Abstract
The aim of the present study was to determine the toxic targets of proteins from Croton tiglium L. and to investigate the potential mechanism of their toxicity. The toxic targets were determined by oral medication and intraperitoneal injection. The median lethal dose of oral medication in mice was calculated using Bliss software (2,752.8–3,407.5 mg/kg), and that of intraperitoneal injection was 195.8–272.69 mg/kg. The results of histopathological examination demonstrated that the kidney was primarily impaired by intraperitoneal injection, with slight degeneration of renal tubular epithelial cells. As to oral medication, the digestive tract was primarily injured, which manifested as congestion, bleeding, serious edema and other symptoms. Oral administration of the proteins caused gastrointestinal edema by increasing the intestinal permeability. Severe edema was associated with the inflammatory response, therefore the association between the toxicity of the proteins and inflammation was investigated. The proinflammatory effects of the crude proteins on the release of inflammatory mediator prostaglandin E2 (PGE2) were evaluated through intraperitoneal injection and the production of proinflammatory cytokines in RAW264.7 macrophages. Maximum PGE2 was released in the mice in vivo following intraperitoneal injection with 400 mg crude protein/kg body weight. Proinflammatory cytokines in macrophages, including tumor necrosis factor-α and interleukin-1β, were produced in dose- and time-dependent manners in vitro. furthermore, the expressions of cell signaling molecules were detected by western blotting. The inflammatory response induced by crude protein in macrophages was associated with the mitogen-activated protein kinase (MAPK) signaling pathway mainly including p38-MAPK, extracellular signal-regulated kinase 1/2 and c-Jun N-terminal kinase 1/2/3 and the activated p38-MAPK signaling pathway. However, extracellular signal-regulated kinase 1/2 and c-Jun N-terminal kinases 1–3 exhibited no significant response.
Keywords: Croton tiglium L. proteins, toxicity, gastrointestinal edema, inflammatory response, macrophages, mitogen-activated protein kinase signaling pathways, p38-mitogen-activated protein kinase
Introduction
In recent years, the toxicity of traditional Chinese medicine, which has attracted increasing attention, restricts it development and modernization. Croton tiglium L., as one of the most poisonous traditional Chinese medicines, is officially prescribed as the dried mature seeds of the Euphorbiaceae C. tiglium L. (1). C. tiglium L. is widely distributed in tropical and subtropical zones, including India, New Guinea, Java, Indonesia, China and the Philippine islands (2). As a traditional herbal medicine, C. tiglium L. has been primarily used to treat indigestion, abdominal distension, visceral pain, constipation and other gastrointestinal disorders (3,4). However, it also causes severe gastrointestinal syndrome and even mortality following ingestion (5). Although the use of C. tiglium L. has a long history, its toxicity remains largely unknown. A preliminary study attributed its toxicity to irritating oils and toxic proteins (6). Oral medication of croton oil is able to cause gastrointestinal edema and diarrhea (7). The toxicity of croton proteins is typically evaluated by intraperitoneal injection; however, C. tiglium L. is orally administrated in clinical practice. Therefore, evaluating the toxicity of croton proteins by intraperitoneal injection is not sufficiently accurate. Chen et al (8) demonstrated that edema and diarrhea of the digestive tract were primarily caused by the inflammatory response.
Inflammation is one of the most important protective defensive reactions to tissue injury. Once the body is stimulated by chemical drugs, appropriate inflammatory responses are activated to maintain homeostasis (9,10). In response to stimulation in the process of inflammation, macrophages serve a key role as defending cells and release large amounts of inflammatory cytokines (11,12). Simultaneously, the injured tissues or cells release inflammatory mediator prostaglandin E2 (PGE2) and a large amount of proinflammatory cytokines, including tumor necrosis factor (TNF)-α and interleukin (IL)-1β, in order to maintain balance (13,14). The release of proinflammatory cytokines is associated with the activation of cellular signaling pathways (15). The mitogen-activated protein kinase (MAPK) signaling pathways, as key signaling molecules, serve an important role in the inflammatory response, mediating the generation of cytokines (16). The MAPK family primarily comprises p38-MAPK, extracellular signal-regulated kinase (ERK)1/2 and c-Jun N-terminal kinases (JNKs), therefore the three primary pathways should be studied to analyze the inflammatory responses (17,18). In the present study, the toxic targets of proteins from C. tiglium L. were determined by oral medication and intraperitoneal injection. The inflammatory response was evaluated by intraperitoneal injection and in RAW264.7 macrophages, and the proinflammatory effects of crude protein on the release of PGE2 were evaluated through intraperitoneal injection and production of proinflammatory cytokines in macrophages. The inflammatory response induced by crude protein was associated with MAPK signaling.
Materials and methods
Materials
C. tiglium L. was collected from Sichuan province (China). The PGE2 kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), penicillin and streptomycin were obtained from Gibco (Thermo Fisher Scientific, Inc.). Primary antibodies, including anti-phosphorylated (p)-ERK (cat. no. 9101S), anti-ERK (cat. no. 9102S), anti-p-JNK (cat. no. 9251S), anti-JNK (cat. no. 9252S), anti-p-p38 (cat. no. 9211S), and anti-p38 antibodies (cat. no. 9212S), were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA). Anti-GAPDH (cat. no. YFPA1059) was obtained from Yi Fei Xue Biotech Co., Ltd. (Nanjing, China). Goat anti-rabbit secondary antibodies (cat. no. 10285-1-AP) were purchased from ProteinTech Group, Inc. (Chicago, IL, USA).
Animals
A total of 230 ICR mice, (115 female and 115 male, aged 8–10 weeks old; weight, 18–22 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). The mice were separated by sex in plastic cages with stainless steel mesh lids in a ventilated room. The room was maintained at standard temperature (20°C) and humidity (50–60%) with a 12 h light-dark cycle. The mice were given sterilized water and food. Prior to treatment, the mice were not fed for >12 h. The mice were fed with a standard diet ad libitum. The present study was approved by the Animal Ethics Committee of Nanjing University of Chinese Medicine (Nanjing, China) and performed according to the Guidelines for the Care and Use of Laboratory Animals (19).
Cell culture
The murine RAW264.7 macrophage cell line was obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China), and cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a humidified atmosphere containing 5% CO2. The cells were sub-cultured every 2–3 days.
Extraction of crude protein
The extraction was performed as previously described (6). Decorticated seeds of C. tiglium L. were powdered using a blender and extracted with petroleum ether until colorless. The petroleum ether was subsequently removed by leaching. The resulting powders were allowed to dry in a ventilated room and extracted with PBS (Concentration=0.01 mol/l; pH 7.2). The mixture was stirred on a magnetic stirrer for 2–3 h and left overnight at 4°C. The supernatant was collected by centrifugation at 10,000 × g for 20 min at 4°C, brought to 80% saturation with saturated (NH4)2SO4 added slowly with constant stirring and subsequently left overnight at 4°C. Protein precipitate was collected by centrifugation at 10,000 × g for 20 min at 4°C, dissolved in the minimum amount of PBS and dialyzed for 48 h against a continuous flow of the same buffer. The supernatant was collected by centrifugation at 10,000 × g for 20 min at 4°C, filtered, freeze-dried and stored in a refrigerator at −80°C, giving crude protein powders.
Animal treatment and sample collection
Acute toxicity study
i) Oral administration. For the oral acute toxicity study, a series of doses were set based on a median lethal dose (LD50) estimated in the present study: Dmax=4,996.59 mg/kg; Dmin=2,094.56 mg/kg. A total of 60 mice of either sex were exposed to 4,996.59, 3,999.67, 3,198.82 2,558.89 2,050.68 and 0 mg/kg crude protein. Crude protein in physiological saline solution was administered. The mice were also intragastrically administrated with sterile physiological saline as a control. Following the intragastric administration, symptoms and mortality were observed and recorded throughout the experiment. At the end of the experiment, all animals were sacrificed for subsequent study and histological analysis of main organs. LD50 was estimated according to the Bliss method (20) using LD50CALC version 2.0, developed by Professor Jianhua Wang (Zhongshan School of Medicine, Guangzhou, China).
ii) Intraperitoneal administration. Acute toxicity was studied using intraperitoneal injection and based on an LD50 estimating study: Dmax=523.64 mg/kg; Dmin=53.46 mg/kg. A total of 90 mice of either sex were exposed to 523.64, 414.95, 331.68, 265.54, 212.24, 169.65, 135.49, 108.22 and 0 mg/kg crude protein. Crude protein in physiological saline solution was administered intraperitoneally. The following steps were the same as those for intragastric administration. LD50 was estimated according to the Bliss method (Bliss, 1935).
Histopathological examination
The digestive tract, heart, liver, spleen, lung, kidney and brain were removed, fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin for histological examination using an optical microscope at ×400 magnification. A professional pathologist was blind to the identity and analysis of the pathological sections, and score for the injuries.
Gut permeability
Following overnight fasting, a total of 40 ICR mice of either sex were orally administrated with 0, 0.75, 1.5 and 3 g/kg crude protein (10 g/0.2 ml). After 1 h, they intragastrically received fluorescein isothiocyanate (FITC)-conjugated dextran (0.2 mg/g body weight; mean molecular weight, 3,000–5,000 kDa; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) by oral administration. Plasma (200 µl) was sampled from the mouse eyes every 1 h. The FITC-dextran concentration in the plasma was determined with a fluorescent microplate reader (excitation, 485 nm; emission, 535 nm).
Inflammatory mediator PGE2 assay
A total of 60 ICR mice of either sex were administrated intraperitoneally with 25, 50, 100, 200 and 400 mg/kg crude protein. The control group was injected with physiological saline. The dose was 0.2 ml/10 g. After 30 min, all animals were sacrificed for subsequent experimentation. The dead mice were dissected, with the abdominal membrane exposed and subsequently injected with 1 ml physiological saline. The peritoneal fluid was thereafter collected in 5 min. The content of inflammatory mediator PGE2 was determined according to the instructions of the PGE2 kit.
TNF-α and IL-1β assay
RAW264.7 macrophages were plated on 48-well plates at a density of 1×105 cells/well and cultured in serum-free DMEM medium (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA). To analyze the dose dependence, the cells were treated with crude protein (0.78125–400 µg/ml) stimulation for 3 h to determine the highest concentration of proinflammatory effect. To study time dependence, the association between the proinflammatory effect and time at the highest concentration was investigated. The release of TNF-α (cat. no. 88-7324-86; eBioscience; Thermo Fisher Scientific, Inc.) and IL-1β (cat. no. EK201B2; Multi Sciences Biotech Co., Ltd.) by the cells was determined by ELISA using commercial kits and following manufacturer's protocol. The culture supernatant (100 µl) was used for the estimation, with its absorbance determined at 450 nm and 570 nm. All estimations were performed in triplicate.
Western blotting
Macrophages were inoculated at a density of 1×107 cells/ml in a cell culture dish and cultured in the serum starvation state. The cultured macrophages were divided into four groups (experiments for each group were performed in triplicate), of which three groups were given croton protein solutions with the same volume but at different concentrations, making the final concentrations 25, 50 and 100 µg/ml. The blank group was given DMEM of the same volume and the cells were cultured for 3 h in 5% CO2 at 37°C following shaking. After 3 h, RAW264.7 cells were washed with cord buffer (10 mM PBS, 0.0067 M (PO4), Ca, Mg; pH 7.4) and subsequently lysed with radioimmunoprecipitation assay buffer (Nanjing KeyGen Biotech Co., Ltd.) containing a protease inhibitor mixture (0.1 mM phenylmethylsulfonyl fluoride, 5 mg/ml aprotinin, 5 mg/ml pepstatin A and 1 mg/ml chymostatin). Following the centrifugation at 825 × g, 4°C for 10 min, the supernatant was collected and protein concentrations were determined using a bicinchoninic acid kit (Thermo Fisher Scientific, Inc.). A total of 40 µg cellular protein was subjected to SDS-PAGE on a 10% gel and transferred to a PVDF membrane, which was activated in methanol. The membrane was incubated with 5% bovine serum albumin (BSA; Biosharp, Hefei, China) for 2 h at room temperature, followed by incubation with primary antibodies at 1:1,000 (v/v) dilution in 5% BSA overnight at 4°C. Blots were washed three times with Tween-20/TBS (TTBS) and incubated with a 1:10,000 (v/v) dilution of horseradish peroxidase (HRP)-conjugated secondary antibody for 2 h at room temperature. GAPDH was used as a loading control. The blots were washed three times again with TTBS, probed by enhanced chemiluminescence HRP substrate and autoradiographed using Image Lab version 3.0 (Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
In order to compare the control and treated groups, the Student's t-test was used. The data are presented as the mean ± standard error and were statistically analyzed using SPSS software (version 19; IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
LD50
According to the Bliss analysis, LD50 of oral medication (Feiller correction) at 95% credibility was 2,752.8–3,407.5 mg/kg (Table I) and that of intraperitoneal injection (Feiller correction) at 95% credibility was 195.8–272.69 mg/kg (Table II).
Table I.
Bliss analysis for the oral median lethal dose of Croton tiglium L. protein.
| Dose, mg/kg | Logarithmic dose, x | Mouse no. | No. of mortalities | Percentage mortality, % | Probability unit, Y | Regression probability, Y |
|---|---|---|---|---|---|---|
| 4996.59 | 3.70 | 10 | 10 | 100.00 | n/a | 7.61 |
| 3999.67 | 3.60 | 10 | 9 | 90.00 | 6.28 | 6.42 |
| 3198.82 | 3.50 | 10 | 6 | 60.00 | 5.25 | 5.23 |
| 2558.89 | 3.41 | 10 | 2 | 20.00 | 4.16 | 4.04 |
| 2050.68 | 3.31 | 10 | 0 | 0.00 | n/a | 2.84 |
n/a, not applicable.
Table II.
Bliss analysis for the intraperitoneal median lethal dose of Croton tiglium L. protein.
| Dose, mg/kg | Logarithmic dose, x | Mouse no. | No. of mortalities | Percentage mortality, % | Probability unit, Y | Regression probability, Y |
|---|---|---|---|---|---|---|
| 523.64 | 2.72 | 10 | 10 | 100.00 | n/a | 6.77 |
| 14.95 | 2.62 | 10 | 9 | 90.00 | 6.28 | 6.27 |
| 331.68 | 2.52 | 10 | 7 | 70.00 | 5.52 | 5.78 |
| 265.54 | 2.42 | 10 | 6 | 60.00 | 5.25 | 5.30 |
| 212.24 | 2.33 | 10 | 4 | 40.00 | 4.75 | 4.81 |
| 169.65 | 2.23 | 10 | 3 | 30.00 | 4.48 | 4.33 |
| 135.49 | 2.13 | 10 | 2 | 20.00 | 4.16 | 3.84 |
| 108.22 | 2.03 | 10 | 0 | 0.00 | n/a | 3.35 |
n/a, not applicable.
Pathological examination
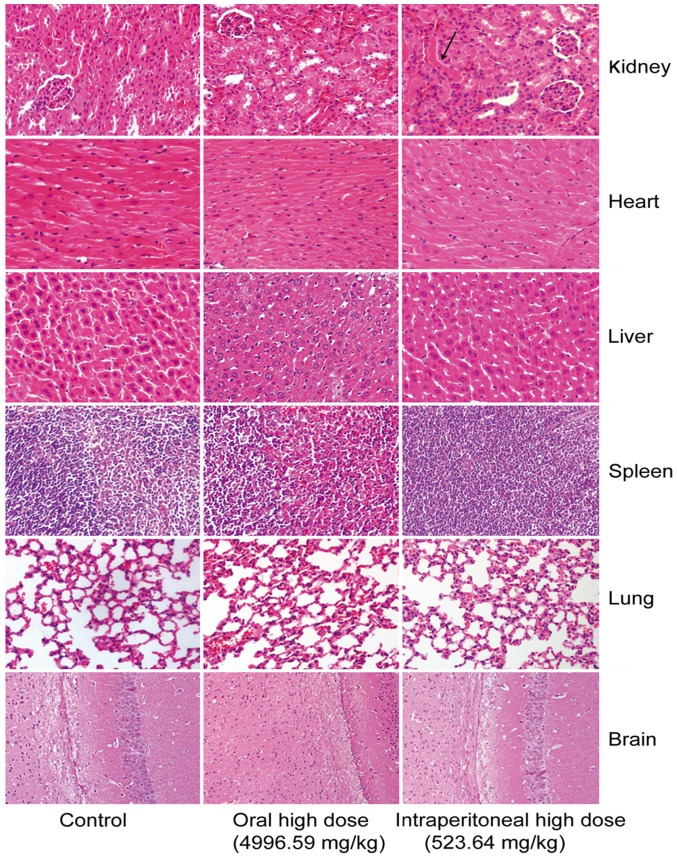
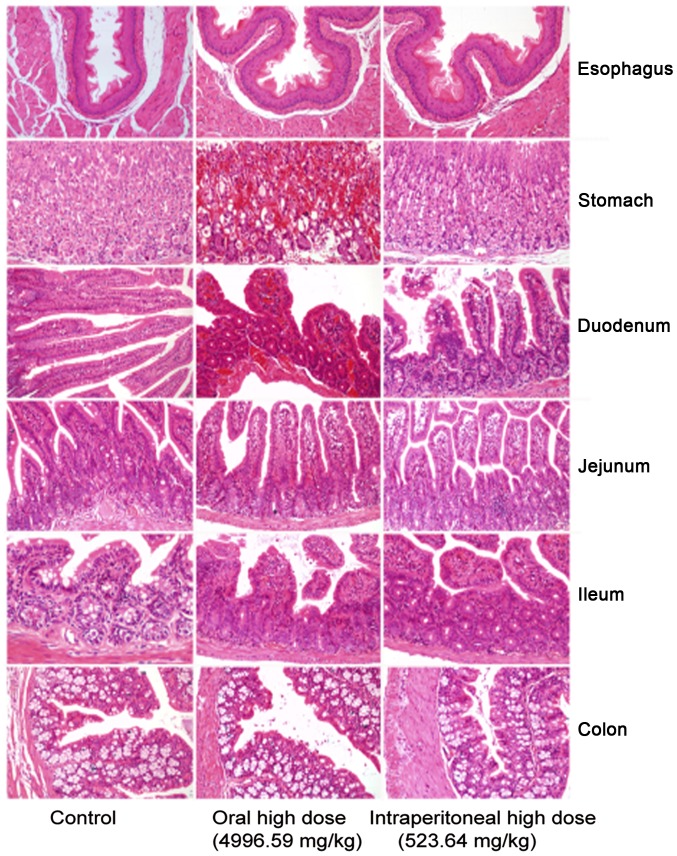
In the control group, gastrointestinal sections exhibited healthy structural features. The oral medication and intraperitoneal injection groups exhibited obvious mucous layer, epithelial cell and interstitial damages. The oral medication and intraperitoneal injection groups primarily suffered from mucosal necrosis, epithelial cell necrosis or erosion, interstitial hyperemia or bleeding, mucosal congestion and villous edema in the intestine. However, they exhibited different extents of gastrointestinal damage (Fig. 1). The mice intraperitoneally administrated with crude protein underwent degeneration of renal tubular epithelial cells in the kidney. No marked difference was observed in other tissues or organs (Fig. 2).
Figure 1.
Histological analysis of the digestive tract in mice. Oral medication and intraperitoneal injection groups exhibited marked mucous layer, epithelial cell and interstitial damage. Mice primarily suffered from mucosal necrosis, epithelial cell necrosis or erosion, interstitial hyperemia or bleeding, mucosal congestion and villous edema in the intestine. However, the groups exhibited different extents of gastrointestinal damage.
Figure 2.
Histological analyses of the kidney, heart, liver, spleen, lung and brain in crude protein-treated mice. The mice intraperitoneally administrated with crude protein underwent degeneration of renal tubular epithelial cells in the kidney. No marked difference was observed in other tissues or organs.
Evaluation of intestinal permeability with FITC-dextran
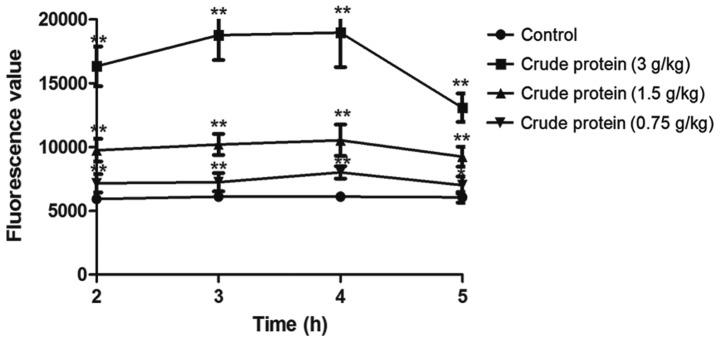
Compared with the control group (Fig. 3), croton proteins significantly increased the intestinal permeability of the oral administration group. When the dose reached 3 g/kg, the intestinal permeability reached maximum. Following 4 h of treatment, the digestive tract was most obviously damaged, and the intestinal permeability gradually recovered. Meanwhile, the toxicity was gradually reduced.
Figure 3.
Evaluation of intestinal permeability. Compared with the control group, treatment with croton proteins significantly increased the intestinal permeability of the oral administration group. Intestinal permeability reached a maximum following treatment with 3 g/kg. After 4 h of treatment, the digestive tract was most noticeably damaged and the intestinal permeability gradually recovered. *P<0.05, **P<0.01 vs. control.
In vivo proinflammatory evaluation
PGE2 assay
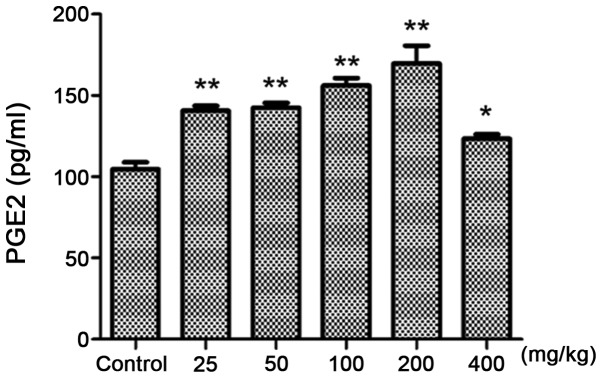
Compared with the control group (Fig. 4), 25 mg/kg croton proteins significantly increased PGE2 in the abdominal fluid (P<0.01). When the dose reached 200 mg/kg, the content of PGE2 reached the maximum. Therefore, the toxicity of croton proteins is associated with inflammation.
Figure 4.
PGE2 contents of mice treated with crude protein. Treatment with crude protein increased PGE2 in the abdominal fluid. *P<0.05, **P<0.01 vs. control.
Croton proteins induces the release of the proinflammatory cytokines TNF-α and IL-1β in RAW264.7 macrophages in a dose-dependent manner
Croton proteins significantly stimulated the production of proinflammatory cytokines dose-dependently (Fig. 5). Compared with the control group, croton proteins (50, 100, 200 and 400 µg/ml) increased cytokines, including TNF-α and IL-1β, significantly after 3 h of treatment (P<0.01). Furthermore, when the concentration of croton proteins reached 100 µg/ml, maximum TNF-α and IL-1β were released. In the presence of croton proteins at >100 µg/ml the TNF-α and IL-1 levels began to decrease.
Figure 5.
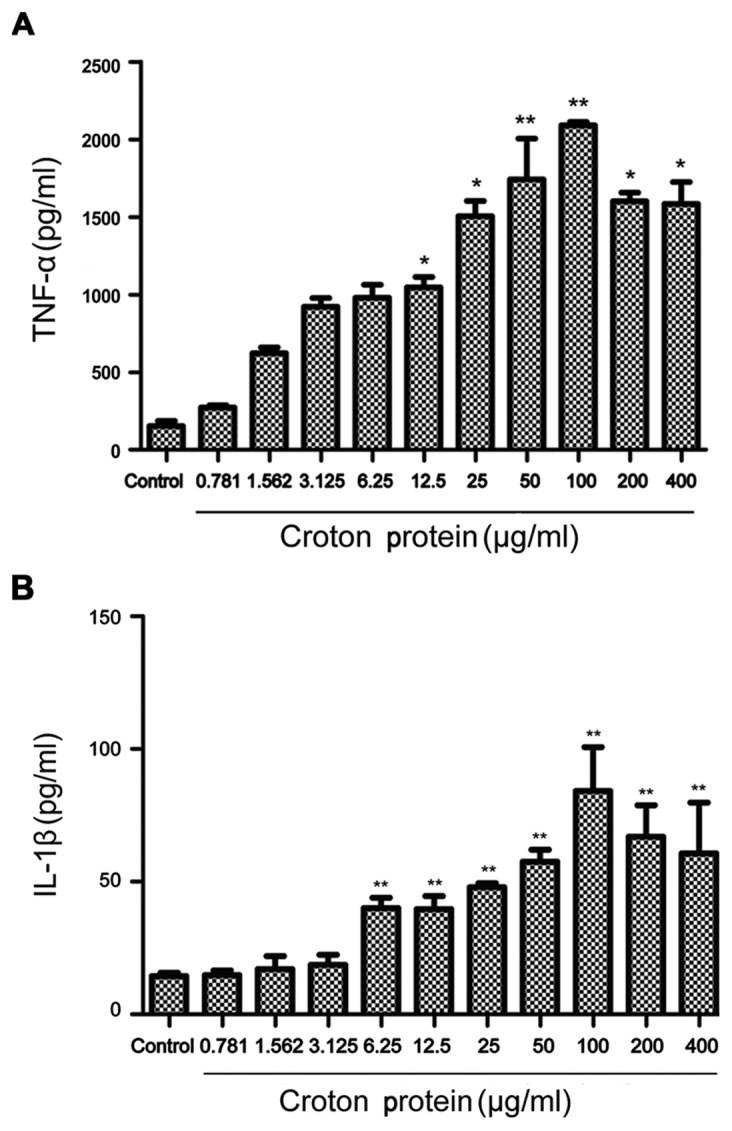
Evaluation of cytokine levels following treatment with crude protein. (A) TNF-α levels following treatment with crude protein. (Β) IL-1β levels following treatment with crude protein. *P<0.05, **P<0.01 vs. control. ΤΝF, tumor necrosis factor; IL, interleukin.
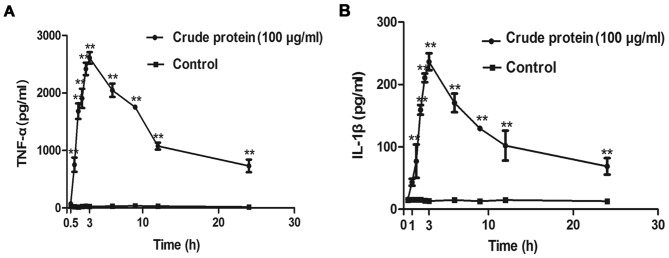
Croton proteins induces the release of the proinflammatory cytokines TNF-α and IL-1β in RAW264.7 macrophages in a time-dependent manner
Croton proteins also stimulated the production of proinflammatory cytokines in a time-dependent manner (Fig. 6). After 3 h of treatment, the release of TNF-α and IL-1β reached maximum.
Figure 6.
Cytokine release following treatment with crude protein. (A) TNF-α release in a time-dependent manner. (B) IL-1β release in a time-dependent manner. *P<0.05, **P<0.01 vs. control. ΤΝF, tumor necrosis factor; IL, interleukin.
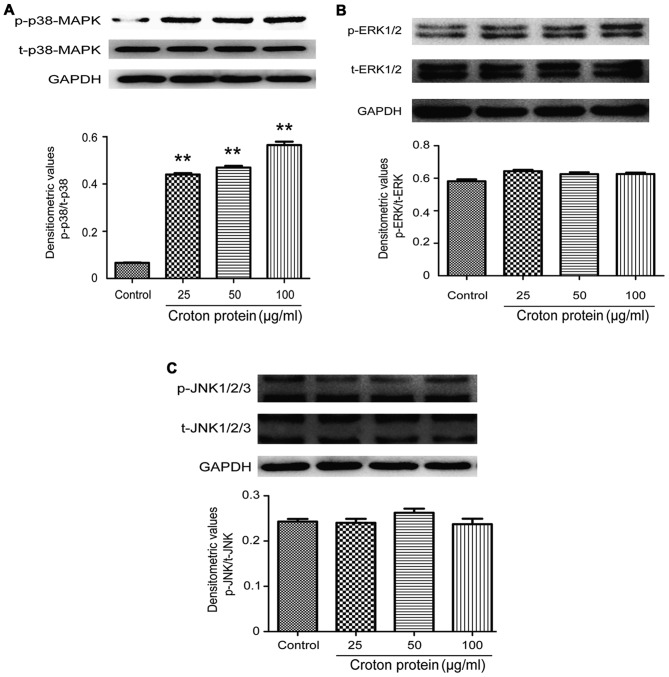
Effect of crude protein on the MAPK signaling pathway in RAW264.7 cells
To understand the molecular mechanisms underlying the proinflammatory effect of crude protein on the production of various cytokines, the effects of crude protein on the MAPK signaling pathway were examined. p38-MAPK, p-p38-MAPK, ERK1/2, p-ERK1/2, JNK1-3 and p-JNK1-3 protein levels were decreased in RAW264.7 cells upon croton proteins treatment (0, 25, 50 and 100 µg/ml) (Fig. 7).
Figure 7.
Effect of crude protein on the MAPK signaling pathway in RAW264.7 cells. Western blotting of (A) p-p38-MAPK and t-p38-MAPK, (B) p-ERK1/2 and t-ERK1/2, and (C) p-JNK1-3 and t-JNK1-3. **P<0.01 vs. control. MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; p, phosphorylated; t, total.
Discussion
C. tiglium L. as a classic traditional Chinese medicine, has been widely used to treat indigestion, abdominal distension, visceral pain, constipation and other gastrointestinal disorders; however, it exhibits toxicity. It is essential to investigate the toxic composition and targets for improved clinical application. To analyze the toxic components, croton oil has been extensively studied, whereas croton proteins has seldom been assessed. Croton oil is an active and toxic ingredient which, following dilution to 18–20%, may be safe used in clinical practice. Nevertheless, the dilution method induces strong gastrointestinal edema and diarrhea (21). Therefore, the toxicity of C. tiglium L. cannot be completely attributed to croton oil. For the dilution method, the toxicity of C. tiglium L. is reduced by first heating with steam and subsequent dilution, to ensure the clinical curative effect and safety. The process of heating, which denatures croton proteins that exhibit the majority of the toxicity, primarily weakens the toxicity of C. tiglium L.; therefore, it is important to study the toxicity of these proteins to the digestive tract. Ricinus communis L. is a plant of the same family as that of C. tiglium and produces ricin, which is able to cause strong toxicity to the digestive tract, manifested as edema and diarrhea primarily associated with inflammation (22). Therefore, the toxicity of orally administrated croton proteins to the digestive tract may be associated with the inflammatory response. Inflammation is one of the most important protective defensive reactions to tissue injury. Furthermore, the proinflammatory signaling pathway in macrophages further determines the production of these factors (23). The MAPK signaling pathway is an important pathway responsible for inflammatory responses. However, the process of inflammatory response is accompanied by activation of other signaling pathways, including nuclear factor (NF)-κB. The activation of the NF-κB signaling pathway is indicated by p65 nuclear translocation (24,25), which will be investigated in future studies.
The present study demonstrated that oral medication of croton proteins caused significant gastrointestinal damage with edema and diarrhea. The toxic reactions of the crude protein were associated with inflammation. In vivo, the crude protein caused the release of inflammatory mediator PGE2 in mice by intraperitoneal injection. In vitro, proinflammatory cytokines, including TNF-α and IL-1β, were produced in macrophages in a dose- and time-dependent manner. Furthermore, the crude protein in macrophages was associated with MAPK signaling pathway and the activated p38-MAPK pathway. In clinical applications, the effective components in C. tiglium L. frequently coexist with toxic ingredients, including croton oil and protein, that may cause gastrointestinal toxicity. Therefore, they may have a synergistic effect, and it is necessary to clarify the toxic ingredients and targets, as well as the synergistic toxicity to ensure safe use of C. tiglium L. in clinical practice. The aim of future studies will be to solve these problems.
References
- 1.National Pharmacopoeia Committee: Pharmacopoeia of the People's Republic of China. Beijing: China Medical Science and Technology Press; 2015. [Google Scholar]
- 2.Shahid M, Tayyab M, Naz F, Jamil A, Ashraf M, Gilani AH. Activity-guided isolation of a novel protein from Croton tiglium with antifungal and antibacterial activities. Phytother Res. 2008;22:1646–1649. doi: 10.1002/ptr.2544. [DOI] [PubMed] [Google Scholar]
- 3.Wang JF, Yang SH, Liu YQ, Li DX, He WJ, Zhang XX, Liu YH, Zhou XJ. Five new phorbol esters with cytotoxic and selective anti-inflammatory activities from Croton tiglium. Bioorg Med Chem Lett. 2015;25:1986–1989. doi: 10.1016/j.bmcl.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Zhang DD, Zhou B, Yu JH, Xu CH, Ding J, Zhang H. Cytotoxic tigliane-type diterpenoids from Croton tiglium. Tetrahedron. 2015;71:9638–9644. doi: 10.1016/j.tet.2015.10.070. [DOI] [Google Scholar]
- 5.State Administration of Traditional Chinese Medicine: Materia Medica of China. Shanghai: Shanghai Scientific Technology Press; 1999. p. 769. [Google Scholar]
- 6.Stirpe F, Pession-Brizzi A, Lorenzoni E, Strocchi P, Montanaro L, Sperti S. Studies on the proteins from the seeds of Croton tiglium and of Jatropha curcas. Toxic properties and inhibition of protein synthesis in vitro. Biochem J. 1976;156:1–6. doi: 10.1042/bj1560001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabral PH, de Morais Campos R, Fonteles MC, Santos CF, Leal Cardoso JH, do Nascimento NR. Effects of the essential oil of Croton zehntneri and its major components, anethole and estragole, on the rat corpora cavernosa. Life Sci. 2014;112:74–81. doi: 10.1016/j.lfs.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Chen P, Zhou X, Zhang L, Shan M, Bao B, Cao Y, Kang A, Ding A. Anti-inflammatory effects of Huangqin tang extract in mice on ulcerative colitis. J Ethnopharmacol. 2015;162:207–214. doi: 10.1016/j.jep.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 9.Baines KJ, Backer V, Gibson PG, Powel H, Porsbjerg CM. Impaired lung function is associated with systemic inflammation and macro-phage activation. Eur Respir J. 2015;45:557–559. doi: 10.1183/09031936.00187514. [DOI] [PubMed] [Google Scholar]
- 10.Wen Z, Fan L, Li Y, Zou Z, Scott MJ, Xiao G, Li S, Billiar TR, Wilson MA, Shi X, Fan J. Neutrophils counteract autophagy-mediated anti-inflammatory mechanisms in alveolar macrophage: Role in posthemorrhagic shock acute lung inflammation. J Immunol. 2014;193:4623–4633. doi: 10.4049/jimmunol.1400899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai DM, editor. The reasons of Inflammation. Beijing: People's Medical Publishing House; 2014. Pathology; pp. 49–52. [Google Scholar]
- 12.Cronstein BN, Weissman G. The adhesion molecules of inflammatio. Arthritis Rheum. 1993;36:147–157. doi: 10.1002/art.1780360204. [DOI] [PubMed] [Google Scholar]
- 13.Noah T. Ashley, Zachary M Weil, Randy J. Nelson. Inflammation: Mechanisms, costs and natural variation. Ann Rev Ecol Evolution and Systemat. 2012;43:385–406. doi: 10.1146/annurev-ecolsys-040212-092530. [DOI] [Google Scholar]
- 14.Galley HF, Webster NR. The immuno-inflammmatory cascade. Brit J Anaesth. 1996;77:11–16. doi: 10.1093/bja/77.1.11. [DOI] [PubMed] [Google Scholar]
- 15.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Qin Y, Mi X. The protective effects of bone marrow-derived mesenchymal stem cell (BMSC) on LPS-induced acute lung injury via TLR3-mediated IFNs, MAPK and NF-κB signaling pathways. Biomed Pharmacother. 2016;79:176–187. doi: 10.1016/j.biopha.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 17.Guo C, Hao LJ, Yang ZH, Chai R, Zhang S, Gu Y, Gao HL, Zhong ML, Wang T, Li JY, Wang ZY. Deferoxamine-mediated up-regulation of HIF-1α prevents dopaminergic neuronal death via the activation of MAPK family proteins in MPTP-treated mice. Exp Neurol. 2016;280:13–23. doi: 10.1016/j.expneurol.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Yıldız MT, Arslanyolu M. In silico identification and characterization of the MAPK family members of unicellular model eukaryoteTetrahymena thermophila. Eur J Protistol. 2014;50:538–550. doi: 10.1016/j.ejop.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Jones-Bolin S. Guidelines for the care and use of laboratory animals in biomedical research. Curr Protoc Pharmacol: Appendix 4: Appendix 4B. 2012 doi: 10.1002/0471141755.pha04bs59. [DOI] [PubMed] [Google Scholar]
- 20.Bliss CI. The calculation of the dosage-mortality curve. Ann Appl Biol. 1935;22:134–167. doi: 10.1111/j.1744-7348.1935.tb07713.x. [DOI] [Google Scholar]
- 21.Yi Wang, Zhang JX. New processing method of croton cream and its acute toxicity test. Chin Herbal Med. 1993;4:24–27. [Google Scholar]
- 22.Linna Liu, Hongwei Gao, Jiping Li, Ying Dong, Ning Liu, Jiayu Wan, Wensen Liu, Yucheng Sun, Ming Xu. Analysis of intestinal injuries induced by ricin in vitro using SPR technology and MS identification. Int J Mol Sci. 2009;5:2431–3439. doi: 10.3390/ijms10052431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang XL. Prarctical molecular pharmacology. Inflammatory mediators and anti-inflammatory drugs. In: Wang XL, editor. Beijing: Peking Union Medical College Publishers; 2005. pp. 433–438. [Google Scholar]
- 24.Kim HR, Shin DY, Chung KH. The role of NF-κB signaling pathway in polyhexamethylene guanidine phosphate induced inflammatory response in mouse macrophage RAW264.7 cells. Toxicol Lett. 2015;233:148–155. doi: 10.1016/j.toxlet.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Bey Williams Y, Boularan C, Vural A, Huang NN, Hwang IY, Shan-Shi C, Kehrl JH. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-κB activation and enhancing autophagy. PLoS One. 2014;9:e97957. doi: 10.1371/journal.pone.0097957. [DOI] [PMC free article] [PubMed] [Google Scholar]