Abstract
Background:
Middle cerebral artery (MCA) aneurysms constitute from 18–40% of all intracranial aneurysms. They are mainly found in the proximal and bifurcation tracts and only in the 1.1-1.7% of cases they are located in the distal segment. The authors report a case of a ruptured saccular cortical MCA aneurysm with unknown etiology.
Case Description:
A 53-year-old female was admitted with a sudden severe headache, nausea, vomiting, and a slight left hemiparesis. The computed tomography (CT) scan showed subarachnoid hemorrhage (SAH) in the left sylvian fissure and intracerebral hemorrhage (ICH) in the left posterior parietal area. The CT angiography (CTA) reconstructed with 3D imaging showed a small saccular aneurysm in the M4 segment in proximity of the angular area. A left parieto-temporal craniotomy was performed, the aneurysm was clipped and the ICH evacuated. The motor deficit was progressively recovered. At 3-month follow-up examination, the patient was asymptomatic and feeling well.
Conclusions:
In our opinion, surgery is the best choice for the treatment of ruptured M4 aneurysms with ICH, because it allows to evacuate the hematoma and to exclude the aneurysm from the intracranial circulation. In addition, we suggest both the use of the neuronavigation technique and of the indocyanine green videoangiography (ICGV) for the aneurismal surgery.
Keywords: Cortical aneurysm, intracerebral hemorrhage, middle cerebral artery
INTRODUCTION
Middle cerebral artery (MCA) aneurysms constitute from 18% to 40% of all intracranial aneurysms.[4] They are mainly found in the proximal and bifurcation tracts, and only in the 1,1–7% of cases they are located in the distal segment.[1,4,9] Even rarer are the aneurysms that arise from the cortical segment of the MCA (M4).[1,4,6] Generally, these aneurysms are secondary to traumatic injuries and inflammatory or infectious diseases.[1] An exceptional occurrence is the presence of a saccular or fusiform aneurysm in the cortical segment without an evident risk factor. The authors report the case of a ruptured saccular cortical MCA aneurysm with unknown etiology.
Clinical aspects, radiological features, surgical treatment, and operative findings are discussed reviewing the pertinent literature.
CASE REPORT
A 53-year-old female was admitted to our institute with a sudden severe headache, accompanied by nausea and vomiting with a slight left hemiparesis. There was no history of traumatic events, infectious or inflammatory diseases, hypertension, or other cardiovascular disorders. The results of the routine hematologic tests showed indices within normal limits. The computed tomography (CT) scan of the head showed subarachnoid hemorrhage (SAH) in the left sylvian fissure and intracerebral hemorrhage (ICH), measured 22 mm × 26 mm, in the left posterior parietal area [Figure 1]. The CT angiography (CTA) reconstructed with 3D imaging software showed a small saccular aneurysm in the M4 segment in proximity of the angular area [Figure 2]. No digital subtraction angiography (DSA) was performed. Due to a sudden decreased level of consciousness, the emergency surgery was necessary. A left parieto-temporal craniotomy was performed. Through the use of neuronavigation it was possible to locate the ICH and the aneurysm. Under microscopic vision, a small corticotomy was made and the ICH was gently evacuated. A small saccular aneurysm was found. Before clipping, the indocyanine green videoangiography (ICGV) was used to identify the perforating arteries close to the aneurysm sac, and to detect the neck. The aneurysm neck was clipped with a mini-clip and the aneurysm sac was coagulated with a bipolar forceps. After clipping, the ICGV showed the patency of all efferent and afferent arteries to the aneurysm and confirmed the aneurysm exclusion. The postoperative CTA showed neither residual aneurysm nor occlusion of the parent vessel [Figure 3]. The motor deficit was progressively recovered and the patient was discharged on the 20th postoperative day without complications. At 3-month follow-up examination, the patient was asymptomatic and feeling well.
Figure 1.
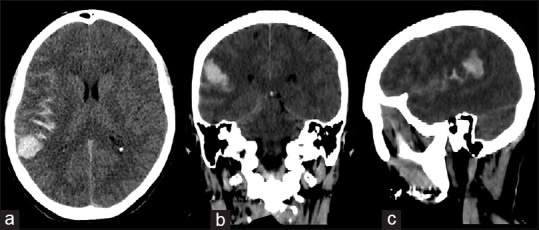
Non enhanced cranial CT scan in axial (a), coronal (b) and sagittal (c) projections showed a subarachnoid haemorrhage associated with a parietal intraparenchymal haemorrhage
Figure 2.
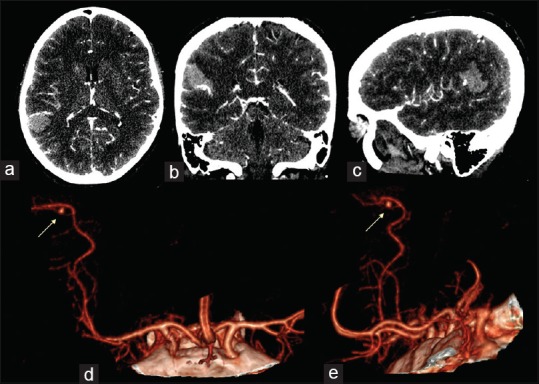
Intracranial angio-CT scan in axial (a), coronal (b) and sagittal (c) projections showed the presence of one aneurysm of the M4 tract of the MCA, in correspondence of the parietal intraparenchymal haemorrhage. CTA with 3-D reconstruction rendering in antero-posterior ad lateral projection (d and e) showed the morphology of the aneurism (yellow arrows)
Figure 3.
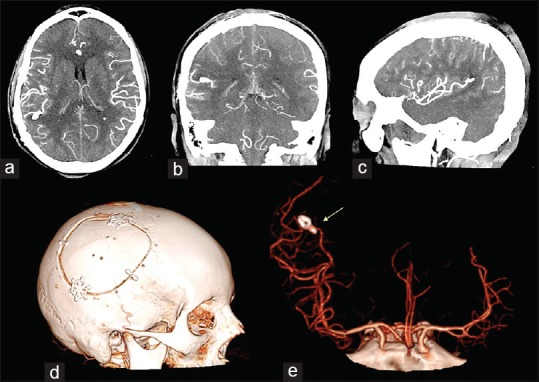
Postoperative intracranial angio-CT scan in axial (a), cronal (b) and sagittal (c) projections showed the results of the aneurysm clipping and the absence of the parietal intraparenchymal haemorrhage. 3D cranial CT rendering of the skull (d) documented the features of the bone flap. 3D angio-CT rendering in antero-posterior ad lateral projection (e) showed the position of the clip and the complete exclusion of the aneurysm (yellow arrow)
DISCUSSION
The MCA is the largest and most complex of the cerebral vessels.[6] According to the anatomic relationships, it is divided into four or five segments: sphenoidal (M1), insular (M2), opercular (M3), cortical (M4), and terminal (M5).[4,6]
Furthermore, the MCA is also one of the most common sites of saccular aneurysms (GIBO). Although several classifications have been proposed for the MCA aneurysms,[1,4,6] classically they are divided into four groups: proximal, bifurcation, distal, and cortical aneurysms.[1,4] Most of the MCA aneurysms occur at the bifurcation, while the cortical aneurysms are very rare. As a rule, the latter ones are manifested clinically after the rupture of their aneurysmal sac.
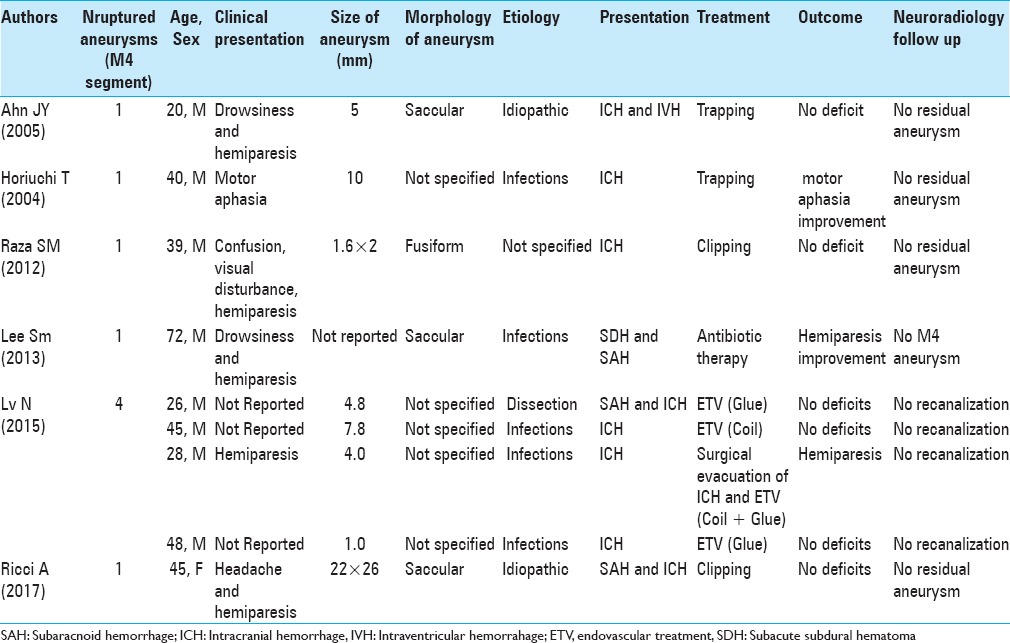
At present, only eight cases of ruptured cortical MCA aneurysms have been described in literature.[1,7,8,9,13]
The patients are all males, except our case. The average age of the reported patients is 40 years. The size of the aneurysms is between 1 mm and 10 mm and, in most cases, they have a saccular or fusiform morphology. In five patients, the aneurysms present infectious etiology. Usually, they occur with ICH, sometimes associated with SAH. The endovascular treatment (EVT) has been performed in four cases, while the surgical treatment has been performed in three cases (two of trapping and one of clipping). In one patient, the infectious aneurysm has resolved spontaneously after antibiotic therapy. In all treatments performed, the patients have improved the neurologic symptoms and no residual aneurysms have been observed in the subsequent neuroradiology follow-up [Table 1].
Table 1.
Review of ruptured cortical middle cerebral artery aneurysms (M4 segment)
Habitually, cerebral aneurysms arise at the bifurcation or trifurcation of the MCA due to hemodynamic stress or congenital factors.[1] On the contrary, for the most part of cases the M4 aneurysms have an infectious etiology and only rarely they have idiopathic origin.[1]
In some authors’ opinion, the etiology of the aneurysm could influence the choice of treatment.[8] Several studies suggest that in the case of mycotic aneurysms, the empirical antibiotic therapy may be sufficient to resolve the aneurysmal lesion, unless the size of the hematoma requires surgery.[8]
Although surgery remains the main choice in the M4 aneurysms, because of the extremely distal location of them over the motor/somatosensory cortices,[13] Lv et al.[9] propose the use of the EVT in all types of the M4 aneurysms, especially after the surgery, when it is impossible to locate the small ruptured aneurysm.
The main difficulty of the surgery is the precise surgical localization of the small M4 aneurysms.[13] An inaccurate localization of these vascular lesions may result in larger craniotomies and unnecessary arachnoid and pial dissections with possible resultant permanent neurological injuries.[13] In cases of aneurysms or arteriovenous malformations located at the sylvian point or at the posterior superior aspect of the insula, especially in dominant hemisphere, to reduce the dissection and open easily sylvian fissure, a logical path would follow the angular artery in the sylvian fissure cutting the arachnoid fibers and retracting only the tissues which are necessary to gain more exposure of the lesion.[2]
The current neuronavigation systems, based on preoperative magnetic resonance imaging and/or CT imaging, could be a remedy to these difficulties. However, according to Raza et al.,[13] these systems could not be sufficient for the intraoperative detection of a small vascular pathology, while the intraoperative CTA (iCTA) integrated with a navigation platform is a more accurate system.
When the iCTA is not available, as in our case, we recommend at least the use of normal neuronavigation techniques. Furthermore, to have a more accurate localization of the ICH and of small aneurysms with these systems, we think that it is important to reduce the use of osmotic diuretics before the dural opening, unless they are strictly necessary due to brain swelling. In this way, it is possible to avoid the brain shift which makes inaccurate the preoperative images used by the neuronavigation.
According to literature data, also for the M4 aneurysms, the use of the ICGV[3,11,12] before the clipping is helpful to identify the aneurismal sac, to define the surgical planning of the clip positioning, to visualize the eventual perforating arteries or to identify atheromas in the vessel and/or sac.[10] After the clipping, the use of the ICGV can intraoperatively detect a residual aneurysm filling, permitting a prompt clip repositioning.[10]
Generally, the preoperative CTA with 3-D reconstructions is an effective instrument for evaluating the ruptured cerebral aneurysms.[5] Nowadays, it provides several advantages with respect to the DSA: it is quick; it is relatively non-invasive; it involves less use of contrast dye compared with a four-vessel angiogram; it is relatively economic; and it involves the mobilization of fewer personnel and resources. Besides, in many cases it seems that the CTA 3-D reconstructions provide a superior image of the aneurysm compared to the DSA, and sometimes provide the surgeon with additional information concerning the aneurysmal anatomy and the relationships to parent vessels.[5] Indeed, Zhao et al.[14] believe that the CTA alone can be safely and effectively used in most patients requiring surgical treatment and they consider additional DSA in patients with small ruptured aneurysms or in those with multiple aneurysms.
However, CTA is only valuable in detecting lesions that are at least 4 mm in diameter. The resolution of the CTA 3-D reconstruction can only be based on the information from the CT. An angiogram will provide detail to 50 microns or 100 microns and the 3D reconstruction can provide more detail than the CT. This may guide the surgeon in his/her approach to the lesion especially if it is not at the sylvan point and is deeper in the fissure.
CONCLUSION
In our opinion, surgery is the best choice for the treatment of ruptured M4 aneurysms with the ICH, because it allows to evacuate the hematoma and exclude the aneurysm from the intracranial circulation. In addition, we suggest both the use of the neuronavigation technique and of the ICGV for the aneurismal surgery.
In some cases, we think that the use of the CTA with 3-D reconstruction alone is sufficient to the preoperative planning of emergency surgery of small M4 aneurysms associated with the ICH.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors thank Maria Silvia Marottoli for her assistance in the translation.
Footnotes
Contributor Information
Alessandro Ricci, Email: alex.ricci@email.it.
Hambra Di Vitantonio, Email: hambra.divitantonio@gmail.com.
Danilo De Paulis, Email: d.depaulis@alice.it.
Mattia Del Maestro, Email: mattiadelmaestro@gmail.com.
Soheila Dehcordi Raysi, Email: soheila.raysi@alice.it.
Domenico Murrone, Email: doflamingo82@gmail.com.
Sabino Luzzi, Email: sabino.luzzi@libero.it.
Renato Juan Galzio, Email: renato.galzio@cc.univaq.
REFERENCES
- 1.Ahn JY, Han IB, Joo JY. Aneurysm in the penetrating artery of the distal middle cerebral artery presenting as intracerebral haemorrhage. Acta Neurochir. 2005;147:1287–90. doi: 10.1007/s00701-005-0622-3. [DOI] [PubMed] [Google Scholar]
- 2.Ausman JI, Diaz FG, Malik GM, Tomecek F. A new microsurgical approach to cerebrovascular lesions of the sylvian point: Report of two cases. Surg Neurol. 1990;34:48–51. doi: 10.1016/0090-3019(90)90172-l. [DOI] [PubMed] [Google Scholar]
- 3.Della Puppa A, Rustemi O, Scienza R. The “ICG Entrapment Sign” in Cerebral Aneurysm Surgery Assisted by Indocyanine Green Videoangiography. World Neurosurg. 2017;97:287–91. doi: 10.1016/j.wneu.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Elsharkawy A, LeheÄka M, Niemelä M, Billon-Grand R, Lehto H, Kivisaari R, et al. A new, more accurate classification of middle cerebral artery aneurysms: Computed tomography angiographic study of 1,009 consecutive cases with 1,309 middle cerebral artery aneurysms. Neurosurgery. 2013;73:94–102. doi: 10.1227/01.neu.0000429842.61213.d5. [DOI] [PubMed] [Google Scholar]
- 5.Farsad K, Mamourian AC, Eskey CJ, Friedman JA. Computed tomographic angiography as an adjunct to digital subtraction angiography for the pre-operative assessment of cerebral aneurysms. Open Neurol J. 2009;3:1–7. doi: 10.2174/1874205X00903010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibo H, Carver CC, Rhoton AL, Jr, Lenkey C, Mitchell RJ. Microsurgical anatomy of the middle cerebral artery. J Neurosurg. 1981;54:151–69. doi: 10.3171/jns.1981.54.2.0151. [DOI] [PubMed] [Google Scholar]
- 7.Horiuchi T, Tanaka Y, Takasawa H, Murata T, Yako T, Hongo K. Ruptured distal middle cerebral artery aneurysm. J Neurosurg. 2004;100:384–8. doi: 10.3171/jns.2004.100.3.0384. [DOI] [PubMed] [Google Scholar]
- 8.Lee SM, Park HS, Choi JH, Huh JT. Ruptured mycotic aneurysm of the distal middle cerebral artery manifesting as subacute subduralhematoma. J Cerebrovasc Endovasc Neurosurg. 2013;15:235–40. doi: 10.7461/jcen.2013.15.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lv N, Zhou Y, Yang P, Li Q, Zhao R, Fang Y, et al. Endovascular treatment of distal middle cerebral artery aneurysms: Report of eight cases and literature review. Interv Neuroradiol. 2016;22:12–7. doi: 10.1177/1591019915617317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Della Puppa A, Rustemi O, Scienza R. The “ICG Entrapment Sign” in Cerebral Aneurysm Surgery Assisted by Indocyanine GreenVideoangiography. World Neurosurg. 2017;97:287–91. doi: 10.1016/j.wneu.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V. Near-infrared indocyanine green video angiography: A new method for intraoperative assessment of vascular flow. Neurosurgery. 2003;52:132–9. doi: 10.1097/00006123-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Raabe A, Beck J, Seifert V. Technique and image quality of intraoperative indocyanine green angiography during aneurysm surgery using surgical microscope integrated near-infrared video technology. Zentralbl Neurochir. 2005;66:1–6. doi: 10.1055/s-2004-836223. [DOI] [PubMed] [Google Scholar]
- 13.Raza SM, Papadimitriou K, Gandhi D, Radvany M, Olivi A, Huang J. Intra-arterial intraoperative computed tomography angiography guided navigation: A new technique for localization of vascular pathology. Neurosurgery. 2012;71:240–52. doi: 10.1227/NEU.0b013e3182647a73. [DOI] [PubMed] [Google Scholar]
- 14.Zhao B, Lin F, Wu J, Zheng K, Tan X, Cao Y, et al. A Multicenter Analysis of Computed Tomography Angiography Alone Versus Digital Subtraction Angiography for the Surgical Treatment of Poor-Grade Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2016;91:106–11. doi: 10.1016/j.wneu.2016.03.099. [DOI] [PubMed] [Google Scholar]


