Abstract
The aim of the present study was to investigate the prognostic significance of stromal interaction molecule 1 (STIM1) expression in gastric cancer (GC) and examine the association between STIM1 and epithelial-mesenchymal transition (EMT). Immunohistochemical staining was performed to detect STIM1, E-cadherin, β-catenin and matrix metalloproteinase-9 (MMP-9) in 170 GC and 35 adjacent healthy gastric tissue samples. Positive staining of STIM1, E-cadherin, β-catenin and MMP-9 in GC tissues was significantly greater compared with adjacent healthy tissues (P<0.05). Clinicopathological analysis revealed that STIM1 expression was significantly associated with LNM (P<0.001) and tumor-node-metastasis stage (P=0.01). The overall survival rate was significantly reduced in STIM1-positive compared with STIM1-negative patients (P=0.043). Cox regression analysis indicated that STIM1 expression and LNM were independent prognostic factors for GC. Chi-square tests suggested that STIM1 expression in GC tissues was significantly associated with E-cadherin (P<0.001) and β-catenin (P<0.001), whereas no association was observed between STIM1 and MMP-9 expression (P>0.05). In conclusion, the results of the present study suggested that STIM1 may be a valuable prognostic marker in GC patients, and that STIM1 may increase GC motility and invasiveness by promoting epithelial-mesenchymal transition.
Keywords: β-catenin, E-cadherin, epithelial-mesenchymal transition, gastric cancer, overall survival, stromal interaction molecule 1, matrix metalloproteinase-9
Introduction
Gastric cancer (GC) is the fourth most common cancer and the second leading cause of cancer-associated mortality worldwide (1). As 5-year survival rates of GC remain <30% (2,3), further understanding of GC is urgently required.
Epithelial-mesenchymal transition (EMT) is an essential early step in tumor metastasis (4). During EMT, tumor cells lose their epithelial characteristics and obtain mesenchymal traits (5–8). It has been demonstrated that EMT is correlated with poor tumor staging, an increased risk of cancer recurrence and decreased survival in various cancer types, including breast (9,10), colorectal (11), bladder (12,13), lung (14) and GC (15).
Stromal interaction molecule 1 (STIM1) is responsible for the activation of store-operated Ca2+ entry (16). Previous studies have reported that STIM1 is important in the growth and migration of cancer cells, and for angiogenesis and progression of cancer (17–20). Furthermore, STIM1 is a key molecule in the process of EMT in various cancer types. Ectopic STIM1 expression induced EMT in colorectal cancer cells (20), and STIM1 silencing reversed EMT initiated by downregulation of the POU class 5 homeobox 1 transcription factor in breast cancer cells (19). Casas-Rua et al (21) demonstrated that STIM1 overexpression increased migration and EMT in endometrial adenocarcinoma cells. However, the role of STIM1 in GC progression and metastasis and its association with EMT remains to be elucidated.
In the present study, immunohistochemistry was performed to detect STIM1, E-cadherin, β-catenin and matrix metalloproteinase-9 (MMP-9) in 170 GC and 35 adjacent healthy gastric tissue samples. STIM1 was overexpressed in GC samples and associated with poor survival of GC patients. STIM1 expression was significantly associated with abnormal cytoplasmic and nuclear expression of E-cadherin and β-catenin in GC cells, which suggested that STIM1 may promote EMT in GC.
Materials and methods
Patients and tissue samples
GC and adjacent healthy tissue samples were obtained from 170 GC patients with histologically confirmed gastric adenocarcinoma between June 2009 and October 2011 at The Fourth Affiliated Hospital of Hebei Medical University (Shijiazhuang, China). All patients underwent surgical resection of the stomach with lymph node dissection, with no chemotherapy or radiotherapy preoperation; no other cancers were diagnosed simultaneously. The present study was approved by the Ethics Committees of The Fourth Affiliated Hospital of Hebei Medical University, and written consent was obtained from all patients. Follow-up data were primarily obtained by telephone and out-patient review. Patients with inadequate follow-up were excluded from the study.
Of the 170 GC patients enrolled in the present study, 127 were male (74.7%) and 43 were female (25.3%), with an average age of 57.85 years (range, 33–81 years). In total, 57 (33.5%) cases were stage I and II tumors at diagnosis, and 113 (66.5%) were stage III and IV. Tumors were evaluated according to the tumor-node-metastasis (TNM) staging system for carcinoma of the stomach (22). A total of 106 patients (62.4%) had regional lymph node metastasis (LNM), whereas 64 (37.6%) had no regional LNM. Tumors were located in the cardia of 54.1% of patients, and in the antrum of 39.4%. Tumors ranged from 2 to 12 cm in size, with a mean size of 7.12 cm. A total of 79 tumors (46.5%) were poorly differentiated and 91 (53.5%) were moderately or well differentiated, according to the criteria proposed by the World Health Organization Classification of Tumors (3rd Edition) (23). The clinicopathological characteristics of patients are presented in Table I.
Table I.
Association of STIM1, E-cadherin, β-cadherin and MMP-9 expression with characteristics of 170 gastric cancer patients.
| STIM1 | E-Cadherin | β-cadherin | MMP-9 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | |||||||||
| Characteristic | (74) | (96) | χ2 | P-value | (89) | (81) | χ2 | P-value | (105) | (65) | χ2 | P-value | (88) | (82) | χ2 | P-value |
| Sex | ||||||||||||||||
| Male (127) | 54 (42.5) | 73 (57.5) | 0.208 | 0.648 | 63 (49.6) | 64 (50.4) | 1.52 | 0.218 | 76 (59.8) | 51 (40.2) | 0.79 | 0.375 | 68 (53.5) | 59 (46.5) | 0.64 | 0.43 |
| Female (43) | 20 (46.5) | 23 (53.5) | 26 (60.5) | 17 (39.5) | 29 (67.4) | 14 (32.6) | 20 (46.5) | 23 (53.5) | ||||||||
| Age (years) | ||||||||||||||||
| ≤60 (96) | 45 (46.9) | 51 (53.1) | 1.004 | 0.316 | 48 (50.0) | 48 (50.0) | 0.49 | 0.484 | 55 (57.3) | 41 (42.7) | 1.87 | 0.171 | 45 (46.9) | 51 (53.1) | 2.11 | 0.15 |
| >60 (74) | 29 (39.2) | 45 (60.8) | 41 (55.4) | 33 (44.6) | 50 (67.6) | 24 (32.4) | 43 (58.1) | 31 (41.9) | ||||||||
| Tumor location | ||||||||||||||||
| Cardia (92) | 43 (46.7) | 49 (79.0) | 1.010 | 0.603 | 44 (47.8) | 48 (52.2) | 1.70 | 0.428 | 58 (63.0) | 34 (37.0) | 0.20 | 0.904 | 49 (53.3) | 43 (46.7) | 5.37 | 0.07 |
| Body (11) | 5 (45.5) | 6 (54.5) | 6 (54.5) | 5 (45.5) | 7 (63.6) | 4 (36.4) | 2 (18.2) | 9 (81.8) | ||||||||
| Antrum (67) | 26 (38.8) | 41 (61.2) | 39 (58.2) | 28 (41.8) | 40 (59.7) | 27 (40.3) | 37 (55.2) | 30 (44.8) | ||||||||
| Tumor differentiation | ||||||||||||||||
| Poor/undifferentiated (79) | 32 (40.5) | 47 (59.5) | 0.549 | 0.459 | 37 (46.8) | 42 (53.2) | 1.80 | 0.180 | 54 (68.4) | 25 (31.6) | 2.71 | 0.099 | 45 (57.0) | 34 (43.0) | 1.60 | 0.21 |
| High/moderate (91) | 42 (46.2) | 49 (53.8) | 52 (57.1) | 39 (42.9) | 51 (56.0) | 40 (44.0) | 43 (47.3) | 48 (52.7) | ||||||||
| Tumor size | ||||||||||||||||
| <5 cm (74) | 34 (45.9) | 40 (54.1) | 0.311 | 0.577 | 36 (48.6) | 38 (51.4) | 0.72 | 0.399 | 48 (64.9) | 26 (35.1) | 25.84b | <0.001 | 32 (43.2) | 42 (56.8) | 3.81 | 0.05 |
| ≥5 cm (96) | 40 (41.7) | 56 (58.3) | 53 (55.2) | 43 (44.8) | 57 (59.3) | 39 (40.6) | 56 (58.3) | 40 (41.7) | ||||||||
| Lymphatic metastasis | ||||||||||||||||
| Negative (64) | 10 (15.6) | 54 (84.4) | 32.513b | <0.001 | 22 (34.4) | 42 (65.6) | 13.30b | <0.001 | 16 (25.0) | 48 (75.0) | 58.75b | <0.001 | 16 (25.0) | 48 (75.0) | 29.45b | <0.001 |
| Positive (106) | 64 (60.4) | 42 (39.6) | 67 (63.2) | 39 (36.8) | 89 (84.0) | 17 (16.0) | 72 (67.9) | 34 (32.1) | ||||||||
| Tumor-node-metastasis stage | ||||||||||||||||
| I–II (57) | 17 (29.8) | 40 (70.2) | 6.552a | 0.010 | 18 (31.6) | 39 (68.4) | 14.84b | <0.001 | 20 (35.1) | 37 (64.9) | 25.84b | <0.001 | 27 (47.4) | 30 (52.6) | 0.66 | 0.42 |
| III–IV (113) | 57 (50.4) | 56 (49.6) | 71 (62.8) | 42 (37.2) | 85 (75.2) | 28 (24.8) | 61 (54.0) | 52 (46.0) | ||||||||
Data are expressed as no. (%).
P<0.05
P<0.01. STIM1, stromal interaction molecule 1; MMP-9, matrix metalloproteinase-9.
170 GC tumor tissues were analyzed in the present study, with 35 adjacent healthy gastric mucosa tissues as negative controls. All tissue samples were fixed in 10% neutral formalin, embedded in paraffin blocks, cut into 4-µm thick serial sections, and placed on glass slides for immunohistochemical staining.
Immunohistochemical staining of STIM1, E-cadherin, β-catenin and MMP-9
Immunohistochemistry was performed using rabbit anti-human STIM1 (ab108994; 1:100; Abcam, Cambridge, UK), mouse anti-human E-cadherin (ab1416; 1:100; Abcam), rabbit anti-human anti-β-catenin (ab32572; 1:500; Abcam) and rabbit anti-human MMP-9 (ab73734; 1:200; Abcam) primary antibodies. Sections were deparaffinized, rehydrated, rinsed in phosphate-buffered saline (PBS; pH 7.4) and autoclaved in EDTA buffer (pH 8.0) for antigen retrieval. Following cooling to room temperature for 20 min, sections were rinsed in PBS and incubated in 3% H2O2 for 15 min to block endogenous peroxidase activity. Sections were again rinsed with PBS and incubated with normal goat serum (Beijing Zhongshan Jinqiao Biological Technology Co., Ltd., Beijing, China) at 37°C for 15 min to block nonspecific antibody binding. Following incubation with primary antibodies at 37°C for 2 h, sections were rinsed in PBS, incubated with a biotinylated secondary antibody (biotinylated goat anti-mouse/rabbit IgG; SP-9000; Beijing Zhongshan Jinqiao Biological Technology Co., Ltd.) at room temperature for 15 min and rinsed with PBS. Following incubation with streptavidin-horseradish peroxidase (Beijing Zhongshan Jinqiao Biological Technology Co., Ltd.) and further rinsing with PBS, proteins were visualized using 3,3′-diaminobenzidine (Beijing Zhongshan Jinqiao Biological Technology Co., Ltd.) and sections were counterstained with hematoxylin. Finally, sections were dehydrated, cleared, covered with coverslips and sealed with neutral gum.
All slides were assessed by two pathologists who were blinded to the patient details. The intensity of STIM1 staining was graded on a 0–3 scale: 0, no staining; 1, weak immunoreactivity; 2, moderate immunoreactivity; 3, strong immunoreactivity. The percentage of immunoreactivity was scored on a 0–3 scale: 0, no positive cells; 1, 0–25% positive cells; 2, 26–50% positive cells; 3, >50% positive cells (24). E-cadherin, β-catenin and MMP-9 staining were classified as abnormal according to the degree of cytoplasmic and nuclear staining and the proportion of positive cells. Abnormal staining intensity was graded on a 0–3 scale: 0, no staining; 1, weak immunoreactivity; 2, moderate immunoreactivity; 3, strong immunoreactivity. The percentage of abnormal immunoreactivity was scored on a 0–4 scale: 0, 0–20% positive cells; 1, 21–40% positive cells; 2, 41–60% positive cells; 3, 61–80% positive cells; 4, >80% positive cells (25). The staining intensity score was multiplied by the percentage immunoreactivity score to obtain a composite score. The composite score was considered the overall expression level: 0–4, negative; 5–6, positive; 6–12, strongly positive.
Statistical analysis
All data were processed with SPSS software version 19.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference. The chi-square test was used to analyze the association between STIM1 expression and patient characteristics. A binary logistical regression model was applied to identify factors associated with STIM1 positive expression. Cohen's kappa statistic was used to determine the association between STIM1 expression and abnormal E-cadherin and β-catenin expression. The Kaplan-Meier method was used to calculate patient survival rate, and the Cox proportional hazards models were employed to identify independent factors associated with patient survival. In this model, X1, Age; X2, Sex; X3, Tumor location; X4, Tumor differentiation; X5, Tumor size; X6, Lymphatic metastasis; X7, Tumor-node-metastasis; and X8, STIM1 expression were used as independent variables; and Y, Survival as a dependent variable.
Results
STIM1 expression and its association with clinicopathological characteristics of GC patients
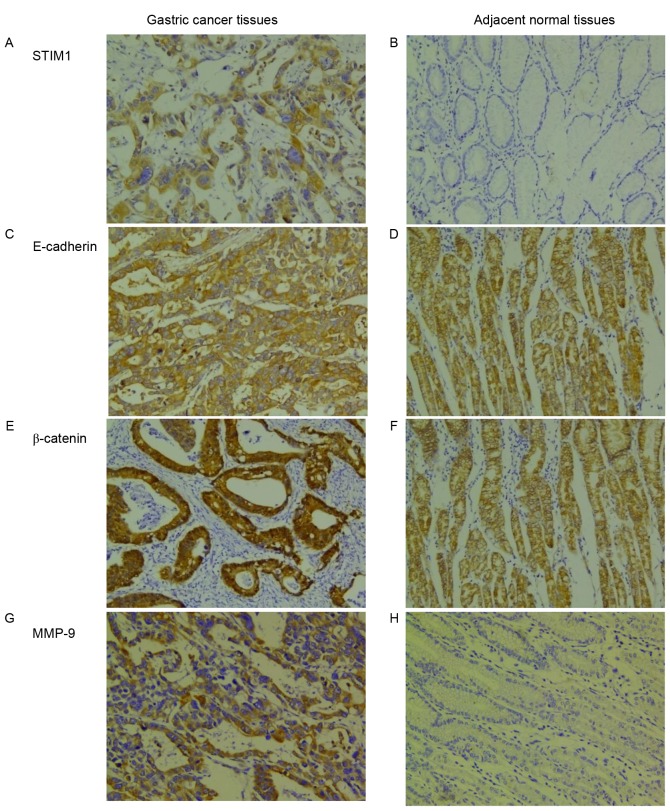
STIM1 expression in GC tissues was predominantly cytoplasmic (Fig. 1A). The STIM1 positive expression rate in GC tissues was 43.5% (74/170), which was significantly greater compared with adjacent healthy tissues (8.60%; 3/35; χ2=15.12; P<0.001; Table II; Fig. 1B). The STIM1 expression rate in GC patients with LNM was significantly greater compared with patients without LNM (P<0.001). STIM1 expression in stage I–II GC tissues was 33.5% (17/57), which was significantly reduced compared with stage III–IV tumors (66.5%; 57/113; P=0.01; Table I). However, STIM1 expression in GC tissues did not correlate with sex, age, the degree of histologic differentiation, location of the tumor or tumor size (P>0.05). Cox risk regression analysis indicated that lymphatic metastasis was the only independent risk factor for STIM1 expression in GC patients (Table III).
Figure 1.
Immunohistochemical staining of STIM1, E-cadherin, β-catenin and MMP-9 in GC and adjacent healthy gastric tissues. STIM1 expression was detected in (A) GC tissues, but not in (B) adjacent healthy gastric tissues. E-cadherin expression was abnormal in (C) GC tissues and normal in (D) adjacent healthy gastric tissues. β-catenin expression was abnormal in (E) GC tissues and normal in (F) adjacent healthy gastric tissues. MMP-9 expression was detected in (G) GC tissues, but not in (H) adjacent healthy gastric tissues. Original magnification, ×200. STIM1, stromal interaction molecule 1; MMP-9, matrix metalloproteinase-9; GC, gastric cancer.
Table II.
STIM1, E-cadherin, β-catenin and MMP-9 expression in GC tissues and adjacent healthy gastric tissues.
| STIM1 | E-cadherin | β-catenin | MMP-9 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue | Positive | Negative | χ2 | P-value | Positive | Negative | χ2 | P-value | Positive | Negative | χ2 | P-value | Positive | Negative | χ2 | P-value |
| GC | 74 (43.5) | 96 (56.5) | 15.12b | <0.001 | 105 (61.8) | 65 (38.2) | 29.53b | <0.001 | 89 (52.4) | 81 (47.6) | 12.20b | <0.001 | 88 (51.8) | 82 (48.2) | 6.26a | 0.012 |
| Adjacent healthy gastric | 3 (8.60) | 32 (91.4) | 4 (11.4) | 31 (88.6) | 7 (20.0) | 28 (80.0) | 10 (28.6) | 25 (71.4) | ||||||||
Data are expressed as no. (%).
P<0.05
P<0.01. STIM1, stromal interaction molecule 1; MMP-9, matrix metalloproteinase-9; GC, gastric cancer.
Table III.
Multivariate analysis of factors associated with stromal interaction molecule 1 expression in gastric carcinoma.
| 95.0% CI for Exp (B) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | B | SE | Wald | df | Sig. | Exp (B) | Lower | Upper | ||
| Sex | 0.690 | 0.429 | 2.584 | 1 | 0.108 | 1.994 | 0.860 | 4.626 | ||
| Age | −0.403 | 0.359 | 1.256 | 1 | 0.262 | 0.669 | 0.331 | 1.352 | ||
| Tumor location | −0.028 | 0.190 | 0.023 | 1 | 0.881 | 0.972 | 0.670 | 1.410 | ||
| Tumor differentiation | 0.162 | 0.358 | 0.205 | 1 | 0.651 | 1.176 | 0.583 | 2.371 | ||
| Tumor size | −0.126 | 0.361 | 0.121 | 1 | 0.728 | 0.882 | 0.434 | 1.790 | ||
| Lymphatic metastasis | 2.171 | 0.446 | 23.734 | 1 | 0.000 | 8.767 | 3.660 | 20.998 | ||
| Tumor-node-metastasis | 0.238 | 0.411 | 0.336 | 1 | 0.562 | 1.269 | 0.567 | 2.840 | ||
| Constant | −2.666 | 1.154 | 5.335 | 1 | 0.021 | 0.070 | ||||
CI, confidence interval. B, regression coefficient; SE, standard error; Wald, the statistic value of the regression; df, degree of freedom; Sig, significance; Exp (B), odds ratio.
E-cadherin, β-catenin and MMP-9 expression, and their association with clinicopathological features of GC patients
In the present study, E-cadherin (Fig. 1C and D) and β-catenin (Fig. 1E and F) were abnormally expressed in the cytoplasm or nucleus of GC cells. The positive expressions of E-cadherin and β-catenin were observed in 61.8% (105/170) and 52.4% (89/170), respectively, of GC tissue samples, and in 11.4% (4/35) and 20.0% (7/35), respectively, of adjacent healthy gastric tissues. Differences in the rates of abnormal E-cadherin and β-catenin expression between GC tissues and adjacent healthy gastric tissues were significant (P<0.001; Table II). MMP-9 expression in GC tissues was additionally predominantly cytoplasmic (Fig. 1G). Greater expression of MMP-9 was observed in GC tissues compared with adjacent healthy gastric tissues (P<0.05; Table II; Fig. 1H). In addition, expression of E-cadherin was positively associated with LNM and a more advanced clinical stage (P<0.001; Table I). β-catenin expression correlated significantly with tumor size, LNM and the clinical stage of GC tissues (P<0.001; Table I); however, there was no correlation between β-catenin expression and other clinicopathological parameters (P>0.05; Table I). Expression of MMP-9 was positively associated with LNM (P<0.001; Table I); however, there was no correlation between MMP-9 expression and other clinicopathological parameters (P>0.05; Table I).
Associations between STIM1, E-cadherin, β-catenin and MMP-9 expression in GC tissues
Potential associations between STIM1, E-cadherin and β-catenin expression patterns in GC were evaluated. Of STIM1-positive tumors, 78.4% (58/74) were E-cadherin positive and 90.5% (67/74) were positive for β-catenin. Chi-square tests revealed that STIM1 expression correlated significantly with abnormal E-cadherin expression (χ2=34.555; P<0.001; κ=0.447) and with abnormal β-catenin expression (χ2=45.947; P<0.001; κ=0.486; Table IV), whereas no correlation was observed between STIM1 and MMP-9 (χ2=1.420; P=0.233; κ=−0.616; Table IV). Furthermore, 79.8% (71/89) of E-cadherin-positive tumors were additionally positive for β-catenin, and this association was statistically significant (P<0.05; Table V).
Table IV.
STIM1, E-cadherin, β-catenin and MMP-9 expression in 170 gastric cancer samples.
| E-cadherin | β-cadherin | MMP-9 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Positive | Negative | χ2 | P-value | Positive | Negative | χ2 | P-value | Positive | Negative | χ2 | P-value |
| STIM1 (+) | 58 (78.4) | 16 (21.6) | 34.555a | <0.001 | 67 (90.5) | 7 (9.5) | 45.947a | <0.001 | 12 (16.2) | 62 (83.8) | 1.420 | 0.233 |
| STIM1 (−) | 31 (32.3) | 65 (67.7) | 38 (39.6) | 58 (60.4) | 76 (79.2) | 20 (20.8) | ||||||
Data are expressed as no. (%).
P<0.01. STIM1, stromal interaction molecule 1; MMP-9, matrix metalloproteinase-9.
Table V.
E-cadherin and β-catenin expression in 170 gastric cancer samples.
| β-cadherin | ||||
|---|---|---|---|---|
| Parameter | Positive | Negative | χ2 | P-value |
| E-cadherin (+) | 71 (79.8) | 18 (20.2) | 4.92a | 0.03 |
| E-cadherin (−) | 34 (42.0) | 47 (58.0) | ||
Data are expressed as no. (%).
P<0.05.
Association between STIM1 expression and survival
Using Kaplan-Meier analysis, it was demonstrated that the overall survival rate was significantly reduced in patients with STIM1-expressing GC tumors compared with patients with GC tumors without STIM1 expression (P=0.043; Fig. 2). Factors that significantly correlated with patient survival rate, including STIM1 expression, LNM and a high TNM stage, were identified by univariate analysis (Table VI). Cox risk regression analysis indicated that STIM1 expression and LNM were independent prognostic factors for GC patients (Table VII).
Figure 2.
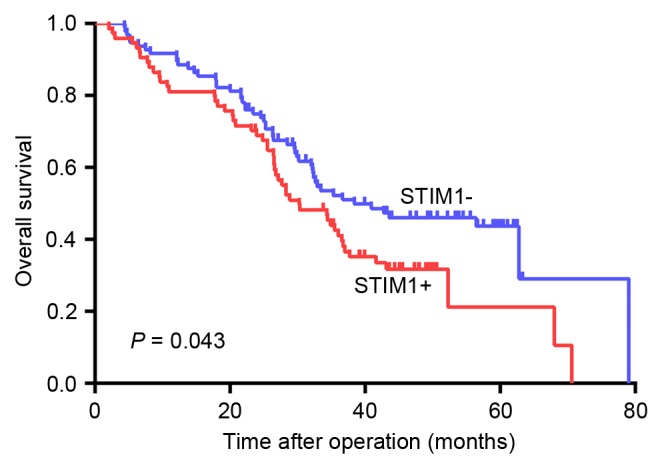
Overall survival of STIM1-positive and -negative patients. The overall survival rate was significantly reduced in patients with STIM1-expressing GC tumors compared with patients with GC tumors without STIM1 expression (P=0.043). STIM1, stromal interaction molecule 1; GC, gastric cancer.
Table VI.
Univariate analysis of prognostic factors for gastric carcinoma.
| Characteristic | Succumbed to disease (104) | Survival rate at 80 months (66) | χ2 | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Male | 79 (76.0) | 48 (72.7) | 0.224 | 0.636 | ||||
| Female | 25 (24.0) | 18 (27.3) | ||||||
| Age (years) | ||||||||
| ≤60 | 58 (55.8) | 38 (57.6) | 0.054 | 0.817 | ||||
| >60 | 46 (44.2) | 28 (42.4) | ||||||
| Tumor location | ||||||||
| Cardia | 58 (55.8) | 34 (51.5) | 0.400 | 0.819 | ||||
| Body | 6 (5.8) | 5 (5.8) | ||||||
| Antrum | 40 (38.5) | 27 (40.9) | ||||||
| Tumor differentiation | ||||||||
| Poor/undifferentiated | 49 (47.1) | 30 (45.5) | 0.045 | 0.832 | ||||
| High/moderate | 55 (52.9) | 36 (54.5) | ||||||
| Tumor size | ||||||||
| <5 cm | 47 (45.2) | 27 (40.9) | 0.301 | 0.583 | ||||
| ≥5 cm | 57 (54.8) | 39 (59.1) | ||||||
| Lymphatic metastasis | ||||||||
| Negative | 15 (14.4) | 49 (74.2) | 61.549b | <0.001 | ||||
| Positive | 89 (85.6) | 17 (25.8) | ||||||
| Tumor-node-metastasis stage | ||||||||
| I–II | 14 (13.5) | 43 (65.2) | 48.404b | <0.001 | ||||
| III–IV | 90 (86.5) | 23 (34.8) | ||||||
| STIM1 expression | ||||||||
| Positive | 52 (50.0) | 22 (33.3) | 4.563a | 0.033 | ||||
| Negative | 52 (50.0) | 44 (66.7) | ||||||
Data are expressed as no. (%).
P<0.05
P<0.01. STIM1, stromal interaction molecule 1.
Table VII.
Multivariate analysis of prognostic factors for gastric carcinoma.
| 95.0% CI for HR | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | B | SE | Wald | df | Sig. | HR | Lower | Upper |
| Age | 0.292 | 0.209 | 1.950 | 1 | 0.163 | 1.339 | 0.889 | 2.018 |
| Sex | −0.221 | 0.259 | 0.728 | 1 | 0.394 | 0.802 | 0.483 | 1.331 |
| Tumor location | −0.030 | 0.107 | 0.079 | 1 | 0.778 | 0.970 | 0.786 | 1.197 |
| Tumor differentiation | 0.047 | 0.208 | 0.050 | 1 | 0.823 | 1.048 | 0.696 | 1.577 |
| Tumor size | 0.017 | 0.211 | 0.006 | 1 | 0.936 | 1.017 | 0.672 | 1.539 |
| Lymphatic metastasis | 1.699 | 0.328 | 26.797 | 1 | 0.000 | 5.468 | 2.874 | 10.403 |
| Tumor-node-metastasis | 1.307 | 0.311 | 17.651 | 1 | 0.000 | 3.695 | 2.008 | 6.799 |
| STIM1 expression | 0.095 | 0.286 | 0.111 | 1 | 0.039 | 1.100 | 1.020 | 1.328 |
CI, confidence interval; STIM1, stromal interaction molecule 1. B, regression coefficient. SE, standard error. Wald, the statistic value of the regression. df, degree of freedom. Sig, significance. HR, hazard ratio.
Discussion
Various studies have demonstrated that STIM1 protein is involved in adhesion, invasion, metastasis and proliferation of cancer cells (17,18,26–29). STIM1 expression has been reported to correlate with lymphatic invasion in colon adenocarcinomas (30). Ectopic STIM1 overexpression in colorectal cancer was revealed to significantly associate with tumor size, depth of invasion and LNM status, and to promote colorectal cancer cell motility (24). It has been reported that STIM1 is upregulated during hepatocarcinoma growth (31), and STIM1 has been suggested to be critical for breast cancer cell migration and metastasis (18). However, certain studies have demonstrated that STIM1 protein serves an opposing role in various cancers. For example, in vitro overexpression of STIM1 in G401 rhabdomyosarcoma cells resulted in morphological alterations and, ultimately, cell death (32,33). Suyama et al (34) revealed that STIM1 has an antimetastatic function. Weidinger et al (35) reported that patients with loss-of-function mutations in the STIM1 gene were immunodeficient and prone to developing virus-associated tumors. The present study demonstrated that STIM1 was highly expressed in GC compared with adjacent healthy tissues, and that STIM1 expression was associated with LNM, TNM stage and poor overall survival rate. Furthermore, LNM was the only independent risk factor for STIM1 expression in GC patients. The results of the present study indicated that STIM1 may serve an important role in the initiation and development of GC, and may contribute to the diagnosis and treatment of GC as a prognostic marker. These results therefore provide novel information on the function of STIM1 in GC progression.
The molecular mechanisms underlying the effect of STIM1 on the process of EMT in GC remain to be fully elucidated. A previous study by Hu et al (19) suggested that STIM1 may be involved in EMT, which is a critical step in immune evasion and metastasis of tumor cells. In addition, STIM1 overexpression has been reported to induce EMT in colorectal cancer cells, whereas STIM1 silencing had the opposite effect (20). Casas-Rua et al (21) demonstrated that STIM1 phosphorylation at extracellular signal-regulated kinase 1/2 target sites mediates EMT triggered by epidermal growth factor in Ishikawa cells. However, the role of STIM1 in cancer cell progression and metastasis and its association with EMT in GC remain to be investigated. In the present study, the association between the expression of STIM1, E-cadherin, β-catenin and MMP-9 proteins in GC tissues was analyzed by immunohistochemical staining. The results of the present study revealed that STIM1 overexpression in GC tissues correlated significantly with abnormal E-cadherin and β-catenin expression in the cytoplasm and nucleus, whereas no association was observed between STIM1 and MMP-9 expression. Therefore, STIM1 may increase GC motility and invasiveness by promoting EMT via E-cadherin and β-cadherin; however, MMP-9 does not appear to be involved in this process.
In conclusion, the results of the present study demonstrated that STIM1 is significantly upregulated in GC and that STIM1 overexpression is associated with a poor prognosis in GC patients with LNM and an advanced TNM stage. Therefore, STIM1 may be a useful prognostic marker for GC.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki R, Yamamoto E, Nojima M, Maruyama R, Yamano HO, Yoshikawa K, Kimura T, Harada T, Ashida M, Niinuma T, et al. Aberrant methylation of microRNA-34b/c is a predictive marker of metachronous gastric cancer risk. J Gastroenterol. 2014;49:1135–1144. doi: 10.1007/s00535-013-0861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bria E, De Manzoni G, Beghelli S, Tomezzoli A, Barbi S, Di Gregorio C, Scardoni M, Amato E, Frizziero M, Sperduti I, et al. A clinical-biological risk stratification model for resected gastric cancer: Prognostic impact of Her2, Fhit, and APC expression status. Ann Oncol. 2013;24:693–701. doi: 10.1093/annonc/mds506. [DOI] [PubMed] [Google Scholar]
- 4.Kraljevic Pavelic S, Sedic M, Bosnjak H, Spaventi S, Pavelic K. Metastasis: New perspectives on an old problem. Mol Cancer. 2011;10:22. doi: 10.1186/1476-4598-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 7.Savagner P, Boyer B, Valles AM, Jouanneau J, Thiery JP. Modulations of the epithelial phenotype during embryogenesis and cancer progression. Cancer Treat Res. 1994;71:229–249. doi: 10.1007/978-1-4615-2592-9_12. [DOI] [PubMed] [Google Scholar]
- 8.Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med. 2013;19:1438–1449. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Chen X, Ward T, Pegram M, Shen K. Combined niclosamide with cisplatin inhibits epithelial-mesenchymal transition and tumor growth in cisplatin-resistant triple-negative breast cancer. Tumour Biol. 2016;37:9825–9835. doi: 10.1007/s13277-015-4650-1. [DOI] [PubMed] [Google Scholar]
- 10.Luo M, Hou L, Li J, Shao S, Huang S, Meng D, Liu L, Feng L, Xia P, Qin T, Zhao X. VEGF/NRP-1axis promotes progression of breast cancer via enhancement of epithelial-mesenchymal transition and activation of NF-κB and β-catenin. Cancer Lett. 2016;373:1–11. doi: 10.1016/j.canlet.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Ma J, Zhao J, Lu J, Wang P, Feng H, Zong Y, Ou B, Zheng M, Lu A. Cadherin-12 enhances proliferation in colorectal cancer cells and increases progression by promoting EMT. Tumour Biol. 2016;37:9077–9088. doi: 10.1007/s13277-015-4555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruyere F, Namdarian B, Corcoran NM, Pedersen J, Ockrim J, Voelzke BB, Mete U, Costello AJ, Hovens CM. Snail expression is an independent predictor of tumor recurrence in superficial bladder cancers. Urol Oncol. 2010;28:591–596. doi: 10.1016/j.urolonc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Chiu KY, Wu CC, Chia CH, Hsu SL, Tzeng YM. Inhibition of growth, migration and invasion of human bladder cancer cells by antrocin, a sesquiterpene lactone isolated from Antrodia cinnamomea, and its molecular mechanisms. Cancer Lett. 2016;373:174–184. doi: 10.1016/j.canlet.2015.11.046. [DOI] [PubMed] [Google Scholar]
- 14.Lee SO, Yang X, Duan S, Tsai Y, Strojny LR, Keng P, Chen Y. IL-6 promotes growth and epithelial-mesenchymal transition of CD133+ cells of non-small cell lung cancer. Oncotarget. 2016;7:6626–6638. doi: 10.18632/oncotarget.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang L, Wu RL, Xu AM. Epithelial-mesenchymal transition in gastric cancer. Am J Transl Res. 2015;7:2141–2158. [PMC free article] [PubMed] [Google Scholar]
- 16.Parekh AB. Store-operated CRAC channels: Function in health and disease. Nat Rev Drug Discov. 2010;9:399–410. doi: 10.1038/nrd3136. [DOI] [PubMed] [Google Scholar]
- 17.Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY, Chang HC, Tang MJ, Shen MR. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci USA. 2011;108:15225–15230. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15:124–134. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Hu J, Qin K, Zhang Y, Gong J, Li N, Lv D, Xiang R, Tan X. Downregulation of transcription factor Oct4 induces an epithelial-to-mesenchymal transition via enhancement of Ca2+ influx in breast cancer cells. Biochem Biophys Res Commun. 2011;411:786–791. doi: 10.1016/j.bbrc.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Liu X, Feng B, Liu N, Wu Q, Han Y, Nie Y, Wu K, Shi Y, Fan D. STIM1, a direct target of microRNA-185, promotes tumor metastasis and is associated with poor prognosis in colorectal cancer. Oncogene. 2015;34:4808–4820. doi: 10.1038/onc.2014.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casas-Rua V, Tomas-Martin P, Lopez-Guerrero AM, Alvarez IS, Pozo-Guisado E, Martin-Romero FJ. STIM1 phosphorylation triggered by epidermal growth factor mediates cell migration. Biochim Biophys Acta. 2015;1853:233–243. doi: 10.1016/j.bbamcr.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 22.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Handbook. Chicago: Springer-Verlag New York; 2010. [Google Scholar]
- 23.World Health Organization Classification of Tumours: Pathology and genetics of tumours of the digestive system. Lyon: IARC Press; 2000. [Google Scholar]
- 24.Wang JY, Sun J, Huang MY, Wang YS, Hou MF, Sun Y, He H, Krishna N, Chiu SJ, Lin S, et al. STIM1 overexpression promotes colorectal cancer progression, cell motility and COX-2 expression. Oncogene. 2015;34:4358–4367. doi: 10.1038/onc.2014.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaw SY, Majeed AA, Dalley AJ, Chan A, Stein S, Farah CS. Epithelial to mesenchymal transition (EMT) biomarkers-E-cadherin, beta-catenin, APC and Vimentin-in oral squamous cell carcinogenesis and transformation. Oral Oncol. 2012;48:997–1006. doi: 10.1016/j.oraloncology.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Ouadid-Ahidouch H, Dhennin-Duthille I, Gautier M, Sevestre H, Ahidouch A. TRP channels: Diagnostic markers and therapeutic targets for breast cancer? Trends Mol Med. 2013;19:117–124. doi: 10.1016/j.molmed.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: New roles for known actors. Nat Rev Cancer. 2011;11:609–618. doi: 10.1038/nrc3105. [DOI] [PubMed] [Google Scholar]
- 28.Bergmeier W, Weidinger C, Zee I, Feske S. Emerging roles of store-operated Ca2+ entry through STIM and ORAI proteins in immunity, hemostasis and cancer. Channels (Austin) 2013;7:379–391. doi: 10.4161/chan.24302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Hughes JD, Rollins S, Chen B, Perkins E. Calcium entry via ORAI1 regulates glioblastoma cell proliferation and apoptosis. Exp Mol Pathol. 2011;91:753–760. doi: 10.1016/j.yexmp.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Wong HS, Chang WC. Correlation of clinical features and genetic profiles of stromal interaction molecule 1 (STIM1) in colorectal cancers. Oncotarget. 2015;6:42169–42182. doi: 10.18632/oncotarget.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Guo B, Xie Q, Ye D, Zhang D, Zhu Y, Chen H, Zhu B. STIM1 Mediates Hypoxia-Driven Hepatocarcinogenesis via Interaction with HIF-1. Cell Rep. 2015;12:388–395. doi: 10.1016/j.celrep.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 32.Sabbioni S, Barbanti-Brodano G, Croce CM, Negrini M. GOK: A gene at 11p15 involved in rhabdomyosarcoma and rhabdoid tumor development. Cancer Res. 1997;57:4493–4497. [PubMed] [Google Scholar]
- 33.Sabbioni S, Veronese A, Trubia M, Taramelli R, Barbanti-Brodano G, Croce CM, Negrini M. Exon structure and promoter identification of STIM1 (alias GOK), a human gene causing growth arrest of the human tumor cell lines G401 and RD. Cytogenet Cell Genet. 1999;86:214–218. doi: 10.1159/000015341. [DOI] [PubMed] [Google Scholar]
- 34.Suyama E, Wadhwa R, Kaur K, Miyagishi M, Kaul SC, Kawasaki H, Taira K. Identification of metastasis-related genes in a mouse model using a library of randomized ribozymes. J Biol Chem. 2004;279:38083–38086. doi: 10.1074/jbc.C400313200. [DOI] [PubMed] [Google Scholar]
- 35.Weidinger C, Shaw PJ, Feske S. STIM1 and STIM2-mediated Ca(2+) influx regulates antitumour immunity by CD8(+) T cells. EMBO Mol Med. 2013;5:1311–1321. doi: 10.1002/emmm.201302989. [DOI] [PMC free article] [PubMed] [Google Scholar]