Abstract
The extracellular signal-regulated protein kinase 1/2 (Erk1/2) and p38 mitogen-activated protein-kinase pathways serve important roles in the regulation of osteogenic and chondrogenic differentiation in mesenchymal stem cells (MSCs). However, the exact mechanism remains unclear, and the effect is controversial. In the present study, the effects of Erk1/2 and p38 on the osteogenic and chondrogenic differentiation of dental pulp stem cells (DPSCs) were compared in vitro. The results indicated that inhibition of Erk1/2 is able to enhance the osteogenic differentiation of DPSCs and inhibit chondrogenic differentiation, whereas inhibition of p38 demonstrated the opposite effect. When compared with previous studies, the present study further confirmed that Erk1/2 and p38 serve important, but complicated, roles in regulating the differentiation of MSCs. Different chemical and physical stimuli, cell types, culture methods, times of inhibitor administration and the dosage of the inhibitor may influence the effect of Erk1/2 and p38 on the differentiation of MSCs. The present study aims to better understand the mechanisms that control the differentiation of MSCs and may be helpful in creating more effective tissue regeneration.
Keywords: extracellular signal-regulated protein kinase 1/2, p38, osteogenic differentiation, chondrogenic differentiation, dental pulp stem cell
Introduction
Mitogen-activated protein-kinases (MAPKs), including extracellular signal-regulated protein kinases (Erks), p38 and c-Jun N-terminal kinases, are a group of well-described serine/threonine-specific protein kinases typically expressed in all cell types, and are essential components of the signal transduction machinery that occupies a central position in regulation of cell survival, motility, apoptosis, proliferation and differentiation (1–4). However, different mitogen-activated protein kinase (MAPK) family members are activated in response to different extracellular stimuli and have different downstream targets, thereby demonstrating distinct biological functions. It has been reported that MAPKs serve crucial and complicated roles in the chondrogenesis and osteogenesis of mesenchymal stem cells (MSCs); however, the exact effects of Erk1/2 and p38 in chondrogenesis and osteogenesis are contradictory in previous studies (5–11). A previous study reported that Erk1⁄ 2 serves a negative role, whereas p38 has a positive role in the chondrogenesis of MSCs, including bone marrow stromal cells, dental follicle stem cells and periodontal ligament stem cells (9). However, another other report determined the opposite results (11). In addition, there are a number of different results, promoting the osteogenesis or inhibiting the osteogenesis, for the effect of Erk1/2 and p38 on the osteogenesis of MSCs (5–8). These data suggested that the biological effects of Erk1/2 and p38 on the osteogenesis/chondrogenesis of MSCs may depend on cell type, culture systems, developmental stage, culture time and stimulating factors.
Dental pulp stem cells (DPSCs) may be easily obtained from extracted third molars, deciduous teeth and other healthy teeth. In addition, they are multipotent, meaning that they are able to differentiate into several different cell types, including neurons, odontoblasts, adipocytes, osteoblasts and chondrocytes under specific stimuli (12,13). These observations suggested that DPSCs may offer an alternative cell source for bone and cartilage regeneration. Erk1/2 and p38 have been demonstrated to be important in the proliferation and differentiation of DPSCs (14,15). A previous study revealed that fibroblast growth factor 9 (FGF9) was able to simultaneously promote the chondrogenesis of DPSCs by activating the phosphorylation of Erk1/2 (16). However, the exact effect of Erk1/2 and p38 on the chondrogenesis and osteognesis of DPSC remains to be completely elucidated. In the present study, the aim was to investigate the function of Erk1/2 and p38 signals on the chondrogenesis and osteognesis of DPSCs.
Materials and methods
Isolation and culture of DPSCs
DPSCs were obtained using direct cell outgrowth from dental pulp tissue explants and cultured as previously reported (16,17). Normal human third molars were obtained from adults (age, 16–25 years) in the Department of Oral and Maxillofacial Surgery, Stomatological Hospital, Shandong University (Jinan, China). The teeth were cleaned, and the pulp chamber was exposed using sterilized dental fissure burs. Subsequently, the dental pulp tissues were dissected into fragments (~0.5 mm), and were placed into a 6-cm dish containing Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 20% fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences, Logan, UT, USA) to incubate at 37°C with 5% CO2 for 2–3 weeks. Following reaching confluence, the DPSCs were continuously passaged, and cells from the third passage (P3) were used for further experiments. The cell morphology was observed under an inverted phase contrast microscope (Nikon Corporation, Tokyo, Japan).
Colony-forming efficiency (CFE) assays
The CFE of the P3 DPSCs was determined as previously reported (16). A total of 1,000 mixed cells were seeded into a 10-cm culture dish, and cultured for a further 14 days in DMEM. Subsequently the cells were fixed in 100% methanol for 20 min at room temperature, and stained with Giemsa for 15 min at room temperature. Colonies containing >50 cells were counted, and the CFE was calculated by dividing the total number of colonies by 1,000.
MTT assay
The vitality of the P3 DPSCs was determined as previously reported (16). Briefly, 104 DPSCs/well were plated in 96-well plates containing 200 µl DMEM with 10% FBS. The proliferation of the DPSCs was evaluated at different time points (1, 3, 5 and 7 days) via an MTT assay.
Osteogenic induction of DPSCs
The osteogenic induction medium was composed of DMEM, 10−8 M dexamethasone, 50 mg/ml L-ascorbic acid, 0.005 M KH2PO4, 50 mg/ml gentamycin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and 10% FBS. The medium was replaced every 3 days for 3 weeks. The conditions used in the different experimental groups were as follows: Control group, DPSCs cultured in DMEM with 10% FBS; Dx group, DPSCs cultured in the osteogenic induction medium dexamethasone (Dx); Dx-PD98059 group, DPSCs cultured in Dx medium with 10 µM PD98059, an inhibitor of Erk1/2; and Dx-SB203580 group, DPSCs cultured in Dx medium with 10 µM SB203580, an inhibitor of p38. The inhibitors, PD98059 (Merck KGaA) and SB203580 (Merck KGaA), were added to the DMEM medium 2 h prior to the shift to osteogenic induction medium (also including PD98059 and SB203580) for continuous culture.
DPSCs pellets cultured in vitro
In vitro chondrogenesis of DPSCs was performed in pellet cultures using P3 DPSCs as previously described (16,18). DPSCs were washed twice with PBS and re-suspended in chondrogenic medium (Cyagen Biosciences, Santa Clara, CA, USA) consisting of DMEM-F12 supplemented with 1% insulin-transferrin-selenium (Sigma-Aldrich; Merck KGaA), 0.1 mM L-ascorbate-2-phosphate, 0.4 mM proline (Sigma-Aldrich; Merck KGaA) and 10 ng/ml transforming growth factor-β3 (TGFβ3; Sigma-Aldrich; Merck KGaA). Subsequently, the cells were centrifuged in 15-ml polypropylene tubes at 500 × g for 5 min at room temperature, and maintained at 37°C under 5% CO2. The caps of the tubes were loosened to allow for air exchange. The medium was changed every 2 days. Following 6 weeks of culture, the pellets were collected and processed for immunohistochemical analysis and analysis of mRNA expression. All pellet groups for the different experiments were prepared as follows: Control group, DPSCs cultured in DMEM with 10% FBS; TGFβ3 group, DPSCs alone cultured in chondrogenic medium with TGFβ3; TGFβ3-PD98059 group, DPSCs cultured in chondrogenic medium with TGFβ3 and PD98059; and TGFβ3-SB203580 group, DPSCs cultured in chondrogenic medium with TGFβ3 and SB203580.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from different cell/pellet samples using the TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) as previously described (16,19). The quality and quantity of the RNA was analyzed. Subsequently, the total RNA was converted to cDNA using PrimeScript-RT reagent kit (Takara Biotechnology Co., Ltd., Dalian, China). GAPDH was applied as an internal control to normalize and quantify the results. The gene-specific primers used for the amplification of Runt-related transcription factor 2 (Runx2) were as follows: CGG AAT GCC TCT GCT GTT ATG (forward) and GGT TCC CGA GGT CCA TCT ACT G (reverse), and primers for GAPDH, alkaline phosphatase (ALP), type II collagen (Col2), aggrecan (ACAN) and SRY-box 9 (Sox9) are described in detail in previous reports (16). The RT-qPCR reactions were performed using the SYBR-Green system (Takara Biotechnology Co., Ltd., Dalian, China) in 96-well microwell plates (total reaction volume, 20 µl) with a MyiQ Single-Color Real-Time PCR Detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The thermocycling parameters were as follows: 95°C for 4 min, then 35 cycles of 95°C for 20 sec, followed by 60°C for 15 sec, and 72°C for 20 sec. The amplification efficiency of target genes was calculated relative to GAPDH (ΔCq=Cq gene-Cq GAPDH). The relative mRNA expression levels of these target genes were calculated compared with the expression of the controls (2−ΔΔCq, ΔΔCq=ΔCq gene-ΔCq control) (20). All the experiments were performed in triplicate.
ALP and alizarin red staining
Following culturing in vitro for 4 weeks, the ALP staining of DPSCs was performed using an alkaline phosphatase staining kit (Biyuntian, Haimen, China). Briefly, the culture dish was washed twice with PBS, and then fixed in 4% paraformaldehyde (PFA), followed by staining with alkaline dye for 30 min at room temperature. Finally, DPSCs underwent thorough washing and scanning. For alizarin red S staining, the DPSCs were fixed in 95% ethanol following 28 days culturing, then stained with 1% alizarin red S (Sigma-Aldrich; Merck KGaA) at room temperature for 20 min, and thoroughly washed prior to scanning.
Western blotting
The protein extraction of DPSCs was performed as previously described (16,19). Protein concentrations were determined using a BCA protein assay kit (cat. no. 23227; Pierce; Thermo Fisher Scientific, Inc.), and 50 µg protein per lane were separated using 10% SDS-PAGE, and transferred to a polyvinylidene difluoride membrane with a semidry transfer apparatus (Bio-Rad Laboratories, Inc.). Subsequently, the membranes were blocked with 5% milk for 2 h at room temperature, and incubated with primary antibodies against Erk1/2 (cat. no. 4695), p-Erk1/2 (cat. no. 4376), p38 (cat. no. 8690) and p-p38 (cat. no. 4511) (all dilution 1:1,000 in PBS Tween-20 buffer; Cell Signaling Technology, Inc., Danvers, MA, USA) overnight at 4°C. Finally, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (cat. no. ab97051; Abcam, Cambridge, UK; dilution, 1:5,000 in PBS) for 2 h at room temperature. Then the membranes were detected with enhanced chemiluminescence reagents (EMD Millipore, Billerica, MA, USA), and were exposed to X-ray film (Fujifilm, Tokyo, Japan).
Immunohistochemistry
DPSCs pellets were fixed with 4% PFA for 4 h, embedded in paraffin, and cut into 5 µm-thick sections. Immunohistochemistry staining of stromal cell antigen-1 (STRO-1) and Col2 in the sections was performed as previously described (16). Antigen retrieval was performed by boiling samples in a sodium citrate solution for 15 min, and pretreating with 0.01% Triton X-100 (Sigma-Aldrich; Merck KGaA) for 20 min, then 3% H2O2 for 20 min and 3% BSA (Sigma-Aldrich; Merck KGaA) for 30 min. Subsequently, the slides were incubated at 4°C overnight with the primary antibody stromal cell antigen-1 (STRO-1; cat. no. sc-47733HRP; Santa Cruz Biotechnology, Dallas, Texas, USA) or Col2 (cat. no. ab21291, Abcam; both dilution, 1:100 in PBS; Abcam) in a humid chamber. Then the samples were washed and incubated with horseradish peroxidase-conjugated secondary antibodies (cat. no. ab97051; Abcam; dilution, 1:150 in PBS) at room temperature for 2 h. The samples were stained with a diaminobenzidine staining kit (Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China) for 3 min, and were counterstained with hematoxylin.
Statistical analysis
The data are presented as the mean ± standard deviation, and an independent samples t-test was chosen to compare the data between two groups using SPSS software (version 18.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was used to indicate a statistically significant difference.
Results
Features of DPSCs
The DPSCs outgrew from dental pulp tissue explants, and exhibited a predominantly long and spindle-shaped morphology (Fig. 1A). DPSCs are able to readily form a colony from a single cell in vitro, and the colony-forming efficiency (CFE) was 7% (Fig. 1B). In addition, DPSCs expressed STRO-1, an early MSCs cell-surface molecule marker (Fig. 1C). At day 2, the P3 DPSCs began to grow exponentially. The population doubling time (PDT) was 1.93 days in the logarithmic phase (Fig. 1D).
Figure 1.
Features of DPSCs. (A) DPSCs direct outgrowth from dental pulp tissue explants. Magnification, ×40. (B) The colony formation of the P3 DPSCs was visualized by Giemsa staining. (C) Immunohistochemical staining results demonstrated that some DPSCs expressed the mesenchymal stem cell marker stromal cell antigen-1. Magnification, ×40. (D) P3 DPSCs present a sigmoidal growth curve. DPSCs, dental pulp stem cells; P3, third passage.
Effect of Erk1/2 and p38 on the osteogenic differentiation of DPSCs
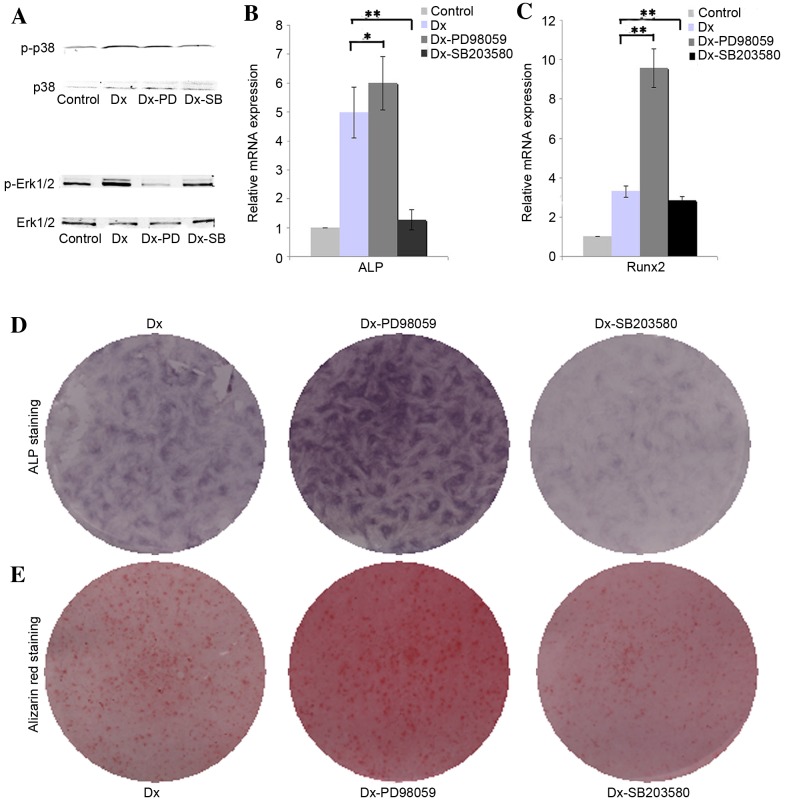
The results of western blotting demonstrated that osteogenic induction medium is able to augment the phosphorylation of ERK1/2 in DPSCs, whereas PD98059 effectively inhibited the phosphorylation of ERK1/2. The presented western blotting results indicated that osteogenic induction medium slightly inhibited the phosphorylation of p38, and SB203580 were able to further inhibit the phosphorylation of p38 (Fig. 2A). The RT-qPCR analysis results revealed that inhibition of phosphorylation of Erk1/2 with PD98059 upregulated the expression of ALP and Runx2 in DPSCs cultured in osteogenic medium when compared with the Dx group (Fig. 2B, P<0.05; Fig. 2C, P<0.01), whereas inhibition of phosphorylation of p38 with SB203580 were able to downregulate the expression of ALP and Runx2 when compared with the Dx group (Fig. 2B and C, P<0.01). The ALP and alizarin red staining results further confirmed that inhibition of phosphorylation of Erk1/2 promote the expression of ALP and ossification, whereas inhibition of phosphorylation of p38 showed opposed effect (Fig. 2D and E).
Figure 2.
Effect of Erk1/2 and p38 on the osteogenic differentiation of DPSCs. (A) The expression of Erk1/2, p-Erk1/2, p38 and p-p38 in DPSCs was detected by western blotting. The expression of (B) ALP and (C) Runx2 mRNAs in treated DPSCs was investigated using the reverse transcription-quantitative polymerase chain reaction. (D) ALP staining and (E) alizarin red staining of DPSCs. The data are presented as the mean ± standard deviation. *P<0.05, **P<0.01. Erk1/2, extracellular signal-regulated protein kinase 1/2; DPSCs, dental pulp stem cells; ALP, alkaline phosphatase; Runx2, Runt-related transcription factor 2; Dx, dexamethasone.
Effect of Erk1/2 and p38 on the chondrogenic differentiation of DPSCs
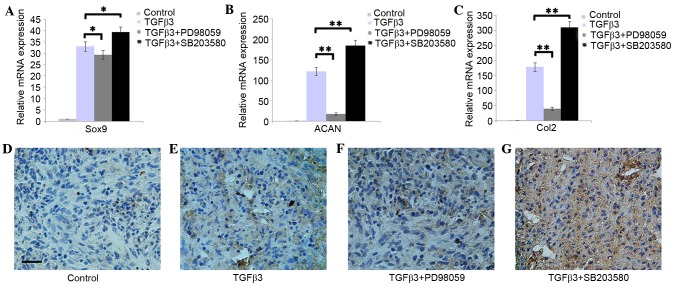
The RT-qPCR results indicated that the addition of PD98059 was able to downregulate the expression of Sox9, ACAN and Col2, whereas SB203580 was able to upregulate the expression of Sox9, ACAN and Col2 in DPSCs pellets cultured in chondrogenic medium (Fig. 3A-C; P<0.05). The immunohistochemistry results demonstrated that DPSCs pellets cultured in chondrogenic medium with PD98059 presented weak Col2 staining. However, SB203580 was able to enhance the Col2 staining (Fig. 3D-G).
Figure 3.
Effect of Erk1/2 and p38 on the chondrogenic differentiation of DPSCs. The expression of (A) Sox9, (B) ACAN and (C) Col2 mRNAs in treated DPSCs was investigated using the reverse transcription-quantitative polymerase chain reaction. (D-G) The expression of Col2 in DPSCs pellets was detected by immunohistochemical staining. Scale=50 µm. The data are presented as the mean ± standard deviation. *P<0.05, **P<0.01. Erk, extracellular signal-regulated protein kinase; Sox9, SRY-box 9; ACAN, aggrecan; DPSCs, dental pulp stem cells; TGFβ, transforming growth factor β.
Discussion
To the best of the authors' knowledge, the present study is one of the first to investigate the effects of Erk1/2 and p38, on the osteogenic and chondrogenic differentiation of DPSCs. The results suggested that Erk1/2 and p38 exhibit an opposing effect on the osteogenic and chondrogenic differentiation of DPSCs. For osteogenic differentiation, previous studies indicated that p38 and Erk1/2 involved in bone morphogenetic factor-9-induced osteogenic differentiation of rat dental follicle stem cells, and inhibition of p38 is able to inhibit the osteogenic differentiation, whereas inhibition of Erk1/2 would promote the osteogenic differentiation (6). Li et al (7) reported that cyclic tensile stress may enhance the osteogenic differentiation of periodontal ligament cells via the Erk1/2 signaling pathway, and inhibition of Erk1/2 may inhibit osteogenic differentiation. Wang et al (21) reported that hypoxia inhibits the osteogenic differentiation of rat bone mesenchymal stem cells, potentially through Erk1/2, but not the p38 signaling pathway. In addition, there are a number of additional reports presenting contradictory results concerning the effects of Erk1/2 and p38 on the osteogenic differentiation of MSCs under different conditions (22–24). These previous findings implied that different chemical and physical stimuli for osteogenic differentiation of MSCs may result in different biochemical responses through different underlying mechanisms, and that different cell types, culture systems, developmental stages and culture times may contribute to the effect (6,7,21–24). In the present study, inhibition of p38 may inhibit the osteogenic differentiation of DPSCs, whereas inhibition of Erk1/2 demonstrated the opposite effect. Although the results were similar with certain previous studies, these findings seem to contradict several other reports. However, the presented results may further confirm a more intricate set of events underlying the differentiation of MSCs.
For chondrogenesis, there are a number of different effects of Erk1/2 and p38 on the chondrogenic differentiation of MSCs. Bobick et al (11) reported that knockdown of ERK1/2 pathway members MEK1 and ERK1 decreased expression of all chondrogenic markers in human bone marrow-derived multipotent progenitor cells cultured in chondrogenic medium with TGF-β3. An additional study suggests that Erk1/2 and p38 serve opposing roles in mediating transcription of cartilage-specific genes in rat bone marrow mesenchymal stem cells cultured in chondrogenic medium with TGF-β1, and inhibition of Erk1/2 may promote chondrogenesis (9). Kim and Im (10) reported that inhibition of Erk1/2 was able to suppress hypertrophy and promote chondrogenesis of bone marrow-derived MSCs and adipose tissue-derived MSCs cultured in chondrogenic medium with bone morphogenetic protein 7 and TGF-β2, whereas inhibition of p38 had little effect. The results of the present study suggest that inhibition of Erk1/2 may inhibit the chondrogenic differentiation of DPSCs, whereas the effect of inhibiting p38 demonstrated an opposing effect. These findings seem to contradict previous reports, suggesting that inhibition of Erk1/2 simultaneously promotes the chondrogenesis and hypertrophy of DPSCs co-cultured with costal chondrocytes in chondrogenic medium with TGF-β3 and FGF9 (16). The present study further demonstrates the complexity of the effect of Erk1/2 and p38 on the differentiation of MSCs.
It is well understood that modifying the signaling pathway during the differentiation process of MSCs may influence the ultimate commitment of the MSCs, and many differentiating stimuli were applied to direct the MSCs to differentiate into the expected cells. However, how these pathways affect the differentiation program of MSCs and how manipulation of these pathways may obtain more efficient differentiation protocols remains unclear. MAPKs have been reported to be one of the most important signaling pathways implicated in chondrogenic and osteogenic differentiation of MSCs (4). However, as discussed above, the exact mechanisms of how Erk1/2 and p38 are involved in these processes remain unclear, and many factors may influence the results. Therefore, future studies should consider the influence of different chemical and physical stimuli, cell types, culture methods, the times of adding the inhibitor and the dosage of inhibitor, on the effect of Erk1/2 and p38 on the differentiation of MSCs. In addition, the potential difference between the in vivo and in vitro effects of Erk1/2 and p38 on the differentiation of MSCs should be taken into consideration. The aim is that these results will help to better understand the underlying mechanisms that control this process, in the search for novel, effective tissue regeneration and stem cell-based regenerative therapies.
References
- 1.Gehart H, Kumpf S, Ittner A, Ricci R. MAPK signalling in cellular metabolism: Stress or wellness? EMBO Rep. 2010;11:834–840. doi: 10.1038/embor.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–218. doi: 10.1016/S0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 3.Peter AT, Dhanasekaran N. Apoptosis of granulosa cells: A review on the role of MAPK-signalling modules. Reprod Domest Anim. 2003;38:209–213. doi: 10.1046/j.1439-0531.2003.00438.x. [DOI] [PubMed] [Google Scholar]
- 4.Stanton LA, Underhill TM, Beier F. MAP kinases in chondrocyte differentiation. Dev Biol. 2003;263:165–175. doi: 10.1016/S0012-1606(03)00321-X. [DOI] [PubMed] [Google Scholar]
- 5.Stanton LA, Beier F. Inhibition of p38 MAPK signaling in chondrocyte cultures results in enhanced osteogenic differentiation of perichondral cells. Exp Cell Res. 2007;313:146–155. doi: 10.1016/j.yexcr.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Yang X, He Y, Ye G, Li X, Zhang X, Zhou L, Deng F. Bone morphogenetic protein-9 induces osteogenic differentiation of rat dental follicle stem cells in P38 and ERK1/2 MAPK dependent manner. Int J Med Sci. 2012;9:862–871. doi: 10.7150/ijms.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Han M, Li S, Wang L, Xu Y. Cyclic tensile stress during physiological occlusal force enhances osteogenic differentiation of human periodontal ligament cells via ERK1/2-Elk1 MAPK pathway. DNA Cell Biol. 2013;32:488–497. doi: 10.1089/dna.2013.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelaez D, Arita N, Cheung HS. Extracellular signal-regulated kinase (ERK) dictates osteogenic and/or chondrogenic lineage commitment of mesenchymal stem cells under dynamic compression. Biochem Biophys Res Commun. 2012;417:1286–1291. doi: 10.1016/j.bbrc.2011.12.131. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Zhao Z, Liu J, Huang N, Long D, Wang J, Li X, Liu Y. MEK/ERK and p38 MAPK regulate chondrogenesis of rat bone marrow mesenchymal stem cells through delicate interaction with TGF-beta1/Smads pathway. Cell Prolif. 2010;43:333–343. doi: 10.1111/j.1365-2184.2010.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HJ, Im GI. The effects of ERK1/2 inhibitor on the chondrogenesis of bone marrow- and adipose tissue-derived multipotent mesenchymal stromal cells. Tissue Eng Part A. 2010;16:851–860. doi: 10.1089/ten.tea.2009.0070. [DOI] [PubMed] [Google Scholar]
- 11.Bobick BE, Matsche AI, Chen FH, Tuan RS. The ERK5 and ERK1/2 signaling pathways play opposing regulatory roles during chondrogenesis of adult human bone marrow-derived multipotent progenitor cells. J Cell Physiol. 2010;224:178–186. doi: 10.1002/jcp.22120. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Walboomers XF, Van Kuppevelt TH, Daamen WF, Van Damme PA, Bian Z, Jansen JA. In vivo evaluation of human dental pulp stem cells differentiated towards multiple lineages. J Tissue Eng Regen Med. 2008;2:117–125. doi: 10.1002/term.71. [DOI] [PubMed] [Google Scholar]
- 13.Grottkau BE, Purudappa PP, Lin YF. Multilineage differentiation of dental pulp stem cells from green fluorescent protein transgenic mice. Int J Oral Sci. 2010;2:21–27. doi: 10.4248/IJOS10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang FM, Hu T, Zhou X. p38 mitogen-activated protein kinase and alkaline phosphatase in human dental pulp cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:114–118. doi: 10.1016/j.tripleo.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Liu S, Zhou Y, Tan J, Che H, Ning F, Zhang X, Xun W, Huo N, Tang L, et al. Natural mineralized scaffolds promote the dentinogenic potential of dental pulp stem cells via the mitogen-activated protein kinase signaling pathway. Tissue Eng Part A. 2012;18:677–691. doi: 10.1089/ten.tea.2011.0269. [DOI] [PubMed] [Google Scholar]
- 16.Dai J, Wang J, Lu J, Zou D, Sun H, Dong Y, Yu H, Zhang L, Yang T, Zhang X, et al. The effect of co-culturing costal chondrocytes and dental pulp stem cells combined with exogenous FGF9 protein on chondrogenesis and ossification in engineered cartilage. Biomaterials. 2012;33:7699–7711. doi: 10.1016/j.biomaterials.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 19.Dai J, Kuang Y, Fang B, Gong H, Lu S, Mou Z, Sun H, Dong Y, Lu J, Zhang W, et al. The effect of overexpression of Dlx2 on the migration, proliferation and osteogenic differentiation of cranial neural crest stem cells. Biomaterials. 2013;34:1898–1910. doi: 10.1016/j.biomaterials.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Li J, Wang Y, Lei L, Jiang C, An S, Zhan Y, Cheng Q, Zhao Z, Wang J, Jiang L. Effects of hypoxia on osteogenic differentiation of rat bone marrow mesenchymal stem cells. Mol Cell Biochem. 2012;362:25–33. doi: 10.1007/s11010-011-1124-7. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Yang Y, Yang P, Gu Y, Zhao Z, Tan L, Zhao L, Tang T, Li Y. The osteogenic differentiation of PDLSCs is mediated through MEK/ERK and p38 MAPK signalling under hypoxia. Arch Oral Biol. 2013;58:1357–1368. doi: 10.1016/j.archoralbio.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Zhang P, Wu Y, Dai Q, Fang B, Jiang L. p38-MAPK signaling pathway is not involved in osteogenic differentiation during early response of mesenchymal stem cells to continuous mechanical strain. Mol Cell Biochem. 2013;378:19–28. doi: 10.1007/s11010-013-1589-7. [DOI] [PubMed] [Google Scholar]
- 24.Yi C, Liu D, Fong CC, Zhang J, Yang M. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano. 2010;4:6439–6448. doi: 10.1021/nn101373r. [DOI] [PubMed] [Google Scholar]