippocampus and protected the cells from transient cerebral ischemic insult. This finding suggested that CIL is promising for the prevention of ischemia-induced neuronal damage.
Keywords: transient cerebral ischemia, Chrysanthemum indicum Linné, neuroprotection, hippocampus, pyramidal cells, antioxidant enzymes
Introduction
Ischemic stroke occurs due to the temporary or permanent blockage of blood circulation in the brain in several circumstances, including brain ischemia, cardiac arrest and cardiovascular surgery (1–3). Transient cerebral ischemia, which is a major cause of ischemic stroke, leads to selective neuronal damage/death in vulnerable brain areas, including the cerebral cortex, the striatum and the hippocampus (3,4). In particular, the most vulnerable area to transient cerebral ischemia is the CA1 region of the hippocampus, in which pyramidal neurons of the stratum pyramidale of the CA1 region die several days following ischemia-reperfusion injury (5,6).
One of mechanisms regarding neuronal damage/death induced by cerebral ischemia is oxidative stress, which is associated with the excessive production of reactive oxygen species (ROS) (7,8). The accumulation of ROS in ischemic conditions induces DNA damage, lipid peroxidation and changes in cellular proteins (8,9). ROS is converted into nontoxic compounds by enzymatic antioxidants including superoxide dismutases (SODs), catalase (CAT) and glutathione peroxidase (GPX); known antioxidant enzymes (8,10). Various antioxidants, including antioxidant enzymes, have been recognized as beneficial in therapies for neurologic diseases (11,12).
Many studies on neuroprotection by plant extracts have been reported using animal models of cerebral ischemic insults (13,14). Chrysanthemum indicum Linné (Compositae; CIL) is a traditional herb used for medicines in East Asia. It has been used for the treatment of immune-related disorders, hypertension, infectious diseases and respiratory illness (15,16). The major components of CIL are bornyl acetate (10.00–21.33%), borneol (8.34–18.34%), camphor (7.75–23.52%) and germacrene D (1.08–12.67%). Significant minor components of CIL include α-terpineol (1.28–3.32%), terpinen-4-ol (0.70–1.59%) and caryophyllene oxide (0.13–2.73%). However, 1,8-cineole is present at 30.41% in fresh flower oil and only 0.12–0.61% in the oil of air-dried and processed flowers (17,18). CIL exhibits anti-bacterial, anti-viral, antioxidant, anti-inflammatory and immunomodulatory functions (17,19). To the best of the authors' knowledge, few studies regarding neuroprotective effects of CIL and its antioxidant mechanism in brain ischemic insults have been published; therefore, the present study investigated the neuroprotective effect of CIL and whether endogenous antioxidant enzymes, including SOD1, SOD2, CAT and GPX, were associated with the neuroprotection in the hippocampus of the gerbil, a good animal model of transient cerebral ischemia (20,21).
Materials and methods
Preparation of extract from CIL
CIL was collected by Professor Jong Dai Kim in Kangwon Province (South Korea), in October 2013 and maintained in a deep freezer (−70°C). The CIL was extracted with 70% ethanol at 70°C for 4 h, and extraction was repeated three times. The extract was filtered through Whatman filter paper (no. 2), concentrated with a vacuum evaporator, and completely dried with a freeze-drier. The extraction yield was 14.5%.
Groups of experimental animals
Male Mongolian gerbils (Meriones unguiculatus; weight, 65–75 g; age, 6 months) were obtained from the Experimental Animal Center, Kangwon National University, Chuncheon, South Korea. The animals were housed in standard conditions under adequate temperature (23°C) and humidity (60%) control with a 12-h:12-h light:dark cycle, and were provided with free access to food and water. All the experimental protocols were approved (approval no. KW-130424-1) by the Institutional Animal Care and Use Committee at Kangwon University and adhered to guidelines that are in compliance with the current international laws and policies (Guide for the Care and Use of Laboratory Animals, The National Academies Press, 8th edition, 2011).
The experimental animals were divided into four groups (n=7 at each time point in each groups): i) Vehicle-sham-group, which was treated with vehicle and underwent no ischemia; ii) CIL-sham-group, which was treated with CIL and underwent no ischemia; iii) vehicle-ischemia-group, which was treated with vehicle and underwent ischemia surgery; and iv) CIL-ischemia-group, which was treated with CIL and underwent ischemia surgery.
Administration with CIL
CIL extract was dissolved in saline and administrated orally using a feeding needle once a day for seven days prior to ischemia surgery, according to previously published procedure (22). The preliminary tests were carried out with 25, 50, 100 and 200 mg/kg CIL. There were no neuroprotective effects in doses of 25, 50 and 100 mg/kg, but protective effects were demonstrated in animals treated with 200 mg/kg. Thus, 200 mg/kg was selected. The last treatment was at 30 min prior to the surgery to maintain the level of CIL in animals prior to and following surgery.
Induction of transient cerebral ischemia
As previously described (23), the experimental animals were anesthetized with a mixture of ~2.5% isoflurane (Baxtor Healthcare Corp., Deerfield, IL, USA) in 33% O2 and 67% NO2. Bilateral common carotid arteries were isolated and occluded for 5 min using non-traumatic aneurysm clips. Rectal temperature was controlled under normothermic (37±0.5°C) conditions during the surgery with a rectal temperature probe (TR-100; Fine Science Tools, Inc., Foster City, CA, USA).
Tissue preparation for histology
As previously described (24), gerbils (n=7 at each time point in each group) were anesthetized with pentobarbital sodium at the designated times and perfused transcardially with 0.1 M phosphate-buffered saline (pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate-buffer (PB; pH 7.4). The brains were removed and postfixed in the same fixative for 6 h, and the brain tissues were sectioned on a cryostat (Leica Microsystems GmbH, Wetzlar, Germany) into 30 µm coronal sections.
Immunohistochemistry
Immunohistochemistry was performed according to the previously published procedure (24). In short, the sections were incubated with diluted mouse anti-neuronal nuclei (NeuN; 1:800; cat. no. MAB377), sheep anti-copper, zinc-superoxide dismutase (SOD1; 1:1,000; cat. no. 574597) and sheep anti-mangan-superoxide dismutase (SOD2; 1:1,000; cat. no. 574596; all from EMD Millipore, Billerica, MA, USA), rabbit anti-catalase (CAT; 1:500; cat. no. ab52477) and sheep anti-glutathione peroxidase (GPX; 1:1,000; cat. no. ab21966; both from Abcam, Cambridge, MA, UK). Thereafter the tissues were exposed to biotinylated horse anti-mouse (1:250; cat. no. BA-2000), rabbit anti-sheep (1:250; cat. no. BA-6000) and goat anti-rabbit immunoglobulin (Ig)G (1:250; cat. no. BA-1000) and streptavidin peroxidase complex (1:200, all from Vector Laboratories, Burlingame, CA, USA) and were visualized with 3,3′-diaminobenzidine (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Fluoro-Jade B histofluorescence staining
Fluoro-Jade B (F-J B) histofluorescence staining was conducted according to a published procedure (25) in order to examine neuronal death. In brief, the sections were immersed in a solution containing 1% sodium hydroxide, transferred to a solution of 0.06% potassium permanganate and transferred to a 0.0004% F-J B (Histo-Chem Inc., Jefferson, AR, USA) staining solution. The stained sections were observed using an epifluorescent microscope (Zeiss AG, Oberkochen, Germany) with a blue (450–490 nm) excitation source and a barrier filter.
Data analysis
Data were analyzed according to published procedure (26). Briefly, to quantitatively analyze immunoreactivities of antioxidant enzymes, the immunoreactivity of SOD1, SOD2, CAT and GPX-immunoreactive structures was evaluated on the basis of optical density (OD), which was obtained following the transformation of the mean gray level using the formula: OD = log (256/mean gray level). A portion of the OD of an image file was calibrated in Adobe Photoshop 8.0 (Adobe Systems, Inc., San Jose, CA, USA) and then analyzed as a percentage, with the sham-operated-group designated as 100%, in ImageJ version 1.59 (National Institutes of Health, Bethesda, MD, USA). For cell counting, NeuN- and F-J B-positive cells were imaged from the stratum pyramidale through an AxioM1 light microscope (Zeiss AG) equipped with a digital camera (Axiocam; Zeiss AG) connected to a PC monitor. The mean number of NeuN- and F-J B-positive cells was counted in a 200×200 µm square applied approximately at the center of the CA1 region. Cell counts were obtained by averaging the total cell numbers from each animal per group and analyzing them as a percentage, with the vehicle-sham-group designated as 100%.
Statistical analysis
The data was presented as mean ± standard error of mean of the means among the groups and were statistically analyzed by analysis of variance with a post hoc Bonferroni's multiple comparisons test, in order to present differences among experimental groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Antioxidant immunoreactivities
SOD1 immunoreactivity
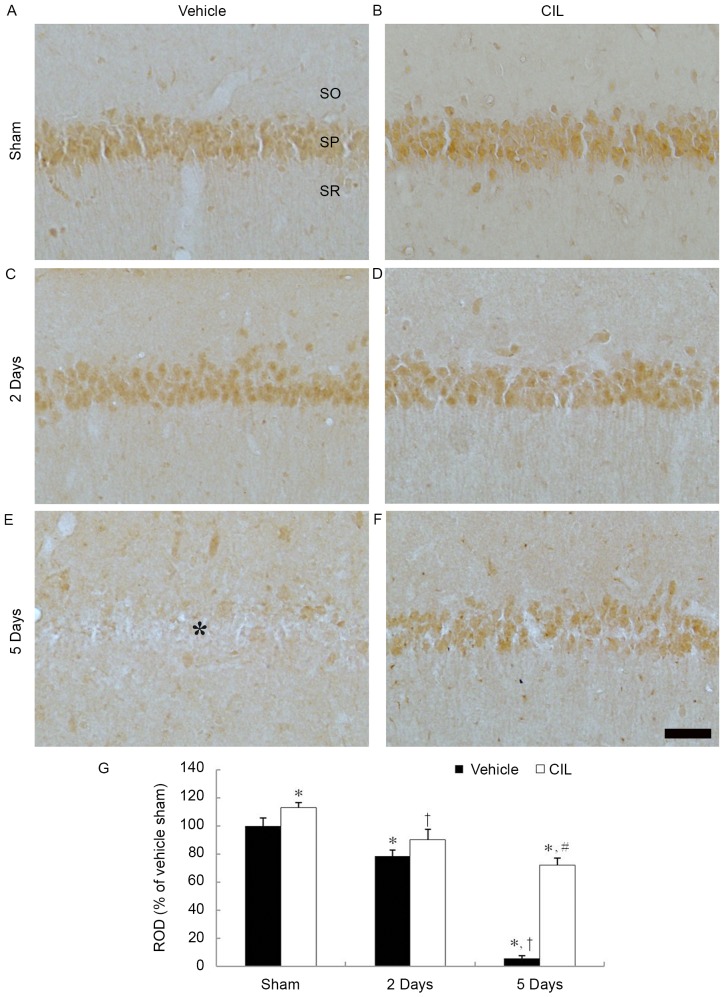
SOD1 immunoreactivity was easily detected in pyramidal cells of the hippocampal CA1 region of the vehicle-sham-group (Fig. 1A). In the vehicle-ischemia-group, SOD1 immunoreactivity was significantly decreased in the CA1 pyramidal cells 2 days following ischemia-reperfusion and SOD1 immunoreactivity was hardly detected in the CA1 pyramidal cells 5 days following ischemia-reperfusion (Fig. 1C, E and G).
Figure 1.
SOD1 immunohistochemistry in the hippocampal CA1 region of the (A) vehicle-sham-, (B) CIL-sham-, (C and E) vehicle-ischemia- and (D and F) CIL-ischemia-groups following ischemia-reperfusion. SOD1 immunoreactivity is easily observed in the SP in the vehicle-sham-group. In the vehicle-ischemia-group, SOD1 immunoreactivity is hardly observed in the SP (asterisk) 5 days following ischemia-reperfusion. In the CIL-sham-group, SOD1 immunoreactivity is significantly increased compared with the vehicle-sham-group, and the immunoreactivity is well detected until 5 days following ischemia-reperfusion. Scale bar, 100 µm. (G) ROD as % values of SOD1 immunoreactivity in the SP of each group (*P<0.05 vs. vehicle-sham-group; #P<0.05 vs. corresponding vehicle-ischemia-group; †P<0.05 vs. respective pre-time point group). The bars indicate the means ± standard error of mean. SP, stratum pyramidale; SOD, superoxide dismutase; CIL, Chrysanthemum indicum Linné extract; ROD, relative optical density; SO, stratum oriens; SR, stratum radiatum.
In the CIL-sham-group, SOD1 immunoreactivity in the CA1 pyramidal cells was significantly higher compared with the vehicle-sham-group (Fig. 1B and G). In the CIL-ischemia-group, SOD1 immunoreactivity in the CA1 pyramidal cells was reduced following ischemia-reperfusion; however, the SOD1 immunoreactivity was significantly higher compared with the corresponding vehicle-ischemia-group (Fig. 1D, F and G). In particular, 5 days following ischemia-reperfusion in the CIL-ischemia-group, numerous SOD1-immunoreactive CA1 pyramidal cells were observed (Fig. 1F).
SOD2 immunoreactivity
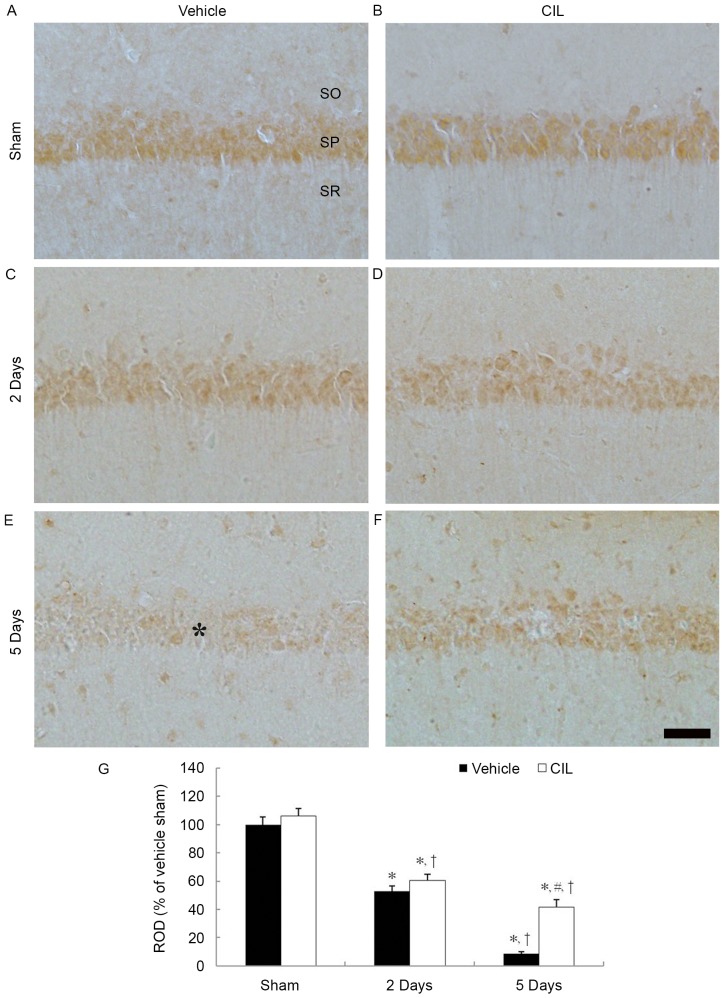
SOD2 immunoreactivity was also clearly identified in the CA1 pyramidal cells in the vehicle-sham-group (Fig. 2A). SOD1 immunoreactivity in the CA1 pyramidal cells was significantly decreased 2 days and barely detected 5 days following ischemia-reperfusion in the vehicle-ischemia-group (Fig. 2C, E and G).
Figure 2.
SOD2 immunohistochemistry in the hippocampal CA1 region of the (A) vehicle-sham-, (B) CIL-sham-, (C and E) vehicle-ischemia- and (D and F) CIL-ischemia-groups following ischemia-reperfusion. SOD2 immunoreactivity is identified in the SP in the vehicle-sham-group, and SOD2 immunoreactivity in the SP (asterisk) is barely observable 5 days following ischemia-reperfusion. In the CIL-sham-group, SOD2 immunoreactivity is similar to that of the vehicle-sham-group, and, in the CIL-ischemia-group, SOD2 immunoreactivity in the SP is higher than that corresponding vehicle-sham-group. Scale bar, 100 µm. (G) ROD as % values of SOD2 immunoreactivity in the SP of each group (*P<0.05 vs. vehicle-sham-group; #P<0.05 vs. corresponding vehicle-ischemia-group; †P<0.05 vs. respective pre-time point group). The bars indicate the means ± standard error of mean. SP, stratum pyramidale; SOD, superoxide dismutase; CIL, Chrysanthemum indicum Linné extract; ROD, relative optical density; SO, stratum oriens; SR, stratum radiatum.
In the CIL-sham-group, SOD2 immunoreactivity in the CA1 pyramidal cells was slightly increased compared with the vehicle-sham-group; however, it was not statistically significant (Fig. 2B and G). In the CIL-ischemia-group, the changing pattern of SOD2 immunoreactivity in the CA1 pyramidal cells was similar to that of the SOD1 immunoreactivity (Fig. 2D, F and G).
CAT immunoreactivity
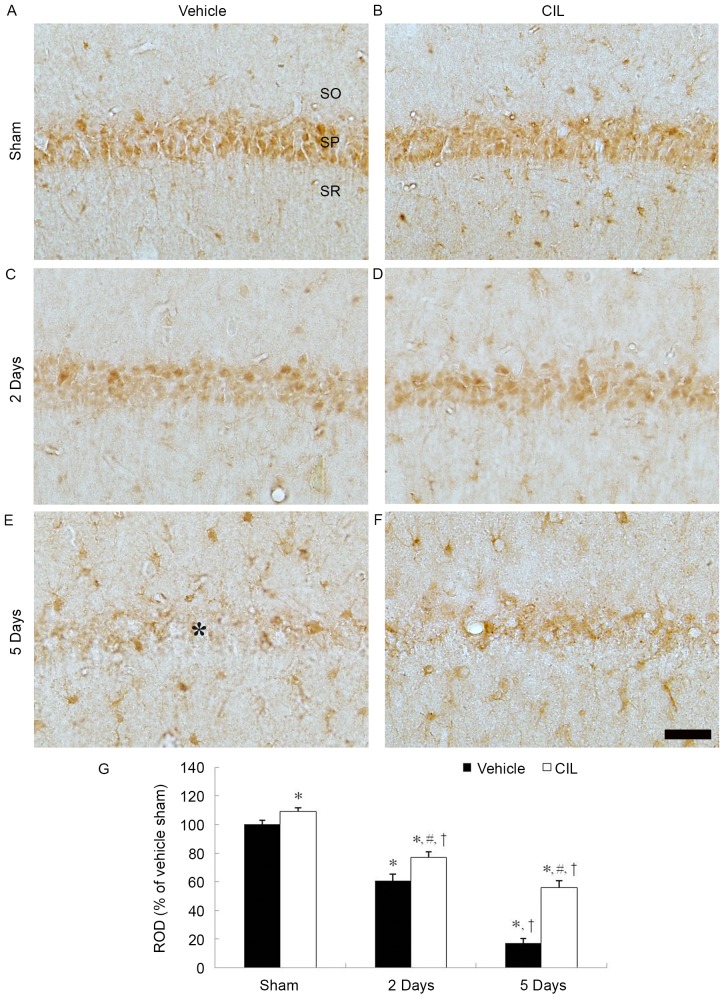
In the vehicle-sham-group, CAT immunoreactivity was clearly observed in the pyramidal cells of the stratum pyramidale layer of the CA1 region (Fig. 3A). In the vehicle-ischemia-group, CAT immunoreactivity was significantly decreased in the CA1 pyramidal cells at 2 days post-ischemia (Fig. 3C and G). At 5 days post-ischemia, CAT immunoreactivity in the CA1 pyramidal cells was barely observable; however, CAT immunoreactivity was newly expressed in non-pyramidal cells in the strata oriens and radiatum of the CA1 region (Fig. 3E and G).
Figure 3.
CAT immunohistochemistry in the hippocampal CA1 region of the (A) vehicle-sham, (B) CIL-sham-, (C and E) vehicle-ischemia- and (D and F) CIL-ischemia-groups following ischemia-reperfusion. CAT immunoreactivity is markedly decreased in the SP (asterisk) of the vehicle-ischemia-group at 5 days following ischemia-reperfusion; however, in the CIL-sham- and CIL-ischemia-groups, CAT immunoreactivity in the SP is significantly higher compared with the vehicle-sham- and vehicle-ischemia-groups. Scale bar, 100 µm. (G) ROD as % values of CAT immunoreactivity in the SP of each group (*P<0.05 vs. vehicle-sham-group; #P<0.05 vs. corresponding vehicle-ischemia-group; †P<0.05 vs. respective pre-time point group). The bars indicate the means ± standard error of mean. SP, stratum pyramidale; CAT, catalase; CIL, Chrysanthemum indicum Linné extract; ROD, relative optical density; SO, stratum oriens; SR, stratum radiatum.
In the CIL-sham-group, CAT immunoreactivity in the CA pyramidal cells was significantly increased compared with the vehicle-sham-group (Fig. 3B and G). In the CIL-ischemia-group, although the CAT immunoreactivity in the CA1 pyramidal cells was decreased following ischemia-reperfusion, the CAT immunoreactivity was significantly higher compared with the corresponding vehicle-ischemia-group (Fig. 3D, F and G).
GPX immunoreactivity
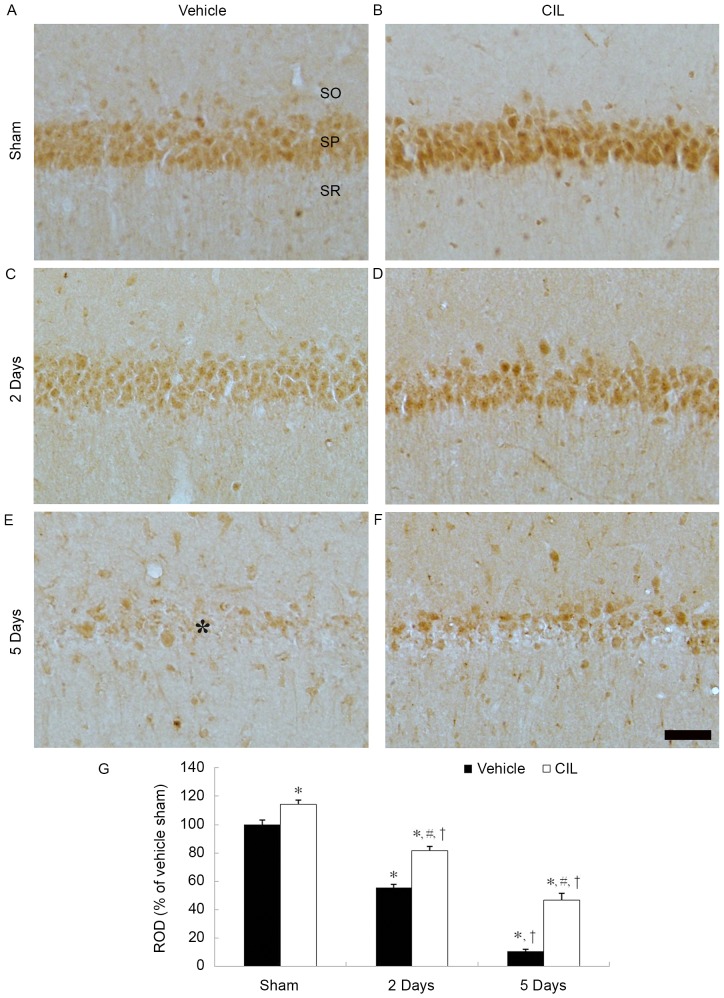
GPX immunoreactivity in the vehicle-sham-group was easily detected in the CA1 pyramidal cells (Fig. 4A). GPX immunoreactivity in the CA1 pyramidal cells was markedly decreased at 2 days post-ischemia and barely identified at 5 days post-ischemia (Fig. 4C, E and G).
Figure 4.
GPX immunohistochemistry in the hippocampal CA1 region of the (A) vehicle-sham-, (B) CIL-sham-, (C and E) vehicle-ischemia- and (D and F) CIL-ischemia-groups following ischemia-reperfusion. GPX immunoreactivity is detected well in the SP of the vehicle-sham-group; the immunoreactivity is significantly increased in the CIL-sham-group. In the vehicle-ischemia-group, GPX immunoreactivity is hardly observed in the SP at 5 days post-ischemia; however, in the CIL-ischemia-group, GPX immunoreactivity is significantly higher than that in the vehicle-ischemia-group. Scale bar, 100 µm. (G) ROD as % values of GPX immunoreactivity in the SP of each group (*P<0.05 vs. vehicle-sham-group; #P<0.05 vs. corresponding vehicle-ischemia-group; †P<0.05 vs. respective pre-time point group). The bars indicate the means ± standard error of mean. GPX, glutathione peroxidase; CIL, Chrysanthemum indicum Linné extract; ROD, relative optical density; SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum.
GPX immunoreactivity in the CA1 pyramidal cells of the CIL-sham-group was significantly higher compared with the vehicle-sham-group (Fig. 4B and G). In the CIL-ischemia-group, GPX immunoreactivity in the CA1 pyramidal cells was decreased following ischemia-reperfusion; however, the GPX immunoreactivity was significantly higher compared with the corresponding vehicle-ischemia-group (Fig. 4D, F and G).
Neuroprotective effects
NeuN-positive neurons
NeuN-positive neurons were identified in the stratum pyramidale of the hippocampus proper (CA1-3 regions) of the vehicle-sham-group (Fig. 5A and B). The distribution of NeuN-positive neurons in the CIL-sham-group was similar to the vehicle-sham-group and the number of NeuN-positive neurons remained unchanged (Fig. 5D, E and M).
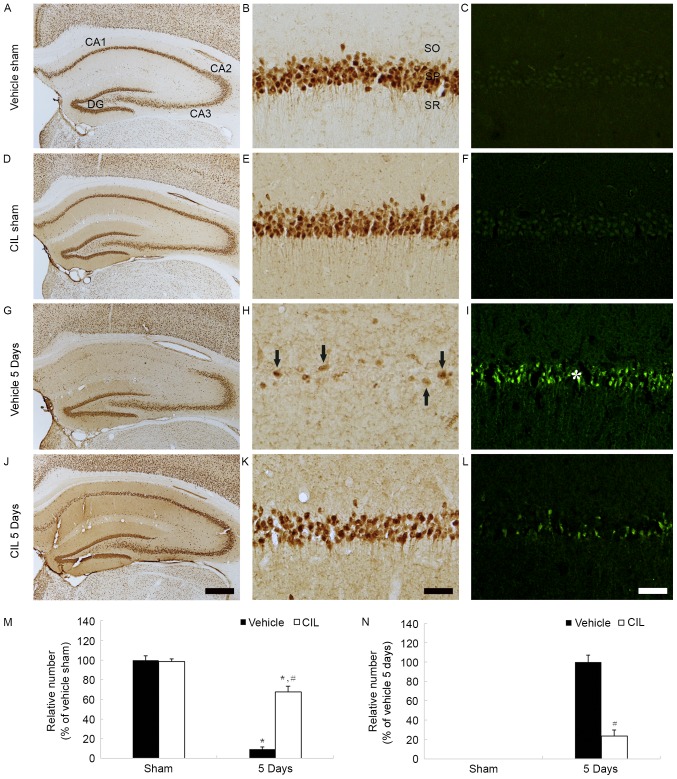
Figure 5.
NeuN-(left and middle columns) and F-J B-(right column) positive cells of (A-C) vehicle-sham-, (D-F) CIL-sham-, (G-I) vehicle-ischemia-(J-L) and CIL-ischemia-groups 5 days following ischemia-reperfusion. In the vehicle-ischemia-group, a few NeuN-(arrows) and numerous F-J B-(asterisk) are detected in the SP of the CA1 region. In the CIL-ischemia-group, numerous NeuN- and few F-J B-positive cells are observed in the SP at 5 days post-ischemia. Scale bar, (A, D, G and J) 50 µm and (B, C, E, F, H, I, K and L) 100 µm. (M and N) Relative analysis as % of the number of NeuN- and F-J B-positive cells in the CA1 region (*P<0.05 vs. respective vehicle-sham-group; #P<0.05 vs. corresponding vehicle-ischemia-group). The bars indicate the means ± standard error of mean. SP, stratum pyramidale; NeuN, neuronal nuclei; CIL, Chrysanthemum indicum Linné extract; F-J B, Fluoro-Jade B; SO, stratum oriens; SR, stratum radiatum.
In the vehicle-ischemia-group, a small number of NeuN-positive neurons were observed in the stratum pyramidale of the CA1 region, and none in the CA2-3 region, 5 days following ischemia-reperfusion (Fig. 5G and H); the relative number of NeuN-positive neurons was ~9% compared with the vehicle-sham-group (Fig. 5M). However, in the CIL-ischemia-group, numerous NeuN-positive neurons were detected in the stratum pyramidale of the CA1 region 5 days following ischemia-reperfusion (Fig. 5J and K); the protected neurons were ~68% of the vehicle-sham-group (Fig. 5M); this finding was identical to our previously study (22).
F-J B-positive cells
In the vehicle-sham- and CIL-sham-groups, F-J B-positive cells were not observed in the stratum pyramidale of the CA1 region (Fig. 5C, F and N). In the vehicle-ischemia-group, however, numerous F-J B-positive cells were detected in the stratum pyramidale of the CA1 region 5 days following ischemia-reperfusion (Fig. 5I and N). However, in the CIL-ischemia-group, only a few F-J B-positive cells were detected, and the relative number of F-J B-positive cells was ~24% that of the vehicle-ischemia-group (Fig. 5L and N); this finding was identical to a previous study of the authors (22).
Discussion
Just five min of transient cerebral ischemia results in the damage/death of pyramidal neurons in the hippocampus and neuronal death occurs selectively in the hippocampal CA1 region (27,28). It has been reported (6,29) that pyramidal cells of the stratum pyramidale in the hippocampal CA1 region die several days following transient cerebral ischemia. Mongolian gerbils have been generally used as a good experimental animal model of transient cerebral ischemia as the posterior communicating arteries in Willis' circle, which connect the vertebrabasilar and carotid arterial system, are lacking in gerbils and transient cerebral ischemia can easily be made by the ligation of bilateral common carotid arteries (24,30,31).
The present study observed the death of pyramidal cells in the hippocampal CA1 region by NeuN immunohistochemistry and F-J B histofluorescence; a noticeable loss of CA1 pyramidal cells was identified in the stratum pyramidale of the CA1 region 5 days following ischemia-reperfusion. This result corresponds to findings of previous studies (23,32,33). In addition, it was identified that the oral pre-treatment of 200 mg/kg CIL to the gerbils protected CA1 pyramidal cells (~67% of the sham-operated gerbils) from 5 min of transient cerebral ischemia; this finding was identical to a previous study of the authors (22).
Although mechanisms regarding neuronal death by transient cerebral ischemic insult are complex, it has been demonstrated that, among the mechanisms, endogenous antioxidant enzymes are associated with neuroprotection via the efficient scavenging of ROS (2,9,34,35). Excessive ROS production is a cause of neuronal damage/death following ischemia-reperfusion injury and has been implicated in the development of numerous neurologic disorders and brain dysfunctions (10,36–38). Accumulated ROS cause the injurious modification of cellular elements including DNA, proteins and lipids; eventually, the accumulated ROS can impair cellular function and result in neuronal damage/death (39,40). In the present study, SOD1, SOD2, CAT and GPX immunoreactivities of the vehicle-ischemia-group were significantly decreased and barely identified in the CA1 pyramidal cells 5 days following ischemia-reperfusion. This result is coincident with the finding of a previous study using gerbils (40).
Kim et al (41) investigated the protective effect of Chrysanthemum indicum ethanol extract against cisplatin-induced nephrotoxicity in vitro. Their findings may be associated with the antioxidative effects of Chrysanthemum indicum ethanol extract since the Chrysanthemum indicum ethanol extract pre-treated group demonstrated a recovery of serum renal function index with ameliorated oxidative stress; the effect has not been investigated in any ischemic stroke model and may be a subject for future studies. In the present study, CIL pre-treatment significantly enhanced the immunoreactivities of SOD1, CAT and GPX, although not SOD2, in the CA1 pyramidal cells of the vehicle-sham-group, and SOD1, SOD2, CAT and GPX immunoreactivities in the CA1 pyramidal cells were significantly higher compared with the vehicle-ischemia-group. These results suggested that the administration of CIL increases antioxidant enzymes and it exhibits neuroprotection following transient cerebral ischemia.
ROS are scavenged by SODs, GPX and CAT, and functions of the antioxidant enzymes have been studied by a number of researchers. It has been reported that SOD1 overexpression demonstrated a neuroprotective effect in the hippocampal CA1 region against cerebral ischemic insults in rodents (42,43). Kondo et al (44) reported that SOD1 knockout mice had demonstrated the increase of cell death and edema of the brain following focal cerebral ischemia, and Murakami et al (45) demonstrated that, in SOD2 knockout mice, exacerbated infarct volume was identified in the brain following permanent focal cerebral ischemia, and suggested that SOD2 was an important enzyme in protecting brain from ischemic injury. It has also been reported that the administration of PEP-1-CAT fusion protein demonstrated significant neuroprotection in the hippocampal CA1 region following transient cerebral ischemia (46). Furthermore, it was recently reported that GPX, which is another antioxidant enzyme contributing to H2O2 scavenging, exhibited a stronger neuroprotective antioxidant against oxidative stress than SOD (8).
It has been demonstrated that CIL is associated with the inhibition of inflammatory responses (15,16,19,22,47). Cheng et al (15) reported that a butanol soluble fraction of CIL possessed anti-inflammatory, immunomodulatory and mononuclear phagocytic activities by the enhancement of serum IgG and IgM levels in response to sheep red blood cells in cyclophosphamide-induced mice, and Cheon et al (19) demonstrated that CIL suppressed the production of inflammatory mediators and proinflammatory cytokines via the downregulation of nuclear factor κB and mitogen-activated protein kinases in RAW264.7 macrophages (15,19). Previously, Kim et al (48) reported that CIL protected against 1-methyl-4-phenylpridinium ions and lipopolysaccharide-induced cytotoxicity in a cellular model of Parkinson's disease. In addition, Yoo et al (22) recently reported that CIL pre-treatment increased anti-inflammatory cytokines in the hippocampus and that the increased anti-inflammatory cytokines were associated with neuroprotection in the gerbil hippocampus induced by transient cerebral ischemia.
In brief, the present study identified that CIL pre-treatment enhanced SOD1, CAT and GPX, although not SOD2, in pyramidal cells in the gerbil hippocampal CA1 region and protected the cells from transient cerebral ischemia. These results indicated that CIL-mediated neuroprotective effect may be associated with increases of antioxidant enzymes in the CA1 pyramidal cells and suggested that CIL may be used for the prevention of ischemic damage in the brain.
Acknowledgements
The present study was supported by the Bio-Synergy Research Project (grant no. NRF-2015M3A9C4076322) of the Ministry of Science, ICT and Future Planning through the National Research Foundation, and by the Bio and Medical Technology Development Program of the NRF funded by the Korean Government, Minister of Science, ICT and Future Planning (grant no. NRF-2015M3A9B6066835).
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, et al. Heart disease and stroke statistics-2011 update: A report from the American heart association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang G, Shi B, Luo W, Yang J. The protective effect of caffeic acid on global cerebral ischemia-reperfusion injury in rats. Behav Brain Funct. 2015;11:18. doi: 10.1186/s12993-015-0064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White BC, Grossman LI, Krause GS. Brain injury by global ischemia and reperfusion: A theoretical perspective on membrane damage and repair. Neurology. 1993;43:1656–1665. doi: 10.1212/WNL.43.9.1656. [DOI] [PubMed] [Google Scholar]
- 4.Butler TL, Kassed CA, Sanberg PR, Willing AE, Pennypacker KR. Neurodegeneration in the rat hippocampus and striatum after middle cerebral artery occlusion. Brain Res. 2002;929:252–260. doi: 10.1016/S0006-8993(01)03371-6. [DOI] [PubMed] [Google Scholar]
- 5.Crain BJ, Westerkam WD, Harrison AH, Nadler JV. Selective neuronal death after transient forebrain ischemia in the Mongolian gerbil: A silver impregnation study. Neuroscience. 1988;27:387–402. doi: 10.1016/0306-4522(88)90276-X. [DOI] [PubMed] [Google Scholar]
- 6.Kirino T. Delayed neuronal death. Neuropathology. 2000;20:95–97. doi: 10.1046/j.1440-1789.2000.00306.x. (Suppl) [DOI] [PubMed] [Google Scholar]
- 7.Lewén A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 8.Lee JC, Won MH. Neuroprotection of antioxidant enzymes against transient global cerebral ischemia in gerbils. Anat Cell Biol. 2014;47:149–156. doi: 10.5115/acb.2014.47.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JH, Park OK, Yan B, Ahn JH, Kim IH, Lee JC, Kwon SH, Yoo KY, Lee CH, Hwang IK, et al. Neuroprotection via maintenance or increase of antioxidants and neurotrophic factors in ischemic gerbil hippocampus treated with tanshinone I. Chin Med J (Engl) 2014;127:3396–3405. [PubMed] [Google Scholar]
- 10.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Delanty N, Dichter MA. Antioxidant therapy in neurologic disease. Arch Neurol. 2000;57:1265–1270. doi: 10.1001/archneur.57.9.1265. [DOI] [PubMed] [Google Scholar]
- 12.Pastore A, Petrillo S, Piermarini E, Piemonte F. Systemic redox biomarkers in neurodegenerative diseases. Curr Drug Metab. 2015;16:46–70. doi: 10.2174/138920021601150702161250. [DOI] [PubMed] [Google Scholar]
- 13.Duan X, Wang W, Liu X, Yan H, Dai R, Lin Q. Neuroprotective effect of ethyl acetate extract from gastrodia elata against transient focal cerebral ischemia in rats induced by middle cerebral artery occlusion. J Tradit Chin Med. 2015;35:671–678. doi: 10.1016/S0254-6272(15)30158-8. [DOI] [PubMed] [Google Scholar]
- 14.Surapaneni S, Prakash T, Ansari M, Manjunath P, Kotresha D, Goli D. Study on cerebroprotective actions of Clerodendron glandulosumleaves extract against long term bilateral common carotid artery occlusion in rats. Biomed Pharmacother. 2016;80:87–94. doi: 10.1016/j.biopha.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 15.Cheng W, Li J, You T, Hu C. Anti-inflammatory and immunomodulatory activities of the extracts from the inflorescence of Chrysanthemum indicum Linné. J Ethnopharmacol. 2005;101:334–337. doi: 10.1016/j.jep.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 16.Lee DY, Choi G, Yoon T, Cheon MS, Choo BK, Kim HK. Anti-inflammatory activity of Chrysanthemum indicum extract in acute and chronic cutaneous inflammation. J Ethnopharmacol. 2009;123:149–154. doi: 10.1016/j.jep.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Shunying Z, Yang Y, Huaidong Y, Yue Y, Guolin Z. Chemical composition and antimicrobial activity of the essential oils of Chrysanthemum indicum. J Ethnopharmacol. 2005;96:151–158. doi: 10.1016/j.jep.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 18.Zhang C, Qin MJ, Shu P, Hong JL, Lu L, He DX. Chemical variations of the essential oils in flower heads of Chrysanthemum indicum L. From China. Chem Biodivers. 2010;7:2951–2962. doi: 10.1002/cbdv.201000034. [DOI] [PubMed] [Google Scholar]
- 19.Cheon MS, Yoon T, Lee DY, Choi G, Moon BC, Lee AY, Choo BK, Kim HK. Chrysanthemum indicum Linné extract inhibits the inflammatory response by suppressing NF-kappaB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophages. J Ethnopharmacol. 2009;122:473–477. doi: 10.1016/j.jep.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 20.Dekanski D, Selaković V, Piperski V, Radulović Z, Korenić A, Radenović L. Protective effect of olive leaf extract on hippocampal injury induced by transient global cerebral ischemia and reperfusion in Mongolian gerbils. Phytomedicine. 2011;18:1137–1143. doi: 10.1016/j.phymed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Rocher MN, Carré D, Spinnewyn B, Schulz J, Delaflotte S, Pignol B, Chabrier PE, Auguet M. Long-term treatment with standardized Ginkgo biloba extract (EGb 761) attenuates cognitive deficits and hippocampal neuron loss in a gerbil model of vascular dementia. Fitoterapia. 2011;82:1075–1080. doi: 10.1016/j.fitote.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Yoo KY, Kim IH, Cho JH, Ahn JH, Park JH, Lee JC, Tae HJ, Kim DW, Kim JD, Hong S, et al. Neuroprotection of Chrysanthemum indicum Linne against cerebral ischemia/reperfusion injury by anti-inflammatory effect in gerbils. Neural Regen Res. 2016;11:270–277. doi: 10.4103/1673-5374.177735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim IH, Yan BC, Park JH, Yeun GH, Yim Y, Ahn JH, Lee JC, Hwang IK, Cho JH, Kim YM, et al. Neuroprotection of a novel synthetic caffeic acid-syringic acid hybrid compound against experimentally induced transient cerebral ischemic damage. Planta Med. 2013;79:313–321. doi: 10.1055/s-0032-1328211. [DOI] [PubMed] [Google Scholar]
- 24.Kim DW, Lee JC, Cho JH, Park JH, Ahn JH, Chen BH, Shin BN, Tae HJ, Seo JY, Cho JH, et al. Neuroprotection of ischemic preconditioning is mediated by anti-inflammatory, not pro-inflammatory, cytokines in the gerbil hippocampus induced by a subsequent lethal transient cerebral ischemia. Neurochem Res. 2015;40:1984–1995. doi: 10.1007/s11064-015-1694-y. [DOI] [PubMed] [Google Scholar]
- 25.Candelario-Jalil E, Alvarez D, Merino N, León OS. Delayed treatment with nimesulide reduces measures of oxidative stress following global ischemic brain injury in gerbils. Neurosci Res. 2003;47:245–253. doi: 10.1016/S0168-0102(03)00184-6. [DOI] [PubMed] [Google Scholar]
- 26.Lee JC, Kim IH, Cho GS, Park JH, Ahn JH, Yan BC, Kwon HM, Kim YM, Cheon SH, Cho JH, et al. Ischemic preconditioning-induced neuroprotection against transient cerebral ischemic damage via attenuating ubiquitin aggregation. J Neurol Sci. 2014;336:74–82. doi: 10.1016/j.jns.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Petito CK, Olarte JP, Roberts B, Nowak TS, Jr, Pulsinelli WA. Selective glial vulnerability following transient global ischemia in rat brain. J Neuropathol Exp Neurol. 1998;57:231–238. doi: 10.1097/00005072-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Sugawara T, Fujimura M, Morita-Fujimura Y, Kawase M, Chan PH. Mitochondrial release of cytochrome c corresponds to the selective vulnerability of hippocampal CA1 neurons in rats after transient global cerebral ischemia. J Neurosci. 1999;19:RC39. doi: 10.1523/JNEUROSCI.19-22-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang QG, Wang R, Han D, Dong Y, Brann DW. Role of Rac1 GTPase in JNK signaling and delayed neuronal cell death following global cerebral ischemia. Brain Res. 2009;1265:138–147. doi: 10.1016/j.brainres.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine S, Sohn D. Cerebral ischemia in infant and adult gerbils. Relation to incomplete circle of Willis. Arch Pathol. 1969;87:315–317. [PubMed] [Google Scholar]
- 31.Martínez NS, Machado JM, Pérez-Saad H, Coro-Antich RM, Berlanga-Acosta JA, Salgueiro SR, Illera GG, Alba JS, del Barco DG. Global brain ischemia in Mongolian gerbils: Assessing the level of anastomosis in the cerebral circle of Willis. Acta Neurobiol Exp (Wars) 2012;72:377–384. doi: 10.55782/ane-2012-1909. [DOI] [PubMed] [Google Scholar]
- 32.Kim IH, Yoo KY, Park JH, Yan BC, Ahn JH, Lee JC, Kwon HM, Kim JD, Kim YM, You SG, et al. Comparison of neuroprotective effects of extract and fractions from Agarum clathratum against experimentally induced transient cerebral ischemic damage. Pharm Biol. 2014;52:335–343. doi: 10.3109/13880209.2013.837074. [DOI] [PubMed] [Google Scholar]
- 33.Park JH, Shin BN, Chen BH, Kim IH, Ahn JH, Cho JH, Tae HJ, Lee JC, Lee CH, Kim YM, et al. Neuroprotection and reduced gliosis by atomoxetine pretreatment in a gerbil model of transient cerebral ischemia. J Neurol Sci. 2015;359:373–380. doi: 10.1016/j.jns.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 34.Numagami Y, Sato S, Ohnishi ST. Attenuation of rat ischemic brain damage by aged garlic extracts: A possible protecting mechanism as antioxidants. Neurochem Int. 1996;29:135–143. doi: 10.1016/0197-0186(95)00117-4. [DOI] [PubMed] [Google Scholar]
- 35.Tu Q, Wang R, Ding B, Zhong W, Cao H. Protective and antioxidant effect of Danshen polysaccharides on cerebral ischemia/reperfusion injury in rats. Int J Biol Macromol. 2013;60:268–271. doi: 10.1016/j.ijbiomac.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 36.Chan PH. Oxygen radicals in focal cerebral ischemia. Brain Pathol. 1994;4:59–65. doi: 10.1111/j.1750-3639.1994.tb00811.x. [DOI] [PubMed] [Google Scholar]
- 37.Rodrigo R, Fernández-Gajardo R, Gutiérrez R, Matamala JM, Carrasco R, Miranda-Merchak A, Feuerhake W. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol Disord Drug Targets. 2013;12:698–714. doi: 10.2174/1871527311312050015. [DOI] [PubMed] [Google Scholar]
- 38.Mantha AK, Sarkar B, Tell G. A short review on the implications of base excision repair pathway for neurons: Relevance to neurodegenerative diseases. Mitochondrion. 2014;16:38–49. doi: 10.1016/j.mito.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Sugawara T, Chan PH. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal. 2003;5:597–607. doi: 10.1089/152308603770310266. [DOI] [PubMed] [Google Scholar]
- 40.Park JH, Cho JH, Kim IH, Ahn JH, Lee JC, Chen BH, Shin BN, Tae HJ, Yoo KY, Hong S, et al. Oenanthe Javanica extract protects against experimentally induced ischemic neuronal damage via its antioxidant effects. Chin Med J (Engl) 2015;128:2932–2937. doi: 10.4103/0366-6999.168063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim TW, Kim YJ, Park SR, Seo CS, Ha H, Shin HK, Jung JY. Chrysanthemum indicum attenuates cisplatin-induced nephrotoxicity both in vivo and in vitro. Nat Prod Commun. 2015;10:397–402. [PubMed] [Google Scholar]
- 42.Murakami K, Kondo T, Epstein CJ, Chan PH. Overexpression of CuZn-superoxide dismutase reduces hippocampal injury after global ischemia in transgenic mice. Stroke. 1997;28:1797–1804. doi: 10.1161/01.STR.28.9.1797. [DOI] [PubMed] [Google Scholar]
- 43.Chan PH, Kawase M, Murakami K, Chen SF, Li Y, Calagui B, Reola L, Carlson E, Epstein CJ. Overexpression of SOD1 in transgenic rats protects vulnerable neurons against ischemic damage after global cerebral ischemia and reperfusion. J Neurosci. 1998;18:8292–8299. doi: 10.1523/JNEUROSCI.18-20-08292.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kondo T, Reaume AG, Huang TT, Carlson E, Murakami K, Chen SF, Hoffman EK, Scott RW, Epstein CJ, Chan PH. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci. 1997;17:4180–4189. doi: 10.1523/JNEUROSCI.17-11-04180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim DW, Kim DS, Kim MJ, Kwon SW, Ahn EH, Jeong HJ, Sohn EJ, Dutta S, Lim SS, Cho SW, et al. Imipramine enhances neuroprotective effect of PEP-1-Catalase against ischemic neuronal damage. BMB Rep. 2011;44:647–652. doi: 10.5483/BMBRep.2011.44.10.647. [DOI] [PubMed] [Google Scholar]
- 47.Kim JE, Jun S, Song M, Kim JH, Song YJ. The extract of Chrysanthemum indicum Linne inhibits EBV LMP1-induced NF-κB activation and the viability of EBV-transformed lymphoblastoid cell lines. Food Chem Toxicol. 2012;50:1524–1528. doi: 10.1016/j.fct.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 48.Kim IS, Ko HM, Koppula S, Kim BW, Choi DK. Protective effect of Chrysanthemum indicum Linne against 1-methyl-4-phenylpridinium ion and lipopolysaccharide-induced cytotoxicity in cellular model of Parkinson's disease. Food Chem Toxicol. 2011;49:963–973. doi: 10.1016/j.fct.2011.01.002. [DOI] [PubMed] [Google Scholar]