Abstract
The present study aimed to investigate the role of Bruton's tyrosine kinase (BTK) in the pathogenesis of lung injury induced by trauma-hemorrhagic shock (THS), and to examine the pulmonary protective effects of BTK inhibition. Male Sprague-Dawley rats were divided into four groups (n=12/group): i) A Sham group, which received surgery without induced trauma; ii) a THS-induced injury group; iii) a THS-induced injury group that also received treatment with the BTK inhibitor LFM-A13 prior to trauma induction; and iv) a Sham group that was pretreated with LFM-A13 prior to surgery but did not receive induced trauma. The expression of phosphorylated-BTK protein in the lungs was measured by immunohistochemistry and western blot analysis. The bronchoalveolar lavage fluid (BALF) protein concentration, total leukocyte and eosinophil numbers, and the expression levels of peripheral blood proinflammatory factors were measured. Morphological alterations in the lungs were detected by hematoxylin and eosin staining. Pulmonary nitric oxide (NO) concentration and inducible NO synthase (iNOS) expression were also assessed. Activities of the nuclear factor (NF)-κB and mitogen-activated protein kinase (MAPK) signaling pathways were determined by western blotting or electrophoretic mobility shift assay. BTK was notably activated in lungs of THS rats. BALF protein concentration, total leukocytes and eosinophils, peripheral blood expression levels of tumor necrosis factor-α, interleukin (IL)-1β, IL-6 and monocyte chemotactic protein 1 were significantly upregulated after THS induction, and each exhibited decreased expression upon LFM-A13 treatment. THS-induced interstitial hyperplasia, edema and neutrophilic infiltration in lungs were improved by the inhibition of BTK. In addition, THS-induced NO release, iNOS overexpression, and NF-κB and MAPK signaling were suppressed by BTK inhibition. Results from the present study demonstrate that BTK may serve a pivotal role in the pathogenesis of THS-related lung injury, and the inhibition of BTK may significantly alleviate THS-induced lung damage. These results provide a potential therapeutic application for the treatment of THS-induced lung injury.
Keywords: trauma-hemorrhagic shock, lung, inflammation, Bruton's tyrosine kinase, LFM-A13
Introduction
Trauma-induced hemorrhage remains the leading cause of mortality for people under the age of 45, and affects almost every community (1,2). The pathophysiological process of trauma and severe hemorrhage-induced shock (THS) is complex; it involves a systemic inflammatory reaction and pathological alterations, such as hypovolemia, hypoxemia, microcirculatory disturbances and oxidative stress (3). Major complications of THS include systemic inflammatory response syndrome, multiple organ dysfunction syndrome and sepsis, which are the main causes of the high mortality rate (4). Since the inflammatory response is a key element in THS-induced injury (5,6), the majority of studies have focused on the regulation of proinflammatory mediators (7,8).
Bruton's tyrosine kinase (BTK) is a prototypical member of the Tec family of protein tyrosine kinases. It serves an essential role in B cell development, and mature B cell activation and survival; BTK gene mutations result in B cell deficiency-related X-linked agammaglobulinemia in humans and X-linked immunodeficiency in mice (9,10). Previous studies have demonstrated BTK to be a crucial effector for B cell receptor-, immunoglobulin (Ig)E receptor-, Toll-like receptor (TLR)- and cytokine receptor-dependent innate and adaptive immunity systems (10–13). The activation (by tyrosine phosphorylation) of BTK may stimulate the nuclear factor (NF)-κB and mitogen-activated protein kinase (MAPK) signaling pathways, and ultimately trigger a series of inflammatory reactions (14–18). Previous studies have reported that BTK inhibition may be efficient in controlling B cell malignancy and B cell-related autoimmune disorders (19–23); however, whether BTK participates in THS-induced lung injury remains to be elucidated.
NF-κB and MAPKs have been reported to be involved in several proinflammatory signaling pathways (24,25). The activation of NF-κB- or MAPK-mediated pathways have been demonstrated to increase the levels of inflammatory mediators, such as nitroc oxide (NO) and inducible NO synthase (iNOS), which serve important roles in THS-induced organ injury (26). Various organs can be severely affected by trauma-induced hemorrhage; however, THS-induced lung injury is one of the main causes of post-traumatic mortality (27). The present study aimed to reveal the potential role of BTK in the progression of THS, investigate the protective effects of BTK inhibition on THS-induced lung injury in vivo, and explore the molecular mechanisms underlying the actions of BTK by assessing the activation of NF-κB and MAPK pathways.
Materials and methods
Animals
Male Sprague-Dawley rats (n=48; age, 10–14 weeks; weight, 360–400 g) were purchased from Liaoning Changsheng Biotechnology Co., Ltd. [permit no. SCXK (Liao) 2015–0001; Benxi, China]. Rats were allowed to acclimate for 1 week in a controlled environment: 22±1°C, 40–50% humidity, under a 12 h light-dark cycle. Food pellets and tap water were available ad libitum throughout the study. Animal care and handling procedures strictly followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th Edition, 2010) and were approved by the Institutional Animal Care and Use Committee of the General Hospital of Shenyang Military Area Command (Shenyang, China).
Development of the THS model
THS was induced in rats as previously described (28). Briefly, rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg), bilateral groins were dissected and the femoral arteries of both sides, and the femoral vein of one side, were cannulated. Bilateral femur fractures were induced by hemostatic forceps, one of the femoral arteries was connected to a multichannel physiology recorder by catheter and the other artery was induced to hemorrhage to a mean arterial pressure <50 mmHg for 1.5 h. Rats were resuscitated with lactated Ringer's solution (Hangzhou Empyrean Animal Health Co., Ltd., Hangzhou, China), at four times the volume of shed blood, through the femoral vein and were maintained under anesthesia for an additional 4.5 h.
Experimental groups
A total of 48 rats were randomly divided into four groups (n=12/group): i) The Sham group, the femoral arteries and veins were cannulated without induced fractures and bloodletting; ii) the THS group, bilateral femur fractures and hemorrhage were induced manually; iii) the THS + LFM-A13 group, rats received a peritoneal injection of the BTK inhibitor LFM-A13 (25 mg/kg; Shanghai Biochempartner Co., Ltd., Wuhan, China) and trauma was immediately induced; and iv) the Sham + LFM-A13 group, rats received a peritoneal injection of LFM-A13 (25 mg/kg) prior to surgery but did not receive induced fractures and bloodletting.
Bronchoalveolar lavage fluid (BALF), blood and lung tissue collection
A total of 6 h following the induction of trauma, 6 rats from each group received endotracheal intubation and the left lungs were lavaged three times with 1.5 ml cold saline; the whole BALF volume was collected. Then, the abdomen was opened and peripheral blood was collected from inferior vena cava using a 10 ml syringe. Both lungs were removed by midline thoracolaparotomy. The left lungs were snap-frozen in liquid nitrogen and stored at −80°C, and the right lungs were fixed in 4% paraformaldehyde at 4°C for 48 h for subsequent histological analysis. The remaining rats were euthanized with anesthetic overdose (supplementary injection with 50 mg/kg sodium pentobarbital), the lungs were removed and the wet weight was measured. Subsequently, lungs were dried at 100°C in a thermostabilized oven for 72 h, the dry weight was measured and the wet/dry ratio was calculated. The BALF was divided into two parts; one part was used for Giemsa staining, as follows: BALF cells were collected by centrifugation at 300 × g for 10 min at 4°C and stained with Giemsa solution (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The total numbers of leukocytes and eosinophils were counted under an optical microscope. The other part of the BALF was centrifuged at 1,000 × g for 10 min at 4°C and the protein concentration of the supernatant was determined using a Bradford Protein Assay kit (Wanleibio, Shenyang, China).
Histological and immunohistochemical analysis
Lung tissues (n=5 rats/group) fixed in paraformaldehyde were embedded in paraffin and cross-sectioned (5 µm). Sections were stained with hematoxylin and eosin, and lung morphology was observed under a light microscope with an Olympus DP73 digital camera (Olympus Corporation, Tokyo, Japan).
For immunohistochemical analysis, the 5 µm-thick lung sections were heated for 10 min in 0.01 mol/l citrate buffer for antigen retrieval, followed by endogenous peroxidase inactivation by incubation in 3% H2O2 for 15 min. Sections were blocked with normal goat serum (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) for 15 min at room temperature, and incubated with rabbit primary antibodies against phosphorylated (p)-BTK (1:200; cat no. bs-3055R; BIOSS, Beijing, China) or inducible nitric oxide synthase (iNOS; 1:200; cat no. BA0362; Wuhan Boster Biological Technology, Ltd., Wuhan, China) at 4°C overnight. Following overnight incubation, slides were washed with PBS and incubated with a secondary biotinylated goat anti-rabbit IgG antibody (1:200; cat no. A0277; Beyotime Institute of Biotechnology, Haimen, China) at 37°C for 30 min. Specific proteins of interest were detected by horseradish peroxidase (HRP)-conjugated streptavidin (cat no. A0303; Beyotime Institute of Biotechnology) and visualized by 3,3′-Diaminobenzidine solution (Beijing Solarbio Science & Technology Co. Ltd.); sections were counterstained with hematoxylin and observed under an optical microscope.
Inflammatory factors and nitric oxide (NO) detection
Peripheral blood samples were centrifuged at 1,000 × g for 10 min at 4°C and the serum was collected. Serum protein expression levels of tumor necrosis factor-α (TNF-α) (Rat TNF Alpha PicoKine™ ELISA kit; cat no. EK0526), interleukin (IL)-1β (Rat IL-1 Beta PicoKine™ ELISA kit; cat no. EK0393), IL-6 (Rat IL-6 PicoKine™ ELISA kit; cat no. EK0412) and monocyte chemotactic protein 1 (MCP-1; Rat MCP-1 PicoKine™ ELISA kit; cat no. EK0902) were determined using commercially available ELISA kits purchased from Wuhan Boster Biological Technology, Ltd., according to the manufacturers' protocol.
Lung tissues were homogenized, freeze-thawed with liquid nitrogen three times and centrifuged at 10,000 × g for 10 min at 4°C. Following centrifugation, supernatants were collected and protein concentrations were measured using the Bicinchoninic Acid Protein Assay kit (Wanleibio). Proteins were diluted to 2 µg/µl in PBS and NO concentrations were measured using the Total Nitric Oxide Assay kit (cat no. S0023; Beyotime Institute of Biotechnology).
Western blot analysis
Total and nuclear proteins were extracted from lung tissue samples (n=5 rats/group) using the Nuclear and Cytoplasmic Protein Extraction kit (Wanleibio) and quantified using a bicinchoninic assay kit (Wanleibio). Equal amounts of extracted protein samples (40 µg) were separated by 5–12% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). Membranes were blocked with 5% fat-free milk for 1 h at room temperature and probed with primary antibodies against BTK (1:500; cat no. bs-2752R; BIOSS), p-BTK (1:500; cat no. bs-3055R; BIOSS), iNOS (1:400; cat no. BA0362; Wuhan Boster Biological Technology, Ltd.), inhibitor of NF-κB (IκB; 1:500; cat no. bs-1287R; BIOSS), p-IκB antibody (1:500; cat no. bs-5515R; BIOSS), NF-κB (1:400; cat no. BA0610; Wuhan Boster Biological Technology, Ltd.), extracellular signal-regulated kinase (ERK; 1:500; cat no. bs-2637R; BIOSS), p-ERK (1:500; cat no. bs-1522R; BIOSS), c-Jun N-terminal kinase (JNK; 1:500; cat no. bs-10562R; BIOSS), p-JNK (1:500; cat no. bs-1640R; BIOSS), p38 (1:500; cat no. bs-0637R; BIOSS) or p-p38 (1:500; cat no. bs-5477R; BIOSS) at 4°C overnight. Following overnight incubation, the membranes were washed with PBS and the specific proteins of interest were detected with secondary HRP-conjugated goat anti-rabbit IgG (1:5,000; cat no. WLA023; Wanleibio) or goat anti-mouse IgG (1:5,000; cat no. WLA024; Wanleibio) at 37°C for 45 min. Protein bands were visualized using an Enhanced Chemiluminescence kit (Wanleibio). Densitometric analysis was performed by Gel-Pro Analyzer version 3.0 (Media Cybernetics, Inc., Rockville, MD, USA), using β-actin (1:1,000; cat no. sc-47778; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and histone H3 (1:500; cat no. bs-17422R, BIOSS) as internal controls.
Electrophoretic mobility shift assay (EMSA)
NF-κB DNA-binding activity was detected using an NF-kB EMSA kit (cat no. BITF001; Viagene Biotech, Inc., Tampa, FL, USA), according to the manufacturer's protocol. Nuclear proteins were extracted and quantified as aforementioned. Proteins (25 µg) were diluted in 5 µl PBS and incubated with 0.5 µl biotin-labeled NF-κB specific probes (0.2 µmol/l; cat no. TF001BP; Viagene Biotech, Inc.) at room temperature for 20 min. The NF-κB-specific recognition sequence is: 5′-AGTTGAGGGGACTTTCCCAGGC-3′. The reaction mixtures (10 µl) were electrophoresed on 6.5% non-denaturing polyacrylamide gel at 180 V for 80 min. Protein-DNA complexes were electrically transferred onto nylon membranes, cross-linked under an ultraviolet lamp for 10 min and specific bands were detected by HRP-conjugated streptavidin and visualized using the Enhanced Chemiluminescence kit (Wanleibio).
Statistical analysis
Data are expressed as the mean ± standard deviation of at least 5 independent experiments. The statistical significance of the differences between groups was assessed by one-way analysis of variance followed by a post hoc Bonferroni test for multiple comparisons. Statistical analysis was performed using SPSS software version 16.0 (SPSS, Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Pulmonary BTK is activated by THS
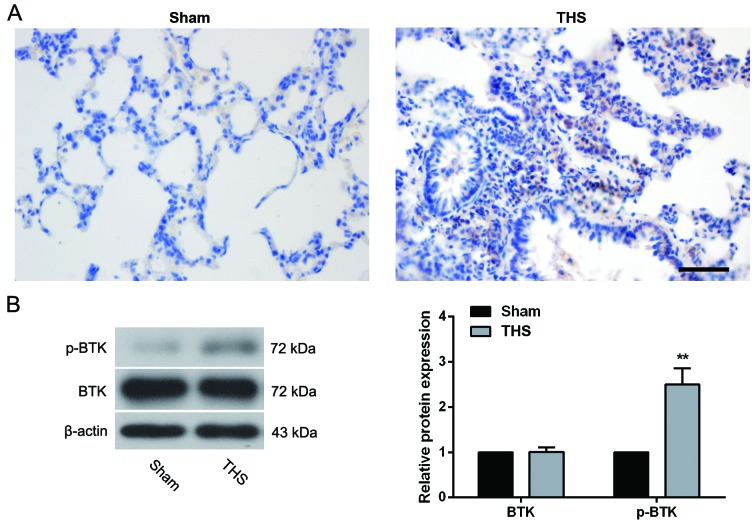
To investigate a potential role for BTK in THS-induced pulmonary injury, the protein expression levels of p-BTK were examined by immunohistochemistry. As shown in Fig. 1A, p-BTK was mainly localized to the membrane of alveolar epithelial cells in the Sham group, and was notably upregulated by THS-induced injury. Western blot analysis also demonstrated that THS rats exhibited a significantly increased expression of p-BTK in the lungs (P<0.01 vs. Sham group); however, the protein expression levels of total BTK were unchanged (Fig. 1B), suggesting that BTK was activated in the lungs of rats with THS-induced injury.
Figure 1.
Expression of p-BTK in THS-induced lung tissue. A total of 4.5 h after THS, lungs were removed and subjected to (A) immunohistochemistry and (B) western blot analysis to determine the expression of p-BTK (n=5 rats/group). Representative images are included. Scale bar, 100 µm. Data are expressed as the mean ± standard deviation. Protein expression levels were quantified relative to the levels in the Sham group, which were set as 100%. **P<0.01 vs. Sham group. BTK, Bruton's tyrosine kinase; p, phosphorylated; THS, trauma-hemorrhagic shock.
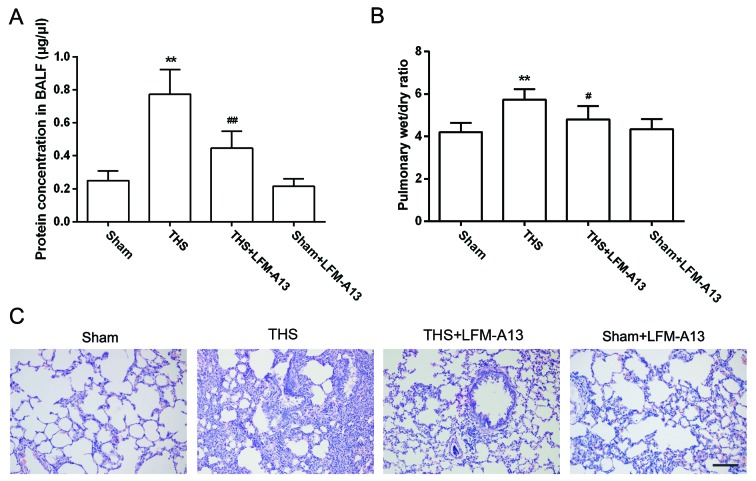
Effects of BTK on pulmonary capillary permeability and morphological alterations
To examine the role of BTK in pulmonary capillary permeability, a specific inhibitor of BTK, LFM-A13, was intraperitoneally injected prior to THS induction. Total protein concentration in the BALF from rats with THS-induced injury was significantly higher compared with the Sham group (P<0.01; Fig. 2A), and this was significantly reduced by LFM-A13 treatment (P<0.05); however, protein concentrations in the Sham + LFM-A13 group remained unaltered. Similarly, the pulmonary wet/dry ratio was significantly increased in the THS group compared with rats in the Sham group (P<0.01; Fig. 2B), and was reduced in THS rats treated with LFM-A13 compared with untreated THS rats (P<0.05). The wet/dry ratio was not altered in the Sham + LFM-A13 group. Histological analysis revealed that the lungs of THS rats exhibited notable interstitial hyperplasia, edema and neutrophil infiltration, which were reduced in LFM-A13-treated THS rats. Converesely, LFM-A13 administration in Sham rats did not produce significant histopathological alterations compared with the Sham group (Fig. 2C).
Figure 2.
Effects of Bruton's tyrosine kinase on THS-induced pulmonary capillary permeability and morphological alterations. (A) BALF protein concentration and (B) pulmonary wet/dry weight ratio were detected from 6 rats of each group. Data are expressed as the mean ± standard deviation; **P<0.01 vs. Sham group; ##P<0.05, ##P<0.01 vs. THS group. (C) Morphological alterations were analyzed by hematoxylin and eosin staining, typical images of 5 rats per group are shown. Scale bar, 50 µm. BALF, bronchoalveolar lavage fluid; LFM-A13, a Bruton's tyrosine kinase inhibitor; THS, trauma-hemorrhagic shock.
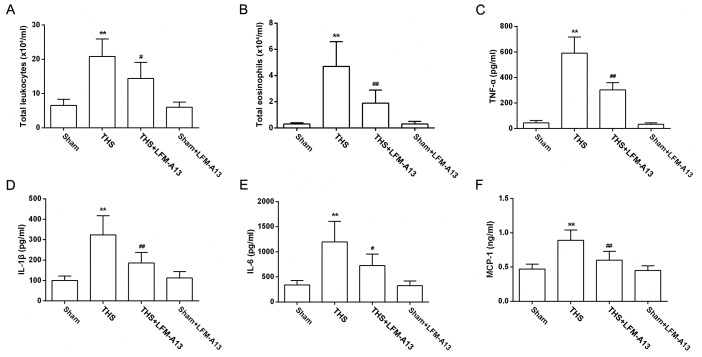
Effects of BTK on the inflammatory response in THS rats
The total number of leukocytes and eosinophils were counted in BALF from each group to examine the effects of BTK on pulmonary inflammatory cell infiltration. As shown in Figs. 3A and B, the number of leukocytes and eosinophils were significantly increased in the BALF of THS rats compared with rats in the Sham group, and were significantly decreased by LFM-A13 treatment. The expression levels of the proteins involved in the inflammatory response were also determined in samples of peripheral blood collected from rats in each group. The results demonstrated that the levels of TNF-α, IL-1β, IL-6 and MCP-1 in THS rats were significantly upregulated (P<0.01 vs. Sham), as expected; LFM-A13 treatment effectively reduced the levels of these inflammatory cytokines in THS rats (P<0.05 vs. untreated THS rats; Fig. 3C-F). Notably, treatment of Sham rats with LFM-A13 did not affect inflammatory cell numbers or the levels of inflammatory factors.
Figure 3.
Effects of Bruton's tyrosine kinase on THS-induced inflammatory response. (A) Numbers of total leukocytes and (B) eosinophils in the bronchoalveolar lavage fluid were counted and the levels of (C) TNF-α, (D) IL-1β, (E) IL-6 and (F) MCP-1 were measured from 6 rats of each group. Data are expressed as the mean ± standard deviation, **P<0.01 vs. Sham group; #P<0.05, ##P<0.01 vs. THS group. IL, interleukin; LFM-A13, a Bruton's tyrosine kinase inhibitor; MCP-1, monocyte chemotactic protein 1; THS, trauma-hemorrhagic shock; TNF-α, tumor necrosis factor-α.
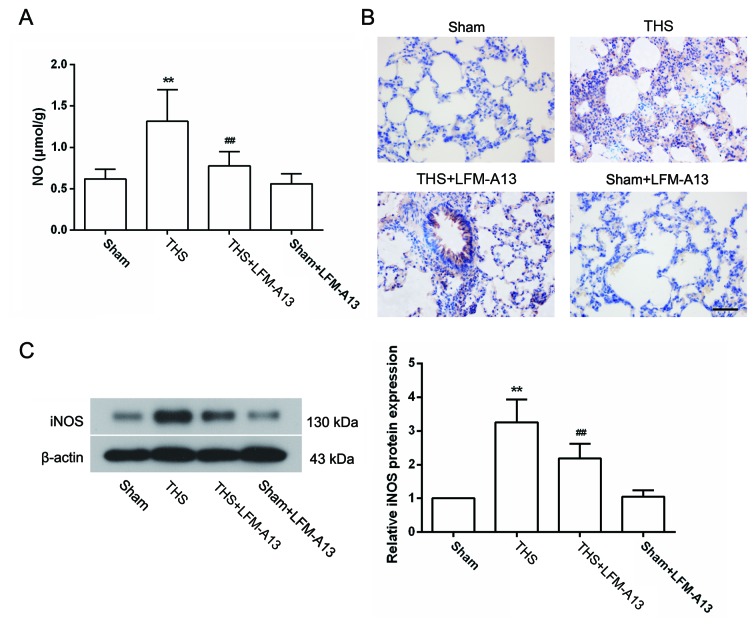
Effects of BTK on the expression of NO and iNOS in THS rats
The excessive production of NO by iNOS has been reported to be involved in the pathogenesis of THS-induced lung injury (29); therefore, the concentration of NO and the expression of iNOS in the lungs were measured. As shown in Fig. 4A, the concentration of NO in the lungs of THS rats was significantly increased compared with rats in the Sham group (P<0.01), and was significantly decreased by LFM-A13 treatment (P<0.01 vs. untreated THS rats). Treatment with LFM-A13 did not affect NO concentration in Sham rats (P>0.05 compared with the Sham group). Immunohistochemical analysis demonstrated that very little iNOS protein expression was detected in the lungs of rats in the Sham group, whereas iNOS was widely expressed in lung tissues of THS rats (Fig. 4B). The protein expression levels of iNOS were notably reduced by LFM-A13 treatment (Fig. 4B). These results were confirmed by western blot analysis (Fig. 4C), which demonstrated that the significant increase in iNOS protein expression induced by THS (P<0.01 vs. Sham) was significantly inhibited by LFM-A13 treatment (Fig. 4C; P<0.01 vs. untreated THS rats). In addition, immunohistochemical and western blot analysis revealed that iNOS expression levels in rats from the Sham + LFM-A13 group were not significantly different compared with rats in the Sham group.
Figure 4.
Effects of Bruton's tyrosine kinase on NO release and iNOS expression in THS rats. (A) Pulmonary NO concentrations were detected using a commercial kit (n=6 rats/group). iNOS protein expression levels were measured by (B) immunohistochemistry and (C) western blot analysis (n=5 rats/group). Data are expressed as the mean ± standard deviation; **P<0.01 vs. Sham group; ##P<0.01 vs. THS group. Scale bar, 50 µm. iNOS, inducible NO synthase; LFM-A13, a Bruton's tyrosine kinase inhibitor; NO, nitric oxide; THS, trauma-hemorrhagic shock.
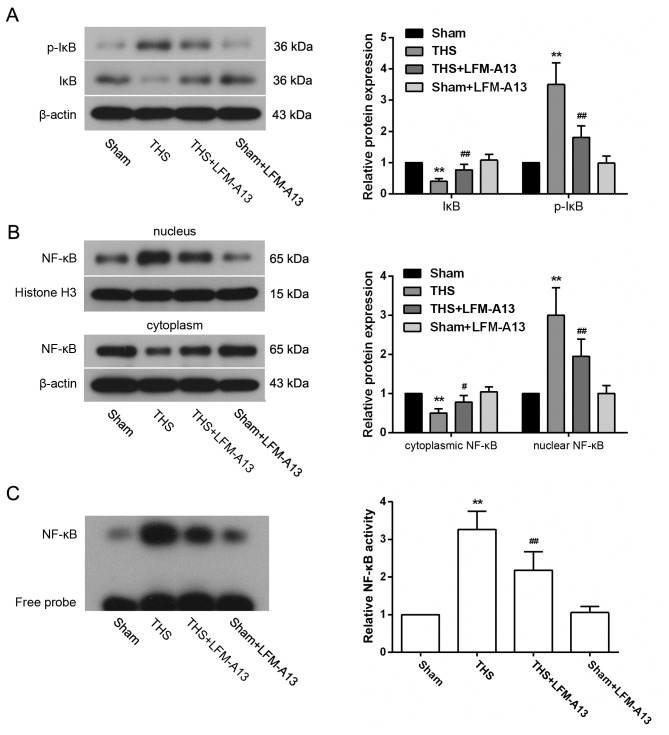
Effects of BTK on NF-κB activity
To investigate the role of NF-κB signaling in the observed protective effects of LFM-A13 on THS-induced lung injury, the activation of NF-κB expression in lungs post-THS induction was examined. p-IκB expression was significantly increased and the level of IκB was significantly decreased in the THS group compared with rats in the Sham group (P<0.01; Fig. 5A); conversely, the cytoplasmic expression of NF-κB was reduced and the nuclear expression levels of NF-κB were increased following THS induction (P<0.05 vs. Sham; Fig. 5B). However, these changes in IκB, p-IκB and NF-κB expression levels were dampened by LFM-A13 treatment in rats with THS-induced injury. p-IkB, IκB and NF-κB levels in rats from the Sham + LFM-A13 group were not significantly different compared with in rats in the Sham group (P>0.05). Similarly, EMSA experiments demonstrated an increase in the binding activity of NF-κB in the THS group, which was strongly reduced by LFM-A13 treatment (P<0.01 vs. Sham or untreated THS rats, respectively; Fig. 5C); treatment with LFM-A13 in Sham rats did not affect the binding activity of NF-κB when compared to the Sham group (P>0.05).
Figure 5.
Effects of Bruton's tyrosine kinase on pulmonary NF-κB activity in THS rats. Protein expression levels of (A) p-IκB and IκB, and (B) nuclear and cytoplasmic NF-κB were detected by western blotting and densitometric analysis. (C) Binding activity of NF-κB was analyzed by electrophoretic mobility shift assay (n=5 rats/group). Typical bands are shown. Data are expressed as the mean ± standard deviation, **P<0.01 vs. Sham group; #P<0.05, ##P<0.01 vs. untreated THS group. IκB, inhibitor of NF-κB; LFM-A13, a Bruton's tyrosine kinase inhibitor; NF-κB, nuclear factor-κB; p, phosphorylated; THS, trauma-hemorrhagic shock.
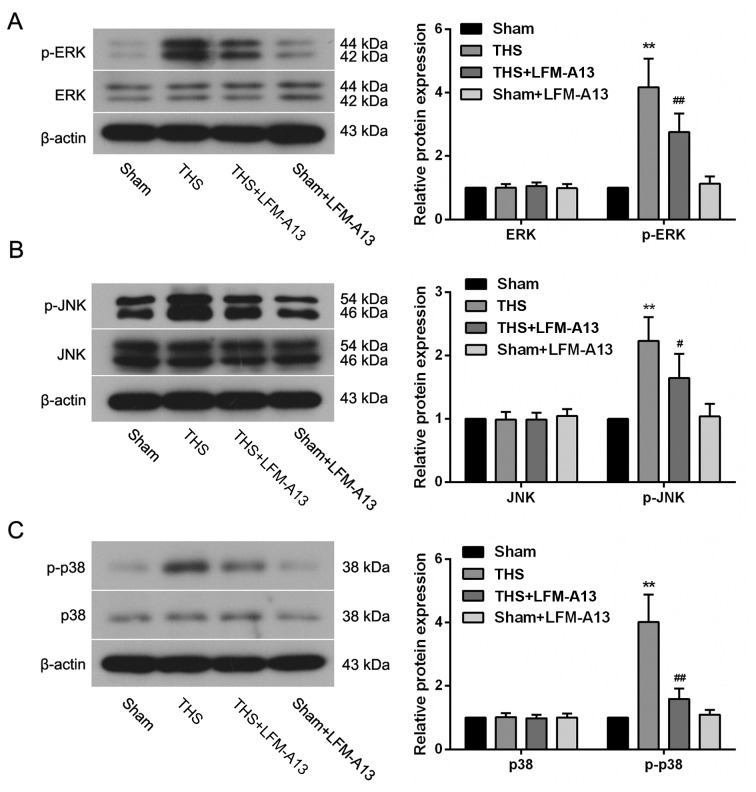
Effects of BTK on MAPK pathways
To further investigate the mechanism by which the inhibition of BTK protected lungs from THS-induced injury, components of the MAPK signaling pathway were examined. THS-induced injury resulted in a pronounced upregulation in the levels of p-ERK, p-JNK and p-p38 expression (P<0.01; Fig. 6A-C); however, treatment with LFM-A13 significantly reversed these changes in THS rats, indicating that inhibition of BTK may be able to suppress the THS-induced activation of MAPK pathways. The activity of MAPK-associated pathways in rats from the Sham + LFM-A13 group was similar compared with in rats from the Sham group (P>0.05),.
Figure 6.
Effects of Bruton's tyrosine kinase on the activation of mitogen-activated protein kinase signaling pathways following THS. The expression of (A) p-ERK, (B) p-JNK and (C) p-p38 was determined by western blotting (n=5 rats/group). Representative protein bands are included. Data are expressed as the mean ± standard deviation, **P<0.01 vs. Sham group; #P<0.05, ##P<0.01 vs. THS group. ERK, extracellular-signal regulated kinase; JNK, c-Jun N-terminal kinase; LFM-A13, a Bruton's tyrosine kinase inhibitor; p, phosphorylated; THS, trauma-hemorrhagic shock.
Discussion
The present study demonstrated that the activation of BTK was significantly increased in the lungs of rats following THS-induced injury. The results revealed that treatment with the BTK-specific inhibitor LFM-A13 appeared to protect pulmonary capillary permeability, suppress inflammatory cell infiltration, inhibit the inflammatory response and alleviate pathological damage. In addition, LFM-A13 treatment suppressed NO production, iNOS expression and the activation of NF-κB and MAPK signaling in rats with THS-induced injury, suggesting that these pathways may be a part of the mechanisms responsible for the pulmonary protective effects of BTK inhibition in THS rats.
Systemic inflammation is a major cause of mortality in patients with THS. A previous study reported that during hemorrhagic shock an overabundance of inflammatory cytokines were produced and severe visceral injury occurred (4). BTK is expressed in all hematopoietic cells, with the exception of plasma cells and T lymphocytes (30), and it is essential for lipopolysaccharide (LPS)-induced TNF-α production in mononuclear cells (31). Additional studies have demonstrated that the downregulation of BTK expression by small interfering RNA conferred strong protective effects against sepsis-induced acute lung injury (32,33). However, whether BTK is involved in THS-induced lung injury remains unknown. The present study revealed that BTK was highly activated in the lungs following THS-induced injury, indicating an essential role for BTK in THS-related pulmonary damage.
Hemorrhage-induced ischemia and subsequent reperfusion may result in the release of toxic mediators, resulting in systemic inflammatory reactions (34). Lung tissues are particularly vulnerable to injury caused by ischemia-reperfusion (I/R) (35). Inflammatory molecules, such as TNF-α, IL-1β, IL-6 and NO, and the infiltration of neutrophils may lead to an increase in microvascular permeability and pulmonary edema (36–38). Results from the present study demonstrated that BTK inhibition via LFM-A13 treatment attenuated pulmonary capillary permeability, reduced the inflammatory response and alleviated pulmonary pathological damage in THS rats. These findings were consistent with a previous study demonstrating that following BTK knockdown, the levels of inflammatory cytokines, as well as the lung pathological scores, were reduced in mice following cecal ligation and puncture-induced sepsis (32).
Low concentrations of NO were reported to be essential for microvascular perfusion and in maintaining organ function during the early phase of hypovolemic shock (39). However, the levels of iNOS expression were revealed to be upregulated following hemorrhage (40), and the resulting overproduction of NO enhanced inflammatory reactions during hemorrhagic shock-induced I/R, and further aggravated lung and liver injury (41). The inhibition of iNOS expression significantly reduced the strength of the inflammatory response and lung injury in hemorrhagic shock model mice (29). In the present study, LFM-A13 treatment significantly reduced the levels of NO concentration and iNOS expression in the lungs of THS rats, suggesting that the inhibition of BTK may protect the lungs from THS-induced injury through the suppression of NO production.
NF-κB and MAPK signaling are important regulatory pathways that have been previously reported to participate in the recruitment of neutrophils and the release of inflammatory cytokines (42,43). Additional reports demonstrated that hemorrhagic shock induced abnormal activation of the NF-κB and MAPK pathways, whereas the suppression of these pathways was revealed to aid in the protection of shock-induced organ damage (44,45). BTK may directly bind to TLR4 and mediate the expression of its downstream targets, such as p38 MAPK and NF-κB (11), and the inhibition of BTK expression significantly weakened LPS-induced NF-κB activation (46). In the absence of BTK, TLR3-triggered activation of MAPK and NF-κB signaling was abrogated (47). In line with these studies, results from the present study demonstrated that BTK inhibition significantly suppressed the THS-induced activation of NF-κB and MAPK signaling pathways. Therefore, the inhibition of BTK may protect lungs from THS-induced damage, in part by suppressing NF-κB and MAPK signaling.
In conclusion, the present study demonstrated that BTK was activated in the lungs of THS model rats and that the inhibition of BTK significantly attenuated pulmonary capillary permeability, reduced inflammatory reactions, improved lung pathological injury, and decreased NO and iNOS levels in THS model rats. The pulmonary protective effects of BTK inhibition appear to be at least partly due to the suppression of NF-κB and MAPK signaling. These data suggested that the inhibition of BTK may be a potential therapeutic method to protect lungs from THS-induced damage.
References
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: An overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. (6 Suppl) [DOI] [PubMed] [Google Scholar]
- 2.Kauvar DS, Wade CE. The epidemiology and modern management of traumatic hemorrhage: US and international perspectives. Crit Care. 2005;9:S1–S9. doi: 10.1186/cc3752. (Suppl 5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angele MK, Schneider CP, Chaudry IH. Bench-to-bedside review: Latest results in hemorrhagic shock. Crit Care. 2008;12:218. doi: 10.1186/cc6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai B, Deitch EA, Ulloa L. Novel insights for systemic inflammation in sepsis and hemorrhage. Mediators Inflamm. 2010;2010:642462. doi: 10.1155/2010/642462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CC, Chang IJ, Yen ZS, Hsu CY, Chen SY, Su CP, Chiang WC, Chen SC, Chen WJ. Delayed fluid resuscitation in hemorrhagic shock induces proinflammatory cytokine response. Ann Emerg Med. 2007;49:37–44. doi: 10.1016/j.annemergmed.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Claridge JA, Schulman AM, Young JS. Improved resuscitation minimizes respiratory dysfunction and blunts interleukin-6 and nuclear factor-kappa B activation after traumatic hemorrhage. Crit Care Med. 2002;30:1815–1819. doi: 10.1097/00003246-200208000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, Huang Y, Xu H, Hu R, Li QF. Inhibition of hypoxia inducible factor-1α ameliorates lung injury induced by trauma and hemorrhagic shock in rats. Acta Pharmacol Sin. 2012;33:635–643. doi: 10.1038/aps.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koscsó B, Trepakov A, Csóka B, Németh ZH, Pacher P, Eltzschig HK, Haskó G. Stimulation of A2B adenosine receptors protects against trauma-hemorrhagic shock-induced lung injury. Purinergic Signal. 2013;9:427–432. doi: 10.1007/s11302-013-9362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang WC, Collette Y, Nunès JA, Olive D. Tec kinases: A family with multiple roles in immunity. Immunity. 2000;12:373–382. doi: 10.1016/S1074-7613(00)80189-2. [DOI] [PubMed] [Google Scholar]
- 10.Mohamed AJ, Yu L, Bäckesjö CM, Vargas L, Faryal R, Aints A, Christensson B, Berglöf A, Vihinen M, Nore BF, Smith CI. Bruton's tyrosine kinase (Btk): Function, regulation, and transformation with special emphasis on the PH domain. Immunol Rev. 2009;228:58–73. doi: 10.1111/j.1600-065X.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 11.Jefferies CA, O'Neill LA. Bruton's tyrosine kinase (Btk)-the critical tyrosine kinase in LPS signalling? Immunol Lett. 2004;92:15–22. doi: 10.1016/j.imlet.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Fluckiger AC, Li Z, Kato RM, Wahl MI, Ochs HD, Longnecker R, Kinet JP, Witte ON, Scharenberg AM, Rawlings DJ. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J. 1998;17:1973–1985. doi: 10.1093/emboj/17.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999;402:B24–B30. doi: 10.1038/35037021. (6760 Suppl) [DOI] [PubMed] [Google Scholar]
- 14.Bajpai UD, Zhang K, Teutsch M, Sen R, Wortis HH. Bruton's tyrosine kinase links the B cell receptor to nuclear factor kappaB activation. J Exp Med. 2000;191:1735–1744. doi: 10.1084/jem.191.10.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu Y, Kung HJ. Signaling network of the Btk family kinases. Oncogene. 2000;19:5651–5661. doi: 10.1038/sj.onc.1203958. [DOI] [PubMed] [Google Scholar]
- 16.Lindvall J, Islam TC. Interaction of Btk and Akt in B cell signaling. Biochem Biophys Res Commun. 2002;293:1319–1326. doi: 10.1016/S0006-291X(02)00382-0. [DOI] [PubMed] [Google Scholar]
- 17.Mueller H, Stadtmann A, Van Aken H, Hirsch E, Wang D, Ley K, Zarbock A. Tyrosine kinase Btk regulates E-selectin-mediated integrin activation and neutrophil recruitment by controlling phospholipase C (PLC) gamma2 and PI3Kgamma pathways. Blood. 2010;115:3118–3127. doi: 10.1182/blood-2009-11-254185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakuma C, Sato M, Takenouchi T, Chiba J, Kitani H. Critical roles of the WASP N-terminal domain and Btk in LPS-induced inflammatory response in macrophages. PLoS One. 2012;7:e30351. doi: 10.1371/journal.pone.0030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kutsch N, Marks R, Ratei R, Held TK, Schmidt-Hieber M. Role of tyrosine kinase inhibitors in indolent and other mature B-Cell neoplasms. Biomark Insights. 2015;10:S15–S23. doi: 10.4137/BMI.S22434. (Suppl 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Rooij MF, Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ, Pals ST, Spaargaren M. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119:2590–2594. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 21.Di Paolo JA, Huang T, Balazs M, Barbosa J, Barck KH, Bravo BJ, Carano RA, Darrow J, Davies DR, DeForge LE, et al. Specific Btk inhibition suppresses B cell- and myeloid cell-mediated arthritis. Nat Chem Biol. 2011;7:41–50. doi: 10.1038/nchembio.481. [DOI] [PubMed] [Google Scholar]
- 22.Akinleye A, Chen Y, Mukhi N, Song Y, Liu D. Ibrutinib and novel BTK inhibitors in clinical development. J Hematol Oncol. 2013;6:59. doi: 10.1186/1756-8722-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, Buggy JJ. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai EW, Toledo-Pereyra LH, Walsh J, Lopez-Neblina F, Anaya-Prado R. The role of MAP kinases in trauma and ischemia-reperfusion. J Invest Surg. 2004;17:45–53. doi: 10.1080/08941930490269646. [DOI] [PubMed] [Google Scholar]
- 25.Jarrar D, Chaudry IH, Wang P. Organ dysfunction following hemorrhage and sepsis: Mechanisms and therapeutic approaches (Review) Int J Mol Med. 1999;4:575–583. doi: 10.3892/ijmm.4.6.575. [DOI] [PubMed] [Google Scholar]
- 26.Kiang JG, Agravante NG, Smith JT, Bowman PD. 17-DMAG diminishes hemorrhage-induced small intestine injury by elevating Bcl-2 protein and inhibiting iNOS pathway, TNF-α increase, and caspase-3 activation. Cell Biosci. 2011;1:21. doi: 10.1186/2045-3701-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy G, Fishman JE, Xu DZ, Dong W, Palange D, Vida G, Mohr A, Ulloa L, Deitch EA. Vagal nerve stimulation modulates gut injury and lung permeability in trauma-hemorrhagic shock. J Trauma Acute Care Surg. 2012;73:338–342. doi: 10.1097/TA.0b013e31825debd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menzel CL, Sun Q, Loughran PA, Pape HC, Billiar TR, Scott MJ. Caspase-1 is hepatoprotective during trauma and hemorrhagic shock by reducing liver injury and inflammation. Mol Med. 2011;17:1031–1038. doi: 10.2119/molmed.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hierholzer C, Harbrecht B, Menezes JM, Kane J, MacMicking J, Nathan CF, Peitzman AB, Billiar TR, Tweardy DJ. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med. 1998;187:917–928. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desiderio S. Role of Btk in B cell development and signaling. Curr Opin Immunol. 1997;9:534–540. doi: 10.1016/S0952-7915(97)80107-0. [DOI] [PubMed] [Google Scholar]
- 31.Horwood NJ, Mahon T, McDaid JP, Campbell J, Mano H, Brennan FM, Webster D, Foxwell BM. Bruton's tyrosine kinase is required for lipopolysaccharide-induced tumor necrosis factor alpha production. J Exp Med. 2003;197:1603–1611. doi: 10.1084/jem.20021845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou P, Ma B, Xu S, Zhang S, Tang H, Zhu S, Xiao S, Ben D, Xia Z. Knockdown of Burton's tyrosine kinase confers potent protection against sepsis-induced acute lung injury. Cell Biochem Biophys. 2014;70:1265–1275. doi: 10.1007/s12013-014-0050-1. [DOI] [PubMed] [Google Scholar]
- 33.Krupa A, Fol M, Rahman M, Stokes KY, Florence JM, Leskov IL, Khoretonenko MV, Matthay MA, Liu KD, Calfee CS, et al. Silencing Bruton's tyrosine kinase in alveolar neutrophils protects mice from LPS/immune complex-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;307:L435–L448. doi: 10.1152/ajplung.00234.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olanders K, Sun Z, Börjesson A, Dib M, Andersson E, Lasson A, Ohlsson T, Andersson R. The effect of intestinal ischemia and reperfusion injury on ICAM-1 expression, endothelial barrier function, neutrophil tissue influx, and protease inhibitor levels in rats. Shock. 2002;18:86–92. doi: 10.1097/00024382-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Kao MC, Yang CH, Sheu JR, Huang CJ. Cepharanthine mitigates pro-inflammatory cytokine response in lung injury induced by hemorrhagic shock/resuscitation in rats. Cytokine. 2015;76:442–448. doi: 10.1016/j.cyto.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Ding R, Han J, Tian Y, Guo R, Ma X. Sphingosine-1-phosphate attenuates lung injury induced by intestinal ischemia/reperfusion in mice: Role of inducible nitric-oxide synthase. Inflammation. 2012;35:158–166. doi: 10.1007/s10753-011-9301-0. [DOI] [PubMed] [Google Scholar]
- 37.Hierholzer C, Harbrecht BG, Billiar TR, Tweardy DJ. Hypoxia-inducible factor-1 activation and cyclo-oxygenase-2 induction are early reperfusion-independent inflammatory events in hemorrhagic shock. Arch Orthop Trauma Surg. 2001;121:219–222. doi: 10.1007/s004020000211. [DOI] [PubMed] [Google Scholar]
- 38.Ishii H, Ishibashi M, Takayama M, Nishida T, Yoshida M. The role of cytokine-induced neutrophil chemoattractant-1 in neutrophil-mediated remote lung injury after intestinal ischaemia/reperfusion in rats. Respirology. 2000;5:325–331. doi: 10.1111/j.1440-1843.2000.00271.x. [DOI] [PubMed] [Google Scholar]
- 39.Cabrales P, Tsai AG, Intaglietta M. Exogenous nitric oxide induces protection during hemorrhagic shock. Resuscitation. 2009;80:707–712. doi: 10.1016/j.resuscitation.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szabo C, Billiar TR. Novel roles of nitric oxide in hemorrhagic shock. Shock. 1999;12:1–9. doi: 10.1097/00024382-199907000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Collins JL, Vodovotz Y, Hierholzer C, Villavicencio RT, Liu S, Alber S, Gallo D, Stolz DB, Watkins SC, Godfrey A, et al. Characterization of the expression of inducible nitric oxide synthase in rat and human liver during hemorrhagic shock. Shock. 2003;19:117–122. doi: 10.1097/00024382-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Partrick DA, Moore FA, Moore EE, Barnett CC, Jr, Silliman CC. Neutrophil priming and activation in the pathogenesis of postinjury multiple organ failure. New Horiz. 1996;4:194–210. [PubMed] [Google Scholar]
- 43.Botha AJ, Moore FA, Moore EE, Kim FJ, Banerjee A, Peterson VM. Postinjury neutrophil priming and activation: An early vulnerable window. Surgery. 1995;118:358–365. doi: 10.1016/S0039-6060(05)80345-9. [DOI] [PubMed] [Google Scholar]
- 44.Jeong KY, Suh GJ, Kwon WY, Kim KS, Jung YS, Kye YC. The therapeutic effect and mechanism of niacin on acute lung injury in a rat model of hemorrhagic shock: Down-regulation of the reactive oxygen species-dependent nuclear factor κB pathway. J Trauma Acute Care Surg. 2015;79:247–255. doi: 10.1097/TA.0000000000000761. [DOI] [PubMed] [Google Scholar]
- 45.Kochanek AR, Fukudome EY, Li Y, Smith EJ, Liu B, Velmahos GC, deMoya M, King D, Alam HB. Histone deacetylase inhibitor treatment attenuates MAP kinase pathway activation and pulmonary inflammation following hemorrhagic shock in a rodent model. J Surg Res. 2012;176:185–194. doi: 10.1016/j.jss.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jefferies CA, Doyle S, Brunner C, Dunne A, Brint E, Wietek C, Walch E, Wirth T, O'Neill LA. Bruton's tyrosine kinase is a Toll/interleukin-1 receptor domain-binding protein that participates in nuclear factor kappaB activation by Toll-like receptor 4. J Biol Chem. 2003;278:26258–26264. doi: 10.1074/jbc.M301484200. [DOI] [PubMed] [Google Scholar]
- 47.Lee KG, Xu S, Kang ZH, Huo J, Huang M, Liu D, Takeuchi O, Akira S, Lam KP. Bruton's tyrosine kinase phosphorylates Toll-like receptor 3 to initiate antiviral response. Proc Natl Acad Sci USA. 2012;109:5791–5796. doi: 10.1073/pnas.1119238109. [DOI] [PMC free article] [PubMed] [Google Scholar]