Abstract
Objective
To sustain the bioactivity of proanthocyanidins-rich plant-derived extracts via encapsulation within biodegradable polymer microcapsules.
Methods
Polylactide microcapsules containing grape seed extract (GSE) were manufactured using a combination of double emulsion and solvent evaporation techniques. Microcapsule morphology, size distribution, and cross-section were examined via scanning electron microscopy. UV-vis measurements were carried out to evaluate the core loading and encapsulation efficiency of microcapsules. The bioactivity of extracts was evaluated after extraction from capsules via solvent partitioning one week or one year post-encapsulation process. Fifteen human molars were cut into 7mm × 1.7 mm μ0.5 mm thick mid-coronal dentin beams, demineralized, and treated with either encapsulated GSE, pristine GSE, or left untreated. The elastic modulus of dentin specimens was measured based on three-point bending experiments as an indirect assessment of the bioactivity of grape seed extracts. The effects of the encapsulation process and storage time on the bioactivity of extracts were analyzed.
Results
Polynuclear microcapsules with average diameter of 1.38 μm and core loading of up to 38wt% were successfully manufactured. There were no statistically significant differences in the mean fold increase of elastic modulus values among the samples treated with encapsulated or pristine GSE (p = 0.333), or the storage time (one week versus one year storage at room temperature, p = 0.967).
Significance
Polynuclear microcapsules containing proanthocyanidins-rich plant-derived extracts were prepared. The bioactivity of extracts was preserved after microencapsulation.
Keywords: Microencapsulation, Polynuclear microcapsules, Polylactide microcapsules, Surface biomodification, Dentin, Proanthocyanidins, Sustained bioactivity
1. Introduction
Dental caries is the most prevalent chronic disease worldwide. Resin composites have been widely used as restorative materials to manage tissue loss due to caries, yet, their service life is limited to only a few years (e.g. average 7 years in posterior teeth) [1-3].
The predominant reason for the replacement of composite restorations is the gradual loss of adhesion at the dentin-resin interface, likely due to the enzymatic degradation of collagen fibrils [4], and the hydrolytic degradation of resin components at the interface [5]. There are two ongoing efforts to improve the long-term stability of the interface materials, and hence, the longevity of composite restorations: advancements in resin composite chemistry for increased stability in the oral environment, and strengthening and stabilization of dentin matrix at the dentin–resin interface. The latter can be achieved by an increase in the number of inter- and intramicrofibrillar cross-links within type I collagen fibrils through the use of exogenous natural and synthetic cross-linkers [6]. The use of plant-derived extracts as collagen cross-linkers is more attractive and promising due to their biocompatibility and wide source availability of these compounds compared to their synthetic counterparts [7].
Recently, the use of plant-derived extracts rich in proanthocyanidins (PA), such as cocoa, pine bark, and grape seed extracts has been reported to effectively improve the mechanical properties and biostability of the dentin matrix as well as the dentin-resin bond strength [8–10]. Simple addition of bioactives into the dental adhesive may prove challenging due to stability of the polyphenols and reactivity with the dental resin chemistry. On-demand release of bioactives at the adhesive interface is an attractive way to deliver and sustain activity over the long-term, or to localize activity where potential flaws are present at the interface.
Herein, we propose an approach for sustaining the long-term bioactivity of natural extracts and protection from potential environmental degradation via sequestration within polymer microcapsules. Microencapsulation is a versatile technique commonly used for isolation, protection, and controlled delivery of active core materials in a wide range of applications and industries including drug delivery [11], self-healing materials [12], nutrient preservation [13], and perfumery [14]. The versatility of microcapsules is exemplified by the ability to release their core material in response to a variety of environmental stimuli including chemical triggers (e.g. change in pH), light irradiation, mechanical rupture, temperature, and time [15]. For dental materials microencapsulation of bioactive or reactive agents has enabled strategies for enhancing the performance of dental tissues, materials, or treatments. For example, self-healing resin composites were demonstrated using core-shell microcapsules that release a healing agent in response to mechanical damage [16-18]. Microcapsules have also been used for the time-release (also known as sustained release) of useful agents for antimicrobial activities [19,20], remineralization [21-23], and regeneration [24-27]. Time-release is generally achieved via the degradation of the encapsulating wall material, diffusion through the shell wall, or a combination of these two mechanisms [28]. Microencapsulation has also been used to enhance the stability of resinous components prior to clinical treatments via the sequestration of the reactive and sensitive components (e.g. polymerization initiators), and releasing them when required for optimal polymerization reactivity [29,30].
In this study, grape seed extract (GSE) was used as a core material for microencapsulation because of its high PA content (up to 97%, [31]) and proven biomodification of the dentin matrix. Polylactide (PLA), a biodegradable and biocompatible polymer, was used as the shell wall material since the encapsulation by PLA does not involve in-situ chemical reactions which could adversely affect the bioactivity of natural extracts, and the biodegradation of PLA offers the ability to control and tailor the sustained release of core materials for optimal delivery to the dentin matrix. The goals of this study were to develop a facile microencapsulation technique for the encapsulation of natural extracts (e.g. GSE) and to demonstrate the sustained bioactivity of the core material after the encapsulation process. Microcapsules containing natural extracts are ultimately envisioned to be embedded in the adhesive dental resin that anchors the bulk composite to the dentin substrate. We hypothesize that the biodegradation of the microcapsule shell wall will enable the sustained delivery of bioactives to the dentin matrix for continued stabilization of collagen fibrils, and consequently, prolonged service life of dental restorations. Consequently, the size of microcapsules is constrained by the maximum thickness of the adhesive layer (ca. 20 μm) for practical clinical applications.
A novel double emulsion-solvent evaporation microencapsulation technique was used to encapsulate GSE within PLA microcapsules. Encapsulation parameters were optimized to produce microcapsules with acceptable morphology, desired size range, and high core loading. Bioactivity of GSE, both pre-and post-encapsulation, was assessed by the pretreatment of demineralized dentin specimens with GSE and the measurement of elastic modulus from three-point bend experiments.
2. Materials and methods
2.1. Materials
Unless otherwise specified, all chemicals and reagents were purchased from Sigma Aldrich and used as received. Polylactide (PLA) pellets were purchased from NatureWorks, LLC (Ingeo 4043D, Mn ∼100,000Da, and Mw ∼150,000Da). Grape seed extract (GSE) was 95% proanthocyanidins-rich Vitis vinifera (MegaNatural™ gold grape seed extract, California, USA).
2.2. Preparation of microcapsules
Microcapsules were fabricated using a combination of water-oil-water (W/O/W) double emulsion and solvent evaporation techniques (Fig. 1). In a typical experiment 600mg GSE was dissolved in 3mL of distilled water to produce the inner water solution (Wi). The middle oil solution (O) comprised 20 g of 1.5 wt% Span 85 oil-soluble surfactant and 3wt% PLA in dichloromethane (DCM), a volatile, water-immiscible organic solvent. The outer aqueous solution (Wo) was 300 mL of 2.5 wt% polyethylene glycol (PEG) and 1 wt% sodium dodecyl sulfate (SDS) surfactants in distilled water. The aqueous surfactant mixture was selected from the experimental trials of various surfactant mixtures in order to obtain microcapsules with good surface morphology and size control.
Fig. 1.
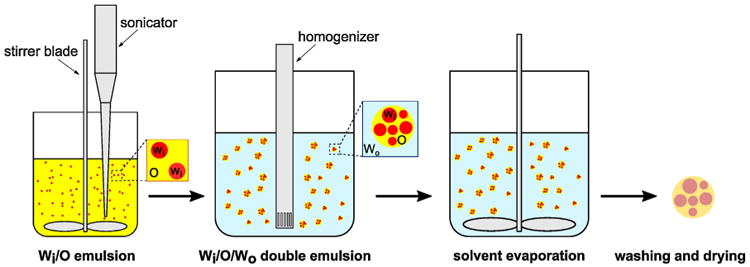
Schematic representation of the microencapsulation technique. First, an aqueous solution of GSE (Wi) is emulsified in an oil solution (O), consisting of PLA and Span 85 surfactant dissolved in DCM. The Wi/O emulsion solution is then further emulsified in an aqueous PVA surfactant solution (Wo) to form a Wi/O/Wo double emulsion. Upon the formation of a stable double emulsion, it is continuously agitated until DCM evaporates from the middle oil layer and a solid polymer shell wall is formed around the aqueous core. Finally, microcapsules are washed and dried to remove any residual surfactant and water from the capsules.
The first emulsion (i.e. Wi/O) was prepared by adding Wi solution dropwise to the oil solution in a 50 mL beaker under continuous agitation at 1500 rpm and simultaneous sonication (tapered 3 mm tip sonication horn in 750 W ultrasonic homogenizer, Cole Parmer) for 10min (20% amplitude, 0.2 s pulse, 0.2 s pause). The beaker was kept in an ice bath to minimize the evaporation of DCM during emulsification. The prepared emulsion was then, in turn, emulsified into the Wo solution in a 600 mL beaker using a homogenizer (OMNI GLH-01) for three minutes. After forming a stable Wi/O/Wo double emulsion, the emulsion was agitated at 800 rpm for five hours to allow the gradual diffusion and evaporation of DCM solvent. Removal of DCM from the middle layer resulted in the consolidation of PLA polymer and the formation of a protective shell wall around the aqueous core (Wi). Microcapsules were then centrifuged (4000rpm for 10min) and washed with distilled water four times to remove any residual surfactants. Finally, collected microcapsules were lyophilized for 24h to remove water from the microcapsules.
2.3. Characterization of microcapsules
Microcapsule morphology, cross-sectional imaging, and size distribution were characterized via scanning electron microscopy (SEM, Quanta 450 FEG ESEM). For cross-sectional imaging, microcapsules were embedded in an epoxy resin, and cured for 24h at 30°C. Specimens were then immersed in liquid nitrogen for a few minutes and then immediately fractured using a razor blade. Before imaging, specimens were sputter-coated with Au/Pd to avoid surface charging.
UV–visible spectroscopy (UV-2401 PC, Shimadzu, Japan) measurements were performed to determine the microcapsules core loading as well as the encapsulation efficiency. A 1g/L solution of microcapsules in a mixture of 60vol% tetrahydrofuran (THF) and 40vol% methanol was prepared, and diluted with distilled water at a 1:9 volume ratio to force the polymer precipitate out of the solution. A linear calibration curve of absorbance (at 280 nm) as a function of GSE concentration in the same solvent mixture was also created by measuring the absorbance of GSE (200–700 nm) at known concentrations. The absorbance of GSE in the microcapsule solution was then measured, and GSE concentration was determined from the calibration curve. Microcapsules core loading, LGSE, was calculated as,
| (1) |
where cGSE is the concentration of GSE determined from calibration curve, Vs is the volume of diluted solution used in the UV–vis measurement, d is the dilution ratio (equal to 10 in this study), and Mcap is the original mass of microcapsules dissolved in the solution. Encapsulation efficiency, ξ, of the microencapsulation procedure was calculated by comparing the actual loading of microcapsules with the theoretical loading of GSE,
| (2) |
and,
| (3) |
where MGSE and MP are the mass of GSE and PLA polymer used to manufacture microcapsules, respectively.
2.4. Modulus of elasticity of demineralized dentin
Fifteen extracted human third molars were obtained under a protocol approved by the Institutional Review Board Committee from the University of Illinois at Chicago (2011-0312). Serial sections in the mid-coronal dentin were performed, resulting in dentin beams with 0.50mm thickness × 1.7 mm width × 7.0 mm length and a dimple was made at the margin of the beam for measurements to be performed on the same surface. The dentin beams were immersed in 10% phosphoric acid solution (Ricca Chemical Company, USA) for 5h for complete demineralization [32]. Specimens were thoroughly rinsed with distilled water for 10 min. The modulus of elasticity (E) of the dentin matrix was assessed at baseline and immediately after the 1-h treatment with bioactive solutions using a non-destructive three-point bending flexural test at a 3% strain, in a universal testing machine (EZ Graph, Shimadzu, Japan) with a 1N load cell at 0.5 mm/min crosshead speed [10]. Solutions containing grape seed extract were freshly prepared at 0.65 wt/v % in HEPES solution at pH 7.2. The encapsulated GSE (1-week post encapsulation and 12 months after room storage) was removed by solvent partitioning using 5:1 chloroform and distilled water with multiple water replenishment. Distilled water was lyophilized, and then, GSE powder was dissolved at the concentration described above. The modulus of elasticity was calculated and statistically analyzed by 2-way ANOVA.
3. Results
PLA microcapsules with GSE as the core material were successfully manufactured. A scanning electron microscope image of microcapsules is shown in Fig. 2a. Manufactured microcapsules are polydisperse in size (Fig. 2a), and possess a smooth surface morphology. In Fig. 2b, microcapsule size distribution obtained from analyzing the SEM images of microcapsules (count 124) is shown. Average microcapsule diameter Davg = 1.38 μm with the maximum diameter Dmax = 14.35 μm, and minimum diameter Dmin = 0.20 μm was determined from the size distribution analysis. Cross-sectional SEM imaging of microcapsules (Fig. 2c) reveals a polynuclear structure with multiple pore regions that are randomly and non-uniformly distributed throughout the encapsulating PLA polymer matrix. GSE loading of microcapsules was evaluated by dissolving the capsules in the organic solvent mixture and measuring the absorbance at 280 nm, where GSE shows a distinctive absorbance peak. The calibration curve of absorbance as a function of concentration of GSE is shown in Fig. 3. Based on these measurements, an average GSE loading of 38.1 ±1.3% was obtained for the PLA microcapsules. In Fig. 4, the results of three-point bend experiments on untreated and treated dentin specimens are presented. Pretreatment of dentin specimens with GSE results in significant enhancement in the modulus of elasticity compared to the baseline groups, indicating the strengthening effect of the extract on the dentin matrix. There were no statistically significant differences in the mean fold increase (the ratio of the elastic modulus after treatment to the baseline elastic modulus) values among the encapsulated and pristine GSE experimental groups (p = 0.333), or as a function of the storage time (one week versus one year storage at room temperature, p = 0.967).
Fig. 2.
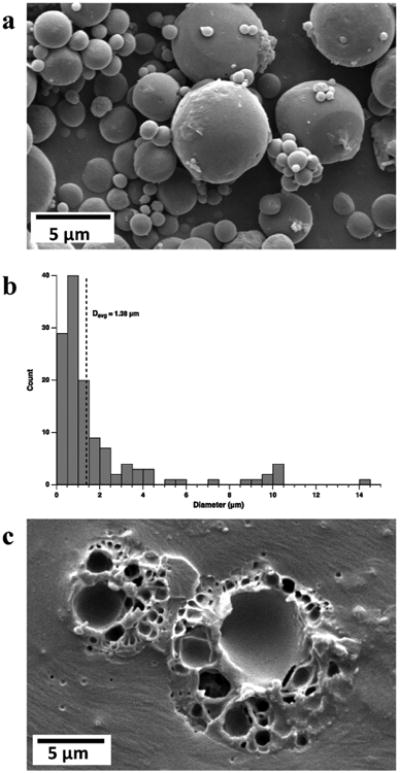
Microcapsule characterization. (a) Scanning electron microscope image of microcapsules reveals a smooth surface morphology and polydisperse size distribution. (b) Representative microcapsule size histogram (Davg = 1.38 μm). (c) Cross-sectional SEM image of two microcapsules embedded in epoxy resin shows the interior polynuclear structure of the capsules.
Fig. 3.

Calibration curve of UV-vis absorbance of GSE as a function of concentration was used to determine core loading and encapsulation efficiency. The red solid diamond represents the absorbance of a solution of microcapsules at Mcap/(Vs.d) = 0.1 g/L, equivalent to LGSE ∼38%. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
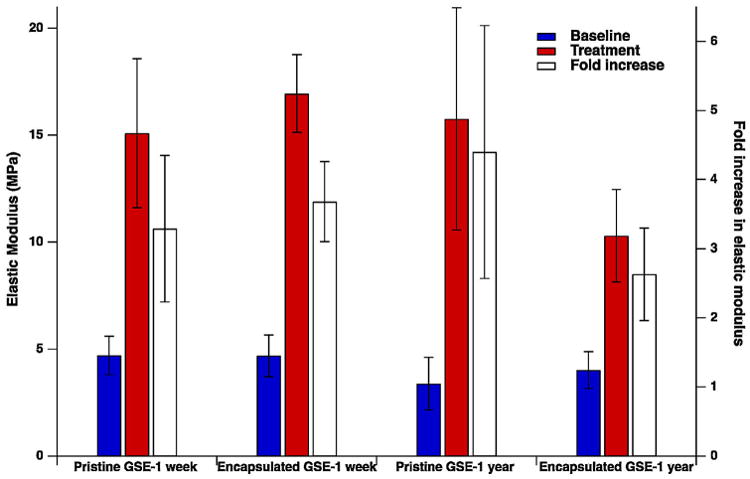
Results of elastic modulus and fold increase in elastic modulus of demineralized dentin specimens prior to (baseline) and after treatment with fresh solutions of pristine GSE and GSE extracted from microcapsules at one week or one year post-encapsulation.
4. Discussion
Microencapsulation offers effective compartmentation, protection, and controlled delivery of bioactive or therapeutic agents in dental material applications. Selection of proper microencapsulation technique is governed by the chemical and physical characteristics of the core material as well as the encapsulating shell wall polymer. The water-solubility of natural extracts, such as the one used in this study, limits the choice of microencapsulation techniques since many conventional techniques generally require a continuous water phase for emulsification. Use of a double emulsion approach may allow for the formation of aqueous emulsion of natural extracts within a middle hydrophobic phase, however, it requires the ability to form a shell wall in the middle layer of the double emulsion. In addition, formation of two stable emulsions makes the control over the microcapsule structure, loading, and size challenging, especially at micron and sub-micron scale.
In the present study, we successfully encapsulated GSE by using a combination of double emulsion and solvent evaporation techniques. Use of sonication in the first emulsification step (Wi/O) results in the formation of nanoscale aqueous droplets within the oil solution, while the homogenization in the second step allows for the formation of micron- and sub-micron scale double emulsion droplets. The combination of these two approaches provides efficient encapsulation of the aqueous core within microcapsules within the desired size range. The influence of using two separate emulsification steps can clearly be seen in the resulting polynuclear structure of microcapsules, where multiple pores of the inner solution are randomly dispersed within the polymer matrix of capsules. In addition, a smooth surface morphology with no surface porosity was observed, a requirement for the proper protection of the core material from the surrounding environment.
A relatively high GSE loading within microcapsules (38.1 ±1.3%) was achieved. In general, microcapsule loading can be adjusted by varying the ratio of core material to shell polymer, as long as the polymer is able to form a complete shell around the core material. In this study, a theoretical loading of 50% was expected based on the ratio of core material and polymer. Thus, an encapsulation efficiency of ξ = 76.2 ± 2.6% was achieved for this microencapsulation technique and condition. Loss of a small fraction of the inner aqueous solution at the interface of the Wi/O emulsion droplets and outer aqueous solution (Wo) during the second emulsification step is likely the cause of the reduction in the encapsulation efficiency.
The bioactivity of encapsulated GSE was evaluated after extraction from capsules via solvent partitioning at one week or one year post-encapsulation storage at room temperature. Demineralized dentin specimens were treated with encapsulated GSE and pristine GSE (stored under similar conditions). The modulus of elasticity of the specimens was measured and compared to provide an indirect assessment of the degree of bioactivity of GSE post-encapsulation. The results of these experiments reveal that the pretreatment of dentin specimens with GSE, either pristine or encapsulated, results in significant enhancement in the modulus of elasticity compared to the baseline groups. This indicates the strengthening effect of the extract on the dentin matrix, which is in good agreement with previous observations on the bioactivity of GSE [32,33]. In addition, these results suggest that the encapsulation process does not adversely influence the bioactivity of the extracts.
The retention of bioactivity of natural extracts after the encapsulation process along with the time-dependent release from degradable PLA microcapsules is the key requirements for the proposed approach to enhance the lifetime of dental restorations. The polynuclear structure of microcapsules is particularly advantageous for time-release, since the core compound is only released from the outermost pores as the capsule shell wall degrades. The degradation rate of the polymer is also a crucial factor when designing time-release microcapsules. The hydrolytic degradation of biodegradable polymers typically depends on several factors, including molecular weight, morphology (i.e. degree of crystallinity), porosity, and environmental conditions [34]. In general, high molecular weight PLA (>20,000 Da), such as the one used in the present study, exhibits a long degradation time ranging from 12 months up to 5 years depending on other described factors [35,36], which is ideal for the expected prolonged release of bioactives to the dentin–resin interface.
5. Conclusion
Grape seed extract was successfully encapsulated within polylactide polymeric microcapsules for use in dental resin applications. Polynuclear microcapsules with high loading, acceptable surface morphology, and micron- and sub-micron size range were manufactured. The bioactivity of GSE was preserved during the microencapsulation process. The use of polylactide as the shell polymer of microcapsules is expected to provide time-dependent release of the core material upon exposure to enzymatic or hydrolytic environmental conditions. Integration of these capsules into the adhesive resin of dental restorations is envisioned to provide sustained delivery of the extracts to the dentin-resin interface for prolonged strengthening of collagen structure, and consequently, enhanced service life of restorations.
Acknowledgments
This work was financially supported by a research grant from NIH-NIDCR (Grant# DE021040). Scanning electron microscopy was performed in the Imaging Technology Group of the Beck-man Institute for Advanced Science and Technology.
References
- 1.Mjor IA, Moorhead JE, Dahl JE. Reasons for replacement of restorations in permanent teeth in general dental practice. Int Dent J. 2000;50:361–6. doi: 10.1111/j.1875-595x.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 2.Bogacki RE, Hunt RJ, del Aguila M, Smith WR. Survival analysis of posterior restorations using an insurance claims database. Oper Dent. 2002;27:488–92. [PubMed] [Google Scholar]
- 3.Sarrett DC. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent Mater. 2005;21:9–20. doi: 10.1016/j.dental.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Yiu CKY, King NM, Pashley DH, Suh BI, Carvalho RM, Carrilho MRO, et al. Effect of resin hydrophilicity and water storage on resin strength. Biomaterials. 2004;25:5789–96. doi: 10.1016/j.biomaterials.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 5.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–21. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 6.Cecchin D, Pin LC, Farina AP, Souza M, Vidal CDP, Dal Bello Y, et al. Bond strength between fiber posts and root dentin treated with natural cross-linkers. J Endod. 2015;41:1667–71. doi: 10.1016/j.joen.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Bedran-Russo AK, Pauli GF, Chen SN, McAlpine J, Castellan CS, Phansalkar RS, et al. Dentin biomodification: strategies, renewable resources and clinical applications. Dent Mater. 2014;30:62–76. doi: 10.1016/j.dental.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Ammar A, Drummond JL, Bedran-Russo AK. The use of collagen cross-linking agents to enhance dentin bond strength. J Biomed Mater Res B. 2009;91b:419–24. doi: 10.1002/jbm.b.31417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bedran-Russo AKB, Pereira PNR, Duarte WR, Drummond JL, Yamauchi M. Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. J Biomed Mater Res B. 2007;80b:268–72. doi: 10.1002/jbm.b.30593. [DOI] [PubMed] [Google Scholar]
- 10.Bedran-Russo AKB, Pashley DH, Agee K, Drummond JL, Miescke KJ. Changes in stiffness of demineralized dentin following application of collagen crosslinkers. J Biomed Mater Res B. 2008;86b:330–4. doi: 10.1002/jbm.b.31022. [DOI] [PubMed] [Google Scholar]
- 11.Meng FH, Zhong ZY, Feijen J. Stimuli-responsive polymersomes for programmed drug delivery. Biomacromolecules. 2009;10:197–209. doi: 10.1021/bm801127d. [DOI] [PubMed] [Google Scholar]
- 12.Blaiszik BJ, Kramer SLB, Olugebefola SC, Moore JS, Sottos NR, White SR. Self-healing polymers and composites. Annu Rev Mater Res. 2010;40:179–211. [Google Scholar]
- 13.Onwulata CI. Microencapsulation and functional bioactive foods. J Food Process Preserv. 2013;37:510–32. [Google Scholar]
- 14.Vaughn J, Wu HH, Efremovska B, Olson DH, Mattai J, Ortiz C, et al. Encapsulated recyclable porous materials: an effective moisture-triggered fragrance release system. Chem Commun. 2013;49:5724–6. doi: 10.1039/c3cc41236a. [DOI] [PubMed] [Google Scholar]
- 15.Esser-Kahn AP, Odom SA, Sottos NR, White SR, Moore JS. Triggered release from polymer capsules. Macromolecules. 2011;44:5539–53. [Google Scholar]
- 16.Ouyang XB, Huang XQ, Pan QH, Zuo CQ, Huang C, Yang XL, et al. Synthesis and characterization of triethylene glycol dimethacrylate nanocapsules used in a self-healing bonding resin. J Dent. 2011;39:825–33. doi: 10.1016/j.jdent.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Wu JL, Weir MD, Melo MAS, Xu HHK. Development of novel self-healing and antibacterial dental composite containing calcium phosphate nanoparticles. J Dent. 2015;43:317–26. doi: 10.1016/j.jdent.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu JL, Weir MD, Zhang Q, Zhou CJ, Melo MAS, Xu HHK. Novel self-healing dental resin with microcapsules of polymerizable triethylene glycol dimethacrylate and N,N-dihydroxyethyl-p-toluidine. Dent Mater. 2016;32:294–304. doi: 10.1016/j.dental.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen F, Liu XM, Rice KC, Li X, Yu F, Reinhardt RA, et al. Tooth-binding micelles for dental caries prevention. Antimicrob Agents Chemother. 2009;53:4898–902. doi: 10.1128/AAC.00387-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han B, Wang XY, Liu JG, Liang FX, Qu XZ, Yang ZZ, et al. The biological performance of calcium hydroxide-loaded microcapsules. J Endod. 2013;39:1030–4. doi: 10.1016/j.joen.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Burbank BD, Slater M, Kava A, Doyle J, McHale WA, Latta MA, et al. Ion release, fluoride charge of and adhesion of an orthodontic cement paste containing microcapsules. J Dent. 2016;45:32–8. doi: 10.1016/j.jdent.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Davidson MT, Greving TA, McHale WA, Latta MA, Gross SM. Ion permeable microcapsules for the release of biologically available ions for remineralization. J Biomed Mater Res A. 2012;100a:665–72. doi: 10.1002/jbm.a.34000. [DOI] [PubMed] [Google Scholar]
- 23.Falbo MM, Elassal P, Greving TA, McHale WA, Latta MA, Gross SM. The control of phosphate ion release from ion permeable microcapsules formulated in to rosin varnish and resin glaze. Dent Mater. 2013;29:804–13. doi: 10.1016/j.dental.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Kuang R, Zhang ZP, Jin XB, Hu J, Gupte MJ, Ni LX, et al. Nanofibrous spongy microspheres enhance odontogenic differentiation of human dental pulp stem cells. Adv Healthc Mater. 2015;4:1993–2000. doi: 10.1002/adhm.201500308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JH, Kim J, Baek HR, Lee KM, Seo JH, Lee HK, et al. Fabrication of an rhBMP-2 loaded porous beta-TCP microsphere-hyaluronic acid-based powder gel composite and evaluation of implant osseointegration. J Mater Sci Mater Med. 2014;25:2141–51. doi: 10.1007/s10856-014-5250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathieu S, Jeanneau C, Sheibat-Othman N, Kalaji N, Fessi H, About I. Usefulness of controlled release of growth factors in investigating the early events of dentin-pulp regeneration. J Endod. 2013;39:228–35. doi: 10.1016/j.joen.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Oliva-Rodriguez R, Perez-Urizar J, Dibildox-Alvarado E, Martinez-Saldana MC, Avelar-Gonzalez FJ, Flores-Reyes H, et al. Design of a controlled release system of OP-1 and TGF-beta 1 based in microparticles of sodium alginate and release characterization by HPLC-UV. In Vitro Cell Dev Biol Anim. 2011;47:681–8. doi: 10.1007/s11626-011-9459-7. [DOI] [PubMed] [Google Scholar]
- 28.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–90. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 29.Fuchigami K, Ikemura K, Ichizawa K. Novel, multi-purpose, PMMA-type adhesive resin with newly synthesized microcapsule of radical polymerization initiators. Dent Mater J. 2008;27:35–48. doi: 10.4012/dmj.27.35. [DOI] [PubMed] [Google Scholar]
- 30.Volz M, Ziener U, Salz U, Zimmermann J, Landfester K. Preparation of protected photoinitiator nanodepots by the miniemulsion process. Colloid Polym Sci. 2007;285:687–92. [Google Scholar]
- 31.dos Santos PH, Karol S, Bedran-Russo AKB. Nanomechanical properties of biochemically modified dentin bonded interfaces. J Oral Rehabil. 2011;38:541–6. doi: 10.1111/j.1365-2842.2010.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aguiar TR, Vidal CMP, Phansalkar RS, Todorova I, Napolitano JG, McAlpine JB, et al. Dentin biomodification potential depends on polyphenol source. J Dent Res. 2014;93:417–22. doi: 10.1177/0022034514523783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castellan CS, Bedran-Russo AK, Karol S, Pereira PNR. Long-term stability of dentin matrix following treatment with various natural collagen cross-linkers. J Mech Behav Biomed. 2011;4:1343–50. doi: 10.1016/j.jmbbm.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 2012;64:72–82. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 35.Auras RA, Lim LT, Selke SE, Tsuji H. Poly (lactic acid): synthesis, structures, properties, processing, and applications. John Wiley & Sons; 2011. [Google Scholar]
- 36.Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21:2335–46. doi: 10.1016/s0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]


