Abstract
Background
Relative risks (RR) for cardiovascular disease (CVD) by smoking rate exhibit a concave pattern, with RRs in low rate smokers exceeding a linear extrapolation from higher rate smokers. However, cigarettes/day does not by itself fully characterize smoking-related risks. A reexamination of the concave pattern using a comprehensive representation of smoking may enhance insights.
Material
Data were from the Atherosclerosis Risk in Communities (ARIC) Study, a prospective cohort enrolled in four areas of the U.S. in 1987–89. Follow-up was through 2008. Analyses included 14,233 participants, 245,915 person-years and 3,411 CVD events.
Results
The concave RRs with cigarettes/day were consistent with cigarettes/day modifying a linear RR association of pack-years with CVD, i.e., strength of the pack-years association depended on cigarettes/day, indicating that the manner of pack-years accrual impacted risk. Smoking fewer cigarettes/day for longer duration was more deleterious than smoking more cigarettes/day for shorter duration (P<0.01). For 50 pack-years (365,000 cigarettes), estimated RRs of CVD were 2.1 for accrual at 20 cigarettes/day and 1.6 for accrual at 50 cigarettes/day. Years since smoking cessation did not alter the diminishing strength of association with increasing cigarettes/day. Analyses that accounted for competing risks did not affect findings.
Conclusion
Pack-years remained the primary determinant of smoking-related CVD risk; however, accrual influenced RRs. For equal pack-years, smoking fewer cigarettes/day for longer duration was more deleterious than smoking more cigarettes/day for shorter duration. This observation provides clues to better understanding the biological mechanisms, and reinforces the importance of cessation rather than smoking less to reduce CVD risk.
Introduction
Although cigarette smoking prevalence has declined in most Western countries, it remains high in many countries and consequently smoking remains an important risk factor for cardiovascular disease (CVD) (WHO Report On The Global Tobacco Epidemic, 2013; available at http://www.who.int/tobacco/global_report/2013/en/). Investigators have consistently reported that relative risks (RR) for CVD by cigarettes/day exhibit a concave pattern, implying that the RR per cigarette/day decreases with greater cigarettes/day 1–3. Several reviews have discussed possible mechanisms that link smoking to CVD, including the non-linear effects 2–9; however, the precise smoking rate-dependent biologic mechanisms responsible for the concave pattern remain uncertain.
Cigarettes/day represents a metric of exposure rate (the time weighted average over the period of active consumption), and thus is not a quantitative measure of cigarette smoke exposure. Furthermore, it is well recognized that analyses based on cigarettes/day alone, or indeed smoking duration alone or pack-years alone, provide an incomplete characterization of smoking-related disease risk. Consequently, a comprehensive description of risks by smoking requires a more complete representation of exposure. Starting with cigarettes/day, duration, and/or pack-years, investigators often adjust one metric for another or cross-tabulate RRs for two factors with never-smokers as the referent group. Less frequently, investigators use a single comprehensive smoking index that simultaneously incorporates multiple smoking-related components 10,11. The typical approach computes the joint RRs with cigarettes/day and smoking duration, although this choice leads to problems of interpretation12–14. For example, in a simple log-linear RR model with cigarettes/day and duration, the cigarettes/day parameter represents the ln(RR) per cigarette/day with duration held fixed. Because duration is fixed, RRs for increasing cigarettes/day necessarily embed increasing total pack-years. For example, for 30 years of smoking, a comparison of RRs at 20 and 30 cigarettes/day reflects not only different smoking rates but also different total exposures, i.e., 30 and 45 pack-years, respectively, or nearly 110,000 (≈15×20×365.25) additional cigarettes. Similarly, at fixed cigarettes/day, RRs at two different durations of smoking include effects of increasing pack-years. Hence, the cigarettes/day and duration parameters are not interpretable as distinct, unrelated effects.
We analyze RRs for pack-years and cigarettes/day, which allows a more direct interpretation of parameters, in which smoking rate serves to modify RR trends with pack-years. In this approach, cigarettes/day represents the relative influence of exposure accrual on the RR for a given pack-years, i.e., the differential in the RRs at a fixed pack-years when delivered at lower smoking rates for longer durations or higher rates for shorter durations. This approach reinterprets the non-linear RRs for cigarettes/day as a “smoking rate effectiveness factor” or “delivery rate effect”. For example, for individuals who smoked 20 cigarettes/day for 50 years or 30 cigarettes/day for 33.3 years or 50 cigarettes/day for 20 years, the analysis below estimates RRs of 2.2, 1.9 and 1.7, respectively, even though exposure for all individuals was 50 pack-years (365,000 cigarettes).
Our analysis considers two issues: (i) the joint RRs by pack-years and cigarettes/day for CVD, coronary heart disease (CHD) and stroke; and (ii) the modification of smoking-related RRs for CVD by smoking-related behaviors, age started smoking, extent of inhalation, years since cessation and additional use of cigars and pipes.
Material and Methods
Study design
The Atherosclerosis Risk in Communities Study (ARIC) is a large prospective cohort study conducted in four areas of the U.S.: Forsyth County, NC; Jackson, MS; Washington County, MD; the northwest suburbs of Minneapolis, MN. Enrollment occurred between 1987 and 1989 using a probability-based sample of adults aged 45–64 years. Study details have been provided previously 15–18. Study personnel collected a wide variety of data from clinical examinations and from personal interviews at baseline enrollment and at three clinic visits: 1990–1992 (visit 2), 1993–1995 (visit 3) and 1996–1998 (visit 4). Annual telephone calls collected information from participants or their surrogates on vital status, hospital visits and other factors.
For this analyses, we followed those participants without a pre-enrollment history of coronary heart disease or stroke through the earliest date of CVD incidence, loss to follow-up, death or 31 December 200818. We ascertained outcome information on CVD through annual telephone interviews and surveillance of hospital discharge records in the study areas and death certificates. Events were validated by examination of hospital records, death certificates and, when available, autopsy records, with outcomes classified according to ARIC Study criteria 18. CVD encompassed CHD and stroke. CHD included a validated, definite or probable hospitalized myocardial infarction, a definite CHD death, an unrecognized myocardial infarction defined by electrocardiographic reading or coronary revascularization 17. A stroke event comprised a validated, definite or probable hospitalized ischemic or hemorrhagic stroke.
Questionnaires administered at baseline and subsequent clinic visits provided information on smoking status and cigarettes/day, while the annual telephone contacts provided additional information on smoking status. For cigarettes/day, we used baseline information only. This choice likely had minimal effect, since few individuals modified their cigarettes/day. At baseline, there were 42% never-smokers, 32% former smokers and 26% current smokers, half of whom stopped smoking by the end of 2008. Among current and former smokers at baseline who had follow-up smoking information from one or more clinic visits, only 15% and 8%, respectively, changed their smoking status. In addition, for continuing smokers, the mean cigarettes/day during follow-up was on average within 1–4 cigarettes/day of their baseline value. Additionally, smoking-years from baseline through visit 4 represented a relatively limited percentage of the lifetime years of consumption. Thus, cigarettes/day at baseline provided a good estimate of the time weighted average cigarettes/day throughout follow-up. The clinic questionnaires and the yearly telephone contacts yielded time-dependent information on smoking status, which enabled time-dependent calculation for duration of smoking, pack-years and time since smoking cessation. For the analyses, we defined former smokers as those who last smoked one or more years prior.
Study personnel obtained three blood pressure (BP) measurements using a random-zero sphygmomanometer with the participant seated, with BP determined as the mean of the last two values. We defined hypertension as systolic blood pressure ≥140mmHg, diastolic blood pressure ≥90mmHg or current use of antihypertensive medication. We conducted a fasting blood collection and measured glucose and plasma total cholesterol by standard enzymatic methods. We designated diabetes at baseline as a self-reported history of, or treatment for, diabetes, a fasting glucose level of 126 mg/dL or greater, or a casual blood glucose level of 200 mg/dL or greater 18.
The initial dataset included 14,878 subjects and 3,603 CVD events. We excluded 751 participants with missing data, including 212 CVD events, leaving 14,127 participants with 3,391 CVD events and 232,002 person-years of follow-up. Exclusions resulted from missing information for smoking (247 subjects and 59 CVD cases), lipid measurements (228 subjects and 64 cases), body mass index (BMI) and diagnosed diabetes mellitus (43 subjects and 15 cases), alcohol use (78 subjects and 24 cases) and other variables (155 subjects and 50 cases). There were 2,321 non-CVD deaths during follow-up, with 1,241 cancers (including 352 lung, 97 breast, 80 pancreas, 80 colon and 65 prostate cancer deaths), 468 diseases of the circulatory system (including 72 atherosclerosis, 34 hypertensive disease, 27 congestive heart failure and 23 aortic aneurisms), 236 respiratory diseases (including 140 chronic obstructive pulmonary disease deaths) and 376 other causes. In addition, there were 297 (2%) participants with missing or unknown status which were censored at last contact.
Data structure
We used Poisson regression to estimate relative risks (RR), with data summarized in a multidimensional person-years table. The cross-classification variables included attained age (<54, 54–55, …, 78–79, ≥80), calendar period (<1990, 1990–94, 1995–1999, 2000–2004, 2005–2009), birth year (<1930, 1930–34, 1935–39, ≥1940), study site, sex, race (White, African-America, Asian-American, Other), BMI (<25.0, 25.0–29.9, 30.0–34.9, ≥50 kg/m2), alcohol intake in gm-ethanol/week (<40, 40–107, ≥108), diagnosed high BP, diagnosed diabetes mellitus, total cholesterol (<5.2, 5.2–6.1, ≥6.2 mmol/L), ever use of cigars/pipes, education (<12 years, high school/vocational school and college, graduate or professional school), cigarettes smoked per day (0, 1–4, 5–9, …, 45–49, ≥50), pack-years (0, 1–9, 10–19, 20–24, …, 55–59, ≥60), years since last smoked (<1, 1–4, 5–9, 10–19, ≥20), age first smoked (<16, 16–17, 18–19, ≥20) and inhalation (never/seldom, moderately, deeply). We used fine categorizations for the primary analytic variables (age, calendar year, cigarettes/day, pack-year, etc.). For other variables, we selected broader categories that both covered the full range of values and allowed for sufficient numbers of CVD cases. For each cell, we accrued person-time, counted disease events and computed person-years weighted means for continuous variables.
Competing risks could influence results since those who accrued longer follow-up or were lighter smokers may be more likely to incur CVD events, while heavier smokers were more likely to be selectively removed from follow-up due to other diseases, in particular lung cancer. We therefore conducted analyses using competing risks methodology that incorporated multiple outcomes, including incident CVD, lung cancer, other selected smoking-related cancers (esophagus, larynx, oropharynx, bladder, kidney, stomach, colon, rectum and pancreas) and mortality from all other causes 19,20. For these analyses, we had to restrict follow-up, since detailed cancer incidence data were available only through 31 December 2006. Consequently, we first compared results for the full follow-up with results for the restricted follow-up, which then served as a basis for comparison of results under competing risks methods.
Relative risk models
We modeled disease rate as r(s, z) = exp(αz) × RR(s), where z and α were vectors of adjustment variables and parameters, respectively. For categories of cigarettes/day, smoking duration or pack-years, denoted s, we modeled RR(s) using indicator variables and the standard exponential form. We computed joint RRs for the cross-tabulation of pack-years and cigarettes/day, relative to never-smokers, and observed that RR trends with pack-years were approximately linear within each category of cigarettes/day. Since a linear slope fully described the pack-years-related RRs within cigarettes/day categories, the goal was to characterize the linear trends and their variations with cigarettes/day.
Our models were based on continuous pack-years, denoted d,. We started with a linear model,
| (1) |
where β was the slope parameter, i.e., the excess RR/pack-year. However, since linearity occurred only within cigarettes/day categories, we extended equation 1 for S categories of cigarettes/day, s=1,…,S:
| (2) |
where ds equaled d within category s and zero otherwise and β1, …, βS were slope parameters.
The slope measured the strength of CVD and pack-years association relative to never-smokers within each cigarette/day category, while variations among the slope parameters (β’s) reflected the influence of smoking rate on the strength of association. We modeled variations of the slope with continuous cigarettes/day (n) using:
| (3) |
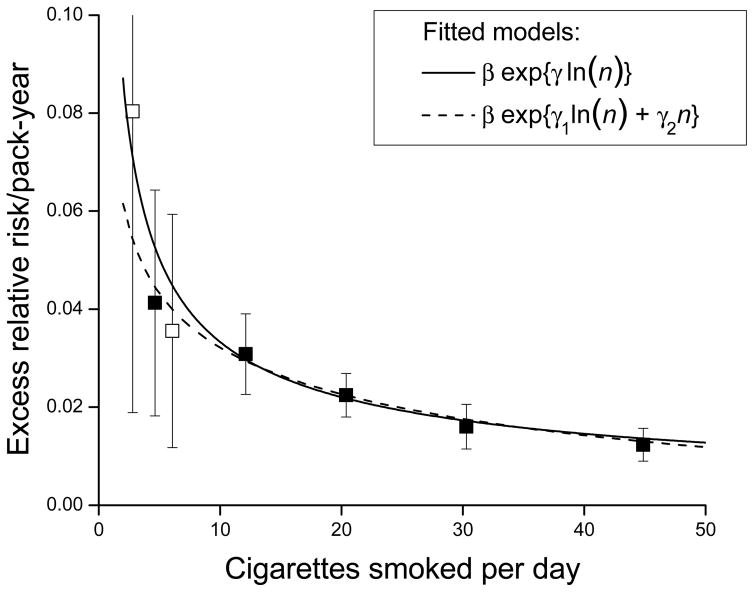
where β g(n) defined the linear slope at n cigarettes/day. We explored various forms for ln[g(.)] including restricted cubic splines and various parametric forms using n, n2, ln(n) and ln(n)2. The simple power function g(n) = exp{γ ln(n)} = nγ, with g(0) = 0, fitted the data well and resulted in the minimum Akaike Information Criterion 21, suggesting it was the preferred form. None of various parametric extensions, including g(n) = exp{γ1 ln(n) + γ2 n}, significantly improved fit.
We evaluated the interactions of several potential smoking-related effect modifiers by extending equation (3) for categories of a factor, e.g., years since smoking cessation. For categorical factor x with levels f = 1,…, F, we fitted
| (4) |
where βfdgf(n) replaced β d g(n). The difference in the deviances of models 3 and 4 provided a likelihood ratio test of no effect modification.
Adjustment variables (z) included study site (4 levels), sex, birth cohort (year of birth categories <1930, 1930–34, 1935–39, 1940–1945), race, BMI, years of schooling, gm-ethanol/week alcohol consumption (never and tertiles based on cases), high BP, diabetes mellitus and total cholesterol. We adjusted for attained age by including four continuous variables, age and its natural logarithm for males and for females. We included an indicator variable for never smokers who used cigars or pipes. For cigarette smokers, we did not define a cigarette-equivalence for cigar and pipe use due to data limitations and the potential for increased misclassification, but rather evaluated cigar or pipe use as an effect modifier.
Analyses used the Epicure software package 22.
The institutional review board of each participating university and the Office of Human Subjects Research Protections of the National Institutes of Health approved the study protocol, and all participants provided informed consent. All authors have no declared conflicts of interest.
Results
Marginal and adjusted relative risks for cigarette smoking variables
RRs with each smoking-related metric—cigarettes/day, duration of cigarette smoking, and pack-years—increased then leveled at higher categories (Table 1). However, a single smoking variable did not fully characterize risk, as model fit improved with inclusion of a second smoking variable (P<0.01). After adjustment for cigarettes/day or for duration, RRs by pack-years (representing 1–9 cigarettes/day or ≥50 years duration, footnoted columns c and d, respectively) continued to exhibit an increasing trend. After adjustment for pack-years, RRs decreased with cigarettes/day and, correspondingly, increased with duration of smoking, indicating a stronger pack-years association at lower cigarettes/day and longer durations.
TABLE 1.
Numbers of cardiovascular disease events, person-years (p-yrs) at risk, relative risks (RR) with 95% confidence intervals (CI) by pack-years of cigarette smoking and cigarettes smoked per day. Data from the Atherosclerosis Risk in Communities Study.
| Casesa | P-yrs | RRb | 95% CI | RRc | 95% CI | RRd | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Never smoker | 1,098 | 103,730.0 | 1.00e | 1.00 e | 1.00 e | |||
| Pack-years of smoking | ||||||||
| 1–19 | 646 | 50,957.8 | 1.25 | (1.1,1.4) | 1.29 | (1.1,1.5) | 1.68 | (1.4,2.0) |
| 20–29 | 350 | 20,341.4 | 1.62 | (1.4,1.8) | 1.75 | (1.4,2.1) | 1.91 | (1.6,2.3) |
| 30–39 | 364 | 19,716.4 | 1.78 | (1.6,2.0) | 1.98 | (1.6,2.4) | 2.02 | (1.7,2.4) |
| 40–49 | 350 | 15,627.8 | 1.94 | (1.7,2.2) | 2.21 | (1.8,2.7) | 2.03 | (1.7,2.5) |
| ≥50 | 583 | 21,628.8 | 2.14 | (1.9,2.4) | 2.73 | (2.2,3.4) | 2.24 | (1.9,2.6) |
| Pf | <0.01 | <0.01 | <0.01 | |||||
| Cigarettes/day | ||||||||
| 1–9 | 262 | 19,638.9 | 1.29 | (1.1,1.5) | 1.00g | |||
| 10–19 | 485 | 32,592.4 | 1.49 | (1.3,1.7) | 0.98 | (0.8,1.2) | ||
| 20–29 | 939 | 48,994.7 | 1.77 | (1.6,1.9) | 0.91 | (0.8,1.1) | ||
| 30–39 | 301 | 14,085.1 | 1.80 | (1.6,2.1) | 0.78 | (0.6,1.0) | ||
| ≥40 | 306 | 12,960.9 | 1.76 | (1.5,2.0) | 0.70 | (0.6,0.9) | ||
| Pf | <0.01 | <0.01 | ||||||
| Cigarette duration (yrs) | ||||||||
| 1–19 | 413 | 33,494.2 | 1.12 | (1.0,1.3) | 0.66 | (0.5,0.8) | ||
| 20–29 | 414 | 26,291.8 | 1.48 | (1.3,1.7) | 0.79 | (0.7,0.9) | ||
| 30–39 | 600 | 34,489.6 | 1.72 | (1.5,1.9) | 0.88 | (0.7,1.0) | ||
| 45–49 | 601 | 25,264.1 | 2.04 | (1.8,2.3) | 1.01 | (0.9,1.2) | ||
| ≥50 | 265 | 8,732.3 | 2.13 | (1.8,2.5) | 1.00g | |||
| Pf | <0.01 | <0.01 | ||||||
Numbers for cases reflect smoking status at cohort exit.
RRs for each smoking metric individually, adjusted for center, race, birth year, age, sex, education, alcohol consumption, high blood pressure, previous diabetes mellitus, total cholesterol, body mass index and use of cigars or pipe exclusively.
Model includes adjustment variables and main effects for both pack-years and cigarettes/day, with the RR for 1–9 cigarettes/day set to one for identifiability.
Model includes adjustment variables and main effects for both pack-years and duration of cigarette smoking, with the RR for ≥50 years set to one for identifiability.
Referent category of never smoked cigarettes with RR set to one.
P-value for score test of no linear trend.
Parameter set to one for identifiability.
Joint relative risks for pack-years and cigarettes/day
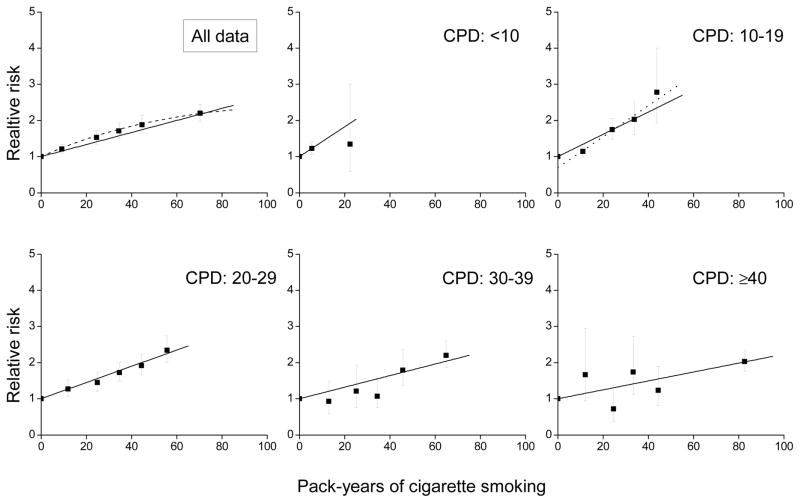
RRs increased with pack-years, but departed from linearity (P<0.01) (Figure 1, upper left panel, dash line). For the joint RRs, relative to never-smokers, RRs by pack-years increased within each cigarettes/day category (Figure 1 and eAppendix, eTable 1). Trends were consistent with linearity within each category, except the 10–19 cigarettes/day category (P=0.01), where the difference between never-smokers and the lowest pack-years category induced non-linearity (dot line, among smokers P=0.25 for the test of non-linearity). The figure highlights the variation in the slopes. The excess RR/pack-year estimates for the five cigarettes/day categories were 0.046, 0.031 (0.065 after adjustment for never/ever smoker), 0.024, 0.017 and 0.011, respectively, revealing a declining strength of association with increasing cigarettes/day (P<0.01 for the test of γ=0 in equation 3).
Figure 1.
Relative risks of cardiovascular disease for categories of pack-years of cigarette smoking (solid symbol) relative to never-smokers for all data and within categories of cigarettes/day (CPD) and fitted models, including: linear (solid line), linear-exponential (dash line) and linear adjusted for ever-smoked cigarettes (dot line). All results adjusted for age, birth year, sex and other factors (see text).
The plot of the slope estimates by mean cigarettes/day revealed a deceasing strength of association across the full range of cigarettes/day and were well-described by equation 3 (Figure 2). For <10 cigarettes/day smokers, there was substantial uncertainty in the excess RR/pack-year estimate, due to a limited range for pack-years (the 25th to 75th percentile interval was 2.6 to 9.6 pack-years). Splitting the category into 1–4 and 5–9 cigarettes/day resulted in excess RR/pack-year estimates of 0.097 and 0.038, respectively, indicting a continued strengthening of the association at lower smoking rates (Figure 2, open symbol). The non-linear excess RR/pack-year pattern with cigarettes/day represented an “inverse smoking rate effect”, suggesting that for equal pack-years, smoking fewer cigarettes/day for longer duration was more deleterious than smoking more cigarettes/day for shorter duration.
Figure 2.
Estimated excess relative risk/pack-year (ERR/PKY) for cardiovascular disease within categories of cigarettes/day (solid symbol), with the lowest category further divided into 1–4 and 5–9 cigarettes/day (open symbol), and fitted models for continuous pack-years and cigarettes/day. All results adjusted for age, birth year, sex and other factors (see text).
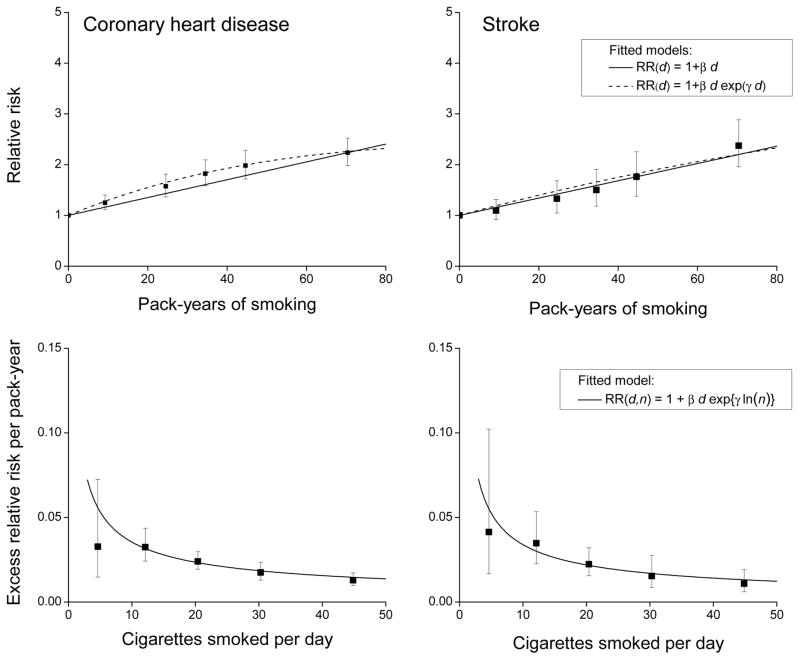
Smoking risks for coronary heart disease and stroke
There were 2,705 CHD and 1,011 stoke events. As in Table 1, RRs by pack-years (Figure 3, upper panels) and by cigarettes/day increased for CHD and for stroke (Table 2). The RRs by cigarettes/day appeared slightly larger for CHD, but homogeneity of the RRs by type of outcome was not rejected (P=0.15). With adjustment for cigarettes/day, RRs by pack-years increased for both outcomes. The inverse smoking rate pattern occurred for both CHD and stroke, although stroke appeared to exhibit a greater rate of decline. The plotted estimates of excess RR/pack-year by mean cigarettes/day and the fitted equation 3 (Figure 3, lower panels) revealed homogeneity of the inverse smoking rate effect by outcome (P=0.57).
Figure 3.
For coronary heart disease (left panels) and stroke (right panels), relative risks for categories of pack-years of cigarette smoking (solid symbol) relative to never-smokers with fitted linear (solid line) and linear-exponential (dash line) model (upper panels) and estimated excess relative risk/pack-year within categories of cigarettes/day (solid symbol) and fitted models for continuous pack-years and cigarettes/day (solid line) (lower panels). All results adjusted for age, birth year, sex and other factors (see text).
TABLE 2.
Numbers of events, relative risks (RR) with 95% confidence intervals (CI) by pack-years of cigarette smoking and cigarettes smoked per day for coronary heart disease and stroke. Data from the Atherosclerosis Risk in Communities Study.
| Coronary heart disease | Stroke | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Casesa | RRb | 95% CI | RRc | 95% CI | Cases | RRb | 95% CI | RRc | 95% CI | |
| Never smoker | 825 | 1.00d | 1.00d | 378 | 1.00d | 1.00d | ||||
| Pack-years of smoking | ||||||||||
| 1–19 | 513 | 1.30 | (1.2,1.5) | 1.28 | (1.1,1.5) | 186 | 1.13 | (0.9,1.4) | 1.28 | (1.0,1.6) |
| 20–29 | 282 | 1.67 | (1.5,1.9) | 1.68 | (1.3,2.1) | 95 | 1.47 | (1.2,1.9) | 1.95 | (1.4,2.8) |
| 30–39 | 304 | 1.89 | (1.6,2.2) | 1.93 | (1.5,2.4) | 96 | 1.60 | (1.3,2.0) | 2.38 | (1.6,3.5) |
| 40–49 | 298 | 2.06 | (1.8,2.4) | 2.16 | (1.7,2.7) | 95 | 1.89 | (1.5,2.4) | 3.05 | (2.1,4.5) |
| ≥50 | 483 | 2.21 | (1.9,2.5) | 2.54 | (2.0,3.2) | 161 | 2.17 | (1.8,2.7) | 4.48 | (3.0,6.7) |
| P-valuee | <0.01 | <0.01 | <0.01 | <0.01 | ||||||
| Cigarettes/day | ||||||||||
| 1–9 | 190 | 1.28 | (1.1,1.5) | 1.00 | 96 | 1.28 | (1.0,1.6) | 1.00 | ||
| 10–19 | 382 | 1.55 | (1.4,1.8) | 1.04 | (0.9,1.3) | 153 | 1.45 | (1.2,1.8) | 0.87 | (0.7,1.2) |
| 20–29 | 779 | 1.85 | (1.7,2.1) | 0.99 | (0.8,1.2) | 250 | 1.64 | (1.4,2.0) | 0.65 | (0.5,0.9) |
| 30–39 | 265 | 1.95 | (1.7,2.3) | 0.89 | (0.7,1.1) | 65 | 1.46 | (1.1,1.9) | 0.43 | (0.3,0.7) |
| ≥40 | 264 | 1.84 | (1.6,2.1) | 0.78 | (0.6,1.0) | 69 | 1.52 | (1.2,2.0) | 0.39 | (0.3,0.6) |
| P-valuee | <0.01 | 0.01 | <0.01 | <0.01 | ||||||
Numbers for cases reflect smoking status at cohort exit.
RRs for pack-years and RRs for cigarettes/day adjusted for center, race, birth year, age, sex, education, alcohol consumption, high blood pressure, diabetes mellitus, total cholesterol, body mass index and use of cigars or pipe exclusively.
Model for the RR includes adjustment variables and main effects for both pack-years and cigarettes/day, with the RR for 1–9 cigarettes/day set to one for identifiability.
Referent category of never smoked cigarettes with RR set to one.
P-value for score test of no linear trend.
Effect modification by smoking-related factors
For age started smoking, depth of inhalation, and additional use of cigars/pipes, the estimated RR by pack-years increased within each category of these variables (Table 3). The RR trends with pack-years were roughly similar within each level. As in Figure 2, the excess RR/pack-year estimates declined smoothly with cigarettes/day within each level of age started smoking, inhalation and use of cigars/pipes (aAppendix eFigures 1–3, with eTable 2 providing parameter estimates). The fitted excess RR/pack-year estimates at 20 cigarettes/day from equation 4 were similar across levels, and hypothesis tests did not reject homogeneous by age started smoking (P=0.50), method of inhalation (P=0.74) or additional use of cigars/pipes (P=0.79) (Table 3).
TABLE 3.
Estimated relative risks (RR) for cardiovascular disease by pack-years with never cigarette smokers as referent and the fitted excess relative risk per pack-year (ERR/PKY) at 20 cigarettes/day (CPD) within level of smoking-related modifiersa. Data from the Atherosclerosis Risk in Communities Study.
| Summary of fitted model | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimated RRs by pack-years | ERR/PKY @ 20 CPDb | ||||||||
| Modifier | Cases | 1–19 | 20–29 | 30–39 | 40–49 | ≥50 | Estimate | 95% CI | Pc |
| Age started smoking | |||||||||
| <16 | 609 | 1.12 | 2.12 | 1.89 | 1.93 | 2.28 | 0.025 | (0.020,0.031) | 0.69 |
| 16–17 | 544 | 1.35 | 1.61 | 1.76 | 2.04 | 2.14 | 0.023 | (0.018,0.029) | |
| 18–19 | 502 | 1.27 | 1.26 | 1.68 | 1.95 | 1.98 | 0.020 | (0.015,0.026) | |
| ≥20 | 638 | 1.23 | 1.66 | 1.82 | 1.87 | 2.03 | 0.023 | (0.018,0.029) | |
| Inhalation | |||||||||
| Never/slightly | 559 | 1.28 | 1.54 | 1.90 | 1.88 | 2.05 | 0.021 | (0.016,0.028) | 0.68 |
| Moderately | 1,003 | 1.22 | 1.71 | 1.72 | 1.93 | 2.13 | 0.022 | (0.018,0.027) | |
| Deeply | 731 | 1.25 | 1.55 | 1.80 | 1.99 | 2.18 | 0.026 | (0.021,0.032) | |
| Smoking cessation (years) | |||||||||
| <1 | 715 | 1.48 | 1.84 | 2.21 | 2.11 | 2.10 | 0.023 | (0.018,0.028) | <0.01 |
| 1–4 | 302 | 1.82 | 2.18 | 2.50 | 2.37 | 3.42 | 0.040 | (0.032,0.049) | |
| 5–9 | 207 | 1.29 | 1.43 | 1.42 | 1.67 | 1.94 | 0.016 | (0.010,0.024) | |
| ≥10 | 1,069 | 1.16 | 1.48 | 1.51 | 1.67 | 1.70 | 0.017 | (0.013,0.023) | |
| Use of cigars/pipesd | |||||||||
| No | 1,742 | 1.20 | 1.66 | 1.80 | 2.00 | 2.15 | 0.023 | (0.019–0.027) | 0.97 |
| Yes | 551 | 1.43 | 1.54 | 1.75 | 1.79 | 2.14 | 0.022 | (0.017–0.030) | |
All models adjusted for center, race, birth year, age, sex, education, alcohol consumption, high blood pressure, diabetes mellitus, total cholesterol, body mass index and use of cigars or pipe exclusively. RRs computed relative to 1,098 never smokers.
For continuous pack-years (d) and cigarettes/day (n) with categorical modifying factor x with F levels, data fitted using: ERR(d, n, X) = Σf βf df exp{γf ln(nf)}, with ERR/PKY estimate at 20 cigarettes/day given as ERR(d=1, n=20, X=xf) = βf exp{γf ln(20)}. For all data without modification, the ERR/PKY at 20 cigarettes/day was 0.023 (0.020,0.028). See Supplemental Material Table S2 for parameter estimates.
P-value for test of homogeneity of smoking effects across factor f, i.e., β1=…=βF and γ1=…= γF,.
Cigar/pipe use in addition to cigarettes.
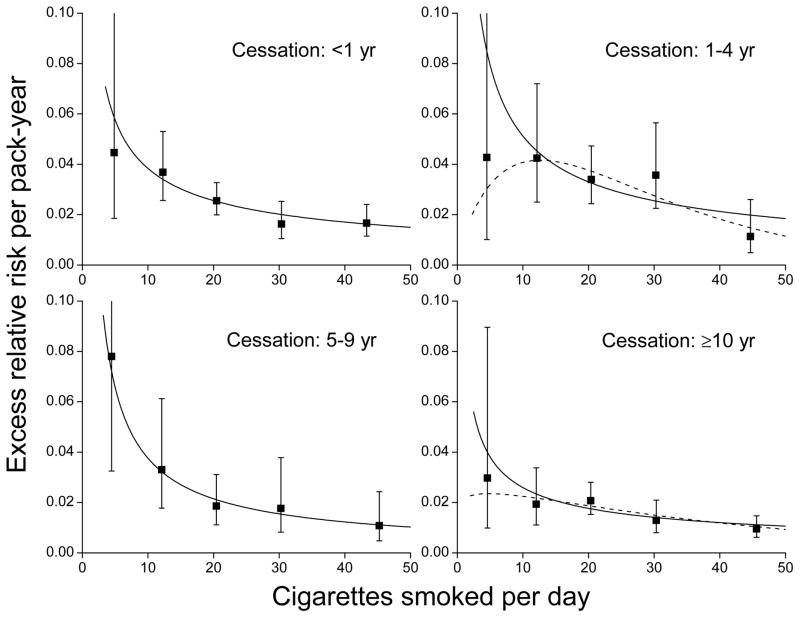
In contrast, RR trends with pack-years significantly diminished with years since cessation of smoking (P<0.01) (Figure 4, left panels). The fitted excess RR/pack-year estimates at 20 cigarettes/day declined with cessation starting five years and more after cessation (P<0.01) (Table 3). Although pack-years-related risks declined with cessation, the inverse smoking rate patterns for each cessation category were similar (Figure 4, right panels).
Figure 4.
For years since cessation of smoking, relative risks for cardiovascular disease by categories of pack-years of cigarette smoking (solid symbol) relative to never-smokers with fitted linear (solid line) and linear-exponential (dash line) models (left panels) and estimated excess relative risk/pack-year within categories of cigarettes/day (solid symbol) and fitted models for continuous pack-years and cigarettes/day (solid line) (right panels). All results adjusted for age, birth year, sex and other factors (see text).
Smoking and CVD under a competing risks model
For the competing risks analysis, follow-up time accrued through 2006. We omitted 803 participants with a pre-enrollment diagnosis of cancer, leaving 13,324 participants, with 200,347 person-years and 4,945 total events, including 2,638 CVD cases, 350 lung cancers, 684 other smoking-related cancers (including 112 bladder, 280 colon and rectum and 114 stomach cancers) and 1,273 deaths from other causes (including 401 other cancers, 363 diseases of the circulatory system and 147 diseases of the respiratory system). The eAppendix presents detailed results. Competing risks adjustment had minimal impact on results. The decreasing smoking rate patterns, i.e., estimates of γ in equation 3, with 95% CI were −0.70 (−0.87, −0.52) for the full data analysis in Figure 3, −0.71 (−0.90, −0.53) for the restricted follow-up through 2006 and −0.75 (−0.96, −0.55) in the competing risks analysis, while the estimated RRs for 50 pack-years accrued at a rate of 20 cigarettes/day were 2.1 (2.0, 2.4), 2.2 (1.2, 2.5) and 2.1 (1.9, 2.4), respectively.
Discussion
In our data, RRs for CVD increased for each smoking metric: cigarettes/day, smoking duration, and pack-years. In addition, there was a concave pattern for the RRs by cigarettes/day, indicating that the RR per cigarette/day decreased with greater cigarettes/day 1–3. However, any interpretation must acknowledge that cigarettes/day represents an exposure rate metric and not a quantitative measure of exposure to cigarette smoke, and that no single metric, cigarettes/day, smoking duration, or pack-years, fully characterizes smoking-related risks. Using joint RRs for pack-years and cigarettes/day, the concave RR pattern with cigarettes/day was consistent with cigarettes/day modifying a linear RR association for CVD and pack-years, i.e., the strength of association declined with smoking rate. Our analyses reaffirmed pack-years as the preeminent smoking-related risk factor for CVD, but also demonstrated that the manner of accrual of pack-years influenced CVD risk levels. There was an inverse smoking rate pattern, whereby smoking fewer cigarettes/day for longer duration was more deleterious than smoking more cigarettes/day for shorter duration. For 50 pack-years (365,000 cigarettes), the estimated RR of CVD was 2.1 if exposure accrued at 20 cigarettes/day and 1.6 if exposure accrued at 50 cigarettes/day. This inverse rate pattern was quantitatively comparable for CHD and stroke.
The observed inverse rate pattern may be due to smoking rate-dependent pathophysiologic mechanisms of CVD risks. Several reviews have described possible mechanisms for smoking-related CVD, and in particular the non-linear RRs for cigarettes/day 1–3,5–9. Factors that link cigarette smoking to CVD which may contribute to the non-linear cigarette/day association include nicotine stimulation with enhanced oxygen demand and vasoconstriction, carbon monoxide (CO) induced hemodynamic effects, increased inflammation arising from reduced anti-oxidant compounds, particulates and other constituents of tobacco smoke, insulin sensitivity, and alterations in lipid profiles 4,8,9. For example, CO, a combustion product of cigarette smoke, has an affinity for hemoglobin and exhibits smoking rate-dependent effects. Among current smokers, the ratio of serum carboxyhemoglobin (COHb) to cigarettes/day decreased with greater smoking intensity 23. Polycyclic aromatic hydrocarbons (PAHs), including benzo[a]pyrene, result from incomplete combustion of tobacco and other organic products, and are associated with increased CVD risk 9. PAH exposure can activate the aryl hydrocarbon receptor (AhR) pathway and thereby induce a vascular inflammatory response, including the progression of atherosclerosis 9,24–26. Investigators reported that DNA adduct levels per unit PAH exposure were higher in environmentally exposed individuals than in workers exposed at occupational levels 27,28.
Cigarette smoking may also influence risk through intensity-dependent variations in its impact on non-tobacco CVD risk factors, although evidence for this is circumstantial. Law and Wald suggested that inflammation-induced platelet aggregation dominates at low smoking intensities while other mechanisms—e.g., increased fibrinogen, reduced high-density lipoprotein cholesterol (HDL), and increased COHb—dominate at higher intensities 3.
Exposure bias may also have contributed to the inverse smoking rate pattern, whereby heavy smokers inhaled less vigorously following nicotine satiation, resulting in “reduced potency”, with the reported cigarettes/day increasingly overestimating internal exposure. Using cotinine as a biomarker of smoking rate, studies have reported that cotinine levels increased approximately linearly through about 15–20 cigarettes/day 23,29–38. However, trends at higher smoking rates have been complex. In some studies, cotinine concentrations increased with cigarettes/day, then leveled and even declined 29,30,32,37. Other studies have reported trends that continued to increase without substantial diminution 30,33,35,36,39,40 or with only modest diminution 23,29–31,34,37. In our data, the inverse smoking rate pattern occurred across the full range of cigarettes/day, and consequently was incompatible with any presumed inhalation bias. A sensitivity analysis in conjunction with a smoking and lung cancer study used urinary cotinine to adjust cigarettes/day and found that the cotinine-adjusted estimates of excess RR/pack-year within smoking rate categories indeed increased, since reported cigarettes/day reflected an overestimate. Nonetheless, the shape of the inverse smoking rate effect was unaffected, since the cotinine-adjusted cigarettes/day at higher rates correspondingly represented lower adjusted rates 41, again suggesting inhalation bias did not greatly influence our results.
Competing risks may have impacted our observed inverse cigarettes/day effects, whereby those who accrued longer follow-up or were lighter smokers may have been more likely to have incurred CVD events, while heavier smokers were selectively removed from follow-up due to other diseases, in particular lung cancer. We conducted competing risks analyses that incorporated incident CVD, lung cancer, other selected smoking-related cancers and mortality from all other causes 19,20. However, we found that consideration of competing risks had no appreciable effect on the results which suggested that any potential bias from competing risk considerations was minimal. This absence of impact was likely due to the relatively small numbers of cancer events, particularly lung cancer, compared to CVD events and to the relatively small number of current smokers (27.4% at enrollment), who had the highest smoking-related risk.
Our results must be interpreted with caution. This analysis is the first to evaluate the effects of exposure accrual, comparing the relative impact on the strength of the association of pack-years and CVD for smoking fewer cigarettes/day for longer duration with more cigarettes/day for shorter duration. At low smoking rates, there was substantial uncertainty due to the limited range of pack-years, and additional analyses are needed to determine whether the inverse smoking rate pattern continues for even lower rate smokers, as in our data, or flattens or decreases. Our analyses did not additionally adjust for exposure to environmental tobacco smoke, either in never-smokers or in smokers, and this may have underestimated smoking risks. In our data, the RR in never-smokers for exposure to any environmental tobacco smoke was of modest magnitude (RR=1.1 with 95% CI 1.0 to 1.3), and additional adjustment for any environmental tobacco smoke exposure had minimal impact on the smoking rate patterns.
Finally, inference for the inverse smoking rate effect arose from the characterization of pack-year trends within categories of cigarettes/day. A comparable analysis could have evolved from the joint RRs of pack-years and duration of smoking, and indeed the RR trends for pack-years increased in strength with increasing duration of smoking. However, this approach was more complex because trends in RRs with pack-years within categories of duration deviated from simple linear relationships and therefore there was no set of linear slope estimates that fully characterize smoking-related RRs.
In summary, the ARIC data confirmed the deleterious consequences of cigarette smoking on CVD risk. While pack-years represented the principal determinant of smoking-related CVD risk, results demonstrated that the manner of exposure accrual had consequences for smoking-related risks. Across the full range of cigarettes/day, smoking fewer cigarettes/day for longer durations was more deleterious than smoking more cigarettes/day for shorter durations. The precise reasons for this inverse smoking intensity pattern remain uncertain.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the Atherosclerosis Risk In Communities (ARIC) study for their important contributions.
Funding: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Dr. Lubin was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics. The authors maintained full control over the management, analysis and interpretation of the data; the preparation, review, and approval of the manuscript; and were independent of funders.
Footnotes
Data sharing: The authors do not have permission to share the data used in this project, which were sourced from the ARIC Coordinating Center (https://www2.cscc.unc.edu/aric/).
Reference List
- 1.Benowitz NL. Cigarette smoking and cardiovascular disease: Pathophysiology and implications for treatment. Prog Cardiovasc Dis. 2003;46(1):91–111. doi: 10.1016/s0033-0620(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 2.Burns DM. Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis. 2003;46(1):11–29. doi: 10.1016/s0033-0620(03)00079-3. [DOI] [PubMed] [Google Scholar]
- 3.Law MR, Wald NJ. Environmental tobacco smoke and ischemic heart disease. Prog Cardiovasc Dis. 2003;46(1):31–38. doi: 10.1016/s0033-0620(03)00078-1. [DOI] [PubMed] [Google Scholar]
- 4.Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P. Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine Tob Res. 2003;5(5):621–624. doi: 10.1080/1462220031000158717. [DOI] [PubMed] [Google Scholar]
- 5.Csordas A, Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol. 2013;10(4):219–230. doi: 10.1038/nrcardio.2013.8. [DOI] [PubMed] [Google Scholar]
- 6.Pant R, Marok R, Klein LW. Pathophysiology of coronary vascular remodeling: relationship with traditional risk factors for coronary artery disease. Cardiol Rev. 2014;22(1):13–16. doi: 10.1097/CRD.0b013e31829dea90. [DOI] [PubMed] [Google Scholar]
- 7.Ambrose JA, Barua RS. The pathophysiology of cigarette C-V smoking and cardiovascular disease - An update. J Am Coll Cardiol. 2004;43(10):1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of Health, Human S. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Washington D.C. 20402: U. S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Superintendent of Documents, U.S. Government Printing Office; 2010. [PubMed] [Google Scholar]
- 9.U.S. Department of Health, Human S. The Health Consequences of Smoking -- 50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Superintendent of Documents, U.S. Government Printing Office; 2014. [Google Scholar]
- 10.Dietrich T, Hoffmann K. A comprehensive index for the modeling of smoking history in periodontal research. J Dental Res. 2004;83(11):859–863. doi: 10.1177/154405910408301107. [DOI] [PubMed] [Google Scholar]
- 11.Leffondre K, Abrahamowicz M, Xiao Y, Siemiatycki J. Modelling smoking history using a comprehensive smoking index: Application to lung cancer. Stat Med. 2006;25(24):4132–4146. doi: 10.1002/sim.2680. [DOI] [PubMed] [Google Scholar]
- 12.Peto J. That effects of smoking should be measured in pack-years: misconceptions 4. Brit J Cancer. 2012;107(3):406–407. doi: 10.1038/bjc.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lubin J, Caporaso N. Misunderstandings in the misconception on the use of pack-years in analysis of smoking. Brit J Cancer. 2013;108(5):1218–1220. doi: 10.1038/bjc.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas DC. Invited Commentary: Is It Time to Retire the “Pack-Years” Variable? Maybe Not! Am J Epidemiol. 2014;179(3):299–302. doi: 10.1093/aje/kwt274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aric Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 16.Rosamond WD, Chambless LE, Heiss G, et al. Twenty-Two-Year Trends in Incidence of Myocardial Infarction, Coronary Heart Disease Mortality, and Case Fatality in 4 US Communities, 1987–2008. Circulation. 2012;125(15):1848–1857. doi: 10.1161/CIRCULATIONAHA.111.047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the atherosclerosis risk in communities (ARIC) study: Methods and initial two years’ experience. J Clin Epidemiol. 1996;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 18.Huxley RR, Yatsuya H, Lutsey PL, Woodward M, Alonso A, Folsom AR. Impact of age at smoking initiation, dosage, and time since quitting on cardiovascular disease in African Americans and Whites. Am J Epidemiol. 2012;175(8):816–826. doi: 10.1093/aje/kwr391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lunn M, McNeil N. Applying Cox Regression to Competing Risks. Biometrics. 1995;51(2):524–532. [PubMed] [Google Scholar]
- 20.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. Wiley; 2015. [Google Scholar]
- 21.Akaike H. A new look at the statistical model identification. IEEE Trans Automatic Control. 1974;19:716–723. [Google Scholar]
- 22.Preston DL, Lubin JH, Pierce DA, McConney ME. Epicure User’s Guide. Seattle, Washington, USA: HiroSoft International Corporation; 2006. [Google Scholar]
- 23.Law MR, Morris JK, Watt HC, Wald NJ. The dose-response relationship between cigarette consumption, biochemical markers and risk of lung cancer. Br J Cancer. 1997;75(11):1690–1693. doi: 10.1038/bjc.1997.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu D, Nishimura N, Kuo V, et al. Activation of Aryl Hydrocarbon Receptor Induces Vascular Inflammation and Promotes Atherosclerosis in Apolipoprotein E−/− Mice. Arterioscler Thromb. 2011;31(6):1260–1237. doi: 10.1161/ATVBAHA.110.220202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewtas J. Air pollution combustion emissions: Characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mutat Res-Rev in Mutat Res. 2007;636(1–3):95–133. doi: 10.1016/j.mrrev.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Alshaarawy O, Zhu M, Ducatman A, Conway B, Andrew ME. Polycyclic aromatic hydrocarbon biomarkers and serum markers of inflammation. A positive association that is more evident in men. Environ Res. 2013;126:98–104. doi: 10.1016/j.envres.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips DH. Smoking-related DNA and protein adducts in human tissues. Carcinog. 2002;23(12):1979–2004. doi: 10.1093/carcin/23.12.1979. [DOI] [PubMed] [Google Scholar]
- 28.Lewtas J, Walsh D, Williams R, Dobias L. Air pollution exposure DNA adduct dosimetry in humans and rodents: evidence for non-linearity at high doses. Mutat Res-Fundam Mol Mech Mutagen. 1997;378(1–2):51–63. doi: 10.1016/s0027-5107(97)00097-3. [DOI] [PubMed] [Google Scholar]
- 29.Joseph AM, Hecht SS, Murphy SE, et al. Relationships between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2963–2968. doi: 10.1158/1055-9965.EPI-04-0768. [DOI] [PubMed] [Google Scholar]
- 30.Blackford AL, Yang G, Hernandez-Avila M, et al. Cotinine concentration in smokers from different countries: relationship with amount smoked and cigarette type. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1799–1804. doi: 10.1158/1055-9965.EPI-06-0427. [DOI] [PubMed] [Google Scholar]
- 31.Campuzano JC, Hernandez-Avila M, Jaakkola MS, et al. Determinants of salivary cotinine levels among current smokers in Mexico. Nicotine Tob Res. 2004;6(6):997–1008. doi: 10.1080/14622200412331324956. [DOI] [PubMed] [Google Scholar]
- 32.Etter JF, Perneger TV. Measurement of self reported active exposure to cigarette smoke. J Epidemiol Community Health. 2001;55(9):674–680. doi: 10.1136/jech.55.9.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis SJ, Cherry NM, Niven RM, Barber PV, Wilde K, Povey AC. Cotinine levels and self-reported smoking status in patients attending a bronchoscopy clinic. Biomarkers. 2003;8(3–4):218–228. doi: 10.1080/1354750031000120125. [DOI] [PubMed] [Google Scholar]
- 34.O’Connor RJ, Giovino GA, Kozlowski LT, et al. Changes in nicotine intake and cigarette use over time in two nationally representative cross-sectional samples of smokers. Am J Epidemiol. 2006;164(8):750–759. doi: 10.1093/aje/kwj263. [DOI] [PubMed] [Google Scholar]
- 35.Olivieri M, Poli A, Zuccaro P, et al. Tobacco smoke exposure and serum cotinine in a random sample of adults living in Verona, Italy. Arch Environ Health. 2002;57(4):355–359. doi: 10.1080/00039890209601421. [DOI] [PubMed] [Google Scholar]
- 36.Richie JP, Carmella SG, Muscat JE, Scott DG, Akerkar SA, Hecht SS. Differences in the urinary metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in black and white smokers. Cancer Epidemiol Biomarkers Prev. 1997;6(10):783–790. [PubMed] [Google Scholar]
- 37.Lubin JH, Caporaso N, Hatsukami DK, Joseph AM, Hecht SS. The association of a tobacco-specific biomarker and cigarette consumption and its dependence on host characteristics. Cancer Epidemiol Biomarkers Prev. 2007;16:1852–1857. doi: 10.1158/1055-9965.EPI-07-0018. [DOI] [PubMed] [Google Scholar]
- 38.Woodward M, Tunstallpedoe H, Smith WCS, Tavendale R. Smoking Characteristics and Inhalation Biochemistry in the Scottish Population. J Clin Epidemiol. 1991;44(12):1405–1410. doi: 10.1016/0895-4356(91)90101-e. [DOI] [PubMed] [Google Scholar]
- 39.Xia Y, Bernert JT, Jain RB, Ashley DL, Pirkle JL. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in smokers in the United States: NHANES 2007–2008. Biomarkers. 2011;16(2):112–119. doi: 10.3109/1354750X.2010.533288. [DOI] [PubMed] [Google Scholar]
- 40.Nagano T, Shimizu M, Kiyotani K, et al. Biomonitoring of Urinary Cotinine Concentrations Associated with Plasma Levels of Nicotine Metabolites after Daily Cigarette Smoking in a Male Japanese Population. Int J Environ Res Pub Health. 2010;7(7):2953–2964. doi: 10.3390/ijerph7072953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lubin JH, Caporaso N, Wichmann HE, Schaffrath-Rosario A, Alavanja MCR. Cigarette smoking and lung cancer: modeling effect modification of total exposure and intensity. Epidemiol. 2007;18:639–648. doi: 10.1097/EDE.0b013e31812717fe. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.