Abstract
Fasudil has been demonstrated to possess a protective effect in neural injury; however, its protective effect on convulsive brain injury remains to be assessed. The aim of the present study was to investigate the latent mechanism and effect of fasudil on cognitive function following status convulsion (SC) in rats. Initially, to determine the effects of SC, the expression levels of Ras homolog gene family, member A (RhoA)/Rho-associated protein kinase (ROCK) signaling pathway-associated proteins were measured by western blot analysis in 16 rats. To investigate the effects of fasudil on cognitive function in SC rats, a further 40 rats were assigned to four groups: Group I (healthy untreated rats), group II (healthy rats treated with fasudil), group III (SC rats) and group IV (SC rats treated with fasudil). An object-in-place memory task and the Morris Water Maze test were subsequently performed. Histopathological alterations in brain tissue and SC latency were additionally analyzed. Following SC, protein expression levels of myelin-associated glycoprotein, myelin oligodendrocyte glycoprotein and leucine rich repeat and immunoglobulin-like domain-containing protein 1 were significantly increased (P<0.05) and levels of neurite outgrowth inhibitor protein A were significantly decreased (P<0.01). SC had no effect on RhoA level (P=0.921); however, it significantly increased the levels of phosphorylated RhoA (P<0.01). Cognitive function was significantly decreased following SC and significantly increased following fasudil intervention. Fasudil intervention improved CA1 structure, which was lost following SC. SC severely impaired cognitive function and affected the expression of neurite growth inhibitory factors. Fasudil treatment improved cognitive function and central nervous system (CNS) injury, and decreased SC susceptibility in rats. Fasudil and SC may regulate the CNS by affecting the expression of neurite growth inhibitory factors in the RhoA/ROCK signaling pathway.
Keywords: status convulsion, fasudil, cognitive function, central nervous system, neurite growth inhibitory factors
Introduction
Status convulsion (SC) is a common neurological emergency in childhood. The incidence of SC in Kenya has been reported to be 268 and 227 per 100,000 per year in children aged 1–11 and 12–59 months, respectively (1). Anticonvulsant drugs are frequently used to treat SC (2). It has been reported that SC is associated with the differentiation of oligodendrocyte precursor cells, which secrete neurite growth inhibitory factors, including neurite outgrowth inhibitor protein A (NogoA), oligodendrocyte myelin glycoprotein (OMgp) and myelin-associated glycoprotein (MAG) (3). In the Ras homolog gene family, member A (RhoA)/Rho-associated protein kinase (ROCK) signaling pathway, the myelin-derived inhibitors OMgp, NogoA and MAG may combine with the leucine rich repeat and immunoglobulin-like domain-containing protein 1 (Lingo-1)-Nogo-p75/tumor necrosis factor receptor superfamily, member 19 (TROY) complex and modulate axon propagation (3). Myelin-derived inhibitors may combine with the Lingo-1-Nogo-p75/TROY complex to activate Rho and its downstream effectors, ultimately resulting in functional alterations in microtubules and actin, and impairment of neuronal function (4–6). As a component of the Nogo-66 receptor/p75 signaling complex, Lingo-1 primarily regulates the RhoA/ROCK signaling pathway via activation of RhoA (4,7). RhoA/ROCK signaling is involved in various diseases of the central nervous system (CNS), including optic nerve and spinal cord injuries, stroke and neurodegenerative diseases. The expression levels of ROCK are upregulated in spinal cord injury, spinal cord nerve inflammation, demyelinating disease, stroke and other CNS insults; ROCK inhibitors promote neurite outgrowth and the recovery of neurological function (8).
Currently, fasudil is the only approved ROCK inhibitor and it has been demonstrated to be neuroprotective against glutamate-associated excitotoxicity (9). In neurology, fasudil is used for the treatment of conditions including vertebral artery insufficiency, acute cerebral infarction and cerebral vasospasm following subarachnoid hemorrhage (10–13). Fasudil specifically blocks the activity of ROCK through competition with adenosine triphosphate (ATP) for the ROCK catalytic domain ATP binding site (14). As a potent inhibitor of ROCK, fasudil may inhibit various other protein kinases, which may counteract each other in the regulation of neuronal excitability (15). Recent studies have demonstrated that fasudil may effectively stimulate neurite proliferation (16) and serves a vital role in curing spinal muscular atrophy in rats (17). ROCK inhibitors, including Y-27632 and fasudil, have additionally been revealed to possess antiepileptic effects (14). However, few studies have reported the application of fasudil in the treatment of SC.
The present study investigated the effects of SC on the RhoA/ROCK signaling pathway using western blot analysis. Subsequently, the beneficial effects of fasudil on cognitive function and brain injury in SC rats were determined using an object-in-place memory task, Morris Water Maze (MWM) test and histopathology.
Materials and methods
Animals
All animal procedures conformed to the National Institutes of Health guidelines (18) and were approved by the Ethics Committee of Chongqing Medical University Animal Center (Chongqing, China). A total of 56 healthy male Sprague Dawley rats [age, 21 days; weight, 42–52 g; animal certificate no. SCXK (Yu) 2012–0001; specific pathogen free (SPF)] were provided by the Chongqing Medical University Animal Center. All rats were housed in an SPF-class animal room at a temperature of 25±2°C, humidity of 50±10%, 12 h light/dark cycle and freely available food and water.
Preparation of SC model rats
The SC model was established by the lithium chloride-pilocarpine method, as described previously (19). Initially, an intraperitoneal injection of 127 mg/kg lithium chloride (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was administered to all test rats. After 18–20 h, 30 mg/kg pilocarpine (Sigma-Aldrich; Merck KGaA) was administered by intraperitoneal injection, which induced SC in the rats. Seizure was divided into five classes according to the Racine classification method (20), and SC was defined as Class 5 or 4 seizures 60 min in duration. All treated rats received 10 mg/kg atropine sulfate (Southwest Pharmaceutical Co., Ltd., Chongqing, China) and 10 mg/kg diazepam (Tianjin Jinyao Amino Acid Co., Ltd., Tianjin, China) by intraperitoneal injection 15 and 60 min following the onset of SC, respectively. A total of 30 min after the intraperitoneal injection of pilocarpine, Sprague Dawley rats that had not experienced Class 4 seizures were treated with a second dose of 10 mg/kg pilocarpine; if class 4 seizures still did not occur, the rats were removed from the study (n=4). In addition, control rats were injected intraperitoneally with lithium, atropine and chloral hydrate, without pilocarpine.
Study design
In the present study, 16 rats were divided into control and SC groups (n=8/group), to investigate the effect of SC on the RhoA/ROCK signaling pathway. On day 1 following establishment of the SC model, rats were sacrificed by decapitation 1–2 min after intraperitoneal injection of 10% chloral hydrate (Dalian Meilun Biotech Co., Ltd., Dalian, China), and subsequently, brain tissues were obtained from rats to analyze protein expression levels of MAG, myelin oligodendrocyte glycoprotein (MOG), NogoA, Lingo-1 and RhoA in the RhoA/ROCK signaling pathway, by western blotting. A further 40 rats were used to investigate the effects of fasudil on cognitive function following SC. These were divided into four groups (n=10/group): Group I (healthy untreated rats), group II (healthy rats treated with fasudil), group III (SC rats), group IV (SC rats treated with fasudil). On day 1 following SC induction, rats in groups II and IV received intraperitoneal injections of 10 mg/kg fasudil (catalog no. HA-1077, Tianjin Chase Sun Pharmaceutical Co. Ltd., Tianjin, China) dissolved in saline. Rats in group I and III received intraperitoneal injections of the same volume of saline. The cognitive function of rats was assessed 30 days later.
Western blot analysis
The hippocampal region was separated from brain tissues of the 16 rats in the first SC model and used for western blot analysis, to determine the protein expression levels of MAG, MOG, NogoA, Lingo-1, RhoA and phosphorylated (p)-RhoA. Brain tissues were homogenized with a lysis buffer solution containing: 50 mM Tris-HCl (pH 7.4), 400 mM NaCl, 2 mM EGTA, 1 mM EDTA, 1 mM dithiothreitol, 10 mM phenylmethylsulphonyl fluoride, 10 µg/ml leupeptin, 1 µg/ml pepstatin and 1 mM benzamidine. The protein concentration was determined using a Bicinchoninic Acid assay. Total cell lysates were prepared in 1X SDS buffer and equal amounts (50 µg/lane) were loaded on 8% gels, subjected to gel electrophoresis and subsequently transferred onto polyvinylidene difluoride membranes (0.22 µm, EMD Millipore, Billerica, MA, USA). Membranes were blocked with 5% bovine serum albumin (BSA; Huayi Bioengineering Co. Ltd., Hubei, China) and incubated overnight at 4°C with the following specific antibodies: Mouse anti MAG (1:5,000; EMD Millipore, catalog no. MAB1567), mouse anti MOG (1:5,000; EMD Millipore; catalog no. MAB5680), rabbit anti NogoA (1:5,000; Abcam, Cambridge, UK; catalog no. ab62024), rabbit anti Lingo-1 (1:5,000; Abcam; catalog no. ab23631), mouse anti RhoA (1:5,000; Abcam; catalog no. ab54835), rabbit anti p-RhoA (1:5,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA); catalog no. sc-32954) or mouse anti β-actin (1:5,000; Sigma-Aldrich; catalog no. A3854). Following this, the membranes were washed with TBS containing Tween-20 and incubated with horse radish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:10,000; Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China; catalog no. bs-0296G-HRP) or goat anti-mouse IgG (1:5,000; Beijing Biosynthesis Biotechnology Co., Ltd., catalog no. bs-0296G-HRP) secondary antibodies for 2 h at room temperature. Target proteins were detected using an Enhanced Chemiluminescence kit (Beyotime Institute of Biotechnology, Haimen, China). The ratios of target proteins to internal control were calculated using ImageJ software version 1.5.0 (National Institutes of Health, Bethesda, MD, USA).
Object-in-place memory task
All 40 rats in the second SC model were assessed on object-in-place recognition memory 30 days following fasudil injection. Exploration occurred in a square and open-topped arena (100 cm3) of black plexiglass. The floor of the arena was covered with sawdust. An overhead camera and video recorder were used to monitor and record the behavior of the rats for subsequent analysis. This procedure lasted for 4 days and comprised an adaptive phase and an acquisition phase separated by a delay from a recognition test. The first two days was the adaptive phase, during which rats were placed into the arena for 10 min at the same time each day.
During the familiarization phase, two identical objects (A1 and A2) were placed near the two corners at either end of one side of the arena (10 cm from each adjacent wall). The rats were placed into the arena facing the center of the opposite wall and allowed 3 min in the arena to find A1 and A2. Exploratory behavior was defined as the animal directing its nose toward the object at a distance of <2 cm. Other behaviors, including looking around while sitting on or resting against the object, were not considered as exploration. The delay between the phases was 5 min or 1 h depending on the experiment. During the delay period, the objects were cleaned with 75% alcohol to remove olfactory cues and any sawdust. For the object-in-place memory test, the two objects were removed and one replaced with a familiar object (A3) and one with a novel object (B) and the subjects were allowed to explore the objects for 3 min. To assess the spatial recognition ability of the rats, the position of object B was altered. The time spent exploring the two objects that had altered position was compared with the time spent exploring the two objects that had remained in the same position. If object-in-place memory is intact, the subject will spend more time exploring the two objects that had altered position, compared with the two objects that are in the same locations. The positions of the objects in the test and the objects used as novel or familiar were counterbalanced among rats. The discrimination ratio was calculated using the following equation: Discrimination ratio (%)=[novel (s)/familiar (s)] ×100.
MWM test
On day 2 following the object-in-place memory task, spatial learning and memory was assessed using the SLY-WMS MWM system version 2.0 (Beijing Sunny Instruments, Co., Ltd., Beijing, China) in the 40 rats of the second SC model, as described previously (21,22). Briefly, the procedure took place in a black circular pool (diameter, 200 cm; depth, 50 cm; inside and outside painted black) filled with 24°C water (dyed with black ink to be opaque) to a depth of 30 cm. The entire procedure was recorded by an overhead camera for subsequent analysis. The MWM tests were performed on six consecutive days and comprised initial spatial training (day 1), spatial reversal training (days 2–5) and probe test (day 6).
Histopathology
On the day following the final MWM test, one rat in each group was randomly selected for hematoxylin and eosin (HE) and Nissl staining. Hippocampal organotypic tissues were obtained from rats, as aforementioned. For cross-sectional observation, tissues were fixed in 4% paraformaldehyde for 24 h and routinely processed into 4-µm thick paraffin-embedded sections. All sections were placed on slides pretreated with 3-aminopropyl-triethoxysilane, and dried overnight in an oven at 60°C. HE staining and Nissl staining were performed using conventional methods.
For HE staining, deparaffinized sections were incubated with hematoxylin solution for 8 sec, differentiated with 0.5% HCl for 10 sec, stained with lithium carbonate solution for 20 sec and counterstained with eosin staining solution for 10 sec. Sections were observed under a light microscope (Eclipse 55i; Nikon Corporation, Tokyo, Japan). For Nissl staining, deparaffinized sections were dehydrated in alcohol, immersed in 1% cresyl fast violet solution for 23–30 min, differentiated in 95% alcohol, hyalinized in xylene and mounted with neutral gum. Sections were observed under a light microscope.
SC susceptibility analysis
SC was subsequently induced for a second time by intraperitoneal injection of pilocarpine, as aforementioned, in second SC model rats two days after the MWM test. The time from the injection of pilocarpine to Class 4 seizures (SC latency) of each rat was recorded for subsequent analysis.
Statistical analysis
Statistical analyses were conducted using SPSS software version 17.0 (SPSS, Inc., Chicago, IL, USA). Data for western-blotting analysis and animal behavior analysis are expressed as the mean ± standard deviation of test rats in each group. The number of times the platform was passed was analyzed by Wilcoxon signed-rank-sum test. The protein expression levels of neurite growth inhibitory factors between-groups were compared using a one-way analysis of variance, followed by the least significant difference post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of neurite growth inhibitory factors in the RhoA/ROCK signaling pathway
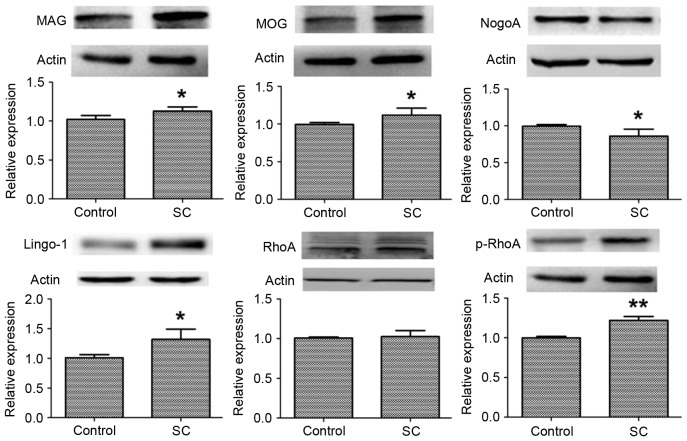
To investigate the effect of SC on the protein expression levels of neurite growth inhibitory factors in the RhoA/ROCK signaling pathway, western blot analysis was performed. On day 1 following SC model establishment, the hippocampal CA1 region was separated from rat brain tissues. Lingo-1 is a transmembrane protein. As presented in Fig. 1, Lingo-1 expression levels were significantly increased in the SC group compared with the control group (P<0.05). In addition, the expression levels of MAG and MOG demonstrated a similar pattern (P<0.05). However, NogoA expression levels were greater in the control group compared with the SC group (P<0.05). In addition, the protein expression levels of RhoA and p-RhoA were measured. The expression levels of RhoA were not significantly altered following SC (P>0.05). However, the protein expression levels of p-RhoA were significantly increased in the SC group compared with the control group (P<0.01).
Figure 1.
Protein expression levels of neurite growth inhibitory factors in the hippocampal CA1 region. The protein expression levels of MAG, MOG, NogoA, Lingo-1, RhoA and p-RhoA were measured by western blotting; β-actin served as an internal control. Data are presented as the mean ± standard deviation of the rats in each group. *P<0.05 and **P<0.01 vs. control. MAG, myelin-associated glycoprotein; MOG, myelin oligodendrocyte glycoprotein; NogoA, neurite outgrowth inhibitor protein A; Lingo-1, leucine rich repeat and immunoglobulin-like domain-containing protein 1; RhoA, Ras homolog gene family, member A; p-, phosphorylated.
Object-in-place memory task
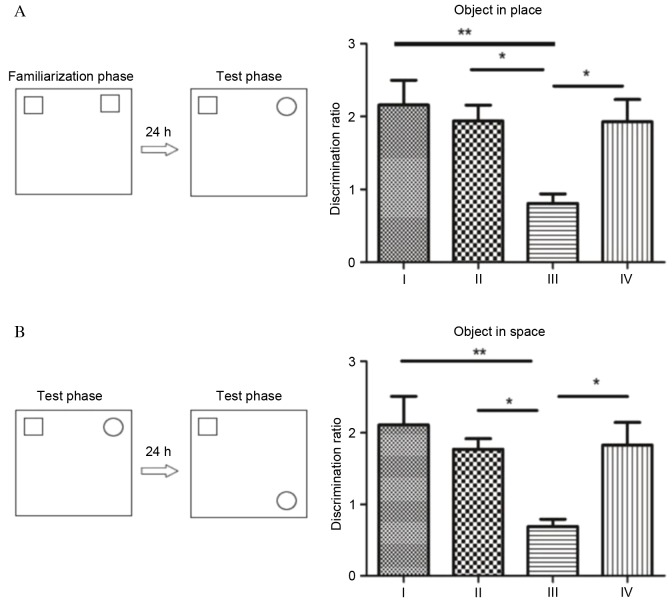
To measure the cognitive function of rats, the object-in-place memory task was performed (Fig. 2). In the object location recognition test, analysis of acquisition phase exploration revealed significant differences between the four groups. As presented in Fig. 2A, no significant differences were observed in object-in-place memory between group I, II and IV (P>0.05). By contrast, rats in group III demonstrated significantly impaired object-in-place memory. The discrimination ratio of rats in group III was significantly decreased compared with groups I (P<0.01) and II (P<0.05). Fasudil significantly improved the object-in-place memory in rats, with the discrimination ratio significantly increased in group IV compared with group III (P<0.05).
Figure 2.
Object-in-place memory task. (A) Novel object recognition test. (B) Spatial recognition. The right panels present the alteration in object identity or position. Data are presented as the mean ± standard deviation of the rats in each group. *P<0.05; **P<0.01.
In the object-in-place memory test, significant memory is inferred when rats spend more time exploring the objects that switched locations compared with the objects that remained in the same location as the sample phase. Analysis of the discrimination ratio during the acquisition phase revealed significant differences among the four groups, which demonstrated a similar profile to the object location recognition test (Fig. 2). Although the discrimination ratio was greater in group I rats compared with groups II and IV, no significant differences were observed between the three groups (P>0.05). Following SC, rats in group III demonstrated impaired object-in-place memory, with a discrimination ratio significantly reduced compared with groups I (P<0.01), II and IV (P<0.05).
MWM test
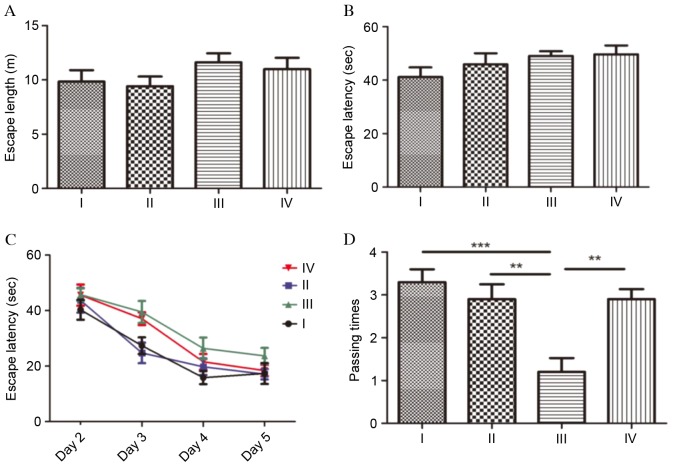
To evaluate spatial learning and the memory of the rats, the MWM test was performed (Fig. 3). The test was performed on 6 consecutive days and comprised initial spatial training (day 1), spatial reversal training (days 2–5) and probe test (day 6). At the initial spatial training phase, no significant differences were observed in escape length (Fig. 3A) or escape latency (Fig. 3B) between the four groups (P>0.05). During the spatial reversal training phase, the escape latencies of group III were longer than the other three groups; however, compared with group III, there were no significant differences between the groups (P>0.05; Fig. 3C). Escape latencies in each group demonstrated a gradually decreasing profile. At the probe test phase, the number of times the platform quadrant area was passed was analyzed during a 60 sec period. Compared with the rats in group I, the number of times the platform quadrant area was passed was significantly decreased in group III (P<0.001), which indicated that cognitive function was severely impaired following SC. The passing times in group III rats were additionally significantly reduced compared with group II rats (P<0.01). Fasudil markedly promoted spatial learning and memory in rats following SC, as the passing times in group IV were significantly increased compared with group III (P<0.01).
Figure 3.
Effects of fasudil on spatial learning of rats in the Morris Water Maze test. (A) Escape latency in initial spatial training. (B) Escape length in initial spatial training. (C) Escape latency in spatial reversal training. (D) Number of times passing the platform area in the probe test. Data are presented as the mean ± standard deviation of the rats in each group. *P<0.05; **P<0.01; ***P<0.001.
SC susceptibility analysis
SC was induced for a second time by intraperitoneal injection of pilocarpine and the time from the injection to Class 4 seizures in each group was recorded as SC latency (Fig. 4). Rats in group II demonstrated the longest SC latency, significantly longer compared with groups I, III (P<0.001) and IV (P<0.05). Rats in group III demonstrated the shortest SC latency. Following treatment with fasudil, SC latency in group IV increased to approximately twice that of group III (P<0.001).
Figure 4.
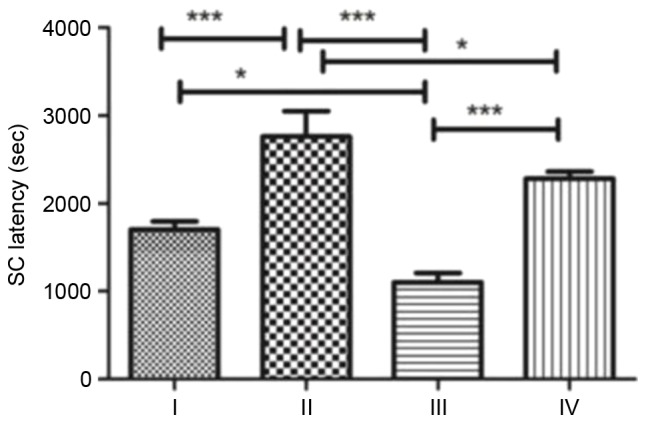
SC susceptibility. Data are presented as the mean ± standard deviation of the rats in each group. *P<0.05; ***P<0.001. SC, status convulsion.
Histopathology
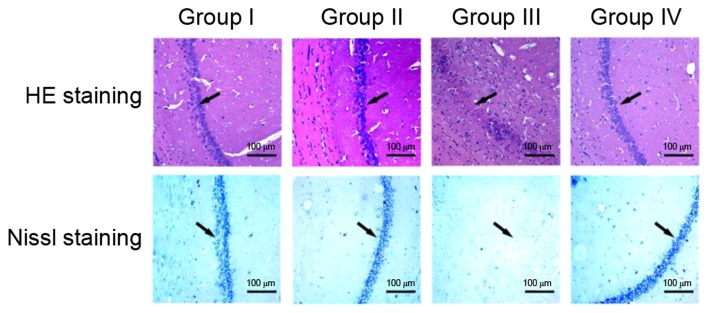
Morphological analysis of the hippocampal organotypic tissue of rats was performed using HE and Nissl staining (Fig. 5). HE and Nissl staining revealed similar results in the four groups. In groups I and II, no clear differences were observed in the hippocampal CA1 region of rats in HE and Nissl staining; the neuronal cells of the hippocampal CA1 region were intact, regularly arranged and evenly distributed. In group III, the number of hippocampal CA1 neurons and Nissl bodies was decreased and HE staining revealed poorly arranged neurons. Nissl staining of the hippocampal CA1 in group III revealed an unclear structure with almost no neurons. In group IV, fasudil treatment markedly promoted the recovery of the CA1 regional structure, including clearly visible Nissl bodies and a relatively regular arrangement of neurons, although a small quantity of irregular dark cellular matter was present.
Figure 5.
Histopathology of the hippocampal CA1 region following status convulsion. HE and Nissl staining of the hippocampal CA1 region. Arrows indicate CA1 area. Scale bars=100 µm. HE, hematoxylin and eosin.
Discussion
A number of studies have demonstrated that SC is closely associated with CNS (23,24). The majority of neurite growth inhibitory factors, including Lingo-1, MAG, NogoA and OMgp, may activate the RhoA/ROCK signaling pathway and further modulate axon propagation (6,7). Generalized seizures exhibit distinct neuroanatomical substrates that initiate and sustain seizure activity (25). The anticonvulsant effects of the MCAT cells on acute SC may be associated with inhibitory factors and immune-modulatory mechanisms assigned to mesenchymal cells in the hippocampus (26). The hippocampus is a structure susceptible to SC and serves an important role in memory function (27). In the present study, rat hippocampal CA1 tissues were selected for the study of fasudil on cognitive function. The protein expression levels of Lingo-1, MAG, MOG and p-RhoA were significantly increased one day after SC. SC had no effect on RhoA expression levels, but significantly reduced NogoA expression levels. These results indicated that the alterations in neurite growth inhibitory factors were not fully synchronized and ultimately resulted in CNS damage, which may be induced by RhoA phosphorylation following SC and the activation of the RhoA/ROCK signaling pathway. Lingo-1 is a leucine-rich repeat transmembrane protein, which may transmit a signal of axon growth inhibition to cells and convert non-activated RhoA-guanosine diphosphate to an activated form of RhoA-guanosine triphosphate, and further activate downstream ROCK (4). The alterations in Lingo-1 expression levels were consistent with those of p-RhoA. The activated ROCK may alter the cytoskeleton dynamics and inhibit axon propagation, leading to the collapse of the neuronal cytoskeleton and CNS damage (5).
Fasudil is a 5-isoquinoline sulfonamide derivative and primarily inhibits ROCK, which is involved in a variety of cellular functions, including apoptosis and smooth muscle contraction. A previous study indicated that fasudil improved CNS injury, promoted axonal regeneration and reduced cell death (28). In a study of other neurological diseases, fasudil was revealed to possess a protective effect against CNS injury (16). Lingor et al (29) applied three inhibitors (Y-27632, fasudil and dimethylfasudil) to suppress the activity of ROCK, which effectively induced nerve cell regeneration following CNS injury. Whether fasudil has a protective effect on seizure-induced brain injury remains to be determined. The results of the present study demonstrated that fasudil may improve the structure of the hippocampus and cognitive function in rats. The hippocampal CA1 region almost disappeared in the SC rats of group III, and recovered to almost normal in the rats of group IV.
The object-in-place memory task and the MWM test were used to analyze cognitive function. The specificity of the recognition memory deficit in the object-in-place test suggested that cognitive functions associated with the prefrontal cortex may be particularly susceptible to alteration following prenatal infection (30). Novel object recognition was used as a facile behavior test for evaluating drug effects in an Alzheimer's disease AbetaPP/PSl mouse model (31). The MWM test is recognized as a method which effectively evaluates learning and memory in animal models (32). Hippocampal long-term depression mediates spatial reversal learning in the MWM test (21). The hippocampus and dorsal striatum serve critical roles in navigation based on spatial or cue-based strategies and the spatial and cue versions of the MWM are impaired by lesions in the dorsal hippocampus and dorsal striatum, respectively (22). The object-in-place memory task and MWM test demonstrated similar results in the present study, which suggested that fasudil significantly improved the cognitive ability of SC rats. Rats in group III exhibited the lowest discrimination ratio, the least number of times passing the platform quadrant area and the longest escape length and latency. Following intraperitoneal injection with fasudil, learning and memory in rats of group IV was significantly improved.
Furthermore, SC susceptibility was analyzed and the time from injection with pilocarpine to Class 4 seizures was recorded in each group. SC latency was the shortest in group III; therefore, rats in group III had the greatest susceptibility to SC, which was consistent with previous studies (33,34). Notably, fasudil lengthened SC latency compared with group III. In addition, the SC latency in rats of group II was greater compared with group I. There are certain limitations to the present study. Lingo-1 is a transmembrane protein, which is located upstream of the signaling pathway of interest. Further studies are required to elucidate whether an improved therapeutic effect may be achieved in rats following SC when the expression of Lingo-1 is inhibited.
In conclusion, the results of the present study demonstrated that fasudil improves cognitive function and CNS injury in rats, and decreases SC susceptibility. Fasudil therefore has a protective effect on convulsive brain injury. Fasudil and SC may regulate the CNS by affecting the expression levels of neurite growth inhibitory factors in the RhoA/ROCK signaling pathway.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81371452), the Natural Science Foundation of Chongqing City (grant no. cstc2013jjB0031), the Foundation of Chongqing Municipal Commission of science and technology (grant no. 2015XMSB000712) and the PhD Programs Foundation of Ministry of Education of China (grant no. 20125503110011).
Glossary
Abbreviations
- MAG
myelin-associated glycoprotein
- MOG
myelin oligodendrocyte glycoprotein
- NogoA
neurite outgrowth inhibitor protein A
- Lingo-1
leucine rich repeat and immunoglobulin-like domain-containing protein 1
- RhoA
Ras homolog gene family, member A
- SC
status convulsion
- ROCK
Rho-associated protein kinase
- OMgp
oligodendrocyte myelin glycoprotein
- CNS
central nervous system
References
- 1.Sadarangani M, Seaton C, Scott JA, Ogutu B, Edwards T, Prins A, Gatakaa H, Idro R, Berkley JA, Peshu N, et al. Incidence and outcome of convulsive status epilepticus in Kenyan children: A cohort study. Lancet Neurol. 2008;7:145–150. doi: 10.1016/S1474-4422(07)70331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appleton R, Martl T, Phillips B. Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Syst Rev: CD001905. 2002 doi: 10.1002/14651858.CD001905. [DOI] [PubMed] [Google Scholar]
- 3.Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N, Perrin S, Sands B, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- 5.Luo L. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu Rev Cell Dev Biol. 2002;18:601–635. doi: 10.1146/annurev.cellbio.18.031802.150501. [DOI] [PubMed] [Google Scholar]
- 6.Stankiewicz TR, Linseman DA. Rho family GTPases: Key players in neuronal development, neuronal survival, and neurodegeneration. Front Cell Neurosci. 2014;8:314. doi: 10.3389/fncel.2014.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita Y, Yamashita T. Axon growth inhibition by RhoA/ROCK in the central nervous system. Front Neurosci. 2014;8:338. doi: 10.3389/fnins.2014.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roloff F, Scheiblich H, Dewitz C, Dempewolf S, Stern M, Bicker G. Enhanced neurite outgrowth of human model (NT2) neurons by small-molecule inhibitors of Rho/ROCK signaling. PLoS One. 2015;10:e0118536. doi: 10.1371/journal.pone.0118536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitaoka Y, Kitaoka Y, Kumai T, Lam TT, Kuribayashi K, Isenoumi K, Munemasa Y, Motoki M, Kobayashi S, Ueno S. Involvement of RhoA and possible neuroprotective effect of fasudil, a Rho kinase inhibitor, in NMDA-induced neurotoxicity in the rat retina. Brain Res. 2004;1018:111–118. doi: 10.1016/j.brainres.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 10.Ishiguro M, Kawasaki K, Suzuki Y, Ishizuka F, Mishiro K, Egashira Y, Ikegaki I, Tsuruma K, Shimazawa M, Yoshimura S, et al. A Rho kinase (ROCK) inhibitor, fasudil, prevents matrix metalloproteinase-9-related hemorrhagic transformation in mice treated with tissue plasminogen activator. Neuroscience. 2012;220:302–312. doi: 10.1016/j.neuroscience.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Saito A, Inoue M, Kon H, Imaruoka S, Basaki K, Midorikawa H, Sasaki T, Nishijima M. Effectiveness of intraarterial administration of fasudil hydrochloride for preventing symptomatic vasospasm after subarachnoid hemorrhage. Acta Neurochir Suppl. 2015;120:297–301. doi: 10.1007/978-3-319-04981-6_50. [DOI] [PubMed] [Google Scholar]
- 12.Iwabuchi S, Hayashi M, Yokouchi T, Sato K, Nakayama H, Harashina J, Iwama J, Ishii M, Hiramoto Y, Hirai N, et al. Prophylactic intra-arterial administration of fasudil hydrochloride for vasospasm following subarachnoid haemorrhage. Acta Neurochir Suppl. 2015;120:167–169. doi: 10.1007/978-3-319-04981-6_28. [DOI] [PubMed] [Google Scholar]
- 13.Liu YH, Zhao Y, Huang FZ, Chen YH, Wang HX, Bonney E, Liu BQ. Combination of early constraint-induced movement therapy and fasudil enhances motor recovery after ischemic stroke in rats. Int J Neurosci. 2016;126:168–173. doi: 10.3109/00207454.2014.998759. [DOI] [PubMed] [Google Scholar]
- 14.Inan S, Büyükafşar K. Antiepileptic effects of two Rho-kinase inhibitors, Y-27632 and fasudil, in mice. Br J Pharmacol. 2008;155:44–51. doi: 10.1038/bjp.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies S, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S, Luo M, Zhao Y, Zhang Y, He M, Cai W, Liu A. Fasudil stimulates neurite outgrowth and promotes differentiation in C17.2 neural stem cells by modulating notch signalling but not autophagy. Cell Physiol Biochem. 2015;36:531–541. doi: 10.1159/000430118. [DOI] [PubMed] [Google Scholar]
- 17.Coque E, Raoul C, Bowerman M. ROCK inhibition as a therapy for spinal muscular atrophy: Understanding the repercussions on multiple cellular targets. Front Neurosci. 2014;8:271. doi: 10.3389/fnins.2014.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals: Guide for the care and use of laboratory animals. 8th. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 19.Lively S, Brown IR. Analysis of the extracellular matrix protein SC1 during reactive gliosis in the rat lithium-pilocarpine seizure model. Brain Res. 2007;1163:1–9. doi: 10.1016/j.brainres.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 20.Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90178-2. [DOI] [PubMed] [Google Scholar]
- 21.Dong Z, Bai Y, Wu X, Li H, Gong B, Howland JG, Huang Y, He W, Li T, Wang YT. Hippocampal long-term depression mediates spatial reversal learning in the Morris water maze. Neuropharmacology. 2013;64:65–73. doi: 10.1016/j.neuropharm.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Miyoshi E, Wietzikoski EC, Bortolanza M, Boschen SL, Canteras NS, Izquierdo I, Da Cunha C. Both the dorsal hippocampus and the dorsolateral striatum are needed for rat navigation in the Morris water maze. Behav Brain Res. 2012;226:171–178. doi: 10.1016/j.bbr.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 23.D'Agostino DP, Pilla R, Held HE, Landon CS, Puchowicz M, Brunengraber H, Ari C, Arnold P, Dean JB. Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am J Physiol Regul Integr Comp Physiol. 2013;304:R829–R836. doi: 10.1152/ajpregu.00506.2012. [DOI] [PubMed] [Google Scholar]
- 24.Chapman CD, Frey WH, II, Craft S, Danielyan L, Hallschmid M, Schiöth HB, Benedict C. Intranasal treatment of central nervous system dysfunction in humans. Pharm Res. 2013;30:2475–2484. doi: 10.1007/s11095-012-0915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Browning RA, Wang C, Nelson DK, Jobe PC. Effect of precollicular transection on audiogenic seizures in genetically epilepsy-prone rats. Exp Neurol. 1999;155:295–301. doi: 10.1006/exnr.1998.6981. [DOI] [PubMed] [Google Scholar]
- 26.Tamura B, Almeida D, Felizardo R, Olanda G, Bocca L, Pinhal N, Alves-de-Moraes L, Covolan L, Cãmara N, Longo BM. Convulsive seizure protection after hippocampal transplantation of mesenchymal cells from adipose tissue in mice. J Stem Cell Res Ther. 2014;4:2. [Google Scholar]
- 27.Scharfman HE. Epileptogenesis in the parahippocampal region. Parallels with the dentate gyrus. Ann N Y Acad Sci. 2000;911:305–327. doi: 10.1111/j.1749-6632.2000.tb06734.x. [DOI] [PubMed] [Google Scholar]
- 28.Nishio Y, Koda M, Kitajo K, Seto M, Hata K, Taniguchi J, Moriya H, Fujitani M, Kubo T, Yamashita T. Delayed treatment with Rho-kinase in-hibior dose not enhance axonal regeneration or functional recovery after spinal cord injury in rats. Exp Neurol. 2006;200:392–397. doi: 10.1016/j.expneurol.2006.02.123. [DOI] [PubMed] [Google Scholar]
- 29.Lingor P, Teusch N, Schwarz K, Mueller R, Mack H, Bähr M, Mueller BK. Inhibition of Rho kinase (ROCK) increases neurite outgrowth on chondroitin sulphate proteoglycan in vitro and axonal regeneration in the adult optic nerve in vivo. J Neurochem. 2007;103:181–189. doi: 10.1111/j.1471-4159.2007.04756.x. [DOI] [PubMed] [Google Scholar]
- 30.Howland JG, Cazakoff BN, Zhang Y. Altered object-in-place recognition memory, prepulse inhibition, and locomotor activity in the offspring of rats exposed to a viral mimetic during pregnancy. Neuroscience. 2012;201:184–198. doi: 10.1016/j.neuroscience.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang R, Xue G, Wang S, Zhang L, Shi C, Xie X. Novel object recognition as a facile behavior test for evaluating drug effects in AβPP/PS1 Alzheimer's disease mouse model. J Alzheimers Dis. 2012;31:801–812. doi: 10.3233/JAD-2012-120151. [DOI] [PubMed] [Google Scholar]
- 32.Vorhees CV, Williams MT. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khazipov R, Khalilov I, Tyzio R, Morozova E, Ben-Ari Y, Holmes GL. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. Eur J Neurosci. 2004;19:590–600. doi: 10.1111/j.0953-816X.2003.03152.x. [DOI] [PubMed] [Google Scholar]
- 34.Moshé SL, Albala BJ, Ackermann RF, Engel J., Jr Increased seizure susceptibility of the immature brain. Brain Res. 1983;283:81–85. doi: 10.1016/0165-3806(83)90083-4. [DOI] [PubMed] [Google Scholar]