Abstract
Sepsis is characterized by the impaired regulation of inflammatory responses. Apoptosis is important in the pathogenesis of sepsis. Cellular FLICE-inhibitory protein (c-FLIP) is a catalytically inactive caspase-8 homologue, which negatively interferes with apoptotic signaling. The role of c-FLIP in sepsis and in endothelial cell apoptosis, a critical step in the pathogenesis of sepsis, remains controversial. In the present study, to investigate the relationship between c-FLIP and sepsis, a rat model of sepsis was induced by cecal ligation and puncture, and western blot analysis was used to detect the expression of c-FLIPL, the long isoform of c-FLIP. Lower protein expression levels of c-FLIPL were found in the brain, intestine and lung of the rat sepsis model, compared with the rats in the sham surgery group. The association between the expression of c-FLIPL and endothelial cell apoptosis was further examined in vitro by c-FLIPL overexpression and flow cytometry, which demonstrated that the expression of c-FLIPL was inversely correlated with endothelial cell apoptosis. These data suggested that c-FLIP may be important in sepsis and shed light on therapeutic strategies.
Keywords: sepsis, cellular FLICE-inhibitory protein, endothelium apoptosis, cecal ligation and puncture
Introduction
Apoptosis is a tightly regulated cell death program, which is actively implemented to ensure proper development. Insufficient or excessive apoptosis can provoke a number of diseases, including autoimmune disease, cancer and disorders in inflammation (1,2). Misregulated apoptosis often leads to severe organ damage in various infectious diseases, including sepsis. The pathogenesis of sepsis involves a complex process of cellular activation at multiple levels resulting in the release of pro-inflammatory cytokines, including tumor necrosis factor-α, interleukin (IL)-6, interferon-γ, IL-1β and high-mobility group box 1, and anti-inflammatory cytokines, including IL-10 (3,4). Other mechanisms include the activation of neutrophils, monocytes and microvascular endothelial cells, in addition to the apoptosis of these cells (3,4). Detailed studies have focused on apoptotic signaling pathways (5), however, the exact molecular mechanism remains to be fully elucidated.
Cellular FLICE-inhibitory protein (c-FLIP) is a catalytically inactive caspase-8 (CASP8) homologue, which negatively interferes with apoptotic signaling (6). The triggering of death-receptors by cognate ligands induces the recruitment of death adaptor proteins, including Fas-associated death domain (FADD) or TRAIL receptor-associated death domain by means of the receptors' intracellular death domain. Death-receptors also possess a death effect domain, which is necessary for the recruitment of the initiator procaspase-8 to form the death inducing signaling complex (DISC). c-FLIP interferes with efficient DISC formation in the extrinsic pathway directly at the receptor level by competing with procaspase-8 to bind FADD. Prevention of the proteolytic cleavage and activation of the procaspase inhibits the transduction of apoptotic signaling, and ultimately promotes cell survival (7). There are three forms of c-FLIP, c-FLIPL (55 KDa), c-FLIPS (26 KDa) and c-FLIPR (24 KDa). All the three forms of c-FLIP (L, S and R) are traditionally accepted as anti-apoptotic proteins. The role of c-FLIPL remains to be elucidated as it has been described to have pro- and anti-apoptotic effects in immune system cells, and this dual role has been found to depend on a variety of parameters, including cellular context and CASP8 to FLIP ratio (6).
There is controversy surrounding the function of c-FLIPL in endothelial cell apoptosis. Bannerman et al found that c-FLIP protected against the apoptosis of human dermal microvessel endothelial cells and suppressed the activation of nuclear factor (NF)-κB induced by lipopolysaccharide (LPS) (8), whereas Karahashi et al found that c-FLIP was not involved in apoptosis induced by LPS or cycloheximide (CHX) (9). As endothelial cell apoptosis is critical in the pathogenesis of sepsis, the aim of the present study was to detect the expression of c-FLIPL in a rat model of sepsis, and examine the association between the expression of c-FLIPL and endothelial apoptosis.
Materials and methods
Materials
LPS was purchased from Sigma-Aldrich; Merck Millipore (Darmstadt, Germany). CHX was purchased from Sangon Biotech Co., Ltd. (Shanghai, China). Sprague-Dawley rats were obtained from the Shanghai Animal Center of the Chinese Academy of Science (Shanghai, China). The human umbilical vein endothelial cells (HUVECs) were purchased from American Type Culture Collection (Manassas, VA, USA).
Rat sepsis model establishment using cecal ligation and puncture (CLP)
A total of 24 Sprague-Dawley rats, male, weighing 250–300 g, were housed under a 12 h daylight cycle at 23–25°C temperature and fed with standard chow and water. The animals were randomly divided into two groups: Sham surgery group and sepsis model group. The sepsis model was induced by CLP (10). Briefly, the animals were deprived of food, but allowed water, for 6 h prior to surgery. To prepare for the surgical procedures, the animals were anesthetized with chloral hydrate (350 mg/kg bodyweight) intraperitoneally. A laparotomy was performed through a midline abdominal incision, and the cecum was exteriorized and ligated halfway between the distal pole and the base of the cecum. Subsequently, the cecum was perforated by a single through-and-through puncture with a 2.5 mm needle and gently compressed until fecal material was extruded. The bowel was then relocated to the abdomen and the abdominal incision was closed in layers. The animals were resuscitated via injection of pre-warmed normal saline (37°C; 5 ml/100 g body weight) subcutaneously. Animals in the sham group received sham surgery, during which the cecum was neither ligated nor punctured. All procedures performed involving animals were in accordance with the guidelines of the Institutional Animal Care and Use Committee. The study protocols were approved by the Research Ethics Committee of Huashan Hospital, Fudan University (Shanghai, China).
Cell culture
The HUVECs were cultured in DMEM (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) enriched with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), bovine brain extract (12 µg/ml), L-glutamine (2 mmol/l) and sodium pyruvate (1 mmol/l) in the presence of penicillin (100 U/ml) and streptomycin (100 µg/ml; Sangon Biotech Co., Ltd).
Western blot analysis
The tissues and HUVECs were homogenized with modified RIPA buffer and stationed on ice for 1 h, following which they were centrifuged at 14,000 × g at 4°C for 5 min. The supernatants were collected, mixed with loading buffer containing 0.1% bromophenol blue and boiled for 10 min, following determination of protein concentration by bicinchoninic acid assay (Beyotime Institute of Biotechnology, Haimen, China). Equal quantities of protein (10 µg) were loaded into a 10% SDS-PAGE gel, followed by electrophoresis, separation under denaturing conditions and electroblotting onto PVDF membranes. The membranes were incubated overnight in Tris-buffered saline containing 7% milk to inhibit nonspecific antibody binding. The proteins of interest were revealed via incubation with specific mouse anti-human monoclonal antibody (anti-FLIPS/L; 1:500 dilution; cat. no. sc-5276; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h at room temperature, followed by incubation with a 1:1,000 dilution of horseradish peroxidase-conjugated goat anti-mouse IgG antibody (cat. no. A0216; Beyotime Insititute of Biotechnology) for 1 h at room temperature. Signals were visualized using chemiluminescence. β-actin antibody was used as a control. All western blots were quantified using densitometry.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Tissues and HUVECs were homogenized using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and total RNA was extracted according to the manufacturer's protocol. Total RNA (1 µg) was then reverse transcribed with reverse transcriptase (Promega Corporation, Madison, WI, USA) for 1 h at 37°C to synthesize cDNA. The following primers, synthesized by Sangon Biotech Co., Ltd., were used: c-FLIP, sense 5′-ATAGGGTGCTGCTGATGG-3′ and antisense 5′-TTGCTTCTTGGCTGGACT-3′. GAPDH, sense 5′-ACCACAGTCCATGCCATCAC-3′ and antisense 5′-CCACCACCCTGTTGCTGTAG-3′. The reactions were performed in a 25-µl volume comprising diluted c-DNA sample, primers and SYBR-Green reagent (Takara Biotechnology Co., Ltd., Dalian, China), according to the manufacturer's protocol. PCR was performed in triplicate as follows: 95°C for 10 min, and 45 cycles of 95°C for 15 sec, 64°C for 30 sec, and 72°C for 30 sec. The qPCR amplifications were performed on an ABI 7500 PCR system (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Relative quantification of the mRNA expression of the target gene was determined using the 2−ΔΔCq method, with GAPDH as an internal reference control (11).
Overexpression of c-FLIP by transfecting HUVECs with PEGFP-N1-CASP8 and FADD-like apoptosis regulator (cflar)
The PEGFP-N1-cflar plasmid was constructed by Shanghai Genechem Co., Ltd. (Shanghai, China) which contained the gene of c-FLIP and kanamycin resistance, and was transfected into HUVECs using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) (12). Briefly, the plasmid was sequenced by Sangon Biotech Co. Ltd. to confirm that the c-FLIP gene was expressed. Subsequently, 1 µg plasmid DNA and 2 µl Lipofectamine 2000 were added to each well of 24-well plates and incubated with HUVECs (105 cells/well) for 4 h at 37°C in 5% CO2. After 4 h, the supernatants were removed and fresh media was added, following which incubation continued for another 32 h. The total transfection time was 36 h and the transfection efficiencies were observed through a fluorescent inverted microscope (Nikon TE2000; Nikon Corporation, Tokyo, Japan).
Induction of HUVEC apoptosis by LPS and CHX
Following transfection with plasmid DNA and Lipofectamine 2000 for 36 h, HUVEC apoptosis was induced by adding LPS (100 ng/ml) or LPS (100 ng/ml)+CHX (40 µg/ml) for 6, 9, 12 and 24 h at 37°C in 5% CO2. The cells were then stained with Annexin V and propidium iodide (PI) using an apoptosis kit (BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer's protocol, and immediately analyzed using flow cytometry.
Statistical analysis
The results are presented in graphs following analysis using Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Comparisons between groups were made using unpaired t tests, or Mann-Whitney U tests. P<0.05 was considered to indicate a statistically significant difference.
Results
Physiological and pathological changes in rats following CLP
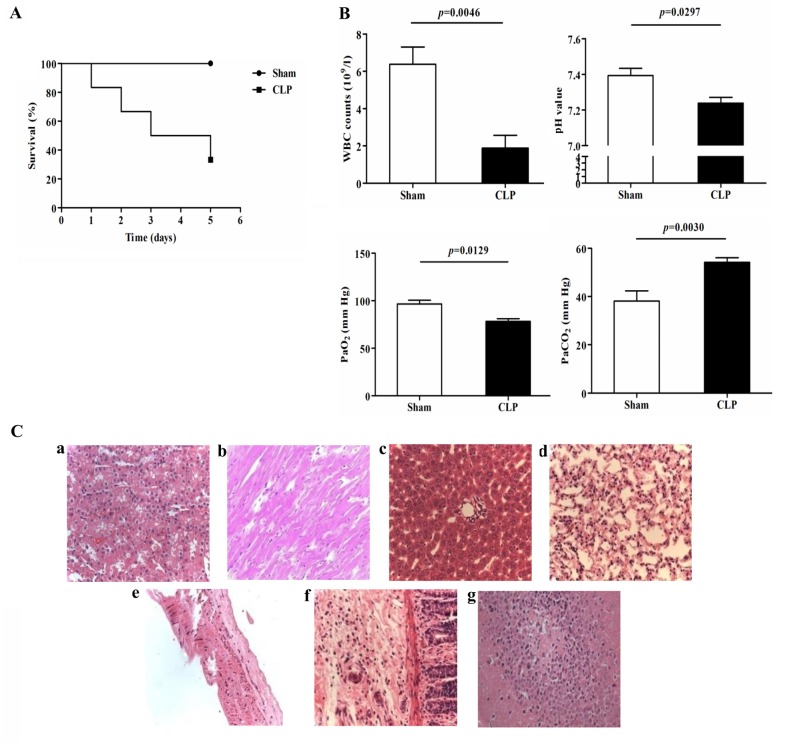
In the present study, the survival rates of the rats in the sham surgery and CLP groups were 100 and 40%, respectively (Fig. 1A), which was consistent with a previous report (10). Significant decreases in white blood counts (WBCs; 6.4±2.3×109/l, vs. 1.9±1.5×109/l; P=0.0046), pH (7.39±0.07, vs. 7.24±0.09; P=0.0297) and PaO2 (96.7±7.0, vs. 78.3±5.9 mm Hg; P=0.0129), and a significant increase in PaCO2 (38.1±7.4, vs. 54.2±5.4 mm Hg; P=0.0030) were observed in the CLP rats, compared to those in the sham group at 24 h post-surgery (Fig. 1B). H&E staining of the organs, including the lungs, liver, kidney, heart, vessel and intestines, revealed a marked inflammatory response (Fig. 1Ca-g). These data confirmed establishment of the CLP-induced rat model of polymicrobial sepsis for subsequent investigations of the mechanism underlying sepsis.
Figure 1.
Physiological and pathological changes in the sepsis model induced by CLP. (A) Survival curves of the Sham and CLP groups (n=6 in each group). (B) WBC counts, pH, PaO2 and PaCO2 of the Sham and CLP groups (n=6 in each group). (C) Pathological changes detected using H&E staining in the (a) kidney, (b) heart, (c) liver, (d) lung, (e) vessel, (f) intestine and (g) brain (magnification, ×200). CLP, cecal ligation and puncture; WBC, white blood cell.
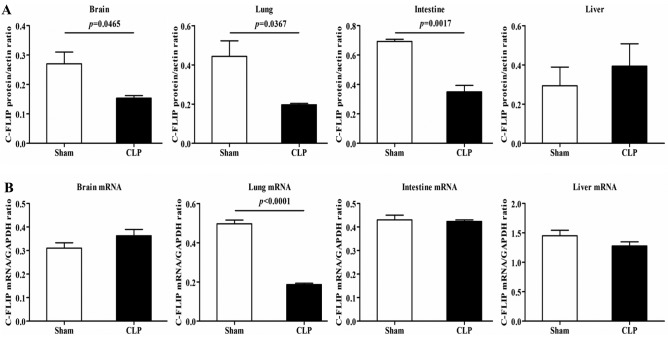
mRNA and protein expression levels of c-FLIP in rats with CLP
The results of the western blot analysis revealed that, compared with the rats in the sham group, the CLP rats showed notable decreases in the protein expression of c-FLIP, primarily c-FLIPL, in the brain, intestine and lungs, but not in the liver (Fig. 2A). In terms of the mRNA expression of c-FLIP, detected using RT-qPCR analysis, a significant decrease was observed in the lung tissues of the CLP rats, compared with the rats in the sham group, with no detectable differences in other organs (Fig. 2B). As c-FLIP functions as an inhibitor of the activation of CASP8 (6), the reduced protein expression of c-FLIP suggested a high induction of apoptosis.
Figure 2.
mRNA and protein expression of c-FLIP in the Sham and CLP groups. (A) Compared with the Sham group, the CLP group showed significantly decreased protein expression levels of c-FLIP in the brain, intestine and lungs, but not in the liver. (B) Compared with the Sham group, the CLP group showed a significant decrease in the mRNA expression of c-FLIP in the lungs, but not in brain, intestine or liver. c-FLIP, cellular FLICE-inhibitory protein; CLP, cecal ligation and puncture.
Transfection of cflar into HUVECs
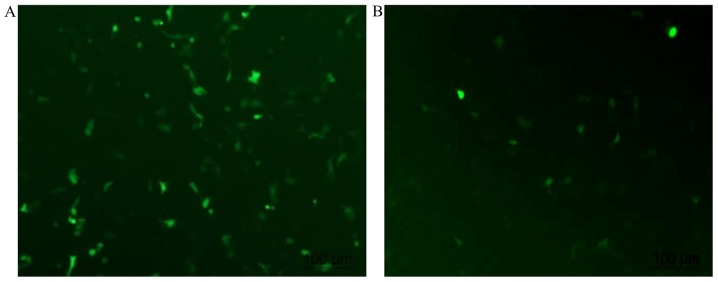
It has been reported that the transfection efficiency of HUVECs varies between 0.45 and 77% (12). When using Lipofectamine 2000, which is recommended as one of the optimal transfection reagents in HUVECs, the reproducible transfection efficiency was previously reported to be 19±9% (12). In the present study, low efficiency was also a problem. As shown in Fig. 3, green signals represent cells transfected with the plasmid. PEGFP-N1 alone was transfected into cells with a transfection efficiency of ~50% (46.8±6.2%; Fig. 3A). The transfection efficiency decreased to <15% (13.7±4.6%) when the cells were transfected with PEGFP-N1+ cflar (Fig. 3B).
Figure 3.
Transfection of human umbilical vein endothelial cells with cflar using Lipofectamine 2000. Transfection was performed after 36 h (magnification, ×100). (A) Transfection efficiency of PEGFP-N1 alone was 46.8±6.2%. (B) Transfection efficiency for PEGFP-N1+ cflar was 13.7±4.6%. cflar, caspase-8 and Fas-associated death domain-like apoptosis regulator.
LPS and CHX following cflar transfection induce the protein expression of c-FLIP in HUVECs
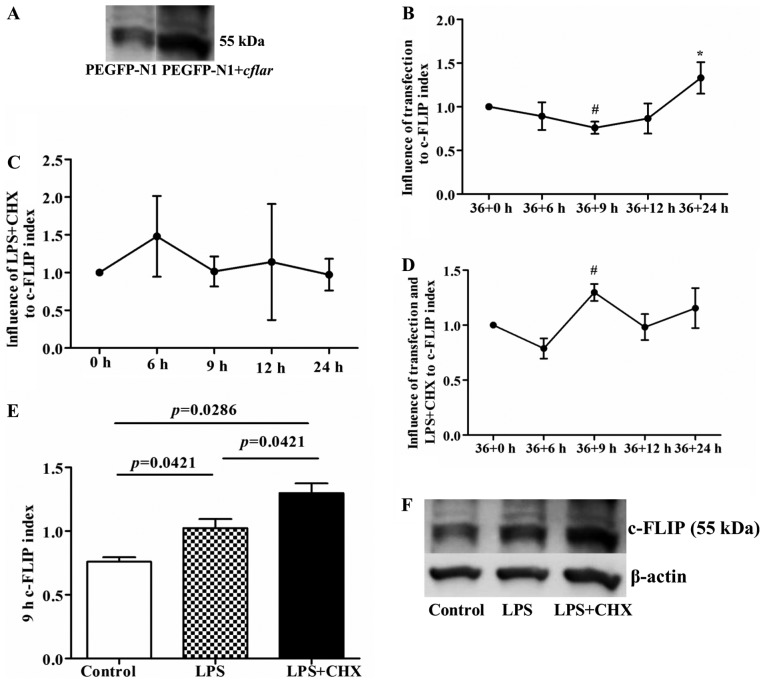
Although the transfection efficiency in the HUVECs was only ~15%, the western blot analysis of c-FLIP protein indicated that the protein expression of c-FLIP in the transfected HUVECs was significantly increased 36 h following cflar transfection (Fig. 4A). Compared with the initial time point (36+0 h), the expression of c-FLIP decreased at 36+9 h (P<0.05), and then gradually increased with increasing duration, reaching a significantly elevated level at 36+24 h (P<0.05), compared with the 36+6, 36+9 and 36+12 h time points, respectively (Fig. 4B). Additionally, previous studies have suggested that LPS induces the expression of c-FLIP in HUVECs, but that CHX suppresses the expression of c-FLIP (7,8). However, in the present study, LPS+CHX exerted a biphasic effect on the expression of c-FLIP, with an initial upregulation, followed by downregulation (Fig. 4C), which was opposite to the change in the protein expression of c-FLIP following transfection. The present study also examined the expression of c-FLIP following sequential stimulation of cflar-transfected cells with LPS and CHX combined. The results of the western blot analysis showed that the overexpression of c-FLIP was only present at the 9 h point following treatment with LPS and CHX (P<0.05, compared with the 36+0 h time point; Fig. 4D) and there were significant differences among the control, LPS and LPS+CHX groups (P<0.05; Fig. 4E and F). At the remaining time points, no significant overexpression of c-FLIP was observed among these three groups (Table I).
Figure 4.
Expression of c-FLIP following cflar transfection and subsequent LPS and CHX induction. (A) Expression of c-FLIP in the transfected cflar human umbilical vein endothelial cells was higher, compared with that in cells transfected with PEGFP-N1 alone, confirmed using western blot analysis. (B) Following transfection only, the expression of c-FLIP decreased at 36+9 h (#P<0.05, compared with 36+0 h), and then gradually increased with increasing transfection duration, particularly at 36+24 h (*P<0.05, compared with the 36+6, 36+9 and 36+12 h groups, respectively). (C) Expression of c-FLIP increased, and then decreased following treatment of untransfected cells with LPS+CHX, but without statistical significance. (D) Expression of c-FLIP increased significantly at 9 h post-transfection in the LPS+CHX group (#P<0.05, compared with 36+0 h). (E) Expression of c-FLIP in cells 9 h post-transfection with LPS or LPS+CHX. The LPS group showed higher protein expression of c-FLIP, compared with the control group (P=0.0421). The LPS+CHX group showed higher protein expression of c-FLIP, compared with the control and LPS groups (P=0.0286 and P=0.0421, respectively). (F) Representative images of western blot analysis of protein expression of c-FLIP and β-actin in the three groups. c-FLIP, cellular FLICE-inhibitory protein; LPS, lipopolysaccharide; CHX, cycloheximide; cflar, caspase-8 and Fas-associated death domain-like apoptosis regulator.
Table I.
Expression of cellular FLICE-inhibitory protein following transfection with CASP8 and Fas-associated death domain-like apoptosis regulator, and subsequent LPS or LPS+CHX-induced apoptosis.
| Time point (h) | Control | LPS | LPS+CHX | P-value |
|---|---|---|---|---|
| 36+6 | 0.89±0.16 | 0.80±0.44 | 0.79±0.19 | 0.857 |
| 36+9 | 0.76±0.07 | 1.02±0.14 | 1.30±0.15 | 0.001 |
| 36+12 | 0.87±0.17 | 1.56±1.10 | 0.98±0.24 | 0.324 |
| 36+24 | 1.94±1.23 | 1.16±0.49 | 1.16±0.36 | 0.317 |
Transfection was performed at 36 h, LPS or LPS+CHX was added for 6, 9, 12 and 24 h. Data are presented as the mean ± standard deviation. LPS, lipopolysaccharide; CHX, cycloheximide.
c-FLIP protein protects HUVECs against apoptosis induced by LPS+CHX
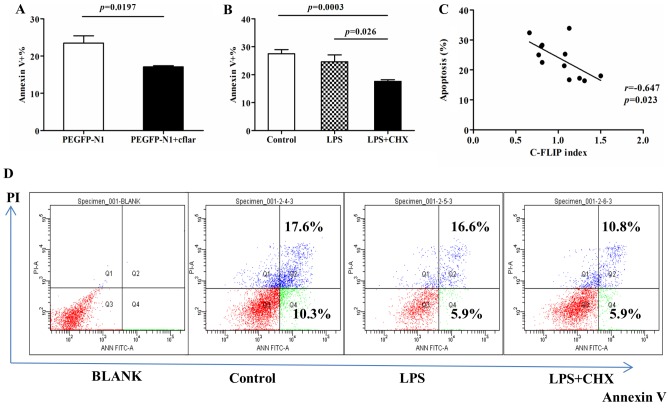
The present study used PI and Annexin V staining to investigate the apoptosis of HUVECs following cflar transfection, and following induction by LPS or LPS+CHX. Compared with the cells transfected with PEGFP-N1 only, the HUVECs transfected with PEGFP+ cflar exhibited a significant decrease in the percentage of Annexin V-positive apoptotic cells following LPS+CHX stimulation, which indicated that transfection with the cflar gene protected the HUVECs against LPS and CHX-induced apoptosis (Fig. 5A). It was shown that LPS+CHX treatment led to the overexpression of c-FLIP (P=0.0286, vs. control group; P=0.0421, vs. LPS group; Fig. 4E). It was subsequently demonstrated that the percentage apoptosis in the cells transfected with cflar and treated with LPS+CHX was significantly lower, compared with that in the control group and LPS group (P=0.0003 and P=0.026, respectively; Fig. 5B). No significant difference was observed between the control group or LPS group (Fig. 5B). The percentage apoptosis was inversely correlated with the expression of c-FLIP (r=−0.0647; P=0.023; Fig. 5C). The representative results of flow cytometry are shown in Fig. 5D. Taken together, these data suggested that c-FLIP protected HUVECs against apoptosis induced by LPS+CHX.
Figure 5.
Apoptosis of human umbilical vein endothelial cells is induced by LPS and CHX. (A) Percentage of Annexin V-positive cells was lower following transfection with PEGFP-N1+ cflar, compared with cells transfected with PEGFP-N1 alone, when exposed to LPS+CHX at 9 h (P=0.0197). (B) Percentage of Annexin V-positive cells transfected with cflar induced by LPS+CHX was lower, compared with the cells in the LPS alone and control groups (P=0.0003 and 0.026, respectively). In all groups, cells were transfected with cflar at 36 h. Subsequently, cells in the control group were incubated in media for 9 h, cells in the LPS group were induced by LPS for 9 h, and cells in the LPS+CHX group were induced by LPS+CHX for 9 h. (C) Percentage of apoptotic cells was inversely correlated with the expression of c-FLIP (r=−0.0647; P=0.023). (D) Representative results of flow cytometric analysis. The percentage of Annexin V-positive cells represents the level of apoptosis. c-FLIP, cellular FLICE-inhibitory protein; LPS, lipopolysaccharide; CHX, cycloheximide; PI, propidium iodide; cflar, caspase-8 and Fas-associated death domain-like apoptosis regulator.
Discussion
CLP is considered to be the gold standard rodent model for the investigations of sepsis and has become the most widely used model for experimental sepsis. However, disease outcome varies depending on several factors, including the length of intestine tied off, the size of the needle and the quantity of fecal material released (10). In the present study, a 50% length ligation was performed, followed by a single through-and-through puncture with a 2.5 mm needle. This model mimicked the sepsis status successfully, resulting in rat mortality, physiological changes in WBC counts and blood gas analysis, and pathological changes in the majority of the rat organs.
In the present study, the expression of c-FLIPL was characterized in different organs of the rat model of sepsis induced by CLP, and found that the expression of c-FLIPL was decreased in the majority of vital organs, including the brain, lungs and intestine. In terms of the mRNA levels, no significant differences in the expression of c-FLIPL were observed. c-FLIP has three forms, c-FLIPL, c-FLIPS and c-FLIPR, and all three forms are widely accepted anti-apoptotic proteins (6). As the effects of c-FLIPL have been described to be pro- and anti-apoptotic, the present study primarily focused on the expression and function of c-FLIPL. The expression of c-FLIPL can be regulated at multiple levels. NF-κB and activator protein 1 family proteins regulate the expression of c-FLIPL at the transcriptional level. At the translational level, regulation of the expression of c-FLIPL also occurs by activation of the Akt-mammalian target rapamycin-p70S6 kinase pathway (13). Therefore, the difference between the protein and mRNA expression levels of c-FLIPL can be interpretative. In the present in vitro study, the expression of c-FLIPL increased following transfection with the plasmid, whereas endothelial apoptosis was attenuated, which indicated that the decreased expression of c-FLIPL in rats with sepsis did not protect cells against apoptosis in vital organs, which may be associated with the poor prognosis of sepsis. Perlman et al (14) found that the overexpression of c-FLIP protected monocytes from Fas-mediated apoptosis, whereas acute c-FLIP inhibition in macrophages induced apoptosis. The addition of an antagonistic Fas ligand antibody to c-FLIP-antisense-treated macrophages rescued the cultures from apoptosis. Therefore, the expression of c-FLIP in macrophages conferred resistance to Fas-mediated apoptosis, which may contribute to the development of inflammatory disease. Bannerman et al (7,8) found that c-FLIP protected against apoptosis and suppressed the activation of NF-κB induced by LPS. These findings are in accordance with those of the present study.
Bacterial LPS is a major component of Gram-negative bacteria, which has been implicated in the pathogenesis of Gram-negative sepsis. To ensure consistency with the rat sepsis model using CLP, which induces abdominal infection, LPS was selected in the present study as an inducer of inflammation in HUVECs. LPS is known as a potent activator of proinflammatory responses in various types of cells. The vascular endothelium is a key host target of LPS and the first host tissue barrier encountered by circulating LPS. Injury to and/or dysfunction of the vascular endothelium has been implicated in the development of sepsis-associated complications, including systemic vascular collapse, disseminated intravascular coagulation, multi-organ failure, and the development of vascular leak syndromes, including acute respiratory distress syndrome (15,16). Therefore, the present study focused on the changes in function of the vascular endothelium, particularly on apoptosis, in the pathogenesis of sepsis. It was described previously that LPS induces apoptosis in human mammary epithelial cells (HMECs) only in the presence of protein synthesis inhibitors, including CHX. Bannerman et al found that c-FLIP protected against HMEC apoptosis and suppressed the activation of NF-κB induced by LPS (8), whereas Karahashi et al found that c-FLIP was not involved in the induction of apoptosis induced by LPS+CHX (9). In order to elucidate the role of c-FLIP in endothelial cell apoptosis, the present study cultured HUVECs with LPS in the presence or absence of CHX, and showed that only LPS and CHX together induced HUVEC apoptosis, and that the expression of c-FLIPL was inversely correlated with apoptosis. Taken together, these data suggested that c-FLIP, primarily c-FLIPL, protected the HUVECs against the apoptosis induced by LPS+CHX.
There were a number of limitations in the present study. Although the effects of upregulated c-FLIPL were addressed, the opposing arm was not investigated. Subsequent investigations aim to examine the role of downregulated c-FLIPL by c-FLIP-specific antisense oligonucleotides or small interfering RNA lipocomplexes. In addition, the present study did not detect the levels of apoptosis in cells of different organs in the rats with sepsis.
Taken together, the present study demonstrated that c-FLIP expression was significantly decreased in organs of septic rats compared with control rats, and that c-FLIP overexpression protected HUVECs from LPS+CHX-induced apoptosis in vitro. These data indicated a protective role of c-FLIP in endothelial cells and suggested that c-FLIP may have potential as a therapeutic target in sepsis treatment in the future.
Acknowledgements
We would like to acknowledge the efforts of the staff at the Central Laboratory, Huashan Hospital (Shanghai, China). The present study was supported by grants from Huashan Hospital (Initiation grants; grant no. 212).
References
- 1.Yu JW, Shi Y. FLIP and the death effector domain family. Oncogene. 2008;27:6216–6227. doi: 10.1038/onc.2008.299. [DOI] [PubMed] [Google Scholar]
- 2.Longley DB, Wilson TR, McEwan M, Allen WL, McDermott U, Galligan L, Johnston PG. c-FLIP inhibits chemotherapy-induced colorectal cancer cell death. Oncogene. 2006;25:838–848. doi: 10.1038/sj.onc.1209122. [DOI] [PubMed] [Google Scholar]
- 3.Yan T, Li Q, Zhou H, Zhao Y, Yu S, Xu G, Yin Z, Li Z, Zhao Z. Gu-4 suppresses affinity and avidity modulation of CD11b and improves the outcome of mice with endotoxemia and sepsis. PLoS One. 2012;7:e30110. doi: 10.1371/journal.pone.0030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Zhao F, Fang Y, Li X, Shen L, Cao T, Zhu H. Glycyrrhizin protects against porcine endotoxemia through modulation of systemic inflammatory response. Crit Care. 2013;17:R44. doi: 10.1186/cc12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krautwald S, Ziegler E, Tiede K, Pust R, Kunzendorf U. Transduction of the TAT-FLIP fusion protein results in transient resistance to Fas-induced apoptosis in vivo. J Biol Chem. 2004;279:44005–44011. doi: 10.1074/jbc.M401327200. [DOI] [PubMed] [Google Scholar]
- 6.Bagnoli M, Canevari S, Mezzanzanica D. Cellular FLICE-inhibitory protein (c-FLIP) signalling: A key regulator of receptor-mediated apoptosis in physiologic context and in cancer. Int J Biochem Cell Biol. 2010;42:210–213. doi: 10.1016/j.biocel.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Bannerman DD, Goldblum SE. Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. Am J Physiol Lung Cell Mol Physiol. 2003;284:L899–L914. doi: 10.1152/ajplung.00338.2002. [DOI] [PubMed] [Google Scholar]
- 8.Bannerman DD, Eiting KT, Winn RK, Harlan JM. FLICE-like inhibitory protein (FLIP) protects against apoptosis and suppresses NF-kappaB activation induced by bacterial lipopolysaccharide. Am J Pathol. 2004;165:1423–1431. doi: 10.1016/S0002-9440(10)63400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karahashi H, Michelsen KS, Arditi M. Lipopolysaccharide-induced apoptosis in transformed bovine brain endothelial cells and human dermal microvessel endothelial cells: The role of JNK. J Immunol. 2009;182:7280–7286. doi: 10.4049/jimmunol.0801376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Hunt MA, Currie MJ, Robinson BA, Dachs GU. Optimizing transfection of primary human umbilical vein endothelial cells using commercially available chemical transfection reagents. J Biomol Tech. 2010;21:66–72. [PMC free article] [PubMed] [Google Scholar]
- 13.Safa AR, Day TW, Wu CH. Cellular FLICE-like inhibitory protein (C-FLIP): A novel target for cancer therapy. Curr Cancer Drug Targets. 2008;8:37–46. doi: 10.2174/156800908783497087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perlman H, Pagliari LJ, Georganas C, Mano T, Walsh K, Pope RM. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to fas-mediated apoptosis. J Exp Med. 1999;190:1679–1688. doi: 10.1084/jem.190.11.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coletta C, Módis K, Oláh G, Brunyánszki A, Herzig DS, Sherwood ER, Ungvári Z, Szabo C. Endothelial dysfunction is a potential contributor to multiple organ failure and mortality in aged mice subjected to septic shock: Preclinical studies in a murine model of cecal ligation and puncture. Crit Care. 2014;18:511. doi: 10.1186/s13054-014-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostrowski SR, Haase N, Müller RB, Møller MH, Pott FC, Perner A, Johansson PI. Association between biomarkers of endothelial injury and hypocoagulability in patients with severe sepsis: A prospective study. Crit Care. 2015;19:191. doi: 10.1186/s13054-015-0918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]