Abstract
G-protein coupled receptors (GPCRs) play critical roles in regulating brain function. Recent advances have greatly expanded our understanding of these receptors as complex signaling machines that can adopt numerous conformations and modulate multiple downstream signaling pathways. While agonists and antagonists have traditionally been pursued to target GPCRs, allosteric modulators provide several mechanistic advantages including the ability to distinguish between closely related receptor subtypes. Recently, the discovery of allosteric ligands that confer bias and modulate some, but not all, of a given receptor's downstream signaling pathways can provide pharmacological modulation of brain circuitry with remarkable precision. In addition, allosteric modulators with unprecedented specificity have been developed that can differentiate between subpopulations of a given receptor subtype based on the receptor's dimerization state. These advances are not only providing insight into the biological roles of specific receptor populations, but hold great promise for treating numerous CNS disorders.
All major functions of the central nervous system require rapid and precise communication between neurons that are organized in circuits that range from simple di-synaptic feedback loops to large networks of interconnected brain nuclei that regulate complex CNS functions. These networks are driven by activation of excitatory and inhibitory ligand-gated ion channels and can be modulated by G protein-coupled receptors (GPCRs; also known as 7 transmembrane spanning receptors). GPCRs represent a large family of receptors that regulate multiple intracellular signaling pathways either through the activation of various G-proteins, or through interactions with β-arrestin and other regulatory proteins (Kristiansen, 2004). GPCRs are critical for fine-tuning transmission through CNS networks and modulate several key neuronal functions such as neurotransmitter release, neuronal excitability, action potential firing patterns, and gene transcription. Given their central role in regulating CNS function, it is not surprising that GPCRs have been among the most successful targets for developing drugs for treatment of CNS disorders (Wise et al., 2002). However, ligands that selectively activate a single receptor subtype exist for only a small fraction of GPCRs and it has been difficult to develop highly selective ligands for most GPCR subtypes. Historically, efforts to develop ligands that target GPCRs have focused on agonists and antagonists that interact with the orthosteric neurotransmitter binding site to either mimic or block the action of the endogenous neurotransmitter. While this strategy has been fruitful, the high conservation of orthosteric binding sites across related receptors can make development of subtype-selective orthosteric ligands challenging.
In recent years, allosteric modulators of GPCRs have emerged as a promising new approach for developing highly selective ligands and potential therapeutic agents for treatment of CNS disorders. Allosteric modulators of GPCRs bind to sites that are often less highly conserved than orthosteric sites (Conn et al., 2009a) and this has allowed optimization of highly selective allosteric modulators of some GPCR subtypes that have been intractable using traditional approaches. Allosteric modulators bind to sites that are topographically distinct from the orthosteric neurotransmitter binding site and can alter receptor signaling (See Table 1). Positive allosteric modulators (PAMs) increase responses to the orthosteric agonist, whereas negative allosteric modulators (NAMs) inhibit responses to orthosteric agonists. PAMs and NAMs often act by modulating the affinity of an orthosteric ligand. Thus, a GPCR PAM can increase the affinity of the endogenous neurotransmitter whereas a NAM may reduce the affinity. However, allosteric modulators can also modulate GPCR coupling to downstream signaling mechanisms independent of the affinity of the orthosteric agonist (Wootten et al., 2013). Major advances have improved our understanding of how allosteric modulators interact with receptors, driven by crystal structures of receptors bound to allosteric modulators (Dore et al., 2014; Kruse et al., 2013; Oswald et al., 2016; Thal et al., 2016; Wu et al., 2014). These studies not only provide detailed information on the molecular interactions between allosteric modulators and their binding sites, but also provide invaluable information on how conformational changes at allosteric sites mechanistically alter orthosteric signaling (Staus et al., 2016). The effects of allosteric ligands on receptor function have been outlined in detail in multiple excellent reviews and can be described and quantified by equations including an “operational model of allosterism” that describes both allosteric modulation of affinity and efficacy (Gregory et al., 2010; May et al., 2007) that has been highly useful in quantifying different aspects of allosteric modulator function.
Table 1. Mechanisms of action observed by allosteric modulators.
| Orthosteric Agonist | Binding of orthosteric agonists (which can be either endogenous neurotransmitters or synthetic agonists) to the neurotransmitter binding site induces a change in receptor confirmation and stabilizes an active state of the receptor. |
| Positive Allosteric Modulator (PAM) | PAMs bind to an allosteric site on the receptor that is distinct from the orthosteric binding pocket and increases the potency and/or efficacy of orthosteric agonists resulting in enhanced receptor activation when an orthosteric agonist is present. |
| Ago-PAM | Binding of Ago-PAMs alone is sufficient to induce receptor activation. In addition, these compounds increase the potency and/or efficacy of orthosteric agonists. |
| Orthosteric Antagonist | Binding of orthosteric antagonists have no effect on receptor activity. These compounds prevent activation of the receptor by preventing neurotransmitter binding. |
| Negative allosteric modulator (NAM) | NAMs bind to an allosteric site distinct from the orthosteric or neurotransmitter binding site and inhibit receptor activation via negative cooperativity to reduce the affinity and/or efficacy of orthosteric agonists. |
| Partial NAM | Partial NAMs do not completely block receptor activation, often due to weak negative cooperativity with respect to agonist binding. Accordingly, even when the allosteric binding pocket is fully occupied, partial NAMs will only partially reduce agonist responses. |
The unique characteristics of allosteric modulators make this mode of GPCR regulation extremely attractive for developing agents with which to interrogate the physiological roles of individual GPCR subtypes and for developing novel treatment strategies for CNS disorders. Allosteric modulators of GPCRs are now being pursued as potential drug candidates for Alzheimer's disease, dystonia, Parkinson's disease, schizophrenia, and other brain disorders (for reviews see Conn et al., 2009a; Kruse et al., 2014). In addition to providing potential new treatment strategies, these compounds are helping drive fundamental insights into the roles of various receptors and specific signaling pathways in modulating identified brain circuits and animal behavior under both physiological and pathological conditions.
Potential antipsychotic and cognition-enhancing effects of mGlu5 receptor PAMs
In recent years, the metabotropic glutamate (mGlu) receptor subtype 5 (mGlu5) has emerged as an exciting new target for the treatment of schizophrenia and improving cognitive function in multiple brain disorders (Nickols and Conn, 2014; Nicoletti et al., 2015). Multiple mGlu5 PAMs have robust efficacy in rodent models used to predict antipsychotic efficacy and the treatment of cognitive disturbances (Conn et al., 2009b; Liu et al., 2008; Parmentier-Batteur et al., 2014; Rook et al., 2013). Interestingly, mGlu5 PAMs enhance induction of both long-term potentiation (LTP) and long-term depression (LTD) at excitatory synapses in the hippocampus and other brain regions (Ayala et al., 2009; Rook et al., 2015; Sarihi et al., 2008; Sun et al., 2016) and can restore deficits in animal models in which synaptic plasticity and cognitive function are impaired (Bhardwaj et al., 2015; Lin et al., 2014; Waung and Huber, 2009; Won et al., 2012).
In addition to potential symptomatic effects, early studies raise the possibility that mGlu5 PAMs could reduce developmental changes that underlie specific deficits in schizophrenia. Deletion of mGlu5 from parvalbumin expressing neurons is sufficient to induce cognitive and sensorimotor gating deficits in rodents (Barnes et al., 2015), and administration of mGlu5 PAMs in adolescence prevented the appearance of delayed cognitive deficits in a developmental model of schizophrenia (Clifton et al., 2013). These findings suggest the exciting possibility of a preventative role for mGlu5 PAM treatment in the development of schizophrenia, and further work will be important to evaluate these findings.
Maintenance of activity-dependence may be important for efficacy of mGlu5 PAMs
An important attribute of pure PAMs is that these compounds have no intrinsic efficacy, but act to enhance activation of the receptor by the endogenous agonist, a property that fundamentally differentiates PAMs from traditional agonists. Recent studies suggest that the ability of mGlu5 PAMs to maintain the spatial and temporal patterning of receptor activation (Figure 1), provides these compounds with the unique ability to enhance both LTP and LTD without altering the afferent activity patterns required for induction of these two forms of synaptic plasticity (Ayala et al., 2009). The strict dependence of LTP and LTD on defined patterns of afferent activity is important for appropriately regulating different domains of cognitive function (see Mockett and Hulme, 2008 for review). The unique ability of mGlu5 PAMs to maintain appropriate activity-dependent plasticity provides an excellent demonstration of the advantages afforded by strictly potentiating responses to endogenous neurotransmitter release compared to use of an agonist. Consistent with the effects of mGlu5 PAMs on synaptic plasticity, systemic administration of selective mGlu5 PAMs improves performance in a broad range of animal models of cognitive function that are dependent on intact function of the hippocampus or prefrontal cortex (PFC) and are disrupted in schizophrenia patients (Bhardwaj et al., 2015; Gilmour et al., 2013; Homayoun et al., 2004; Horio et al., 2013; Moghaddam, 2004; Uslaner et al., 2009). Thus, mGlu5 PAMs have the potential to improve both positive symptoms and cognitive disturbances in schizophrenia patients.
Figure 1. Positive allosteric modulators modulate receptor activity while maintaining spatial and temporal dynamics associated with neurotransmitter release.
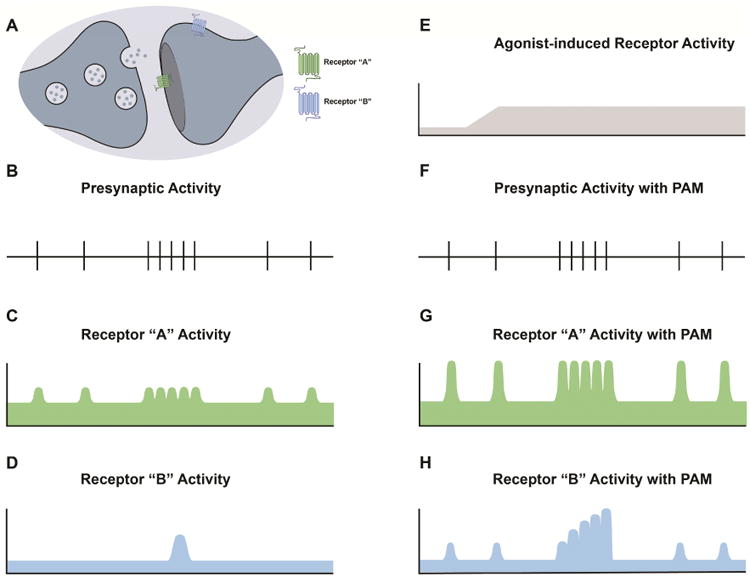
Communication between neurons is commonly encoded by neurotransmitter release events emanating from presynaptic terminals resulting in postsynaptic receptor activation (A). Presynaptic activity patterns (B), and the proximity of a receptor from a neurotransmitter release site, both play a key role in determining postsynaptic receptor activity patterns. In receptor populations that are present in the synaptic cleft, the site of neurotransmitter release is sufficiently proximal to the receptor such that each release event may induce receptor activation (C). Receptor populations that are expressed in extrasynaptic or perisynaptic areas, which are further removed from neurotransmitter release sites, may not be exposed to sufficient neurotransmitter levels following a single release event to become active. However these receptors may be activated following bursts of high-frequency activity when neurotransmitter levels are sufficiently elevated to spill out from the synapse (D). Exogenously applied agonists activate receptors with a temporal profile (E) that is very different from presynaptic activity patterns (B) and will activate receptors regardless of their proximity to presynaptic inputs. In the simplest case, positive allosteric modulators (PAMs) of post-synaptic receptors do not affect presynaptic firing rates (F), but potentiate responses to the endogenous neurotransmitter while maintaining temporally and spatially coded information with respect to receptor activity patterns (G and H). This activity-dependence of allosteric modulators can avoid detrimental effects due to excessive receptor activation and preserve complex physiology such as spike-timing dependent plasticity.
Minimizing Ago-PAM activity reduces the side-effect liability of mGlu5 PAMs
The combined effects of mGlu5 PAMs in preclinical models raise the possibility that these compounds could provide a fundamental advance in treatment of schizophrenia and other disorders that impair cognitive function. However, some mGlu5 PAMs induce severe seizure activity (Bridges et al., 2013; Rook et al., 2013) and excitotoxicity leading to cell death in the forebrain (Parmentier-Batteur et al., 2014; Rook et al., 2015). Interestingly, recent studies suggest that a major factor contributing to the adverse effect liability of some mGlu5 PAMs is the ability of some PAMs to also directly activate the receptor. While prototypical or pure PAMs do not alter receptor activity on their own, some allosteric modulators do possess intrinsic efficacy (Conn et al., 2009a). Allosteric modulators that possess both intrinsic efficacy and potentiate responses to orthosteric agonists are referred to as Ago-PAMs (Table 1). While both pure PAMs and Ago-PAMs can induce a shift in the concentration curve of an orthosteric agonist, only an Ago-PAM will induce receptor activation in the absence of orthosteric agonist. These differences in mechanism of action can profoundly impact both the side-effect profiles and therapeutic potential of a given compound.
Interestingly, systematic comparison of structurally related mGlu5 PAMs that possess Ago-PAM activity, relative to pure PAMs that do not possess agonist activity, revealed that mGlu5 Ago-PAMs induce seizures and behavioral convulsions, whereas closely related pure PAMs do not (Rook et al., 2013). Furthermore, appropriate activity-dependence of LTP and LTD was maintained with pure mGlu5 PAMs but not with Ago-PAMs (Rook et al., 2013). Based on the striking impact of Ago-PAM activity, it is critical to optimize mGlu5 compounds for clinical development that strictly avoid Ago-PAM activity. Based on the propensity of over-activation of mGlu5 to induce seizures and excitotoxicity, recent efforts have focused on developing mGlu5 PAMs that have the minimal positive cooperativity with glutamate required for achieving efficacy (Parmentier-Batteur et al., 2014; Rook et al., 2013). However, avoiding high cooperativity and Ago-PAM activity is not likely to be a universal guideline for all allosteric modulators and should be evaluated independently for each target as well as disease state. For example, in disease states where endogenous neurotransmitter levels are sufficiently attenuated, a PAM may lack sufficient efficacy and optimizing Ago-PAMs may be preferred.
Novel mGlu5 PAMs that induce stimulus bias reduce adverse effect liability
In recent years it has become increasingly clear that agonists can stabilize multiple active states of GPCRs that can engage different signaling pathways (Digby et al., 2010; Kenakin and Christopoulos, 2013). In the simplest case, an allosteric modulator would induce similar inhibition or amplification of all signaling pathways that are activated by an agonist. In this case, the allosteric modulator would not induce a qualitative change in receptor signaling but would potentiate or inhibit all responses that are normally induced by activation of the receptor. However, some allosteric modulators can selectively modulate the ability of agonists to stabilize specific active conformations of the receptor and thereby introduce a “stimulus bias” that differentially alters the effects of the endogenous agonist on specific signaling pathways (Figure 2). Recent studies provide exciting new insights into the potential for optimizing mGlu5 PAMs that display stimulus bias for achieving robust efficacy in the absence of adverse effect liability.
Figure 2. Ability of allosteric modulators to confer bias of GPCR signaling.
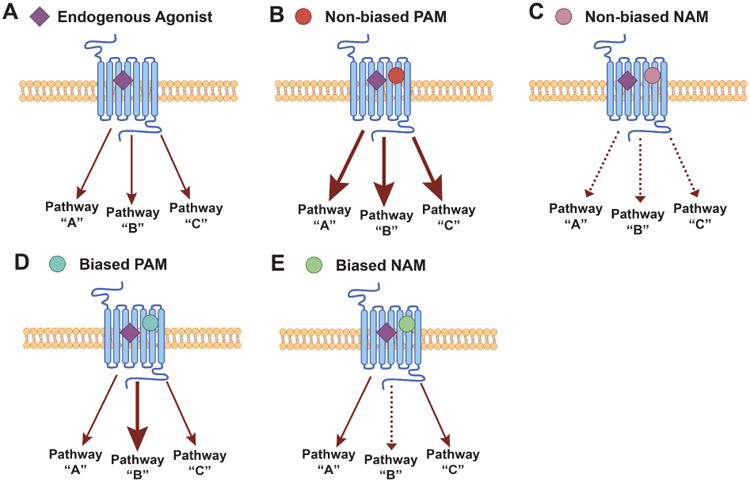
GPCRs can adopt multiple conformations upon neurotransmitter binding that can lead to activation of numerous signaling pathways (A). Non-biased allosteric modulators equally potentiate (B) or inhibit (C) all the signaling pathways that are activated by an agonist. However, some PAMs and NAMs can confer bias to GPCR signaling and selectively modulate coupling of GPCRs to specific signaling pathways while having little or no effect on others (D and E). These tools are affording the opportunity to determine the outcome of modulating a specific receptor-mediated signaling pathway, and hold great therapeutic potential by allowing receptor activation to be steered in maximally beneficial directions.
While the presence of Ago-PAM activity at mGlu5 is known to lead to severe adverse effects, avoiding Ago-PAM activity does not completely eliminate adverse effect liability of these compounds. For instance, an mGlu5 PAM termed 5PAM-523 lacks agonist activity but can induce seizure activity and excitotoxicity after chronic administration (Parmentier-Batteur et al., 2014). While the adverse effects are less pronounced than those seen with mGlu5 Ago-PAMs, this finding suggests that other aspects of mGlu5 signaling can contribute to the adverse effect liabilities. Interestingly, mGlu5 is a close signaling partner with the N-Methyl-D-aspartate subtype of glutamate receptor (NMDAR; Attucci et al., 2001; Awad et al., 2000; Doherty et al., 2000; Ehlers, 1999; Mannaioni et al., 2001; Marino and Conn, 2002; Ugolini et al., 1999) and the ability of mGlu5 to potentiate NMDAR currents in forebrain regions has been commonly viewed as a key mechanism by which mGlu5 PAMs exert their efficacy in rodent models (Darrah et al., 2008; Kinney et al., 2005; Niswender and Conn, 2010; Stefani and Moghaddam, 2010; Won et al., 2012). However, it is well established that excessive activity of NMDARs can induce seizures and excitotoxicity raising the possibility that potentiation of NMDAR signaling could contribute to the adverse effect liability of some mGlu5 PAMs. To directly evaluate the importance of potentiation of NMDAR currents for the observed in vivo efficacy of mGlu5 PAMs, a novel mGlu5 PAM (VU0409551) was developed that displays stimulus bias and potentiates mGlu5 coupling to Gαq and related signaling pathways but does not enhance mGlu5 modulation of NMDAR currents (Rook et al., 2015). Interestingly, VU0409551 produces robust antipsychotic-like and cognition-enhancing effects in rodent models (Balu et al., 2016; Conde-Ceide et al., 2015; Rook et al., 2015), suggesting that the efficacy of mGlu5 PAMs in these models does not require potentiation of NMDAR currents. Furthermore, chronic administration of VU0409551 had no adverse effects at doses over 100× those required to achieve in vivo efficacy (Rook et al., 2015). These studies raise the exciting possibility that it will be possible to develop mGlu5 PAMs that have robust efficacy but are devoid of adverse effects that can be associated with modulation of NMDAR currents. However, these findings also raise the question of what signaling pathways and physiological responses are critical for specific in vivo actions of mGlu5 PAMs. As new tools are further developed that selectively modulate specific signaling pathways by mGlu5, they will provide opportunities to develop a full understanding of the specific signaling pathways that are critical for mediating the efficacy of mGlu5 PAMs in preclinical models of numerous CNS disorders including schizophrenia, fragile X syndrome, Rett syndrome, autistic spectrum disorders, obsessive compulsive disorder, Parkinson's disease, substance abuse (Ade et al., 2016; Gass et al., 2016; Gogliotti et al., 2016; Gould et al., 2016; Michalon et al., 2012; Rylander et al., 2010; Vicidomini et al., 2016), and others.
In addition to discovery of biased mGlu5 PAMs, examples are beginning to emerge in which allosteric modulators induce biased signaling for multiple other GPCR subtypes. These include selective PAMs for multiple mGlu receptor subtypes (Noetzel et al., 2013; Rook et al., 2015; Sheffler and Conn, 2008; Zhang et al., 2005), muscarinic acetylcholine receptors (mAChRs; Leach et al., 2010; Leach et al., 2007; Marlo et al., 2009), dopamine receptors (Free et al., 2014), and cannabinoid receptors (Ahn et al., 2012). In addition, biased or functionally selective NAMs have been developed for mGlu7 (Niswender et al., 2010), prostaglandin D2 CHTH2 receptors (Mathiesen et al., 2005), and neurokinin NK2 receptors (Maillet et al., 2007). While the ability of orthosteric agonists to induce biased GPCR signaling is well established (Digby et al., 2010; Furness et al., 2016), the unique potential of GPCR NAMs to selectively inhibit specific signaling pathways is not shared by orthosteric antagonists and provides an exciting opportunity to develop biased NAMs that target pathways that are most critical for achieving a therapeutic effect. As these new tools continue to emerge, this will provide unprecedented opportunities to develop a more complete understanding of the specific signaling pathways responsible for modulation of different physiological responses in identified neuronal populations and brain circuits.
Potential utility of mGlu1 PAMs for treatment of schizophrenia
In addition to mGlu5, the closely related mGlu1 receptor may also be a potential therapeutic target for treating schizophrenia. Genetic studies have identified multiple mutations in the mGlu1 gene (Grm1) in schizophrenic patients (Ayoub et al., 2012). Interestingly, a recent study revealed that each of the mutations associated with schizophrenia leads to a loss of mGlu1 signaling and that highly selective mGlu1 PAMs can potentiate signaling through these mutant receptors (Cho et al., 2014). At present, the functional impact of these mutations in circuits that may be relevant for the pathophysiology of schizophrenia are not understood. However, mGlu1 regulates many of the same circuits that are relevant for potential mGlu5– mediated antipsychotic efficacy, including modulation of NMDAR signaling (Benquet et al., 2002; Heidinger et al., 2002) and hippocampal plasticity (Aiba et al., 1994). In addition, mGlu1 knock out mice show disrupted prepulse inhibition similar to that seem with mGlu5 knock out mice (Brody et al., 2003; Brody et al., 2004). Future studies with newly developed mGlu1 tools (Cho et al., 2014; Lovell et al., 2013) will provide critical insights into the biological roles and therapeutic potential of mGlu1 in treating schizophrenia, and other disorders in which mGlu1 has been implicated, including ataxia, substance abuse, and autism spectrum disorders (Bariselli et al., 2016; Lum et al., 2014; Power et al., 2016).
Selective mGlu2 and mGlu3 PAMs for treatment of schizophrenia
Over the past two decades there have been intensive efforts targeting Group II mGlu receptors (mGlu2 and mGlu3) for the treatment of schizophrenia. Orthosteric agonists that activate both mGlu2 and mGlu3 have robust antipsychotic-like effects in preclinical models (Muguruza et al., 2016; Niswender and Conn, 2010). Unfortunately, while group II mGlu receptor agonists showed significant improvements in both positive and negative symptoms in an initial phase II trial (Patil et al., 2007), subsequent larger clinical studies failed to demonstrate significant efficacy of these agents compared to placebo (Kinon et al., 2011). However, preclinical studies have demonstrated that the antipsychotic-like activity of group II mGlu receptor agonists are absent in mGlu2, but not mGlu3, receptor knock out mice, suggesting that mGlu2 activation may be sufficient to provide therapeutic benefit (Spooren et al., 2000). The discovery of mGlu2-selective PAMs allowed direct testing of this hypothesis and several of these compounds demonstrated antipsychotic-like efficacy and pro-cognitive effects in multiple preclinical models (Galici et al., 2005; Galici et al., 2006; Griebel et al., 2016). However, a recent report revealed that the mGlu2 PAM AZD8529 had no significant effects on positive or negative symptoms in schizophrenic patients when administered as a monotherapy (Litman et al., 2016). Another mGlu2 PAM, JNJ-40411813/ADX71149, had promising beneficial effects in patients with residual negative symptoms (Hopkins, 2013) and showed potential efficacy in improving some aspects of cognition and reducing negative symptoms after administration of ketamine in healthy volunteers (Salih et al., 2015). However, it is not yet known whether this compound will show efficacy in larger trials in schizophrenia patients. As discussed below several factors including disease etiology, disease progression, and prior medication history may be critical determinants of what compounds are most likely to provide therapeutic benefit.
mGlu2 PAMs may be effective in select patient subpopulations
While the underpinnings of schizophrenia are diverse, great strides have been made in identifying genetic and environmental risk factors as well as understanding the clinical and physiological correlates associated with progression of the disease (Millan et al., 2016). In addition to disease progression, it is important to consider the potential impact of prior medication when assessing a given therapy. Interestingly, some recent studies suggest that group II mGlu agonists, or mGlu2 PAMs, may have a higher likelihood of providing significant efficacy in patients in which treatment is initiated soon after diagnosis and prior to long-term exposure to atypical antipsychotics (Kinon et al., 2015), which may repress activity of the mGlu2 gene promoter (Kurita et al., 2012). Thus, it is possible that stratification of patients will help in identifying patients that would be most responsive to mGlu2 PAMs. In addition, recent advances in stratifying patients using biomarkers, such as PET or functional imaging, could aid in identifying patients that could be most responsive to mGlu2 PAMs or agonists. Such approaches have been effective in correlating treatment responses of atypical antipsychotics with D2 receptor occupancy (Kapur et al., 2000), and similar strategies including genetic and functional screening have been applied with great success with regards to cancer treatment (Vargas and Harris, 2016). While future work is needed to determine the utility of similar strategies in treating schizophrenia and other CNS disorders it is possible that stratification of patient populations could help identify what patients are most likely to respond to a given therapy.
Potential role of heterodimers in mediating mGlu2-mediated antipsychotic efficacy
In addition to the potential importance of segregating patient populations, it is also important to consider the possibility that mGlu2 heterodimers could impact the in vivo effects of mGlu2 PAMs. Recent studies suggest that mGlu2 forms functional heterodimers with mGlu4 that are expressed in the CNS and that some PAMs can selectively activate homomeric relative to heteromeric forms of the receptor (Figure 3; Kammermeier, 2012; Niswender et al., 2016; Yin et al., 2014). Interestingly some mGlu4 PAMs have antipsychotic-like effects in rodent models (Kalinichev et al., 2014; Slawinska et al., 2013), including Lu AF21934, which acts as a robust PAM of mGlu2/4 heterodimers (Niswender et al., 2016; Yin et al., 2014), raising the possibility that mGlu2/4 heterodimers could play a key role in mediating the antipsychotic efficacy seen with mGlu4 and mGlu2 PAMs. Furthermore, mGlu2 and mGlu3 readily form mGlu2/mGlu3 heterodimers in cell lines (Levitz et al., 2016) and these receptors are highly co-expressed in the PFC and other brain regions (Petralia et al., 1996). Interestingly, while neither mGlu2 nor mGlu3 knock out mice show overt behavioral deficits, dual mGlu2/3 knock out mice display blunted responses to amphetamine and cognitive deficits (Lyon et al., 2011), raising the possibility that mGlu2/2, mGlu3/3, and mGlu2/3 complexes could all play roles in regulating schizophrenia-associated circuitry. Finally, recent studies revealed that both mGlu2-mediated signaling in the cortex (Moreno et al., 2016) and the antipsychotic-like effects of Group II mGlu receptor agonists (Fribourg et al., 2011) are absent in 5HT2A knock-out mice, suggesting an important role for cross-talk between these receptors in mediating antipsychotic effects. This cross talk has been postulated to be mediated by formation of mGlu2/5HT2A heterodimers and to be critical for the antipsychotic-like effects of mGlu2 and 5HT2A ligands. Thus, in future studies, it will be important to consider the possibility that regulating signaling through certain heterodimers of mGlu2 could be crucially important in determining antipsychotic-like or cognition-enhancing effects of these compounds. With the exception of PAMs that are known to be active at mGlu2/4 heterodimers, there are no studies detailing if specific pharmacological compounds can distinguish between different Group II mGlu receptor complexes. Furthering our understanding regarding the importance of these various group II receptor complexes to regulating signaling in both physiological and pathological contexts could provide important insights that could translate into improved therapeutics targeting these receptors.
Figure 3. Allosteric modulators can differentially modulate receptor populations based on their dimerization.
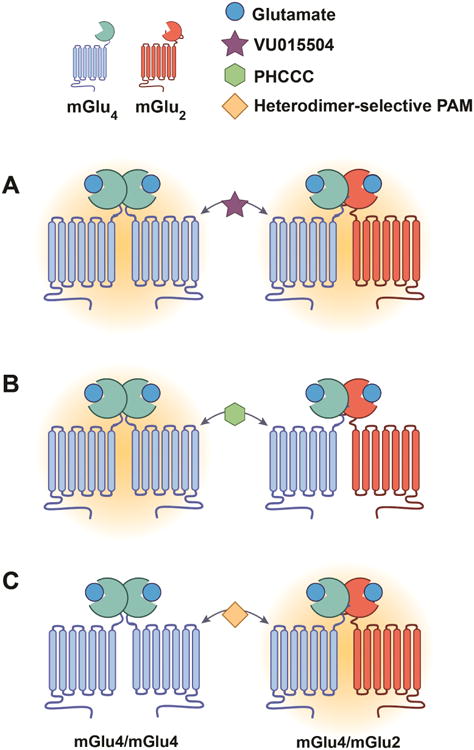
Many GPCRs can form oligomers either as homodimers (containing two receptors of the same subtype) or heterodimers (containing two different receptor subtypes). As depicted above using mGlu4 and mGlu2 receptors as an example, some PAMs (such as VU0155041) are active at both mGlu4/mGlu4 homodimer and mGlu4/mGlu2 heterodimer complexes (A; Potentiation of receptor activity is depicted by orange shading). Other PAMs (such as PHCCC) selectively activate mGlu4 homodimers but are inactive at mGlu4/mGlu2 heterodimers (B). Furthermore, while no examples have been described to date, it is possible that other classes of PAMs could be active at the heterodimer, but inactive at the homodimer (C). These novel tools that can distinguish between receptor complexes will shed light onto the physiological significance of homodimer and heterodimer complexes and could provide therapeutic benefits through incredibly precise modulation of circuitry that targets not only a given receptor subtype, but can target specific receptor complexes.
The discovery of allosteric modulators that differentiate between mGlu2/4 relative to mGlu4 and mGlu2 raises the exciting potential of developing allosteric modulators for homomeric versus heteromeric forms of other GPCR subtypes. GPCRs belonging to each major subclass form functional homomeric as well as heteromeric complexes (Oldham and Hamm, 2008; Smith and Milligan, 2010). However, the majority of studies of GPCR heterodimers have been performed in cell lines and the extent to which many GPCRs function as heterodimers in native systems is not yet clear (Milligan, 2013). Discovery of additional selective modulators could provide critical information on the molecular determinants of signaling through homo- vs heterodimer complexes and pave the way for understanding the unique roles of specific heteromeric GPCR complexes in regulating CNS function. However, at present, it is still unclear how allosteric modulators differentiate between these complexes. The mGlu2/4 allosteric modulators outlined above appear to act at a conserved binding site on a single protomer (mGlu2 or mGlu4) of the mGlu2/4 complex (Niswender et al., 2016). Future studies will be needed to fully understand why some modulators that bind at a single protomer modulate mGlu2/4 signaling, whereas others do not. In addition, other approaches using bivalent compounds to target heterodimer complexes have shown promise (Hubner et al., 2016), and it is possible that allosteric modulators could be developed that bind at the interface between the two subunits. Future work will be needed to determine the structural and molecular mechanisms through which allosteric modulation of heterodimer complexes occurs. However, the approach of developing compounds that not only target a given receptor subtype but specifically target a given heterodimeric complex (Figure 3), has the potential to allow incredibly precise pharmacological modulation of neuronal communication.
Selective mGlu3 PAMs for improving PFC-dependent cognitive function
In addition to potential antipsychotic effects of mGlu2-selective PAMs, recent studies also point to the potential utility of mGlu3 PAMs in improving cognitive function in schizophrenia and other brain disorders. Several studies have identified single nucleotide polymorphisms (SNPs) in the human gene encoding mGlu3 (GRM3) that are associated with poor performance on cognitive tests that are dependent on function of the prefrontal cortex (Egan et al., 2004; Harrison et al., 2008; Tan et al., 2007), and GRM3 has been identified as a risk locus for schizophrenia in genome wide association studies (Schizophrenia Working Group of the Psychiatric Genomics, 2014). The recent discovery of selective mGlu3 NAMs enabled studies in mice that revealed that mGlu3, but not mGlu2, mediates clear postsynaptic effects in the PFC and could regulate synaptic plasticity as well as mediate pro-cognitive effects (Walker et al., 2015). This is consistent with recent studies in non-human primates demonstrating that mGluR2/3 agonists have unexpected postsynaptic actions in the dorsolateral PFC that strengthen synaptic connections and improve cognitive function (Jin et al., 2016). Collectively, these studies suggest that mGlu3 plays a key role in regulating PFC-dependent cognition. Future studies with current and next generation group II modulators will broaden our understanding of these receptors and their potential utility in treating numerous CNS disorders such as schizophrenia, substance abuse, depression (Chaki et al., 2004; Dhanya et al., 2014), and others.
Potential utility of muscarinic receptor PAMs for treatment of schizophrenia
Preclinical and clinical studies suggest that potentiation of specific mAChR subtypes can improve symptoms in patients suffering from schizophrenia and Alzheimer's disease (AD). A large multicenter trial examined the effects of the M1/M4-preferring mAChR agonist xanomeline in patients suffering from AD. While the primary endpoint was improved cognitive function, secondary measures surprisingly revealed that this compound had robust efficacy in reducing psychotic symptoms such as suspiciousness, delusions, and hallucinations in AD patients (Bodick et al., 1997). A subsequent study revealed that xanomeline induced robust improvements in positive symptoms, negative symptoms, and specific domains of cognitive function in patients suffering from schizophrenia (Shekhar et al., 2008). Despite these exciting advances, the clinical utility of xanomeline and other mAChR agonists is restricted by dose-limiting adverse effects (bradycardia, GI distress, salivation, sweating) that are mediated by activation of peripheral M2 and M3 mAChRs (Bymaster et al., 2003). Interestingly, M1 and M4 are the primary mAChR subtypes thought to be involved in the therapeutic effects of mAChR agonists in schizophrenia patients (Langmead et al., 2008). Thus, it is possible that highly selective activators of M1 and/or M4 could provide therapeutic efficacy in these patients in the absence of the peripheral adverse effects associated with less selective mAChR agonists.
M4 PAMs reduce dopamine release and have antipsychotic-like effects in animal models
Despite major investments, previous efforts to develop highly selective agonists of M1 or M4 mAChRs have failed due to high conservation in the orthosteric ACh site across subtypes. However, more recent efforts to develop subtype-selective mAChR PAMs have been highly successful and have yielded multiple selective PAMs for M1 and M4 mAChRs that have excellent pharmacokinetic (PK) profiles and brain penetration, providing excellent tools for evaluating the effects of M1 and M4 PAMs in preclinical animal models for numerous CNS disorders (Conn et al., 2014; Kruse et al., 2014).
The discovery of two structurally distinct M4 PAMs (VU10010 and LY2033298) represented a milestone in the development of M4-selective molecules (Chan et al., 2008; Shirey et al., 2008). Since then, medicinal chemistry efforts have provided compounds with improved brain exposure and properties that are ideal for in vivo use. Studies utilizing these novel M4–selective PAMs have demonstrated robust effects, similar to those seen with xanomeline, in multiple animal models used to predict antipsychotic-like activity including reversal of psychostimulant-induced changes in conditioned avoidance responding, prepulse inhibition, and locomotor activity (Bubser et al., 2014; Chan et al., 2008; Leach et al., 2010; Suratman et al., 2011). Importantly, M4 PAMs do not display any of the detrimental peripheral effects that are seen after administration of non-selective mAChR compounds (Bubser et al., 2014), suggesting that M4 PAMs may provide a novel strategy for treating positive symptoms in schizophrenia patients.
Psychotic symptoms associated with schizophrenia are thought to be intimately associated with hyperactive dopaminergic signaling in the striatum and nucleus accumbens and all currently available antipsychotics act as antagonists of dopamine (DA) receptors (Sawa and Snyder, 2003). Interestingly, M4 PAMs decrease amphetamine-induced DA levels in the dorsal striatum and nucleus accumbens and induce a profound reduction in amphetamine-induced activation of forebrain regions in vivo as assessed by fMRI (Byun et al., 2014), raising the possibility that M4 PAMs mediate their effects through modulating DA release. Cholinergic regulation of DA signaling is complex and cholinergic interneurons in the striatum can exert bidirectional control over dopaminergic signaling through both nicotinic acetylcholine receptors (nAChR) and mAChRs (Rice et al., 2011). Studies using fast scan cyclic voltammetry demonstrated that M4 knock out mice display attenuated mAChR agonist-induced reductions in DA release (Threlfell et al., 2010) and selective M4 PAMs induce a robust inhibition of DA release in striatal slices that persists well after receptor activation (Foster et al., 2016). This sustained inhibition of DA release following M4 activation was distinct from the acute inhibition observed following application of mAChR agonists, which is mediated by primarily by mAChR autoreceptors expressed on cholinergic interneurons (Shin et al., 2015). M4 is not expressed on DA neurons (Weiner et al., 1990), but is highly expressed on D1-containing direct pathway spiny projection neurons (D1-SPNs; Ince et al., 1997). Interestingly, selective deletion of M4 from D1-expressing neurons (D1-M4-/- mice) eliminated the sustained reductions in DA release and antipsychotic-like effects induced by M4 PAMs and xanomeline (Dencker et al., 2011; Foster et al., 2016), suggesting that M4 expressed on D1-containing neurons mediate these effects. The finding that M4 PAMs act at D1-SPNs to inhibit DA release suggests that M4 activation must act by inducing release of a local messenger that acts on neighboring DA terminals to inhibit DA release. Interestingly, M4-mediated effects on DA release are blocked by a CB2 endocannabinoid (eCB) receptor antagonist, absent in CB2 knock out mice, and are occluded by inhibition of the eCB synthetic enzyme diacylglycerol lipase (Foster et al., 2016). Taken together, these data suggest that the effects of M4 PAMs on DA release in the striatum are mediated by activation of eCB synthesis in D1-SPNs and activation of CB2 receptors, possibly expressed on neighboring DA terminals.
The ability of M4 to reduce DA through the local release of eCBs provides a mechanism that may afford a spatially restricted modulation of DA signaling in the limbic forebrain. This could provide a major advantage over clinically available antipsychotics that act as DA receptor antagonists in that it may allow reduced DA signaling in striatal regions that are thought to be important for antipsychotic efficacy, without reducing DA signaling in the hippocampus and cortical regions that may impair cognitive function (Davis et al., 1991; Reilly et al., 2007; see Figure 4). Consistent with this, early reports suggest that M4 PAMs can improve some aspects of cognitive function in animal models that are impaired by DA receptor blockade (Bubser et al., 2014).
Figure 4. The potential therapeutic benefits of selectively regulating dopaminergic signaling in the basal ganglia.
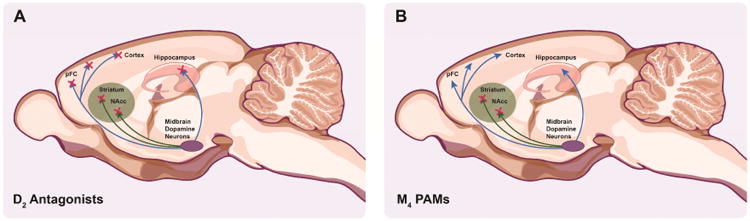
Midbrain dopamine (DA) neurons project to several nuclei including the striatum, nucleus accumbens (NAcc), prefrontal cortex (PFC), cortex, and hippocampus. DA signaling is dysregulated in schizophrenia manifesting in excessive DA release in the striatum and NAcc that is associated with the positive symptoms such as hallucinations and delusions (depicted as green shaded areas). However, DA signaling is not hyperactive in all brain regions and hypoactive disruptions in cortical and hippocampal DA signaling are thought to contribute to the negative symptoms such as anhedonia as well as the cognitive deficits. Currently available antipsychotics act by blocking DA D2 receptors across all brain regions, including the areas that are already DA deficient, potentially worsening negative and cognitive symptoms (A). By depressing DA release through the release of local messengers in the hyperactive limbic brain regions, M4 PAMs have the potential to correct hyperactive DA signaling without further depressing DA signaling in other areas (B), demonstrating how circuit-selective therapeutics have the potential to provide efficacy with reduced adverse effect liability.
In addition to regulation of DA release, recent studies reveal that M4 PAMs can decrease glutamatergic transmission at cortico-striatal synapses (Pancani et al., 2014) and induce spike-timing dependent LTD on D1-SPNs (Shen et al., 2016). M4 receptors are present on cortico-striatal terminals, and activation of these presynaptic M4 receptors may contribute to M4 PAM effects at these excitatory synapses (Pancani et al., 2014). However, as with the effect of M4 PAMs on DA release, the ability of M4 PAMs to induce LTD in D1-SPNs is absent in D1-M4-/-mice and, surprisingly, was blocked by a CB1 receptor antagonist (Shen et al., 2016), suggesting M4 PAM-induced eCB release from D1-SPNs can inhibit excitatory transmission in the striatum. Extensive studies in rodents and non-human primates suggest that these actions of M4 PAMs on striatal plasticity could provide therapeutic benefits in treating L-DOPA-induced dyskinesia in Parkinson's disease (Shen et al., 2016). Furthermore, M4 PAMs can normalize excessive excitatory transmission at cortico-striatal synapses in rodent models of Huntington's disease and chronic administration of M4 PAMs prevents the appearance of motor deficits in these animals (Pancani et al., 2015). Finally, the ability of M4 PAMs to reduce behavioral effects of cocaine suggest that these agents may be useful for the treatment of substance abuse disorders (Dencker et al., 2012). Thus, recent optimization of highly selective M4 PAMs is providing important insights into the roles of this receptor in regulating CNS function and may lead to novel treatment strategies for multiple CNS disorders.
M1 PAMs may enhance specific domains of cognitive function and reduce negative symptoms
In addition to a potential role for M4 in regulating dopaminergic systems that are relevant for positive symptoms in schizophrenia, the M1 receptor may be important for reducing cognitive impairments and negative symptoms in AD and schizophrenia patients. Cholinergic signaling is disrupted in the prefrontal cortex (PFC) of schizophrenia patients (Berman et al., 2007; Raedler et al., 2007) and a subset of schizophrenia patients display profound decreases in M1 levels in PFC, hippocampus, and other forebrain regions (Dean et al., 2002; Scarr et al., 2013). The recent discovery of highly selective M1 PAMs enabled studies that reveal that these agents can enhance both PFC- and hippocampal-dependent forms of cognitive function in rodents (Chambon et al., 2012; Digby et al., 2012; Gould et al., 2015; Ma et al., 2009; Shirey et al., 2009) and non-human primates (Lange et al., 2015; Vardigan et al., 2015) which is consistent with a large literature suggesting that selective M1 receptor activation can have cognition-enhancing effects.
Recent studies are providing important new insights into the possible mechanisms by which M1 PAMs could provide specific benefits to some schizophrenia patients. An emerging body of clinical and preclinical research has led to the hypothesis that deficits in LTD at hippocampo-PFC synapses (Strube et al., 2015; Thomases et al., 2014) and excessive activation of the PFC by excitatory projections from the hippocampus (Jodo, 2013; Woodward et al., 2013) contribute to the cognitive deficits and negative symptoms observed in schizophrenia patients. This is especially interesting in light of the findings that M1 plays a major role in induction of long-term depression (LTD) at hippocampo-PFC synapses (Caruana et al., 2011; Ghoshal et al., 2016), and that M1 knock-out mice display deficits in forms of cognitive function that involve interactions between the hippocampus and PFC (Anagnostaras et al., 2003). Interestingly, M1 receptor-mediated LTD at the hippocampo-PFC synapse is completely lost in rodent models that pharmacologically or genetically inhibit NMDAR function during juvenile development, and M1 PAMs can restore deficits in synaptic plasticity, cognitive function and social interaction in these rodent models (Ghoshal et al., 2016; Grannan et al., 2016). Taken together with multiple studies demonstrating robust effects of M1 PAMs on other aspects of cognitive function, these studies support the exciting possibility that highly selective M1 PAMs may provide a novel approach for reducing cognitive deficits and negative symptoms associated with changes in cortical plasticity in schizophrenia patients. Furthermore, while M4 PAMs are likely to have more robust antipsychotic-like effects, M1 PAMs can augment the antipsychotic-like effects of atypical antipsychotics in wild type, but not M1 knock-out mice (Choy et al., 2016). Accordingly, M1 PAMs either alone or in combination with current antipsychotics, have the potential to provide comprehensive relief across symptom clusters.
Surprisingly, a recent study suggested that some M1-selective Ago-PAMs can induce cholinergic side-effects in animal models that are typically associated with M2/M3 activation (Alt et al., 2016; Davoren et al., 2016). However, several studies with other M1-selective PAMs did not observe any of the side-effects seen with broad spectrum cholinergic mimetics (Chambon et al., 2012; Jones et al., 2008; Vardigan et al., 2015), suggesting that it is possible to develop M1 PAMs that have a desirable side-effect profile. The exact mechanism underlying the adverse effects of M1 Ago-PAMs are not entirely clear. However, it is possible that subtle variations in properties of different M1–selective compounds could influence their in vivo effects in a manner similar to that outlined above for mGlu5 PAMs. M1 PAMs have been reported to possess distinct differences in signaling bias and allosteric agonist activity, and the impact of these differences has not been fully explored. For instance, while M1 PAMs can potentiate ACh-induced activation of both phospholipase D (PLD) and Ca2+ mobilization in cell lines, a novel M1 PAM (VU0029767) selectively potentiates M1-induced Ca2+ mobilization but has no effect on M1-mediated activation of PLD (Marlo et al., 2009). M1-mediated activation of phospholipase C and Ca2+ mobilization involves signaling through Gαq, whereas PLD typically involves the activation of Gα12 or small G-proteins such as R-Ras (Lopez De Jesus et al., 2006). Thus, it is possible that VU0029767 stabilizes a conformation of M1 that couples to Gαq but not Gα12 or small G-proteins. While the precise roles of these signaling pathways in different physiological and behavioral responses to M1 activation are not known, closely related M1 PAMs that display such striking differences in their effects on M1 signaling could have fundamentally different effects on animal behavior greatly modifying their potential therapeutic efficacy or adverse effect liability.
The robust pro-cognitive effects of M1–selective PAMs could prove useful for treatment of multiple disorders in which cognitive function is impaired. Acetylcholinesterase inhibitors, which boost cholinergic signaling, have well-established efficacy in improving cognitive function in patients with early to moderate AD and other neurodegenerative disorders. Current evidence suggests that the M1 receptor plays a vital role in mediating cholinergic pro-cognitive effects. In addition to the M1 PAM efficacy observed in healthy rodents and schizophrenia models discussed above, M1 PAMs have pro-cognitive effects in numerous rodent models of AD (Puri et al., 2015; Shirey et al., 2009), and can reverse cognitive deficits and prolong survival in a mouse model of prion disease that shows AD-like pathology (Bradley et al., 2016). As discussed above in the context of mGlu2 PAMs for schizophrenia, it is possible that careful patient stratification will be critical for evaluating the potential efficacy of M1 PAMs in patient populations. For instance, developing an appropriate PET ligand or other approaches for identifying and recruiting the subset of schizophrenia patients that display decreases in cortical M1 levels (Dean et al., 2002; Scarr et al., 2013) could enrich for patients that would receive the greatest benefit from M1 PAMs. Collectively, these studies call attention to the potential utility of M1 PAMs in correcting cognitive and attentional deficits in a wide range of neurological disorders including schizophrenia, attention deficit hyperactivity disorder, AD, Parkinson's disease, and others.
Allosteric modulation of dopamine receptors as potential treatments of schizophrenia
Since all currently available antipsychotics act via downregulation of D2 signaling it is tempting to hypothesize that a D2-selective NAM (or partial NAM) that possessed desirable signal bias, or selectively modulates only certain D2 dimer complexes, could provide antipsychotic efficacy with reduced cognitive and motor side-effects. The discovery of the first NAM of the D2 receptor represented a breakthrough in efforts to achieve allosteric regulation of this target (Lane et al., 2014). Interestingly, this D2 NAM (SB269652) possessed a bitopic mode of action, binding to both the orthosteric and an allosteric site, and only displayed NAM activity at functional D2 receptor homodimers. Excitingly, medicinal chemistry efforts have recently succeeded at fragmenting SB269652 and have resulted in the first purely allosteric compound possessing NAM activity at the D2 receptor (Mistry et al., 2015). While the hypothesis that a D2 NAM could provide efficacy with a preferred side-effect profile has yet to be tested, these advances suggest that such approaches could be testable in the near future.
Conversely, in preclinical animal models D1 agonists have been shown to mediate cognitive enhancing effects, although with an inverted U-shaped dose-response curve suggesting that too little or too much D1 activation can be detrimental (Arnsten et al., 2016). However, a proof of principle study demonstrated that administration of the D1-preferring agonist dihydrexidine improved working memory in patients with schizotypal personality disorder (Rosell et al., 2015). Unfortunately dihydrexidine, which is only moderately selective for D1 over D5, has poor bioavailability and is rapidly metabolized, limiting the clinical utility of this compound. Excitingly, new D1-selective PAMs have recently been developed that demonstrate enhanced specificity over D5 receptors (Lewis et al., 2015). D1 PAMs, due to their mechanism of action, have the potential to avoid the adverse effects seen excessive D1 receptor activation. Consistent with this D1 PAMs, unlike D1 agonists, induced changes in locomotion in a humanized mouse line that plateaued at high doses without inducing stereotypies (Svensson et al., 2016). The recent discovery of both D2- and D1-selective allosteric scaffolds represents an important advance and hopefully will lead to optimized compounds that can provide therapeutically desirable outcomes with fewer side-effects than those observed with DA receptor agonists and antagonists.
Conclusions and Future Directions
The discovery of allosteric modulators initially provided the ability to target binding sites on specific receptor subtypes that were less conserved than the neurotransmitter binding site allowing allosteric compounds to act with unprecedented selectivity. Given the vast number of possible allosteric binding sites there is reason to believe that many of these sites have not been identified or targeted from a medicinal chemistry perspective. Numerous examples have been detailed where multiple distinct allosteric binding sites have been found for a single GPCR subtype (Gregory and Conn, 2015), and the recent discovery of a cytoplasmic allosteric binding site in chemokine CCR9 represents one example of a previously unappreciated binding site that could allow therapeutic modulation of a previously intractable target (Oswald et al., 2016). As we continue to advance our understanding of the mechanisms underlying allosteric modulation of GPCRs, we have come to appreciate the immense number of conformations and higher-order complexes that these receptors can adopt (Changeux and Christopoulos, 2016; Latorraca et al., 2016), and are beginning to elucidate how these conformations differentially regulate various signaling pathways. In addition to differences in terms of stimulus bias, activity at heterodimers, and presence or absence of ago-PAM activity, it has been possible to develop NAMs that possess weak negative cooperativity can act as partial NAMs that only partially inhibit the response to an orthosteric agonist (Kenakin, 2004; Nickols et al., 2016; Rodriguez et al., 2010; Rodriguez et al., 2005). These partial NAMs could provide a key mechanistic advantage that cannot be achieved using orthosteric antagonists and have the potential to maintain efficacy similar to full NAMs in animal models, but have fewer adverse effects than agents that completely block GPCR signaling (Gould et al., 2016).
Other recent advances include the use of allosteric modulators in combination with optogenetic and chemogenetic technologies to allow selective modulation of specific receptor subpopulations with unprecedented spatial and temporal specificity. For instance, engineered chimeras of GPCRs with rhodopsin can adopt active conformations in the presence of light, allowing optical control over receptor activity (Airan et al., 2009). In addition, introduction of targeted mutations into GPCRs allows for labeling of receptor populations using coordination chemistry approaches that introduce either metal binding domains (Kiyonaka et al., 2016), or photoswitchable tethered ligands (Levitz et al., 2013). By utilizing mutated GPCRs that can be expressed in specific neuronal populations, it is possible to assess the physiological and behavioral consequences of activating a particular receptor subtype in a specific location. More recently, this approach has refined through the use of non-tethered photoswitchable allosteric ligands that possess the properties of an allosteric modulator, but adopt inactive conformations when exposed to light. Discovery of photoswitchable allosteric modulators for the mGlu5 (Pittolo et al., 2014) and mGlu4 receptors (Rovira et al., 2016) allows fast and direct modulation of endogenous receptor subtypes in a particular brain region. Collectively, these tools have the promise to provide unprecedented insights into the biology and circuitry underlying numerous CNS diseases. These discoveries will inform drug discovery efforts on how to optimally steer receptor signaling in a given patient population to provide maximal efficacy with minimal side-effects and provide exciting opportunities for the treatment of CNS orders.
Acknowledgments
We would like to thank Rachel Chandler for illustrating the figures in this manuscript. This work was supported by grants from the NIH (NIMH and NINDS) to PJC and DJF and Brain & Behavior Research Foundation to DJF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ade KK, Wan Y, Hamann HC, O'Hare JK, Guo W, Quian A, Kumar S, Bhagat S, Rodriguiz RM, Wetsel WC, et al. Increased Metabotropic Glutamate Receptor 5 Signaling Underlies Obsessive-Compulsive Disorder-like Behavioral and Striatal Circuit Abnormalities in Mice. Biol Psychiatry. 2016;80:522–533. doi: 10.1016/j.biopsych.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn KH, Mahmoud MM, Kendall DA. Allosteric modulator ORG27569 induces CB1 cannabinoid receptor high affinity agonist binding state, receptor internalization, and Gi protein-independent ERK1/2 kinase activation. J Biol Chem. 2012;287:12070–12082. doi: 10.1074/jbc.M111.316463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba A, Chen C, Herrup K, Rosenmund C, Stevens CF, Tonegawa S. Reduced hippocampal long-term potentiation and context-specific deficit in associative learning in mGluR1 mutant mice. Cell. 1994;79:365–375. doi: 10.1016/0092-8674(94)90204-6. [DOI] [PubMed] [Google Scholar]
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- Alt A, Pendri A, Bertekap RL, Jr, Li G, Benitex Y, Nophsker M, Rockwell KL, Burford NT, Sum CS, Chen J, et al. Evidence for Classical Cholinergic Toxicity Associated with Selective Activation of M1 Muscarinic Receptors. J Pharmacol Exp Ther. 2016;356:293–304. doi: 10.1124/jpet.115.226910. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 2003;6:51–58. doi: 10.1038/nn992. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Girgis RR, Gray DL, Mailman RB. Novel Dopamine Therapeutics for Cognitive Deficits in Schizophrenia. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attucci S, Carla V, Mannaioni G, Moroni F. Activation of type 5 metabotropic glutamate receptors enhances NMDA responses in mice cortical wedges. Br J Pharmacol. 2001;132:799–806. doi: 10.1038/sj.bjp.0703904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci. 2000;20:7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones PJ, et al. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 2009;34:2057–2071. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub MA, Angelicheva D, Vile D, Chandler D, Morar B, Cavanaugh JA, Visscher PM, Jablensky A, Pfleger KD, Kalaydjieva L. Deleterious GRM1 mutations in schizophrenia. PLoS One. 2012;7:e32849. doi: 10.1371/journal.pone.0032849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Li Y, Takagi S, Presti KT, Ramikie TS, Rook JM, Jones CK, Lindsley CW, Conn PJ, Bolshakov VY, Coyle JT. An mGlu5-Positive Allosteric Modulator Rescues the Neuroplasticity Deficits in a Genetic Model of NMDA Receptor Hypofunction in Schizophrenia. Neuropsychopharmacology. 2016;41:2052–2061. doi: 10.1038/npp.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariselli S, Tzanoulinou S, Glangetas C, Prevost-Solie C, Pucci L, Viguie J, Bezzi P, O'Connor EC, Georges F, Luscher C, Bellone C. SHANK3 controls maturation of social reward circuits in the VTA. Nat Neurosci. 2016;19:926–934. doi: 10.1038/nn.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Pinto-Duarte A, Kappe A, Zembrzycki A, Metzler A, Mukamel EA, Lucero J, Wang X, Sejnowski TJ, Markou A, Behrens MM. Disruption of mGluR5 in parvalbumin-positive interneurons induces core features of neurodevelopmental disorders. Mol Psychiatry. 2015;20:1161–1172. doi: 10.1038/mp.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benquet P, Gee CE, Gerber U. Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes. J Neurosci. 2002;22:9679–9686. doi: 10.1523/JNEUROSCI.22-22-09679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JA, Talmage DA, Role LW. Cholinergic circuits and signaling in the pathophysiology of schizophrenia. Int Rev Neurobiol. 2007;78:193–223. doi: 10.1016/S0074-7742(06)78007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj SK, Ryan RT, Wong TP, Srivastava LK. Loss of dysbindin-1, a risk gene for schizophrenia, leads to impaired group 1 metabotropic glutamate receptor function in mice. Front Behav Neurosci. 2015;9:72. doi: 10.3389/fnbeh.2015.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, Shannon HE, Tollefson GD, Rasmussen K, Bymaster FP, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54:465–473. doi: 10.1001/archneur.1997.00550160091022. [DOI] [PubMed] [Google Scholar]
- Bradley SJ, Bourgognon JM, Sanger HE, Verity N, Mogg AJ, White DJ, Butcher AJ, Moreno JA, Molloy C, Macedo-Hatch T, et al. M1 muscarinic allosteric modulators slow prion neurodegeneration and restore memory loss. J Clin Invest. 2016 doi: 10.1172/JCI87526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges TM, Rook JM, Noetzel MJ, Morrison RD, Zhou Y, Gogliotti RD, Vinson PN, Xiang Z, Jones CK, Niswender CM, et al. Biotransformation of a novel positive allosteric modulator of metabotropic glutamate receptor subtype 5 contributes to seizure-like adverse events in rats involving a receptor agonism-dependent mechanism. Drug Metab Dispos. 2013;41:1703–1714. doi: 10.1124/dmd.113.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody SA, Conquet F, Geyer MA. Disruption of prepulse inhibition in mice lacking mGluR1. Eur J Neurosci. 2003;18:3361–3366. doi: 10.1111/j.1460-9568.2003.03073.x. [DOI] [PubMed] [Google Scholar]
- Brody SA, Dulawa SC, Conquet F, Geyer MA. Assessment of a prepulse inhibition deficit in a mutant mouse lacking mGlu5 receptors. Mol Psychiatry. 2004;9:35–41. doi: 10.1038/sj.mp.4001404. [DOI] [PubMed] [Google Scholar]
- Bubser M, Bridges TM, Dencker D, Gould RW, Grannan M, Noetzel MJ, Lamsal A, Niswender CM, Daniels JS, Poslusney MS, et al. Selective activation of M4 muscarinic acetylcholine receptors reverses MK-801-induced behavioral impairments and enhances associative learning in rodents. ACS Chem Neurosci. 2014;5:920–942. doi: 10.1021/cn500128b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Carter PA, Yamada M, Gomeza J, Wess J, Hamilton SE, Nathanson NM, McKinzie DL, Felder CC. Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur J Neurosci. 2003;17:1403–1410. doi: 10.1046/j.1460-9568.2003.02588.x. [DOI] [PubMed] [Google Scholar]
- Byun NE, Grannan M, Bubser M, Barry RL, Thompson A, Rosanelli J, Gowrishankar R, Kelm ND, Damon S, Bridges TM, et al. Antipsychotic drug-like effects of the selective M4 muscarinic acetylcholine receptor positive allosteric modulator VU0152100. Neuropsychopharmacology. 2014;39:1578–1593. doi: 10.1038/npp.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruana DA, Warburton EC, Bashir ZI. Induction of activity-dependent LTD requires muscarinic receptor activation in medial prefrontal cortex. J Neurosci. 2011;31:18464–18478. doi: 10.1523/JNEUROSCI.4719-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki S, Yoshikawa R, Hirota S, Shimazaki T, Maeda M, Kawashima N, Yoshimizu T, Yasuhara A, Sakagami K, Okuyama S, et al. MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology. 2004;46:457–467. doi: 10.1016/j.neuropharm.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Chambon C, Jatzke C, Wegener N, Gravius A, Danysz W. Using cholinergic M1 receptor positive allosteric modulators to improve memory via enhancement of brain cholinergic communication. Eur J Pharmacol. 2012;697:73–80. doi: 10.1016/j.ejphar.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Chan WY, McKinzie DL, Bose S, Mitchell SN, Witkin JM, Thompson RC, Christopoulos A, Lazareno S, Birdsall NJ, Bymaster FP, Felder CC. Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc Natl Acad Sci U S A. 2008;105:10978–10983. doi: 10.1073/pnas.0800567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP, Christopoulos A. Allosteric Modulation as a Unifying Mechanism for Receptor Function and Regulation. Cell. 2016;166:1084–1102. doi: 10.1016/j.cell.2016.08.015. [DOI] [PubMed] [Google Scholar]
- Cho HP, Garcia-Barrantes PM, Brogan JT, Hopkins CR, Niswender CM, Rodriguez AL, Venable DF, Morrison RD, Bubser M, Daniels JS, et al. Chemical modulation of mutant mGlu1 receptors derived from deleterious GRM1 mutations found in schizophrenics. ACS Chem Biol. 2014;9:2334–2346. doi: 10.1021/cb500560h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy KH, Shackleford DM, Malone DT, Mistry SN, Patil RT, Scammells PJ, Langmead CJ, Pantelis C, Sexton PM, Lane JR, Christopoulos A. Positive Allosteric Modulation of the Muscarinic M1 Receptor Improves Efficacy of Antipsychotics in Mouse Glutamatergic Deficit Models of Behavior. J Pharmacol Exp Ther. 2016;359:354–365. doi: 10.1124/jpet.116.235788. [DOI] [PubMed] [Google Scholar]
- Clifton NE, Morisot N, Girardon S, Millan MJ, Loiseau F. Enhancement of social novelty discrimination by positive allosteric modulators at metabotropic glutamate 5 receptors: adolescent administration prevents adult-onset deficits induced by neonatal treatment with phencyclidine. Psychopharmacology (Berl) 2013;225:579–594. doi: 10.1007/s00213-012-2845-3. [DOI] [PubMed] [Google Scholar]
- Conde-Ceide S, Martinez-Viturro CM, Alcazar J, Garcia-Barrantes PM, Lavreysen H, Mackie C, Vinson PN, Rook JM, Bridges TM, Daniels JS, et al. Discovery of VU0409551/JNJ-46778212: An mGlu5 Positive Allosteric Modulator Clinical Candidate Targeting Schizophrenia. ACS Med Chem Lett. 2015;6:716–720. doi: 10.1021/acsmedchemlett.5b00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009a;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CK. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci. 2009b;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Meiler J, Niswender CM. Opportunities and challenges in the discovery of allosteric modulators of GPCRs for treating CNS disorders. Nat Rev Drug Discov. 2014;13:692–708. doi: 10.1038/nrd4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrah JM, Stefani MR, Moghaddam B. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behav Pharmacol. 2008;19:225–234. doi: 10.1097/FBP.0b013e3282feb0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- Davoren JE, Lee CW, Garnsey M, Brodney MA, Cordes J, Dlugolenski K, Edgerton JR, Harris AR, Helal CJ, Jenkinson S, et al. Discovery of the Potent and Selective M1 PAM-Agonist N-[(3R,4S)-3-Hydroxytetrahydro-2H-pyran-4-yl]-5-methyl-4-[4-(1,3-thiazol-4-yl)ben zyl]pyridine-2-carboxamide (PF-06767832): Evaluation of Efficacy and Cholinergic Side Effects. J Med Chem. 2016;59:6313–6328. doi: 10.1021/acs.jmedchem.6b00544. [DOI] [PubMed] [Google Scholar]
- Dean B, McLeod M, Keriakous D, McKenzie J, Scarr E. Decreased muscarinic1 receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2002;7:1083–1091. doi: 10.1038/sj.mp.4001199. [DOI] [PubMed] [Google Scholar]
- Dencker D, Weikop P, Sorensen G, Woldbye DP, Wortwein G, Wess J, Fink-Jensen A. An allosteric enhancer of M(4) muscarinic acetylcholine receptor function inhibits behavioral and neurochemical effects of cocaine. Psychopharmacology (Berl) 2012;224:277–287. doi: 10.1007/s00213-012-2751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencker D, Wortwein G, Weikop P, Jeon J, Thomsen M, Sager TN, Mork A, Woldbye DP, Wess J, Fink-Jensen A. Involvement of a subpopulation of neuronal M4 muscarinic acetylcholine receptors in the antipsychotic-like effects of the M1/M4 preferring muscarinic receptor agonist xanomeline. J Neurosci. 2011;31:5905–5908. doi: 10.1523/JNEUROSCI.0370-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanya RP, Sheffler DJ, Dahl R, Davis M, Lee PS, Yang L, Nickols HH, Cho HP, Smith LH, D'Souza MS, et al. Design and synthesis of systemically active metabotropic glutamate subtype-2 and -3 (mGlu2/3) receptor positive allosteric modulators (PAMs): pharmacological characterization and assessment in a rat model of cocaine dependence. J Med Chem. 2014;57:4154–4172. doi: 10.1021/jm5000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digby GJ, Conn PJ, Lindsley CW. Orthosteric- and allosteric-induced ligand-directed trafficking at GPCRs. Curr Opin Drug Discov Devel. 2010;13:587–594. [PMC free article] [PubMed] [Google Scholar]
- Digby GJ, Noetzel MJ, Bubser M, Utley TJ, Walker AG, Byun NE, Lebois EP, Xiang Z, Sheffler DJ, Cho HP, et al. Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. J Neurosci. 2012;32:8532–8544. doi: 10.1523/JNEUROSCI.0337-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty AJ, Palmer MJ, Bortolotto ZA, Hargreaves A, Kingston AE, Ornstein PL, Schoepp DD, Lodge D, Collingridge GL. A novel, competitive mGlu(5) receptor antagonist (LY344545) blocks DHPG-induced potentiation of NMDA responses but not the induction of LTP in rat hippocampal slices. Br J Pharmacol. 2000;131:239–244. doi: 10.1038/sj.bjp.0703574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore AS, Okrasa K, Patel JC, Serrano-Vega M, Bennett K, Cooke RM, Errey JC, Jazayeri A, Khan S, Tehan B, et al. Structure of class C GPCR metabotropic glutamate receptor 5 transmembrane domain. Nature. 2014;511:557–562. doi: 10.1038/nature13396. [DOI] [PubMed] [Google Scholar]
- Egan MF, Straub RE, Goldberg TE, Yakub I, Callicott JH, Hariri AR, Mattay VS, Bertolino A, Hyde TM, Shannon-Weickert C, et al. Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc Natl Acad Sci U S A. 2004;101:12604–12609. doi: 10.1073/pnas.0405077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Synapse structure: glutamate receptors connected by the shanks. Curr Biol. 1999;9:R848–850. doi: 10.1016/s0960-9822(00)80043-3. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Wilson JM, Remke DH, Mahmood MS, Uddin MJ, Wess J, Patel S, Marnett LJ, Niswender CM, Jones CK, et al. Antipsychotic-like Effects of M4 Positive Allosteric Modulators Are Mediated by CB2 Receptor-Dependent Inhibition of Dopamine Release. Neuron. 2016;91:1244–1252. doi: 10.1016/j.neuron.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Free RB, Chun LS, Moritz AE, Miller BN, Doyle TB, Conroy JL, Padron A, Meade JA, Xiao J, Hu X, et al. Discovery and characterization of a G protein-biased agonist that inhibits beta-arrestin recruitment to the D2 dopamine receptor. Mol Pharmacol. 2014;86:96–105. doi: 10.1124/mol.113.090563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fribourg M, Moreno JL, Holloway T, Provasi D, Baki L, Mahajan R, Park G, Adney SK, Hatcher C, Eltit JM, et al. Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell. 2011;147:1011–1023. doi: 10.1016/j.cell.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness SG, Liang YL, Nowell CJ, Halls ML, Wookey PJ, Dal Maso E, Inoue A, Christopoulos A, Wootten D, Sexton PM. Ligand-Dependent Modulation of G Protein Conformation Alters Drug Efficacy. Cell. 2016 doi: 10.1016/j.cell.2016.09.021. [DOI] [PubMed] [Google Scholar]
- Galici R, Echemendia NG, Rodriguez AL, Conn PJ. A selective allosteric potentiator of metabotropic glutamate (mGlu) 2 receptors has effects similar to an orthosteric mGlu2/3 receptor agonist in mouse models predictive of antipsychotic activity. J Pharmacol Exp Ther. 2005;315:1181–1187. doi: 10.1124/jpet.105.091074. [DOI] [PubMed] [Google Scholar]
- Galici R, Jones CK, Hemstapat K, Nong Y, Echemendia NG, Williams LC, de Paulis T, Conn PJ. Biphenyl-indanone A, a positive allosteric modulator of the metabotropic glutamate receptor subtype 2, has antipsychotic- and anxiolytic-like effects in mice. J Pharmacol Exp Ther. 2006;318:173–185. doi: 10.1124/jpet.106.102046. [DOI] [PubMed] [Google Scholar]
- Gass JT, McGonigal JT, Chandler LJ. Deficits in the extinction of ethanol-seeking behavior following chronic intermittent ethanol exposure are attenuated with positive allosteric modulation of mGlu5. Neuropharmacology. 2016 doi: 10.1016/j.neuropharm.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal A, Rook JM, Dickerson JW, Roop GN, Morrison RD, Jalan-Sakrikar N, Lamsal A, Noetzel MJ, Poslusney MS, Wood MR, et al. Potentiation of M1 Muscarinic Receptor Reverses Plasticity Deficits and Negative and Cognitive Symptoms in a Schizophrenia Mouse Model. Neuropsychopharmacology. 2016;41:598–610. doi: 10.1038/npp.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour G, Broad LM, Wafford KA, Britton T, Colvin EM, Fivush A, Gastambide F, Getman B, Heinz BA, McCarthy AP, et al. In vitro characterisation of the novel positive allosteric modulators of the mGlu(5) receptor, LSN2463359 and LSN2814617, and their effects on sleep architecture and operant responding in the rat. Neuropharmacology. 2013;64:224–239. doi: 10.1016/j.neuropharm.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Gogliotti RG, Senter RK, Rook JM, Ghoshal A, Zamorano R, Malosh C, Stauffer SR, Bridges TM, Bartolome JM, Daniels JS, et al. mGlu5 positive allosteric modulation normalizes synaptic plasticity defects and motor phenotypes in a mouse model of Rett syndrome. Hum Mol Genet. 2016;25:1990–2004. doi: 10.1093/hmg/ddw074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RW, Amato RJ, Bubser M, Joffe ME, Nedelcovych MT, Thompson AD, Nickols HH, Yuh JP, Zhan X, Felts AS, et al. Partial mGlu(5) Negative Allosteric Modulators Attenuate Cocaine-Mediated Behaviors and Lack Psychotomimetic-Like Effects. Neuropsychopharmacology. 2016;41:1166–1178. doi: 10.1038/npp.2015.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RW, Dencker D, Grannan M, Bubser M, Zhan X, Wess J, Xiang Z, Locuson C, Lindsley CW, Conn PJ, Jones CK. Role for the M1 Muscarinic Acetylcholine Receptor in Top-Down Cognitive Processing Using a Touchscreen Visual Discrimination Task in Mice. ACS Chem Neurosci. 2015;6:1683–1695. doi: 10.1021/acschemneuro.5b00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grannan MD, Mielnik CA, Moran SP, Gould RW, Ball J, Lu Z, Bubser M, Ramsey AJ, Abe M, Cho HP, et al. Prefrontal Cortex-Mediated Impairments in a Genetic Model of NMDA Receptor Hypofunction Are Reversed by the Novel M1 PAM VU6004256. ACS Chem Neurosci. 2016 doi: 10.1021/acschemneuro.6b00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory KJ, Conn PJ. Molecular Insights into Metabotropic Glutamate Receptor Allosteric Modulation. Mol Pharmacol. 2015;88:188–202. doi: 10.1124/mol.114.097220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory KJ, Sexton PM, Christopoulos A. Overview of receptor allosterism. Curr Protoc Pharmacol. 2010;Chapter 1:21. doi: 10.1002/0471141755.ph0121s51. Unit 1. [DOI] [PubMed] [Google Scholar]
- Griebel G, Pichat P, Boulay D, Naimoli V, Potestio L, Featherstone R, Sahni S, Defex H, Desvignes C, Slowinski F, et al. The mGluR2 positive allosteric modulator, SAR218645, improves memory and attention deficits in translational models of cognitive symptoms associated with schizophrenia. Sci Rep. 2016;6:35320. doi: 10.1038/srep35320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Lyon L, Sartorius LJ, Burnet PW, Lane TA. The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J Psychopharmacol. 2008;22:308–322. doi: 10.1177/0269881108089818. [DOI] [PubMed] [Google Scholar]
- Heidinger V, Manzerra P, Wang XQ, Strasser U, Yu SP, Choi DW, Behrens MM. Metabotropic glutamate receptor 1-induced upregulation of NMDA receptor current: mediation through the Pyk2/Src-family kinase pathway in cortical neurons. J Neurosci. 2002;22:5452–5461. doi: 10.1523/JNEUROSCI.22-13-05452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B. Functional Interaction Between NMDA and mGlu5 Receptors: Effects on Working Memory, Instrumental Learning, Motor Behaviors, and Dopamine Release. Neuropsychopharmacology. 2004;29:1259–1269. doi: 10.1038/sj.npp.1300417. [DOI] [PubMed] [Google Scholar]
- Hopkins CR. Is there a path forward for mGlu(2) positive allosteric modulators for the treatment of schizophrenia? ACS Chem Neurosci. 2013;4:211–213. doi: 10.1021/cn400023y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio M, Fujita Y, Hashimoto K. Therapeutic effects of metabotropic glutamate receptor 5 positive allosteric modulator CDPPB on phencyclidine-induced cognitive deficits in mice. Fundam Clin Pharmacol. 2013;27:483–488. doi: 10.1111/j.1472-8206.2012.01045.x. [DOI] [PubMed] [Google Scholar]
- Hubner H, Schellhorn T, Gienger M, Schaab C, Kaindl J, Leeb L, Clark T, Moller D, Gmeiner P. Structure-guided development of heterodimer-selective GPCR ligands. Nat Commun. 2016;7:12298. doi: 10.1038/ncomms12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince E, Ciliax BJ, Levey AI. Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse. 1997;27:357–366. doi: 10.1002/(SICI)1098-2396(199712)27:4<357::AID-SYN9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Jin LE, Wang M, Yang ST, Yang Y, Galvin VC, Lightbourne TC, Ottenheimer D, Zhong Q, Stein J, Raja A, et al. mGluR2/3 mechanisms in primate dorsolateral prefrontal cortex: evidence for both presynaptic and postsynaptic actions. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodo E. The role of the hippocampo-prefrontal cortex system in phencyclidine-induced psychosis: a model for schizophrenia. J Physiol Paris. 2013;107:434–440. doi: 10.1016/j.jphysparis.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Jones CK, Brady AE, Davis AA, Xiang Z, Bubser M, Tantawy MN, Kane AS, Bridges TM, Kennedy JP, Bradley SR, et al. Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J Neurosci. 2008;28:10422–10433. doi: 10.1523/JNEUROSCI.1850-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinichev M, Le Poul E, Bolea C, Girard F, Campo B, Fonsi M, Royer-Urios I, Browne SE, Uslaner JM, Davis MJ, et al. Characterization of the novel positive allosteric modulator of the metabotropic glutamate receptor 4 ADX88178 in rodent models of neuropsychiatric disorders. J Pharmacol Exp Ther. 2014;350:495–505. doi: 10.1124/jpet.114.214437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ. Functional and pharmacological characteristics of metabotropic glutamate receptors 2/4 heterodimers. Mol Pharmacol. 2012;82:438–447. doi: 10.1124/mol.112.078501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157:514–520. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Allosteric modulators: the new generation of receptor antagonist. Mol Interv. 2004;4:222–229. doi: 10.1124/mi.4.4.6. [DOI] [PubMed] [Google Scholar]
- Kenakin T, Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat Rev Drug Discov. 2013;12:205–216. doi: 10.1038/nrd3954. [DOI] [PubMed] [Google Scholar]
- Kinney GG, O'Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK, Chen TB, Wisnoski DD, Lindsley CW, Tiller PR, et al. A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. J Pharmacol Exp Ther. 2005;313:199–206. doi: 10.1124/jpet.104.079244. [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Millen BA, Zhang L, McKinzie DL. Exploratory analysis for a targeted patient population responsive to the metabotropic glutamate 2/3 receptor agonist pomaglumetad methionil in schizophrenia. Biol Psychiatry. 2015;78:754–762. doi: 10.1016/j.biopsych.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Zhang L, Millen BA, Osuntokun OO, Williams JE, Kollack-Walker S, Jackson K, Kryzhanovskaya L, Jarkova N, Group HS. A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia. J Clin Psychopharmacol. 2011;31:349–355. doi: 10.1097/JCP.0b013e318218dcd5. [DOI] [PubMed] [Google Scholar]
- Kiyonaka S, Kubota R, Michibata Y, Sakakura M, Takahashi H, Numata T, Inoue R, Yuzaki M, Hamachi I. Allosteric activation of membrane-bound glutamate receptors using coordination chemistry within living cells. Nat Chem advance. 2016 doi: 10.1038/nchem.2554. online publication. [DOI] [PubMed] [Google Scholar]
- Kristiansen K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther. 2004;103:21–80. doi: 10.1016/j.pharmthera.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Kruse AC, Kobilka BK, Gautam D, Sexton PM, Christopoulos A, Wess J. Muscarinic acetylcholine receptors: novel opportunities for drug development. Nat Rev Drug Discov. 2014;13:549–560. doi: 10.1038/nrd4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse AC, Ring AM, Manglik A, Hu J, Hu K, Eitel K, Hubner H, Pardon E, Valant C, Sexton PM, et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature. 2013;504:101–106. doi: 10.1038/nature12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita M, Holloway T, Garcia-Bea A, Kozlenkov A, Friedman AK, Moreno JL, Heshmati M, Golden SA, Kennedy PJ, Takahashi N, et al. HDAC2 regulates atypical antipsychotic responses through the modulation of mGlu2 promoter activity. Nat Neurosci. 2012;15:1245–1254. doi: 10.1038/nn.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane JR, Donthamsetti P, Shonberg J, Draper-Joyce CJ, Dentry S, Michino M, Shi L, Lopez L, Scammells PJ, Capuano B, et al. A new mechanism of allostery in a G protein-coupled receptor dimer. Nat Chem Biol. 2014;10:745–752. doi: 10.1038/nchembio.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange HS, Cannon CE, Drott JT, Kuduk SD, Uslaner JM. The M1 Muscarinic Positive Allosteric Modulator PQCA Improves Performance on Translatable Tests of Memory and Attention in Rhesus Monkeys. J Pharmacol Exp Ther. 2015;355:442–450. doi: 10.1124/jpet.115.226712. [DOI] [PubMed] [Google Scholar]
- Langmead CJ, Watson J, Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther. 2008;117:232–243. doi: 10.1016/j.pharmthera.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Latorraca NR, Venkatakrishnan AJ, Dror RO. GPCR Dynamics: Structures in Motion. Chem Rev. 2016 doi: 10.1021/acs.chemrev.6b00177. [DOI] [PubMed] [Google Scholar]
- Leach K, Loiacono RE, Felder CC, McKinzie DL, Mogg A, Shaw DB, Sexton PM, Christopoulos A. Molecular mechanisms of action and in vivo validation of an M4 muscarinic acetylcholine receptor allosteric modulator with potential antipsychotic properties. Neuropsychopharmacology. 2010;35:855–869. doi: 10.1038/npp.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach K, Sexton PM, Christopoulos A. Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol Sci. 2007;28:382–389. doi: 10.1016/j.tips.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Levitz J, Habrian C, Bharill S, Fu Z, Vafabakhsh R, Isacoff EY. Mechanism of Assembly and Cooperativity of Homomeric and Heteromeric Metabotropic Glutamate Receptors. Neuron. 2016 doi: 10.1016/j.neuron.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz J, Pantoja C, Gaub B, Janovjak H, Reiner A, Hoagland A, Schoppik D, Kane B, Stawski P, Schier AF, et al. Optical control of metabotropic glutamate receptors. Nat Neurosci. 2013;16:507–516. doi: 10.1038/nn.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MA, Hunihan L, Watson J, Gentles RG, Hu S, Huang Y, Bronson J, Macor JE, Beno BR, Ferrante M, et al. Discovery of D1 Dopamine Receptor Positive Allosteric Modulators: Characterization of Pharmacology and Identification of Residues that Regulate Species Selectivity. J Pharmacol Exp Ther. 2015;354:340–349. doi: 10.1124/jpet.115.224071. [DOI] [PubMed] [Google Scholar]
- Lin CW, Chen CY, Cheng SJ, Hu HT, Hsueh YP. Sarm1 deficiency impairs synaptic function and leads to behavioral deficits, which can be ameliorated by an mGluR allosteric modulator. Front Cell Neurosci. 2014;8:87. doi: 10.3389/fncel.2014.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman RE, Smith MA, Doherty JJ, Cross A, Raines S, Gertsik L, Zukin SR. AZD8529, a positive allosteric modulator at the mGluR2 receptor, does not improve symptoms in schizophrenia: A proof of principle study. Schizophr Res. 2016;172:152–157. doi: 10.1016/j.schres.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Liu F, Grauer S, Kelley C, Navarra R, Graf R, Zhang G, Atkinson PJ, Popiolek M, Wantuch C, Khawaja X, et al. ADX47273 [S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piperidin-1- yl}-methanone]: a novel metabotropic glutamate receptor 5-selective positive allosteric modulator with preclinical antipsychotic-like and procognitive activities. J Pharmacol Exp Ther. 2008;327:827–839. doi: 10.1124/jpet.108.136580. [DOI] [PubMed] [Google Scholar]
- Lopez De Jesus M, Stope MB, Oude Weernink PA, Mahlke Y, Borgermann C, Ananaba VN, Rimmbach C, Rosskopf D, Michel MC, Jakobs KH, Schmidt M. Cyclic AMP-dependent and Epac-mediated activation of R-Ras by G protein-coupled receptors leads to phospholipase D stimulation. J Biol Chem. 2006;281:21837–21847. doi: 10.1074/jbc.M604156200. [DOI] [PubMed] [Google Scholar]
- Lovell KM, Felts AS, Rodriguez AL, Venable DF, Cho HP, Morrison RD, Byers FW, Daniels JS, Niswender CM, Conn PJ, et al. N-Acyl-N'-arylpiperazines as negative allosteric modulators of mGlu1: identification of VU0469650, a potent and selective tool compound with CNS exposure in rats. Bioorg Med Chem Lett. 2013;23:3713–3718. doi: 10.1016/j.bmcl.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum EN, Campbell RR, Rostock C, Szumlinski KK. mGluR1 within the nucleus accumbens regulates alcohol intake in mice under limited-access conditions. Neuropharmacology. 2014;79:679–687. doi: 10.1016/j.neuropharm.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon L, Burnet PW, Kew JN, Corti C, Rawlins JN, Lane T, De Filippis B, Harrison PJ, Bannerman DM. Fractionation of spatial memory in GRM2/3 (mGlu2/mGlu3) double knockout mice reveals a role for group II metabotropic glutamate receptors at the interface between arousal and cognition. Neuropsychopharmacology. 2011;36:2616–2628. doi: 10.1038/npp.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Seager MA, Wittmann M, Jacobson M, Bickel D, Burno M, Jones K, Graufelds VK, Xu G, Pearson M, et al. Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc Natl Acad Sci U S A. 2009;106:15950–15955. doi: 10.1073/pnas.0900903106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet EL, Pellegrini N, Valant C, Bucher B, Hibert M, Bourguignon JJ, Galzi JL. A novel, conformation-specific allosteric inhibitor of the tachykinin NK2 receptor (NK2R) with functionally selective properties. FASEB J. 2007;21:2124–2134. doi: 10.1096/fj.06-7683com. [DOI] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci. 2001;21:5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MJ, Conn PJ. Direct and indirect modulation of the N-methyl D-aspartate receptor. Curr Drug Targets CNS Neurol Disord. 2002;1:1–16. doi: 10.2174/1568007023339544. [DOI] [PubMed] [Google Scholar]
- Marlo JE, Niswender CM, Days EL, Bridges TM, Xiang Y, Rodriguez AL, Shirey JK, Brady AE, Nalywajko T, Luo Q, et al. Discovery and characterization of novel allosteric potentiators of M1 muscarinic receptors reveals multiple modes of activity. Mol Pharmacol. 2009;75:577–588. doi: 10.1124/mol.108.052886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen JM, Ulven T, Martini L, Gerlach LO, Heinemann A, Kostenis E. Identification of indole derivatives exclusively interfering with a G protein-independent signaling pathway of the prostaglandin D2 receptor CRTH2. Mol Pharmacol. 2005;68:393–402. doi: 10.1124/mol.104.010520. [DOI] [PubMed] [Google Scholar]
- May LT, Leach K, Sexton PM, Christopoulos A. Allosteric modulation of G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2007;47:1–51. doi: 10.1146/annurev.pharmtox.47.120505.105159. [DOI] [PubMed] [Google Scholar]
- Michalon A, Sidorov M, Ballard TM, Ozmen L, Spooren W, Wettstein JG, Jaeschke G, Bear MF, Lindemann L. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron. 2012;74:49–56. doi: 10.1016/j.neuron.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Andrieux A, Bartzokis G, Cadenhead K, Dazzan P, Fusar-Poli P, Gallinat J, Giedd J, Grayson DR, Heinrichs M, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15:485–515. doi: 10.1038/nrd.2016.28. [DOI] [PubMed] [Google Scholar]
- Milligan G. The prevalence, maintenance, and relevance of G protein-coupled receptor oligomerization. Mol Pharmacol. 2013;84:158–169. doi: 10.1124/mol.113.084780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry SN, Shonberg J, Draper-Joyce CJ, Klein Herenbrink C, Michino M, Shi L, Christopoulos A, Capuano B, Scammells PJ, Lane JR. Discovery of a Novel Class of Negative Allosteric Modulator of the Dopamine D2 Receptor Through Fragmentation of a Bitopic Ligand. J Med Chem. 2015;58:6819–6843. doi: 10.1021/acs.jmedchem.5b00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockett BG, Hulme SR. Metaplasticity: new insights through electrophysiological investigations. J Integr Neurosci. 2008;7:315–336. doi: 10.1142/s0219635208001782. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology (Berl) 2004;174:39–44. doi: 10.1007/s00213-004-1792-z. [DOI] [PubMed] [Google Scholar]
- Moreno JL, Miranda-Azpiazu P, Garcia-Bea A, Younkin J, Cui M, Kozlenkov A, Ben-Ezra A, Voloudakis G, Fakira AK, Baki L, et al. Allosteric signaling through an mGlu2 and 5-HT2A heteromeric receptor complex and its potential contribution to schizophrenia. Sci Signal. 2016;9:ra5. doi: 10.1126/scisignal.aab0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muguruza C, Meana JJ, Callado LF. Group II Metabotropic Glutamate Receptors as Targets for Novel Antipsychotic Drugs. Front Pharmacol. 2016;7:130. doi: 10.3389/fphar.2016.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickols HH, Conn PJ. Development of allosteric modulators of GPCRs for treatment of CNS disorders. Neurobiol Dis. 2014;61:55–71. doi: 10.1016/j.nbd.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickols HH, Yuh JP, Gregory KJ, Morrison RD, Bates BS, Stauffer SR, Emmitte KA, Bubser M, Peng W, Nedelcovych MT, et al. VU0477573: Partial Negative Allosteric Modulator of the Subtype 5 Metabotropic Glutamate Receptor with In Vivo Efficacy. J Pharmacol Exp Ther. 2016;356:123–136. doi: 10.1124/jpet.115.226597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F, Bruno V, Ngomba RT, Gradini R, Battaglia G. Metabotropic glutamate receptors as drug targets: what's new? Curr Opin Pharmacol. 2015;20:89–94. doi: 10.1016/j.coph.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Johnson KA, Miller NR, Ayala JE, Luo Q, Williams R, Saleh S, Orton D, Weaver CD, Conn PJ. Context-dependent pharmacology exhibited by negative allosteric modulators of metabotropic glutamate receptor 7. Mol Pharmacol. 2010;77:459–468. doi: 10.1124/mol.109.058768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Jones CK, Lin X, Bubser M, Thompson Gray A, Blobaum AL, Engers DW, Rodriguez AL, Loch MT, Daniels JS, et al. Development and Antiparkinsonian Activity of VU0418506, a Selective Positive Allosteric Modulator of Metabotropic Glutamate Receptor 4 Homomers without Activity at mGlu2/4 Heteromers. ACS Chem Neurosci. 2016 doi: 10.1021/acschemneuro.6b00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noetzel MJ, Gregory KJ, Vinson PN, Manka JT, Stauffer SR, Lindsley CW, Niswender CM, Xiang Z, Conn PJ. A novel metabotropic glutamate receptor 5 positive allosteric modulator acts at a unique site and confers stimulus bias to mGlu5 signaling. Mol Pharmacol. 2013;83:835–847. doi: 10.1124/mol.112.082891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- Oswald C, Rappas M, Kean J, Dore AS, Errey JC, Bennett K, Deflorian F, Christopher JA, Jazayeri A, Mason JS, et al. Intracellular allosteric antagonism of the CCR9 receptor. Nature. 2016;540:462–465. doi: 10.1038/nature20606. [DOI] [PubMed] [Google Scholar]
- Pancani T, Bolarinwa C, Smith Y, Lindsley CW, Conn PJ, Xiang Z. M4 mAChR-mediated modulation of glutamatergic transmission at corticostriatal synapses. ACS Chem Neurosci. 2014;5:318–324. doi: 10.1021/cn500003z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancani T, Foster DJ, Moehle MS, Bichell TJ, Bradley E, Bridges TM, Klar R, Poslusney M, Rook JM, Daniels JS, et al. Allosteric activation of M4 muscarinic receptors improve behavioral and physiological alterations in early symptomatic YAC128 mice. Proc Natl Acad Sci U S A. 2015;112:14078–14083. doi: 10.1073/pnas.1512812112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier-Batteur S, Hutson PH, Menzel K, Uslaner JM, Mattson BA, O'Brien JA, Magliaro BC, Forest T, Stump CA, Tynebor RM, et al. Mechanism based neurotoxicity of mGlu5 positive allosteric modulators--development challenges for a promising novel antipsychotic target. Neuropharmacology. 2014;82:161–173. doi: 10.1016/j.neuropharm.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Pittolo S, Gomez-Santacana X, Eckelt K, Rovira X, Dalton J, Goudet C, Pin JP, Llobet A, Giraldo J, Llebaria A, Gorostiza P. An allosteric modulator to control endogenous G protein-coupled receptors with light. Nat Chem Biol. 2014;10:813–815. doi: 10.1038/nchembio.1612. [DOI] [PubMed] [Google Scholar]
- Power EM, English NA, Empson RM. Are Type 1 metabotropic glutamate receptors a viable therapeutic target for the treatment of cerebellar ataxia? J Physiol. 2016;594:4643–4652. doi: 10.1113/JP271153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri V, Wang X, Vardigan JD, Kuduk SD, Uslaner JM. The selective positive allosteric M1 muscarinic receptor modulator PQCA attenuates learning and memory deficits in the Tg2576 Alzheimer's disease mouse model. Behav Brain Res. 2015;287:96–99. doi: 10.1016/j.bbr.2015.03.029. [DOI] [PubMed] [Google Scholar]
- Raedler TJ, Bymaster FP, Tandon R, Copolov D, Dean B. Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry. 2007;12:232–246. doi: 10.1038/sj.mp.4001924. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Harris MS, Khine TT, Keshavan MS, Sweeney JA. Antipsychotic drugs exacerbate impairment on a working memory task in first-episode schizophrenia. Biol Psychiatry. 2007;62:818–821. doi: 10.1016/j.biopsych.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Rice ME, Patel JC, Cragg SJ. Dopamine release in the basal ganglia. Neuroscience. 2011;198:112–137. doi: 10.1016/j.neuroscience.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AL, Grier MD, Jones CK, Herman EJ, Kane AS, Smith RL, Williams R, Zhou Y, Marlo JE, Days EL, et al. Discovery of novel allosteric modulators of metabotropic glutamate receptor subtype 5 reveals chemical and functional diversity and in vivo activity in rat behavioral models of anxiolytic and antipsychotic activity. Mol Pharmacol. 2010;78:1105–1123. doi: 10.1124/mol.110.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AL, Nong Y, Sekaran NK, Alagille D, Tamagnan GD, Conn PJ. A close structural analog of 2-methyl-6-(phenylethynyl)-pyridine acts as a neutral allosteric site ligand on metabotropic glutamate receptor subtype 5 and blocks the effects of multiple allosteric modulators. Mol Pharmacol. 2005;68:1793–1802. doi: 10.1124/mol.105.016139. [DOI] [PubMed] [Google Scholar]
- Rook JM, Noetzel MJ, Pouliot WA, Bridges TM, Vinson PN, Cho HP, Zhou Y, Gogliotti RD, Manka JT, Gregory KJ, et al. Unique signaling profiles of positive allosteric modulators of metabotropic glutamate receptor subtype 5 determine differences in in vivo activity. Biol Psychiatry. 2013;73:501–509. doi: 10.1016/j.biopsych.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook JM, Xiang Z, Lv X, Ghoshal A, Dickerson JW, Bridges TM, Johnson KA, Foster DJ, Gregory KJ, Vinson PN, et al. Biased mGlu5-Positive Allosteric Modulators Provide In Vivo Efficacy without Potentiating mGlu5 Modulation of NMDAR Currents. Neuron. 2015;86:1029–1040. doi: 10.1016/j.neuron.2015.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell DR, Zaluda LC, McClure MM, Perez-Rodriguez MM, Strike KS, Barch DM, Harvey PD, Girgis RR, Hazlett EA, Mailman RB, et al. Effects of the D1 dopamine receptor agonist dihydrexidine (DAR-0100A) on working memory in schizotypal personality disorder. Neuropsychopharmacology. 2015;40:446–453. doi: 10.1038/npp.2014.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira X, Trapero A, Pittolo S, Zussy C, Faucherre A, Jopling C, Giraldo J, Pin JP, Gorostiza P, Goudet C, Llebaria A. OptoGluNAM4.1, a Photoswitchable Allosteric Antagonist for Real-Time Control of mGlu4 Receptor Activity. Cell Chem Biol. 2016 doi: 10.1016/j.chembiol.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Rylander D, Iderberg H, Li Q, Dekundy A, Zhang J, Li H, Baishen R, Danysz W, Bezard E, Cenci MA. A mGluR5 antagonist under clinical development improves L-DOPA-induced dyskinesia in parkinsonian rats and monkeys. Neurobiol Dis. 2010;39:352–361. doi: 10.1016/j.nbd.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Salih H, Anghelescu I, Kezic I, Sinha V, Hoeben E, Van Nueten L, De Smedt H, De Boer P. Pharmacokinetic and pharmacodynamic characterisation of JNJ-40411813, a positive allosteric modulator of mGluR2, in two randomised, double-blind phase-I studies. J Psychopharmacol. 2015;29:414–425. doi: 10.1177/0269881115573403. [DOI] [PubMed] [Google Scholar]
- Sarihi A, Jiang B, Komaki A, Sohya K, Yanagawa Y, Tsumoto T. Metabotropic glutamate receptor type 5-dependent long-term potentiation of excitatory synapses on fast-spiking GABAergic neurons in mouse visual cortex. J Neurosci. 2008;28:1224–1235. doi: 10.1523/JNEUROSCI.4928-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa A, Snyder SH. Schizophrenia: neural mechanisms for novel therapies. Mol Med. 2003;9:3–9. [PMC free article] [PubMed] [Google Scholar]
- Scarr E, Craig JM, Cairns MJ, Seo MS, Galati JC, Beveridge NJ, Gibbons A, Juzva S, Weinrich B, Parkinson-Bates M, et al. Decreased cortical muscarinic M1 receptors in schizophrenia are associated with changes in gene promoter methylation, mRNA and gene targeting microRNA. Transl Psychiatry. 2013;3:e230. doi: 10.1038/tp.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffler DJ, Conn PJ. Allosteric potentiators of metabotropic glutamate receptor subtype 1a differentially modulate independent signaling pathways in baby hamster kidney cells. Neuropharmacology. 2008;55:419–427. doi: 10.1016/j.neuropharm.2008.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, Potter WZ, Lightfoot J, Lienemann J, Dube S, Mallinckrodt C, Bymaster FP, McKinzie DL, Felder CC. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165:1033–1039. doi: 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- Shen W, Plotkin JL, Francardo V, Ko WK, Xie Z, Li Q, Fieblinger T, Wess J, Neubig RR, Lindsley CW, et al. M4 Muscarinic Receptor Signaling Ameliorates Striatal Plasticity Deficits in Models of L-DOPA-Induced Dyskinesia. Neuron. 2016;90:1139. doi: 10.1016/j.neuron.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Adrover MF, Wess J, Alvarez VA. Muscarinic regulation of dopamine and glutamate transmission in the nucleus accumbens. Proc Natl Acad Sci U S A. 2015;112:8124–8129. doi: 10.1073/pnas.1508846112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirey JK, Brady AE, Jones PJ, Davis AA, Bridges TM, Kennedy JP, Jadhav SB, Menon UN, Xiang Z, Watson ML, et al. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J Neurosci. 2009;29:14271–14286. doi: 10.1523/JNEUROSCI.3930-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirey JK, Xiang Z, Orton D, Brady AE, Johnson KA, Williams R, Ayala JE, Rodriguez AL, Wess J, Weaver D, et al. An allosteric potentiator of M4 mAChR modulates hippocampal synaptic transmission. Nat Chem Biol. 2008;4:42–50. doi: 10.1038/nchembio.2007.55. [DOI] [PubMed] [Google Scholar]
- Slawinska A, Wieronska JM, Stachowicz K, Marciniak M, Lason-Tyburkiewicz M, Gruca P, Papp M, Kusek M, Tokarski K, Doller D, Pilc A. The antipsychotic-like effects of positive allosteric modulators of metabotropic glutamate mGlu4 receptors in rodents. Br J Pharmacol. 2013;169:1824–1839. doi: 10.1111/bph.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NJ, Milligan G. Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes. Pharmacol Rev. 2010;62:701–725. doi: 10.1124/pr.110.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooren WP, Gasparini F, van der Putten H, Koller M, Nakanishi S, Kuhn R. Lack of effect of LY314582 (a group 2 metabotropic glutamate receptor agonist) on phencyclidine-induced locomotor activity in metabotropic glutamate receptor 2 knockout mice. Eur J Pharmacol. 2000;397:R1–2. doi: 10.1016/s0014-2999(00)00269-7. [DOI] [PubMed] [Google Scholar]
- Staus DP, Strachan RT, Manglik A, Pani B, Kahsai AW, Kim TH, Wingler LM, Ahn S, Chatterjee A, Masoudi A, et al. Allosteric nanobodies reveal the dynamic range and diverse mechanisms of G-protein-coupled receptor activation. Nature. 2016;535:448–452. doi: 10.1038/nature18636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Activation of type 5 metabotropic glutamate receptors attenuates deficits in cognitive flexibility induced by NMDA receptor blockade. Eur J Pharmacol. 2010;639:26–32. doi: 10.1016/j.ejphar.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strube W, Bunse T, Nitsche MA, Wobrock T, Aborowa R, Misewitsch K, Herrmann M, Falkai P, Hasan A. Smoking restores impaired LTD-like plasticity in schizophrenia: a transcranial direct current stimulation study. Neuropsychopharmacology. 2015;40:822–830. doi: 10.1038/npp.2014.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Lipton JO, Boyle LM, Madsen JR, Goldenberg MC, Pascual-Leone A, Sahin M, Rotenberg A. Direct current stimulation induces mGluR5-dependent neocortical plasticity. Ann Neurol. 2016;80:233–246. doi: 10.1002/ana.24708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suratman S, Leach K, Sexton P, Felder C, Loiacono R, Christopoulos A. Impact of species variability and ‘probe-dependence’ on the detection and in vivo validation of allosteric modulation at the M4 muscarinic acetylcholine receptor. Br J Pharmacol. 2011;162:1659–1670. doi: 10.1111/j.1476-5381.2010.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson KA, Heinz BA, Schaus JM, Beck JP, Hao J, Krushinski JH, Reinhard MR, Cohen MP, Hellman SL, Getman BG, et al. An Allosteric Potentiator of the Dopamine D1 Receptor Increases Locomotor Activity in Human D1 Knock-in Mice without Causing Stereotypy or Tachyphylaxis. J Pharmacol Exp Ther. 2016 doi: 10.1124/jpet.116.236372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HY, Chen Q, Sust S, Buckholtz JW, Meyers JD, Egan MF, Mattay VS, Meyer-Lindenberg A, Weinberger DR, Callicott JH. Epistasis between catechol-O-methyltransferase and type II metabotropic glutamate receptor 3 genes on working memory brain function. Proc Natl Acad Sci U S A. 2007;104:12536–12541. doi: 10.1073/pnas.0610125104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DM, Sun B, Feng D, Nawaratne V, Leach K, Felder CC, Bures MG, Evans DA, Weis WI, Bachhawat P, et al. Crystal structures of the M1 and M4 muscarinic acetylcholine receptors. Nature. 2016;531:335–340. doi: 10.1038/nature17188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomases DR, Cass DK, Meyer JD, Caballero A, Tseng KY. Early adolescent MK-801 exposure impairs the maturation of ventral hippocampal control of basolateral amygdala drive in the adult prefrontal cortex. J Neurosci. 2014;34:9059–9066. doi: 10.1523/JNEUROSCI.1395-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Clements MA, Khodai T, Pienaar IS, Exley R, Wess J, Cragg SJ. Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J Neurosci. 2010;30:3398–3408. doi: 10.1523/JNEUROSCI.5620-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugolini A, Corsi M, Bordi F. Potentiation of NMDA and AMPA responses by the specific mGluR5 agonist CHPG in spinal cord motoneurons. Neuropharmacology. 1999;38:1569–1576. doi: 10.1016/s0028-3908(99)00095-7. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JS, McNaughton CH, Jacobson MA, Hutson PH. Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology. 2009;57:531–538. doi: 10.1016/j.neuropharm.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Vardigan JD, Cannon CE, Puri V, Dancho M, Koser A, Wittmann M, Kuduk SD, Renger JJ, Uslaner JM. Improved cognition without adverse effects: novel M1 muscarinic potentiator compares favorably to donepezil and xanomeline in rhesus monkey. Psychopharmacology (Berl) 2015;232:1859–1866. doi: 10.1007/s00213-014-3813-x. [DOI] [PubMed] [Google Scholar]
- Vargas AJ, Harris CC. Biomarker development in the precision medicine era: lung cancer as a case study. Nat Rev Cancer. 2016;16:525–537. doi: 10.1038/nrc.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicidomini C, Ponzoni L, Lim D, Schmeisser MJ, Reim D, Morello N, Orellana D, Tozzi A, Durante V, Scalmani P, et al. Pharmacological enhancement of mGlu5 receptors rescues behavioral deficits in SHANK3 knock-out mice. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AG, Wenthur CJ, Xiang Z, Rook JM, Emmitte KA, Niswender CM, Lindsley CW, Conn PJ. Metabotropic glutamate receptor 3 activation is required for long-term depression in medial prefrontal cortex and fear extinction. Proc Natl Acad Sci U S A. 2015;112:1196–1201. doi: 10.1073/pnas.1416196112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Huber KM. Protein translation in synaptic plasticity: mGluR-LTD, Fragile X. Curr Opin Neurobiol. 2009;19:319–326. doi: 10.1016/j.conb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci U S A. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise A, Gearing K, Rees S. Target validation of G-protein coupled receptors. Drug Discov Today. 2002;7:235–246. doi: 10.1016/s1359-6446(01)02131-6. [DOI] [PubMed] [Google Scholar]
- Won H, Lee HR, Gee HY, Mah W, Kim JI, Lee J, Ha S, Chung C, Jung ES, Cho YS, et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–265. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Duffy B, Karbasforoushan H. Prefrontal cortex activity during response selection predicts processing speed impairment in schizophrenia. J Int Neuropsychol Soc. 2013;19:782–791. doi: 10.1017/S1355617713000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootten D, Christopoulos A, Sexton PM. Emerging paradigms in GPCR allostery: implications for drug discovery. Nat Rev Drug Discov. 2013;12:630–644. doi: 10.1038/nrd4052. [DOI] [PubMed] [Google Scholar]
- Wu H, Wang C, Gregory KJ, Han GW, Cho HP, Xia Y, Niswender CM, Katritch V, Meiler J, Cherezov V, et al. Structure of a class C GPCR metabotropic glutamate receptor 1 bound to an allosteric modulator. Science. 2014;344:58–64. doi: 10.1126/science.1249489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S, Noetzel MJ, Johnson KA, Zamorano R, Jalan-Sakrikar N, Gregory KJ, Conn PJ, Niswender CM. Selective actions of novel allosteric modulators reveal functional heteromers of metabotropic glutamate receptors in the CNS. J Neurosci. 2014;34:79–94. doi: 10.1523/JNEUROSCI.1129-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Rodriguez AL, Conn PJ. Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. J Pharmacol Exp Ther. 2005;315:1212–1219. doi: 10.1124/jpet.105.090308. [DOI] [PubMed] [Google Scholar]


