Abstract
The present study aimed to investigate the effect of metformin on the induction of autophagy in the liver and adipose tissues of a mouse model of obesity. C57BL/6J mice were fed a high-fat diet (HFD) for 12 weeks to induce obesity-associated hepatic steatosis, and treated with metformin (150 mg/kg/d) by intraperitoneal injection for the final 4 weeks of HFD feeding. Body weight was recorded weekly, and the food intake of the mice was recorded daily during the treatment period. Liver and adipose tissues were harvested for histological and molecular analyses. The results revealed that metformin significantly reduced body weight without altering food intake in the HFD mice, particularly in the epididymal white adipose tissue (eWAT). Metformin treatment ameliorated HFD-induced hepatic steatosis and serum levels of triglycerides, which was consistent with a marked increase in the expression levels of microtubule-associated protein 1 light chain 3 (LC3) and AMP-activated protein kinase (AMPK) in the liver following metformin treatment. However, metformin suppressed the expression of LC3 in the eWAT without altering the expression of AMPK, compared with that in the HFD mice. In conclusion, metformin reduced the body weight and hepatic steatosis of the HFD-induced obese mice, without altering food intake. The effects of metformin treatment may be attributable to the improved induction of hepatic autophagy and the inhibited induction of adipose tissue autophagy.
Keywords: metformin, hepatic steatosis, autophagy, microtubule-associated protein 1 light chain 3
Introduction
Over the last 40 years, the incidence of obesity in children and adolescents has increased significantly (1), and has become a major chronic disease endangering the health of children (2). Obesity was recognized as a disease by the American Medical Association in 2013 (3). Childhood and adolescent obesity can adversely affect almost every organ system, including the liver and adipose tissue, and often causes serious consequences, including dyslipidemia and fatty liver disease. Obesity, as a chronic disease requiring long-term management, has led to the increasing focus on the role of adjunctive therapies for obesity, particularly pharmacotherapy (4).
Metformin is a widely used fist-line antidiabetic drug, which effectively lowers plasma glucose and improves hepatic insulin sensitivity, particularly for overweight and obese individuals. An increasing number of studies have shown that metformin can reduce the body weight of obese individuals, and ameliorate lipid accumulation in the liver in hepatic steatosis (5–7). Although the mechanisms underlying the action of metformin are complicated, there is accumulating evidence that demonstrates that the beneficial effects of metformin are associated with autophagy (8,9).
Autophagy is a cellular self-digestion process, which removes damaged macromolecules and organelles, and maintains cellular homeostasis (10). Under physiological conditions, autophagy is involved in the basal turnover of lipids by engulfing and degrading lipid droplets. Autophagy genes are activated by AMP-activated protein kinase (AMPK), a central energy sensor, as a novel Unc-51-like kinase 1 binding partner, which is a protein kinase activated by an increase in the AMP/ATP ratio (11). Several studies have demonstrated that the level of autophagy can be decreased in hepatocytes in obesity (9). The pharmacological inhibition of autophagy using 3-methyladenine (3MA) or the knockdown of the autophagy gene, autophagy related 5 (Atg5), in hepatocytes has been found to significantly increase hepatocyte triglyceride (TG) content in the absence or presence of exogenous lipid supplementation with oleate (12). In addition, Kovsan et al reported that autophagy is upregulated in the adipose tissue of obese individuals, particularly in omental adipose tissue, correlating with the degree of obesity, visceral fat distribution and adipocyte hypertrophy (13). The knockdown of Atg7 or Atg5 in preadipocytes inhibited lipid accumulation, and the adipocyte-specific mouse knockout of Atg7 generated lean mice with decreased white adipose mass (14). These experimental data indicate that autophagy is closely associated with obesity and lipid metabolism. Based on these findings, the present study investigated whether metformin affects lipid metabolism in the liver and adipose tissue of obese individuals, and whether it is associated with ameliorating autophagy.
Materials and methods
Animals
Male C57BL/6 mice (3-week-old) were obtained from the Animal Center of Xi'an Jiaotong University (Xi'an, China). The mice were housed in a specific pathogen-free environment with controlled room temperature and humidity (22°C; 60% relative humidity) prior to commencement of the experiments. The mice were fed adaptively for 1 week, and then randomly divided into three groups: Normal chow diet group (NCD), n=8; High fat diet (HFD) group, n=16; HFD+metformin group, n=13. NCD mice were fed a normal chow diet containing 20.8% fat, 18.3% protein and 60.9% carbohydrates for 12 weeks. HFD mice were fed a high fat diet containing 47.5% fat, 18.5% protein and 34.3% carbohydrates for 12 weeks. HFD+metformin mice were treated with metformin (150 mg/kg/d) by intraperitoneal injection for the final 4 weeks of HFD feeding. All mice were maintained on a 12:12-h light-dark cycle (lights on at 06:00 a.m.). Food and water were provided ad libitum. During the 12-week feeding/treatment period, the body weights of the mice were recorded weekly. The food intake of the mice was recorded daily during the 4-week treatment period. Following the feeding/treatment regimen, the mice were fasted for 8 h prior to sacrifice for the collection of blood and tissue samples. Epididymal, perinephric and brown fat depots were dissected and weighed. The liver was also dissected and weighed. Following weighing, epididymal adipose and liver tissue samples were either fixed and embedded for histological evaluation and immunohistochemistry, or were frozen in liquid nitrogen and stored at −80°C for further analyses. All the mice were fasted similarly and used for serum TG and transaminase assessment. All experiments were performed according to the guidelines of the Committee of Animal Research at Xi'an Jiaotong University and the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (15).
Drugs
Metformin (1,1-dimethylbiguanide hydrochloride; Bristol-Myers Squibb Company, Princeton, NJ, USA) was dissolved in 0.9% saline, and administered daily by intraperitoneal injection at 09:00 a.m.
Histological analyses with hematoxylin and eosin (H&E) staining, and immunohistochemistry
The paraffin-embedded liver and adipose tissue specimens were cut into sections of 5 µm thickness and H&E stained to assess the severity of lipid accumulation in the liver tissues. The surplus sections were used for immunohistochemical analysis. The sections were dewaxed and rehydrated with dimethylbenzene and different concentrations of ethanol, respectively. Subsequently, sections were processed by microwave-based antigen retrieval for 10 min, and treated with 3% H2O2 solution to block endogenous peroxidase activity. After 3 washes in PBS, the sections were treated with goat serum (ZSGB-BIO, Beijing, China), 37°C for 15 min to block nonspecific sites and incubated overnight at 4°C with rabbit polyclonal antibody against LC3 (1:200; bs-8878R) and AMPKα2 (1:100; bs-2771R) (both from BIOSS, Beijing, China) diluted in double distilled water. Following several rinses in PBS, peroxidase-labeled goat anti-rabbit secondary antibody working solution (SP-9001; ZSGB-BIO) was added for 15 min at 37°C. The peroxidase activity was observed with a Q550CW Image Acquiring & Analysis System (Leica Microsystems GmbH, Wetzlar, Germany), using a diaminobenzidine substrate, which yielded a yellow/brown deposit. Negative controls included sections with the primary antibodies omitted. The immunohistochemical-stained slides were selected randomly and were analyzed using Image-Pro plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA). For each selected field, the positive area was calculated automatically using the software. These results were expressed by volume fractions, as the percentage of positive area in relation to the total\area, and expressed as the mean ± standard error of the mean.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from ~50 mg samples of adipose and liver tissue according to the manufacturer's protocol using Ambion TRIzol (Invitrogen cat. no. 15596-026; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The concentration of total RNA was measured using ultraviolet spectrophotometry (ND-1000; Thermo Fisher Scientific, Inc.). First strand-cDNA synthesis was performed with oligo (dT) primer using a PrimeScript RT regent kit (cat. no. RR820A; Takara Bio, Inc., Otsu, Japan). The RT-qPCR analysis was performed with a Bio-Rad iQ5q-PCR system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using SYBR Premix Ex Taq II (Takara Bio, Inc.). The primers used are shown in Table I. Data were normalized to the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). qPCR reactions contained 25 ng cDNA template, 10 µM forward and reverse primers, 10 µl 2X SYBR Premix Ex Tag II (RR820A; Takara Bio, Inc., Otsu, Japan) and 6.4 µl dH2O (Takara Bio, Inc.) in a total reaction volume of 20 µl. Following a pre-denaturation step at 95°C for 30 s, qPCR was performed using 40 cycles of denaturation at 95°C for 5 s, annealing at 60°C for 20 s and elongation at 72°C for 30 s. The results were expressed as the number of cycles (Cq value) at which the fluorescence signal exceeded a defined threshold. The difference in Cq values of the target cDNA and GAPDH were expressed as ΔCq values. The 2-∆∆Cq method was used for quantification of the results (16).
Table I.
Primers used for reverse transcription-quantitative polymerase chain reaction analysis in the control and treatment mice.
| Gene | Primer sequence | Product length (bp) | Gene bank no. |
|---|---|---|---|
| LC3 | F: 5′-CCCCAGTGGATTAGGCAGAG-3′ | 127 | NM_025735.3 |
| R: 5′-CAGCCAGCACCCAAAAGAG-3′ | |||
| AMPK | F: 5′-CCACATTCGAAGCCCATGTT-3′ | 199 | NM_178143.2 |
| R: 5′-AGGGACAGGTAGGTCACAGAGA-3′ | |||
| GAPDH | F: 5′-TGTGTCCGTCGTGGATCTGA-3′ | 150 | NM_008084.2 |
| R: 5′-TTGCTGTTGAAGTCGCAGGAG-3′ |
LC3, microtubule-associated protein 1 light chain 3; AMPK, AMP-activated protein kinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; F, forward; R, reverse.
Statistical analysis
Numeric data are presented as the mean ± standard error of mean. All statistical analyses were performed using Student's t test, and analyzed using two-way repeated measures analysis of variance followed by Bonferroni multiple comparisons using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of metformin on body weight and food intake in HFD-induced obese mice
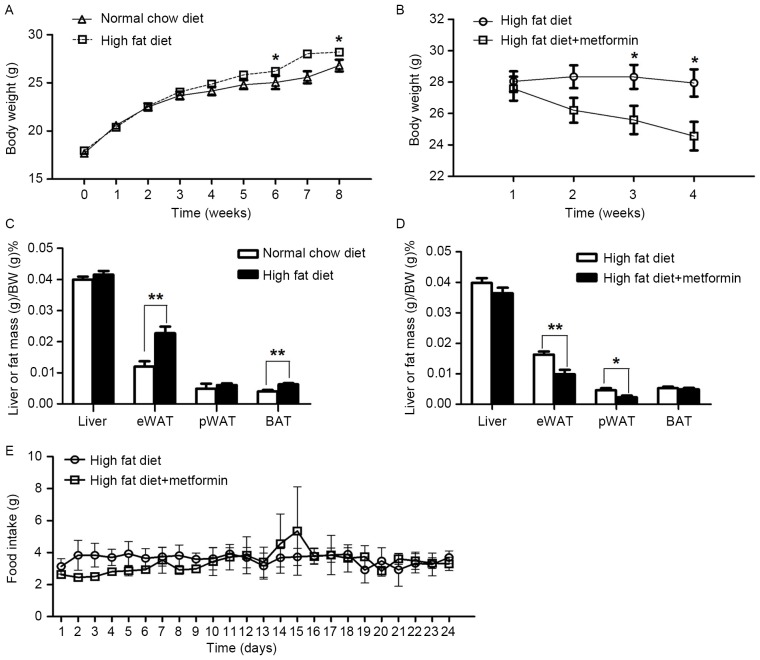
The mice maintained on the HFD for 8 weeks exhibited significantly increased body weight, compared with the NCD mice (P<0.05; Fig. 1A). Metformin treatment at 150 mg/kg daily for 4 weeks significantly decreased body weight in the obese mice maintained on a HFD (P<0.05; Fig. 1B). In addition, the percentage of body fat in the eWAT was significantly reduced in the HFD+metformin mice following the 4 weeks, compared with that in the HFD mice (Fig. 1C and D). However, no significant difference in daily food intake was observed between the HFD mice and the HFD+metformin mice (P>0.05; Fig. 1E).
Figure 1.
Effects of metformin on body weight and food intake of obese mice. Changes in body weight of (A) mice fed an HFD or (B) treated with metformin. Percentage change in liver weights of (C) mice fed an HFD or (D) treated with metformin. (E) Changes in food intake of the mice treated with metformin. Data are presented as the mean ± standard error of the mean. *P<0.05; **P<0.01. eWAT, epididymal white adipose tissue; pWAT, perinephric white adipose tissue; BAT, brown adipose tissue.
Effect of metformin on serum TG
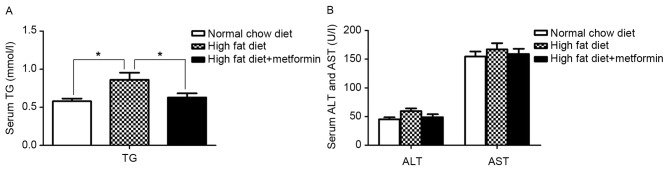
The metformin-treated mice exhibited a significant decrease in the severity of the HFD-induced high TG levels (P<0.05; Fig. 2A). No differences in the serum levels of aspartate aminotransferase (AST) or alanine aminotransferase (ALT) were observed between the NCD mice and HFD mice (P>0.05; Fig. 2B).
Figure 2.
Effect of metformin on serum levels of TG and transaminase. (A) Changes in fasting serum concentrations of (A) TG, and (B) AST and ALT in mice in the normal chow diet, high fat diet and high fat diet+metformin groups during the feeding/treatment period. Data are presented as the mean ± standard error of the mean. *P<0.05. TG, triglyceride; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Metformin treatment ameliorates HFD-induced hepatic lipid accumulation
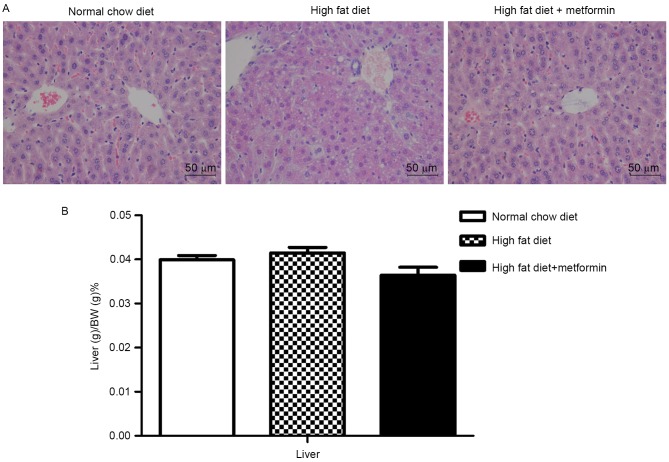
The HFD mice exhibited lipid accumulation surrounding the perisinusoidal areas, indicated by changes in the liver sections stained with H&E (Fig. 3A). In contrast to the increased body weights, the liver weights of the HFD mice were only marginally increased, with no significant difference between the NCD mice and HFD mice (P>0.05; Fig. 3B). Compared with the HFD mice, the HFD+metformin mice exhibited a marginal decrease in liver weight, which was accompanied by a marked decrease in lipid accumulation surrounding the perisinusoidal areas (Fig. 3).
Figure 3.
Metformin treatment ameliorates HFD-induced hepatic lipid accumulation. (A) Representative images of liver lipid accumulation in tissue sections from mice in the normal chow diet, high fat diet and high fat diet+metformin groups following hematoxylin and eosin staining. (B) Percentage changes in liver weight during the feeding/treatment period. Data are presented as the mean ± standard error of the mean.
Effects of metformin on the expression of LC3 in liver and adipose tissues of HFD-induced obese mice
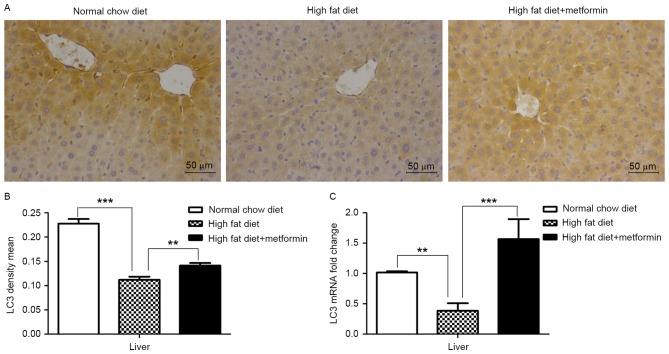
The present study also examined the expression of LC3 in the liver tissue and eWAT of the HFD mice maintained with metformin, respectively. Significantly decreased mRNA and protein levels of LC3 were observed in the liver of the HFD mice, compared with those of the NCD mice (P<0.001), whereas metformin treatment upregulated the expression of LC3 in the liver of the HFD mice (P<0.01; Fig. 4A and B). However, increased mRNA and protein levels of LC3 were observed in the adipose tissues of the HFD mice compared with those of the NCD mice (P<0.01; Fig. 5A-C). Of note, metformin treatment suppressed the expression of LC3 in the eWAT, compared with that in the HFD mice (P<0.05; Fig. 5).
Figure 4.
Metformin treatment upregulates the expression of LC3 in the liver of HFD-induced obese mice. (A) Microphotographs of liver tissues from mice in the normal chow diet, high fat diet and high fat diet+metformin groups following immunohistochemical staining of LC3. (B) Quantitative analysis of LC3-positive regions in the liver tissue sections. (C) mRNA levels of LC3 in the liver, measured using reverse transcription-quantitative polymerase chain reaction analysis and normalized to glyceraldehyde-3-phosphate dehydrogenase. Data are presented as the mean ± standard error of the mean **P<0.01; ***P<0.001. LC3, microtubule-associated protein 1 light chain 3.
Figure 5.
Metformin treatment suppresses the expression of LC3 in the eWAT of HFD-induced obese mice. (A) eWAT sections from mice in the normal chow diet, high fat diet and high fat diet+metformin groups, following immunohistochemical staining of LC3. (B) Quantitative analysis of LC3-positive areas in the eWAT sections. (C) mRNA levels of LC3 in eWAT sections, measured using reverse transcription-quantitative polymerase chain reaction analysis and normalized to glyceraldehyde-3-phosphate dehydrogenase. Data are presented as the mean ± standard error of the mean. *P<0.05; **P<0.01. eWAT, epididymal white adipose tissue; LC3, microtubule-associated protein 1 light chain 3.
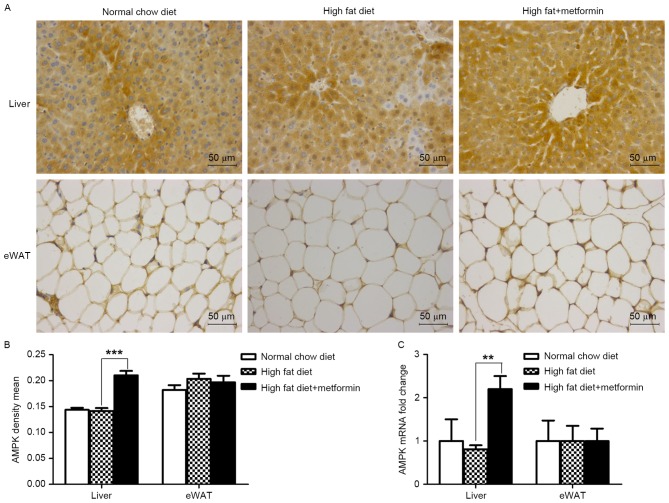
Metformin treatment upregulates the expression of AMPK in the liver, but not the eWAT of HFD-induced obese mice
The present study further investigated the effects of metformin on the protein and mRNA expression levels of AMPK in the liver and eWAT. As shown in Fig. 6A and B, metformin treatment significantly upregulated the expression of AMPK in the liver (P<0.001), which was consistent with the increased expression of LC3 in the liver of the HFD mice. However, this effect of metformin was not observed in the eWAT of the HFD+metformin mice.
Figure 6.
Metformin increases the expression of AMPK in the liver of obese mice. (A) Liver and eWAT sections of mice following immunohistochemical staining of AMPK. (B) Quantitative analysis of AMPK-positive areas in the liver and eWAT sections. (C) mRNA levels of AMPK in the liver and eWAT, analyzed using reverse transcription-quantitative polymerase chain reaction analysis. Data are presented as the mean ± standard error of the mean. **P<0.01; ***P<0.001. eWAT, epididymal white adipose tissue; AMPK, AMP-activated protein kinase.
Discussion
Following feeding of the HFD, C57BL/6 mice developed obesity and hepatic steatosis. Accordingly, the HFD mice were used in the present study as a model of obesity and obesity-induced hepatic steatosis to assess the therapeutic effects of metformin. Consistent with the results obtained from human subjects and rodent models (6,7,17,18), metformin treatment not only decreased the body weight of the HFD-induced obese mice, particularly adipose tissue weight, it also caused a marked decrease in the severity of HFD-induced hepatic steatosis. However, metformin did not alter the food intake of the obese mice. The results also demonstrated that the protein and mRNA levels of autophagy markers were significantly decreased in the liver of the HFD-induced obese mice, but only marginally increased in adipose tissue. Notably, metformin treatment ameliorated the decreased protein and mRNA levels of autophagy marker LC3 in the liver of HFD-induced obese mice, and inhibited the increased expression of LC3 in the adipose tissue of HFD-induced obese mice. Therefore, the present study provided evidence to support the hypothesis that metformin treatment ameliorates obesity and obesity-associated hepatic steatosis in an adipose tissue phenotype-dependent manner, without altering food intake.
Metformin is considered to be an activator of AMPK. The latter, when activated, results in an increase in the induction of autophagy through inhibition of the mammalian target of rapamycin (mTOR) pathway (11,19). Autophagy facilitates the removal of excess lipid, thereby maintaining cellular lipid homeostasis. In the present study, two lines of evidence support the hypothesis that metformin acts through the induction autophagy to reduce hepatic steatosis in HFD-induced obese mice. Firstly, a decrease in the induction of autophagy was observed in mice with obesity-induced hepatic steatosis. Compared with the NCD mice, the protein and mRNA levels of autophagy marker LC3 were significantly decreased in the liver of HFD mice. Secondly, metformin treatment significantly upregulated the expression of LC3 in the HFD mice, and was consistent with the increase in the expression of AMPK.
The results of the present study also suggested a potential link between adipose tissue phenotype and autophagy, which is altered by metformin. As described in widely accepted concepts, dysfunctional adipose tissue in obesity contributes to the pathogenesis of hepatic steatosis by increasing the delivery of fatty acids to the liver to exacerbate hepatic fat deposition (20). In accordance, the present study hypothesized that improved adipose tissue phenotype contributes to the anti-hepatic steatosis effect of metformin. In the present study, adipose tissue weight was significantly decreased in response to metformin treatment in the HFD mice. However, the action of metformin on adipose tissue phenotype remains controversial. Metformin has been shown to reduce body weight (adiposity) in human and rodent models, whereas several studies have demonstrated that metformin does not alter body weight, including in rodents fed an HFD (21–23). A number of studies have also shown that the weight-loss effect of metformin is closely associated with a decrease in food intake (6). However, in the present study, it was found that metformin treatment reduced the body weight of HFD mice without altering the food intake of mice. This raises the question of whether metformin directly acts on adipose tissue. The underlying mechanisms remain to be elucidated, however, they may be attributable to the effect of metformin treatment in altering the induction of adipose tissue autophagy. Therefore, the protein and mRNA levels of the autophagy marker, LC3, in adipose tissues were examined in the present study. Notably, unlike the liver, HFD-induced autophagy was increased in the eWAT, and metformin treatment inhibited the induction of autophagy in the adipose tissue of the HFD mice.
As mentioned above, metformin directly improved the induction of hepatic autophagy and suppressed the induction of autophagy in the adipose tissues of the HFD mice. As the metformin-induced activation of AMPK is associated with inhibition of mTOR signaling in various cells, which induces autophagy (8,19), the protein and mRNA levels of AMPK were detected in the liver and eWAT in the HFD and HFD+metformin mice. Consistent with the increased expression of LC3 in hepatic tissue, the expression level of AMPK in the liver of the HFD mice was upregulated by metformin. When the adipose and liver tissues were compared, metformin treatment had no effect on the expression level of AMPK in the adipose tissue of HFD mice. Therefore, it is possible that a potential anti-autophagy action of metformin acts on adipose tissue via an AMPK-independent pathway. Although this requires further validation, the results suggested that metformin directly targeted the liver to improve features of hepatic steatosis, and this effect of metformin may be dependent on adipose tissue phenotype.
In conclusion, the present study provided evidence to support the beneficial effects of metformin on reducing the body weight/adiposity and hepatic steatosis of HFD-induced obese mice, with no significant effect on food intake, and these effects were dependent on alterations in adipose tissue phenotype. Mechanistically, the actions of metformin were attributable to its effects on improving the induction of hepatic autophagy and inhibiting the induction of adipose tissue autophagy. These results provide experimental evidence for the inhibition of obesity-associated hepatic steatosis in mice treated with metformin. Therefore, metformin supplementation may be an effective approach for the treatment and/or prevention of HFD-induced obesity and hepatic steatosis.
Acknowledgements
The authors would like to thank Dr. Yin Chunyan (Pediatrics department, 2nd Affiliated Hospital of Xi'an Jiaotong University) for their advice in study design and data analysis; Dr. Tan Xinrui (Pediatrics department, 2nd Affiliated Hospital of Xi'an Jiaotong University) for their literature sharing and assistance; and Dr. Antara Sharma (Health Science Center, Xi'an Jiaotong University) as a native-English speaker for their editorial corrections. The authors would also like to thank Professor Zhao Xiaoge and Dr. Huang Shanlong (Health Science Center, Xi'an Jiaotong University) for their assistance with pathological analysis and statistical analysis, respectively. This study was funded by the National Natural Science Foundation of China (grant no. 81172689).
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reed M, Cygan H, Lui K, Mullen M. Identification, prevention, and management of childhood overweight and obesity in a pediatric primary care center. Clin Pediatr (Phila) 2016;55:860–866. doi: 10.1177/0009922815614350. [DOI] [PubMed] [Google Scholar]
- 3.Deiuliis JA. MicroRNAs as regulators of metabolic disease: Pathophysiologic significance and emerging role as biomarkers and therapeutics. Int J Obes (Lond) 2016;40:88–101. doi: 10.1038/ijo.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweeting AN, Hocking SL, Markovic TP. Pharmacotherapy for the treatment of obesity. Mol Cell Endocrinol. 2015;418:173–183. doi: 10.1016/j.mce.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes Res. 1998;6:47–53. doi: 10.1002/j.1550-8528.1998.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, Zhang XH, Park EY, Shin KH, Choi SH, Chun BG, Kim DH. Metformin decreases meal size and number and increases c-Fos expression in the nucleus tractus solitarius of obese mice. Physiol Behav. 2013;110–111:213–220. doi: 10.1016/j.physbeh.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Woo SL, Xu H, Li H, Zhao Y, Hu X, Zhao J, Guo X, Guo T, Botchlett R, Qi T, et al. Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity. PLoS One. 2014;9:e91111. doi: 10.1371/journal.pone.0091111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song YM, Lee YH, Kim JW, Ham DS, Kang ES, Cha BS, Lee HC, Lee BW. Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy. 2015;11:46–59. doi: 10.4161/15548627.2014.984271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavallard VJ, Gual P. Autophagy and non-alcoholic fatty liver disease. Biomed Res Int. 2014;2014:120179. doi: 10.1155/2014/120179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 11.Lee JW, Park S, Takahashi Y, Wang HG. The association of AMPK with ULK1 regulates autophagy. PLoS One. 2010;5:e15394. doi: 10.1371/journal.pone.0015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovsan J, Blüher M, Tarnovscki T, Klöting N, Kirshtein B, Madar L, Shai I, Golan R, Harman-Boehm I, Schön MR, et al. Altered autophagy in human adipose tissues in obesity. J Clin Endocrinol Metab. 2011;96:E268–E277. doi: 10.1210/jc.2010-1681. [DOI] [PubMed] [Google Scholar]
- 14.Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, Czaja MJ. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest. 2009;119:3329–3339. doi: 10.1172/JCI39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Research Council (US) Committee for the update of the guide for the care and use of laboratory animals: Guide for the care and use of laboratory animals. 8th. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–894. doi: 10.1016/S0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 18.Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, Rizzetto M, Marchesini G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–1090. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 19.Shi WY, Xiao D, Wang L, Dong LH, Yan ZX, Shen ZX, Chen SJ, Chen Y, Zhao WL. Therapeutic metformin/AMPK activation blocked lymphoma cell growth via inhibition of mTOR pathway and induction of autophagy. Cell Death Dis. 2012;3:e275. doi: 10.1038/cddis.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh R, Cuervo AM. Lipophagy: Connecting autophagy and lipid metabolism. Int J Cell Biol. 2012;2012:282041. doi: 10.1155/2012/282041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song S, Andrikopoulos S, Filippis C, Thorburn AW, Khan D, Proietto J. Mechanism of fat-induced hepatic gluconeogenesis: Effect of metformin. Am J Physiol Endocrinol Metab. 2001;281:E275–E282. doi: 10.1152/ajpendo.2001.281.2.E275. [DOI] [PubMed] [Google Scholar]
- 22.Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae JW. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 23.Anthony J, Kelkar A, Wilankar C, Ranjith V, Bhumra SK, Mutt SJ, Deka N, Sivaramakrishnan H, Sharma S, Marita AR. Discovery of p1736, a novel antidiabetic compound that improves peripheral insulin sensitivity in mice models. PLoS One. 2013;8:e77946. doi: 10.1371/journal.pone.0077946. [DOI] [PMC free article] [PubMed] [Google Scholar]