Abstract
Purpose
The Diabetic Macular Edema Treated with Ozurdex (DMEO) Trial measured aqueous pro-permeability factors (PPFs) in diabetic macular edema (DME) patients before and after injection of dexamethasone implant or vascular endothelial growth factor (VEGF)-neutralizing protein and correlated changes in levels with changes in excess foveal thickness (EFT) to identify potential PPFs contributing to DME.
Design
Prospective, randomized cross-over clinical trial
Methods
Twenty DME patients randomized to dexamethasone implant or VEGF-neutralizing protein had aqueous taps and spectral domain-optical coherence tomography (SD-OCT) at baseline and every 4 weeks for 28 weeks. Aqueous levels of 55 vasoactive proteins were measured with protein array. Cross-over at week 16 provided changes in protein levels after each intervention in all 20 patients.
Results
After dexamethasone implant there was significant correlation between changes in levels of 13 vasoactive proteins with changes in EFT, including three known PPFs, angiopoietin-2 (r=0.40, p=0.001), hepatocyte growth factor (HGF, r=0.31, p=0.02), and endocrine gland-VEGF (EG-VEGF, r=0.43, p<0.001). Reduction of prolactin, insulin-like growth factor binding protein-3, and matrix metalloproteinase-9 correlated with edema reduction after injection of a VEGF-neutralizing protein as well as dexamethasone implant suggesting their modulation is likely secondary to changes in edema rather than causative.
Conclusions
Correlation of edema reduction with reduction in the PPFs angiopoietin-2, HGF, and EG-VEGF provides potential insight into the multi-factorial molecular mechanism by which dexamethasone implants reduce edema and suggest that additional study is needed to investigate the contributions of these 3 factor to chronic DME.
Introduction
Diabetic macular edema (DME) is a common complication of diabetes, estimated to be present in 3.8% or 746,000 people ≥40 years in the US in 2010.1 Due to increasing obesity and demographic changes, the world-wide prevalence of diabetes is rapidly increasing2 resulting in large increases in the prevalence of DME. Therefore DME is a large public health problem that is getting larger.
Retinal hypoxia plays an important role in the pathogenesis of DME.3 In hypoxic retina, stabilization of hypoxia-inducible factor-1α (HIF-1α) results in more binding to HIF-1β to form elevated levels of HIF-1 heterodimer and upregulation of vascular endothelial growth factor (VEGF) and other hypoxia-regulated gene products.4 A pilot trial demonstrated that suppression of VEGF caused remarkable reductions in DME, implicating VEGF as an important contributor to DME.5 Multiple large multicenter trials have confirmed that VEGF plays a key role in DME and determined that intraocular injections of a VEGF-neutralizing protein provide substantial visual benefit in most patients,6–9 but there are some patients in whom anti-VEGF injections are not sufficient to eliminate edema.10, 11 This suggests that other pro-permeability factors in addition to VEGF contribute in some patients with DME.
Corticosteroids bind to cytoplasmic receptors that translocate to the nucleus and cause transcriptional repression of a large number of genes whose products promote inflammation, vascular leakage, and/or angiogenesis.12–14 The ability to reduce a large number of vasoactive factors provides a potential advantage particularly when the identity of all of the contributors is unknown. The dexamethasone implant (Ozurdex) causes significant reduction in DME and improvement in visual acuity,15 but its precise mechanism of action in DME is unknown. In this study, we investigated the mechanism of action of the dexamethasone implant in DME by measuring changes in aqueous vasoactive factors and correlating them with changes in edema. As a comparator, changes in aqueous vasoactive factors were correlated with changes in edema after intraocular injections of a VEGF-neutralizing protein.
Methods
Study Procedures
The Diabetic Macular Edema Treated with Ozurdex (DMEO) Study was an investigator-initiated study sponsored by Allergan, Inc. (Irvine, CA). The protocol was designed by the investigators who controlled all aspects of the study with no influence from the sponsor. The protocol was approved by the Institutional Review Board of the Johns Hopkins Medical Institutions and was conducted in compliance with the Declaration of Helsinki, US Code 21 of Federal Regulations, and the Harmonized “Tripartite Guidelines for Good Clinical Practice (1996). The study was registered on February 8, 2013 at www.clinicaltrials.gov (NCT01790685). All patients provided informed consent. Twenty subjects with DME were randomized to group 1 (dexamethasone implant/anti-VEGF) or group 2 (anti-VEGF/dexamethasone implant) by the Reading Center and remained masked to group assignment. Randomization sequence was generated using Stata 9.0 (StataCorp, College Station, TX) statistical software and was stratified by the central subfield thickness (CST >450μm or <450μm) with a 1:1 allocation by the Reading Center.
Disease duration was determined from patient reporting and review of records, but only injections documented in records were used to determine the number of prior anti-VEGF injections. Response to prior anti-VEGF therapy was graded as good, moderate, or poor depending upon whether all intraretinal fluid could be eliminated by monthly injections or how frequently anti-VEGF injections had to be given to maintain a dry macula. At baseline and all subsequent visits, subjects had measurement of best-corrected visual acuity (BCVA) using the Early Treatment Diabetic Retinopathy Study (ETDRS) protocol with examiners masked with regard to treatment group, ophthalmologic examination including measurement of intraocular pressure, spectral domain-optical coherence tomography (SD-OCT) using the Spectralis machine (Heidelberg Engineering, Inc., Carlsbad, CA), and an anterior chamber tap. Aqueous samples were stored at −80°C. At baseline, group 1 patients were given an intraocular injection of a dexamethasone implant in the study eye and group 2 patients were given an anti-VEGF injection. For dexamethasone implant injections, povidone-iodine was used to clean the conjunctiva and 2% lidocaine was injected under the conjunctiva. The 22-gauge needle of the injector was inserted through the pars plana and the dexamethasone implant was injected into the vitreous cavity. The procedure was similar for anti-VEGF injections except topical anesthesia and a 30-gauge needle were used. Patients in group 1 were crossed-over to prn anti-VEGF injections and patients in group 2 were crossed-over to dexamethasone implant at the first visit after week 12 at which there was recurrent/persistent edema.
Anatomic and functional outcomes
The major anatomic outcome was excess foveal thickness (EFT) which provides an assessment of the amount of edema: it is calculated by subtracting edema-free central subfield thickness (CST) from measured CST. Normal CST varies among patients, which is particularly true for patients with chronic macular edema who often experience some atrophy from the chronic edema resulting in a relatively thin edema-free CST. Using a single CST threshold such as 320μm as an indicator of a dry retina could substantially over-estimate edema reduction in many patients. Therefore, we used all information at our disposal to obtain the most accurate assessment of edema-free CST and hence change in EFT in each patient. For most patients, it was possible to identify one or more OCT scan obtained during the study or prior to enrollment on which there was no or minimal edema, but for the few subjects in whom such information was not available, 320μm was used for normal CST. The functional outcome measure was change from baseline BCVA in ETDRS letter score at each visit. Mixed effects regression models with a random intercept for eyes to account for the correlation among the repeated measures from the same eye were used to determine if BCVA or CST at each follow up visit was different from baseline.
Vasoactive protein arrays
The levels of vasoactive proteins in aqueous at baseline and each subsequent visit were measured using the Human Angiogenesis Antibody Array Kit (catalog number ARY007, R&D Systems, Inc., Minneapolis, MN). Aqueous samples (115 μl) were blotted on the membrane which was stored for 16 hours at 4°C and further processed according to the manufacturer’s instructions. Each array membrane was exposed to X-ray film for short-term and long-term exposures. The positive signals detected on the developed X-ray film were quantitated using TotalLab Quant software (Gentel Biosciences, Inc., Madison, WI).
Correlation of changes in vasoactive proteins with changes in edema
The diversity of protein levels and center subfield thickness were calculated as fold change(log2) in their measurements at each visit relative to appropriate baseline.
Where, FCProtein and FCEFT are the fold changes(log2) in protein level and excess foveal thickness, ProteinBaseline and ProteinVisit are the protein levels at baseline and visit of interest, and EFTBaseline and EFTVisit are the excess foveal thickness at baseline and the visit of interest. The Kolmogorov-Smirnov test demonstrated a normal distribution of FCProtein and FCEFT for each of the factors; therefore, Pearson correlation coefficients were calculated using R.
Results
Patient Demographics and Baseline Characteristics
Most of the patients enrolled in the DMEO study had chronic/recurrent DME. The mean duration of DME was 48.1 months and the mean number of prior anti-VEGF injections was 13.3 (Table 1). Medical records and OCT images were sufficient to assess prior response to anti-VEGF injections in 15 of the 20 patients. Two patients had a good response defined as elimination of 80–90% of the intraretinal fluid with anti-VEGF injections, 2 patients had moderate response defined as elimination of 30–80% of intraretinal fluid during frequent injections and 11 had poor response defined as <30% reduction in intraretinal fluid during periods of monthly injections. Twenty-five % of patients had previously been treated with intraocular steroids, 30% had received focal/grid laser photocoagulation, and 30% had received scatter photocoagulation for proliferative diabetic retinopathy. The mean BCVA at baseline was 56.1 ETDRS letter score (20/80) and the mean CST at baseline was 464.4 μm. Baseline intraretinal fluid was qualitatively graded as mild in 3 patients, moderate in 14 patients, and severe in 3 patients.
Table 1.
Patient Demographics and Baseline Characteristics
| Variable | (N=20) |
|---|---|
|
| |
| Age in years mean (range) | 61.8(47–75) |
|
| |
| Gender/Females n (%) | 6(30) |
|
| |
| Mean disease duration (months) | 48.1 |
|
| |
| Baseline Intraretinal Fluid, n (%) | |
| Mild | 3(15) |
| Moderate | 14(70) |
| Severe | 3(15) |
|
| |
| Prior anti-VEGF injections* | |
| Mean (range) | 13.3(0–56) |
|
| |
| Response to anti-VEGF injections, n (%) | |
| Gooda | 2(10) |
| Moderateb | 1(5) |
| Poorc | 12(60) |
| Indeterminated | 5(25) |
|
| |
| Prior Intraocular Steroidse n (%) | 5(25) |
|
| |
| Grid laser (%) | 6(30) |
| Scatter Laser photocoagulation (%) | 6(30) |
|
| |
| Baseline BCVA (letter score) | |
| Mean (range) | 56.1(30–74) |
|
| |
| Baseline CST (μm) | |
| Mean (range) | 464.3(280–779) |
Based upon verified observed data and electronic patient records
Good response = elimination of 80–90% of the intraretinal fluid
Moderate response = elimination of 30–80% of intraretinal fluid during frequent injections
Poor Response = <30% reduction in intraretinal fluid during periods of monthly injections
Indeterminate= Unable to determine from available data
Patient 1 received 3 intravitreal triamcinolone acetonide (IVTA) injections 108, 113 and 118 months prior to enrollment.
Patient 2 received 2 IVTA 117 and 110 months prior to enrollment, 1 fluocinolone acetonide implant 102 months prior to enrollment and 1 dexamethasone implant 25 months prior to enrollment. Patient 3 received 1 injection of IVTA 15 months prior to enrollment. Patient 4 received 1 injection of IVTA 66 months prior to enrollment and 2 dexamethasone implants 10 and 6 months prior to enrollment. Patient 5 received 1 injection of IVTA 105 months prior to enrollment, a flucinolone acetonide implant 99 months prior to enrollment and injection of IVTA 40 months prior to enrollment.
Anatomic and Functional Outcomes
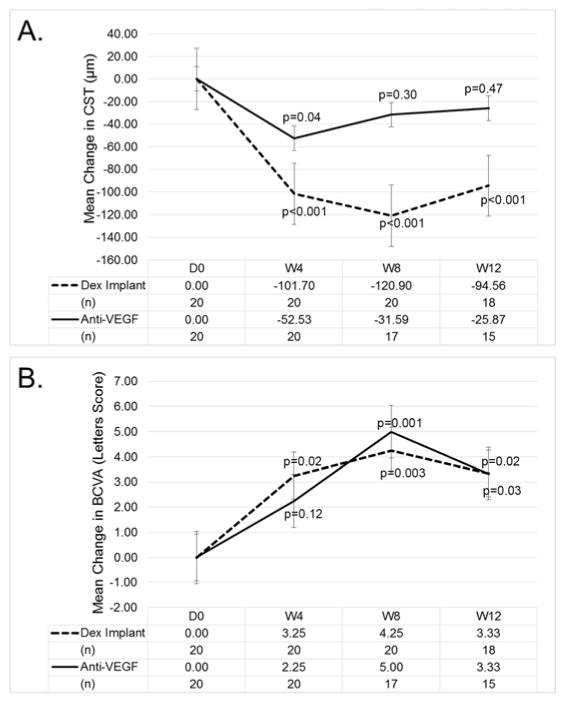
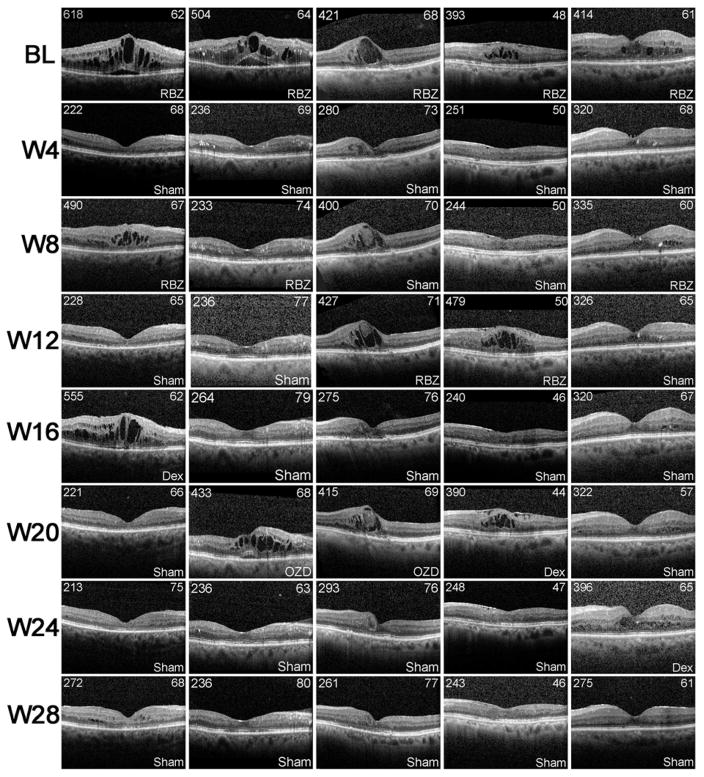
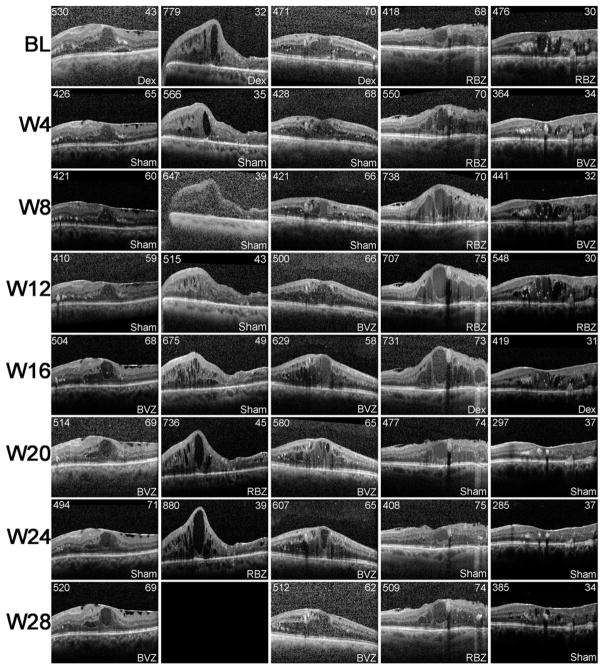
The DMEO study was not designed to compare the efficacy of dexamethasone implant with that of anti-VEGF injections in DME. In fact there was an attempt to enroll patients with DME who did not have elimination of edema despite suppression of VEGF, because such patients are likely to have other factors in addition to VEGF contributing to edema. Figure 1A confirms that the patient population had only a modest reduction in edema from suppression of VEGF. At 4, 8, and 12 weeks after initiating prn anti-VEGF injections, mean CST was reduced 52.5, 31.6, and 21.9 μm. After injection of a dexamethasone implant, mean CST was reduced 101.7, 120.9, and 94.6 μm (Figure 1A). Thus, in the DMEO study population, the mean reduction in CST was significantly greater after dexamethasone implant than after specific suppression of VEGF, but it is also useful to assess individual subject responses. The variation in CST among patients with chronic/recurrent edema is often substantial because of differences in duration of disease, past treatments, and responses to past treatments. This is accounted for in change from baseline EFT. After dexamethasone implant, the number of patients with EFT reduced >90%, >50%, >10%, or ≤10% was 7, 16, 20, 0. During prn anti-VEGF injections the number of patients with EFT reduced >90%, >50%, >10%, or ≤10% was 4, 8, 13, 5, while 2 patients could not be graded (1 due to missed visits eliminating measurement of EFT one month after an anti-VEGF injection, and 1 because a patient randomized to first receive a dexamethasone implant had no recurrent edema throughout the remainder of the trial and did not receive any anti-VEGF injections). Five of the 20 patients had an optimal response to VEGF suppression defined as elimination of 80–90% of the pre-treatment intraretinal fluid. An OCT scan from each visit is shown for these 5 patients in Figure 2 and interestingly, these patients had an equally good response to injection of a dexamethasone implant. As noted above, the response to VEGF suppression could not be assessed in 2 of the remaining 15 patients, but the other 13 patients had a poor response to VEGF suppression. One of these patients also had a poor response to a dexamethasone implant, but the other 12 had at least a moderate response after a single dexamethasone implant defined as elimination of >50% EFT. Figure 3 shows an OCT scan through the fovea at each study visit for 5 representative patients in this group of 12. Thus, as suggested by pre-enrollment data, the DMEO patient population was enriched for those who had little reduction of edema from suppression of VEGF, and many of these patients had a greater reduction in edema after dexamethasone implant, suggesting that proteins other than VEGF that were reduced by dexamethasone implant contributed to edema. While improvements in EFT were accompanied by substantial improvements in BCVA in some patients, this was not the case in other patients due to the chronicity of the DME (Figures 2 and 3). Limited visual potential in some patients and lack of EFT reduction in some others resulted in modest mean improvements from baseline BCVA (Figure 1B).
Figure 1. Improvement in central subfield thickness (A) and best-corrected visual acuity (B) after injection of dexamethasone implant or anti-vascular endothelial growth factor injections in patients with diabetic macular edema.
Patients were randomized to initially receive a dexamethasone implant or pro re nata (prn) anti-vascular endothelial growth factor (anti-VEGF) injections and then crossover at week 12. Mean (±standard error of the mean) change from baseline central subfield thickness (CST, A) and best-corrected visual acuity (BCVA, B) were improved after dexamethasone implant injection or during prn anti-VEGF injections.Mixed effects regression models with a random intercept for eyes to account for the correlation among the repeated measures from the same eye were used to determine whether BCVA or CST was different from baseline at each follow up visit.
Figure 2. Spectral domain-optical coherence tomography horizontal scans through the fovea at each study visit for patients with diabetic macular edema who had a good response to intraocular injection of an anti-vascular endothelial growth factor agent.
Horizontal scans through the fovea at each study visit are shown for 5 patients who had a marked reduction of edema after intraocular injections of ranibizumab (RBZ) or bevacizumab (BVZ). These were the only patients in the trial who showed a good response with regard to edema reduction from anti-VEGF injections. After cross-over, these 5 patients received a dexamethasone implant (Dex) and had elimination of recurrent edema. Treatment (Dex, RBZ, BVZ, or sham injection) received at each visit is indicated in the lower right of each box. Central subfield thickness is shown in the upper left and best-corrected visual acuity in Early Treatment Diabetic Retinopathy Study letter score is shown in the upper right of each box. Blank boxes indicate a missed visit.
Figure 3. Spectral domain-optical coherence tomography horizontal scans through the fovea at each study visit for representative patients with diabetic macular edema who showed substantial reduction in edema after injection of a dexamethasone implant, but little or no edema reduction from anti-vascular endothelial growth factor injections.
Horizontal scans through the fovea at each study visit are shown for 5 patients (representative of the majority of patients in the trial) who showed a substantial reduction in edema after injection of a dexamethasone implant (Dex), but little or no edema reduction after ranibizumab (RBZ) or bevacizumab (BVZ). Treatment (Dex, RBZ, BVZ, or sham injection) received at each visit are indicated in the lower right of each box. Central subfield thickness is shown in the upper left and the best-corrected visual acuity in Early Treatment Diabetic Retinopathy Study letter score is shown in the upper right of each box. Blank boxes indicate a missed visit.
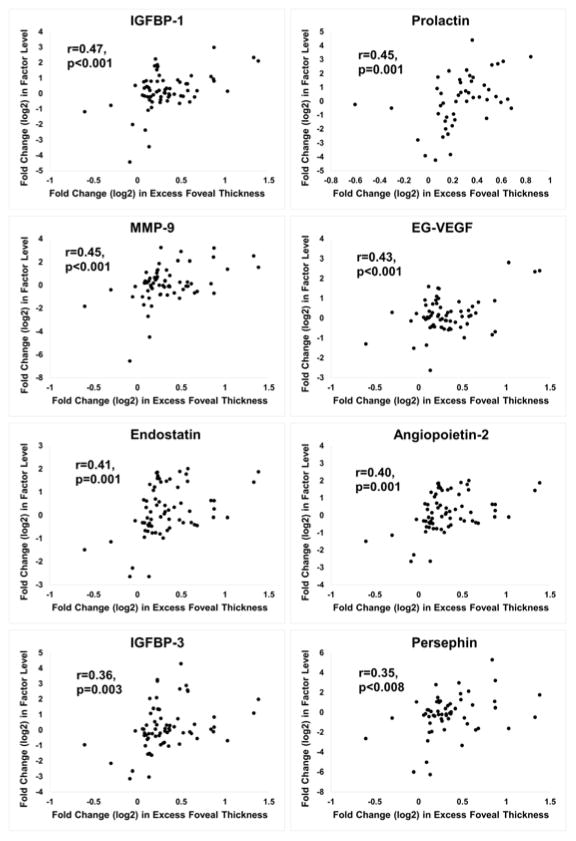
Changes in aqueous levels of vasoactive proteins after injection of dexamethasone implant or anti-VEGF agents
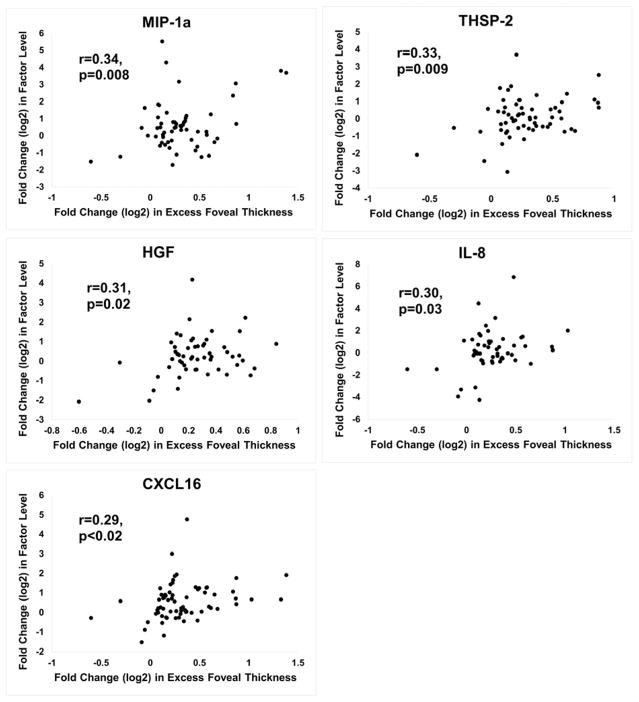
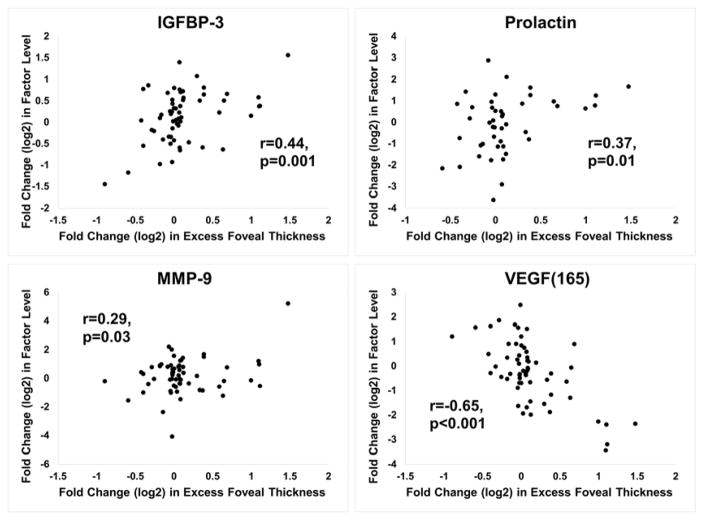
The protein array measured levels for 55 vasoactive proteins (Table 2). Measurements for each of the proteins were made on aqueous samples obtained at baseline and at 4, 8, and 12 weeks after injection of dexamethasone implant or the initiation of prn anti-VEGF injections. For each protein, the FCProtein was plotted against the FCEFT at the visit the sample was obtained. For samples obtained before and after dexamethasone implant, significant positive correlations were identified for insulin-like growth factor binding protein-1 (IGFBP-1), prolactin, matrix metalloproteinase-9 (MMP-9), endocrine gland-VEGF (EG-VEGF), endostatin, angiopoietin 2 (ANGPT2), IGFBP-3, persephin, macrophage inhibitory protein-1α (MIP-1α), thrombospondin-2 (THSP-2), hepatocyte growth factor (HGF), interleukin-8 (IL-8), and C-X-C motif ligand 16 (CXCL16, Figure 4). For samples obtained at baseline and subsequent visits during prn anti-VEGF injections, significant positive correlations were obtained for IGFBP-3, prolactin, and MMP-9, while a strong negative correlation was obtained for VEGF165 (Figure 5). All statistically significant positive and negative correlations are shown in Supplemental Table 1 (available at AJO.com).
Table 2.
Proteins on array used to test aqueous of patients with Diabetic Macular Edema
| Proteins detected | |
|---|---|
|
| |
| Activin A | IL-8 |
| ADAMTS-1 | Leptin |
| Angiogenin | MCP-1 |
| Angiopoietin-1 | MIP-1a |
| Angiopoietin-2 | MMP-8 |
| Angiostatin | MMP-9 |
| Amphiregulin | NRG-1β1 |
| Artemin | PAI-1 |
| CXCL4 | PEDF |
| CXCL16 | Pentraxin3 |
| DPPIV | Persephin |
| EGF | PF4 |
| EG-VEGF | PD-ECGF |
| Endoglin | PDGF-AA |
| Endostatin | PlGF |
| Endothelin-1 | Prolactin |
| FGF Acidic | Serpin B5/Maspin |
| FGF Basic | TIMP-1 |
| FGF-4 | TIMP-4 |
| FGF-7 | TF |
| GDNF | TGF-β 1 |
| GM-CSF | THSP-1 |
| HB-EGF | THSP-2 |
| HGF | uPA |
| IGFBP-1 | Vasohibin |
| IGFBP-2 | VEGF-A (165) |
| IGFBP-3 | VEGF-C |
| IL-1β | |
Abbreviations: ADAMTS-1 = A Disintegrin and Metalloproteinase with Thrombospondin motif protein-1, CXCL = C-X-C motif ligand, DPP-IV = dipeptidyl peptidase-4, EGF = epidermal growth factor, EG-VEGF = endocrine gland-vascular endothelial growth factor, FGF = fibroblast growth factor, GDNF = glial cell linederived neurotrophic factor, GM-CSF = granulocyte macrophage colony-stimulating factor, HB-EGF = heparin binding-epidermal growth factor, HGF = hepatocyte growth factor, IGFBP = insulin-like growth factor binding protein, IL = interleukin, MCP-1 = monocyte chemoattractant protein-1, MIP-1α = macrophage inhibitory protein-1α, MMP = matrix metalloproteinase, NRG-1β1 = neuroregulin-1β1, PAI-1 = plasminogen activator inhibitor-1, PEDF = pigment epithelium-derived factor, PF4 = platelet factor 4, PD-ECGF = platelet-derived endothelial cell growth factor, PDGF = platelet-derived growth factor, PlGF = placental growth factor, TIMP = tissue inhibitor of metalloproteinases, TF = tissue factor, TGF-β1 = transforming growth factor-β1, THSP = thrombospondin, uPA = urokinase, VEGF = vascular endothelial growth factor
Figure 4. Proteins with a significant positive correlation between fold change(log2) in aqueous protein level and fold change(log2) excess foveal thickness after dexamethasone implant injection.
Fold change(log2) from baseline protein level was plotted versus fold change(log2) from baseline excess foveal thickness (EFT) for each protein. The plots for 13 proteins (A, 8 proteins and B, additional 5 proteins) showed a significant positive Pearson correlation coefficient (r) after dexamethasone implant injection: 1) Insulin-like growth factor binding protein-1 (IGFBP-1), 2) Prolactin, 3) Matrix metalloproteinase-9 (MMP-9), 4) Endocrine gland-VEGF (EG-VEGF), 5) Endostatin, 6) Angiopoietin-2, 7) IGFBP-3, 8) Persephin, 9) Macrophage inhibitory protein-1α (MIP-1α), 10) Thrombospondin-2 (THSP-2), 11) Hepatocyte growth factor (HGF), 12) Interleukin-8 (IL-8), and 13) C-X-C motif ligand 16.
Figure 5. Proteins with a significant correlation between fold change(log2) in aqueous protein level and fold change(log2) excess foveal thickness during anti-vascular endothelial growth factor injections.
Fold change(log2) from baseline protein level was plotted versus fold change(log2) from baseline excess foveal thickness (EFT) for each protein. The plots for 4 proteins showed a significant Pearson correlation coefficient (r) during anti-vascular endothelial growth factor injections: A) Insulin-like growth factor binding protein-3 (IGFBP-3), B) Prolactin C) Matrix metalloproteinase-9 (MMP-9) D) Vascular endothelial growth factor165
Intraocular Pressure
Two patients had an increase in intraocular pressure (IOP) to >25mm after a dexamethasone implant which normalized after institution of a topical IOP lowering medication that was discontinued within 3 months. No patients had IOP >25mm during anti-VEGF treatment.
Discussion
VEGF is an important contributor to DME and intraocular injections of VEGF neutralizing proteins provide substantial benefit. However, it is not possible to eliminate all intraretinal fluid in all patients with DME even when anti-VEGF injections are given every month. Frequent episodes of persistent/recurrent intraretinal fluid have been associated with reduced visual outcomes over a 3 year period11 and therefore it is worthwhile to consider alternative treatments to help minimize persistent/recurrent intraretinal fluid. The dexamethasone implant is a reasonable alternative or adjunct because it has a longer duration of action than anti-VEGF agents and a broader spectrum of action, reducing many other vasoactive proteins in addition to VEGF. In this study, we sought to identify molecular targets of dexamethasone implant that may contribute to its DME-reducing activity. The strategy was to measure aqueous levels of vasoactive proteins at baseline and at 4, 8, and 12 weeks after injection of dexamethasone implant and correlate changes in levels with edema reduction and recurrence. As a comparator, similar measurements were done during a 12 week period of prn anti-VEGF injections.
After injection of a dexamethasone implant, there was significant correlation between changes in levels of 13 proteins with changes in amount of edema. Three of the proteins, HGF, EG-VEGF, and ANGPT2 are particularly interesting, because they have known pro-permeability activity. In a similar study, the ORVO study,16 in which protein arrays were done on aqueous samples taken before and 4 weeks after a dexamethasone implant in patients with chronic/recurrent macular edema due to retinal vein occlusion, HGF and EG-VEGF levels were reduced in association with edema reduction in a substantial number of patients. Retinal hypoxia has been implicated in the pathogenesis of DME3 and HGF is a hypoxia-regulated gene product that increases leakage from retinal vessels.17 Thus, evidence suggesting that HGF may be a contributor to macular edema in ischemic retinopathies continues to increase. In normal, unstressed animals, EG-VEGF is detectable primarily in endocrine glands,18, 19 but the current study is the second to demonstrate its presence in eyes with ischemic retinopathy and find that changes in its levels correlate with changes in edema after dexamethasone implant. Additional studies are needed to determine if EG-VEGF has pro-permeability effects in the eye as it does in endocrine tissues. ANGPT2 is a hypoxia-regulated gene product that inhibits Tie 2 and enhances the effects of VEGF and other pro-permeability factors.20–22 The Tie 2 pathway is currently being targeted by two strategies, antagonism of ANGPT2 and activation of Tie2 by blocking a phosphatase, vascular endothelial-protein tyrosine phosphatase (VE-PTP) that inactivates Tie 2.23 Clinical studies using a small molecule antagonist of VE-PTP have provided proof-of-concept for the strategy of Tie 2 activation in DME24 and the strategy of ANGPT2 blockade is being tested in patients with neovascular AMD using a bispecific antibody that binds both VEGF and ANGPT2 (NCT02484690). Aqueous levels of persephin also correlated with changes in edema in RVO patients16 similar to what was seen in DME patients in the current study, but the effects of persephin on retinal vascular permeability are unknown and require additional study. Three of the proteins that were reduced after dexamethasone implant, MIP-1a, IL-8, and CXCL16, are pro-inflammatory. It is reasonable to hypothesize that inflammation exacerbates persistent/recurrent edema and might reduce the effectiveness of anti-VEGF agents. The reduction in endostatin, a proteolytic fragment of collagen 18, could be related to the reduction in MMP-9, but its significance is unknown; gene transfer of endostatin resulting in superphysiologic levels suppresses vascular permeability and neovascularization in the eye in mouse models,25 but it is unknown what role endogenous levels of endostatin play. Thrombospondins are antiangiogenic in the eye,26 but they have not been demonstrated to alter vascular permeability.
Aqueous levels of 3 factors, IGFBP-3, prolactin, and MMP-9, correlated with changes in edema after anti-VEGF injections. Since a VEGF-neutralizing protein does not reduce levels of these proteins, their reduction may be secondary to the reduction in edema rather than a cause for edema reduction. Collection of fluid in the retina displaces and distorts retinal neurons and it is not surprising that stressed neurons might exhibit altered protein expression, but it is unknown why this would be the case for IGFBP-3, prolactin, and MMP-9. Due to its proteolytic activity, the increased expression of MMP-9 in edematous retina could contribute to structural damage over time. Interestingly, aqueous levels of VEGF165 showed a strong negative correlation with changes in EFT. The patient population was enriched in patients who showed little reduction in edema after anti-VEGF injections which likely drove this negative correlation, and it may have been accentuated by the presence of residual VEGF neutralizing protein in aqueous samples which through competition for VEGF binding with the anti-VEGF antibody on the array artificially reduced measured levels of VEGF.
While our data identify several possible contributors to DME in addition to VEGF, the correlation coefficient for each of the candidates is modest suggesting heterogeneity among DME patients with regard to contributors. This is also suggested by differences in contribution of VEGF among the patients as shown by the range of edema reduction seen with specific VEGF antagonists in our study population. While the array contained a large number of vasoactive proteins, there are other proteins that may affect permeability that were not included on the array and this is a limitation of the study.
Sohn et al.27 found elevated aqueous levels of IL-8, interferon-induced protein-10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), and VEGF in 22 eyes of 11 patients with DME compared to 6 control eyes. In the 11 patients with DME, one eye was injected with 4 mg of triamcinolone acetonide and the other was injected with 1.25 mg of bevacizumab; 4 weeks later, steroid-injected eyes showed a reduction in IP-10, MCP-1, VEGF, IL-6, and platelet-derived growth factor-AA (PDGF-AA) and only VEGF was reduced in bevacizumab-injected eyes. The duration of DME was not provided, but only treatment-naïve patients were eligible for the study, suggesting that compared to our patients, the duration of DME was probably shorter. Many other studies have identified vasoactive proteins that are elevated in the aqueous or vitreous of patients with DME compared with controls including IL-628–31, IL-830–32, IP-1030–32, MCP-130, 31, IL-1β30, PDGF-AA31, semaphorin3E33, and angiopoietin-like 433, which is also increased in eyes with proliferative diabetic retinopathy.34 Of this group of proteins, only antibodies for IL-8, MCP-1, and PDGF-AA were present on the arrays used in our study; changes in IL-8 correlated with changes in edema, but changes in MCP-1 and PDGF-AA did not.
In summary, multiple vasoactive proteins are reduced in the aqueous of patients with chronic DME after dexamethasone implant and correlate with changes in edema. Two proteins with known pro-permeability activity, HGF and ANGPT2, are strong candidates for contributors to edema and while the effect of EG-VEGF in the eye is unknown, its pro-permeability effects in other vascular beds makes it a candidate that deserves additional study. The proinflammatory proteins, MIP-1a, IL-8, and CXCL16, may contribute to chronic DME by recruitment of leukocytes that release pro-permeability factors and also deserve further study.
Supplementary Material
Acknowledgments
This study was supported by Allergan Inc., Irvine, CA
The authors gratefully acknowledge the assistance of Jiangxia Wang of the Wilmer Institute Biostatistics Core for statistical analyses and Wilmer Biostatistics Core Grant EY01765.
Funding/Support: This study was supported by a grant from Allergan Inc., Irvine, CA.
Footnotes
Potential Conflicts of Interest are listed after the discussion
Supplemental material available at AJO.com
Disclosure
Financial Disclosures: none for Gulnar Hafiz, Tahreem A. Mir, Adrienne W. Scott, Ingrid Zimmer-Galler, Syed M. Shah, Adam S. Wenick, Christopher J. Brady, Ian Han, Roomasa Channa, David Poon, Catherine Meyerle, Mary Beth Aronow, Akrit Sodhi, Saleema Kherani, Yong Han, Raafay Sophie, Guohua Wang, Jiang Qian
Peter A. Campochiaro-Consulting Agreements-Alimera, Applied Genetic Technologies, Eleven Biotherapeutics, AsclipiX, Kala Pharmaceuticals, Rxi Pharmaceuticals, Akebia, Allegro, Intrexon, RegenX, Institutional Consulting Agreements-Aerpio Therapeutics, Genentech/Roche, Regeneron, Research Support-AbbVie, Allergan, Aerpio Therapeutics, Genentech/Roche, Genzyme, GlaxoSmithKline, Oxford Biomedica, Regeneron, RegenX, Rxi Pharmaceuticals, Equity-Alimera, Allegro, Graybug
James T. Handa-Research Support-Bayer Pharmaceuticals
Lingmin He-Consulting Agreement-Oculeve Inc, Auris Surgical Robotics, Patent-EyeGo, Equity-Oculeve
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Varma R, Bressler NM, Doan QV, et al. Prevalence of risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132(11):1334–1340. doi: 10.1001/jamaophthalmol.2014.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen QD, Shah SM, Van Anden E, et al. Supplemental inspired oxygen improves diabetic macular edema; a pilot study. Invest Ophthalmol Vis Sci. 2003;45(2):617–624. doi: 10.1167/iovs.03-0557. [DOI] [PubMed] [Google Scholar]
- 4.Ozaki H, Yu A, Della N, et al. Hypoxia inducible factor-1a is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci. 1999;40(1):182–189. [PubMed] [Google Scholar]
- 5.Nguyen QD, Tatlipinar S, Shah SM, et al. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol. 2006;142(6):961–969. doi: 10.1016/j.ajo.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen QD, Shah SM, Heier JS, et al. Primary End Point (Six Months) Results of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) Study. Ophthalmology. 2009;116(11):2175–2181. doi: 10.1016/j.ophtha.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for Diabetic Macular Edema. Results from 2 Phase III Randomized Trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 8.Heier JS, Clark WL, Boyer DS, et al. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: two-year results from the COPERNICUS study. Ophthalmology. 2014;121(7):1414–1420. doi: 10.1016/j.ophtha.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 9.The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193–1203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Do DV, Nguyen QD, Khwaja AA, et al. Ranibizumab for edema of the macula in diabetes study: 3-year outcomes and the need for prolonged frequent treatment. JAMA Ophthalmol. 2013;131(2):139–145. doi: 10.1001/2013.jamaophthalmol.91. [DOI] [PubMed] [Google Scholar]
- 11.Channa R, Sophie R, Khwaja AA, et al. Factors affecting visual outcomes in patients with diabetic macular edema treated with ranibizumab. Eye (Lond) 2013;28(3):269–278. doi: 10.1038/eye.2013.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang-Yen HF, Chambard JC, Sun YL, et al. Transciptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- 13.Schule R, Rangarajan P, Kliewer S, et al. Runctional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990;62(6):1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- 14.Heck S, Kullmann M, Gast A, et al. A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP1. EMBO J. 1994;13(17):4087–4095. doi: 10.1002/j.1460-2075.1994.tb06726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyer DS, Yoon YH, Belfort RJ, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904–1914. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Campochiaro PA, Hafiz G, Mir TA, et al. Pro-Permeability Factors After Dexamethasone Implant in Retinal Vein Occlusion; the Ozurdex for Retinal Vein Occlusion (ORVO) Study. Am J Ophthalmol. 2015;160(2):313–321. doi: 10.1016/j.ajo.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clermont AC, Cahill M, Salti H, et al. Hepatocyte growth factor induces retinal vascular permeability via MAP-kinase and PI-3 kinase without altering retinal hemodynamics. Invest Ophthalmol Vis Sci. 2006;47(6):2701–2708. doi: 10.1167/iovs.05-0071. [DOI] [PubMed] [Google Scholar]
- 18.LeCouter J, Kowalski J, Foster J, et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412(6850):877–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- 19.LeCouter J, Lin R, Ferrara N. Endocrine gland-derived VEGF and the emerging hypothesis of organ-specific regulation of angiogenesis. Nature Med. 2002;8(9):913–917. doi: 10.1038/nm0902-913. [DOI] [PubMed] [Google Scholar]
- 20.Hackett SF, Ozaki H, Strauss RW, et al. Angiopoietin 2 expression in the retina: upregulation during physiologic and pathologic neovascularization. J Cell Physiol. 2000;184(3):275–284. doi: 10.1002/1097-4652(200009)184:3<275::AID-JCP1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Hackett SF, Wiegand SJ, Yancopoulos G, Campochiaro P. Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol. 2002;192(2):182–187. doi: 10.1002/jcp.10128. [DOI] [PubMed] [Google Scholar]
- 22.Oshima Y, Deering T, Oshima S, et al. Angiopoietin-2 enhances retinal vessel sensitivity to vascular endothelial growth factor. J Cell Physiol. 2004;199(3):412–417. doi: 10.1002/jcp.10442. [DOI] [PubMed] [Google Scholar]
- 23.Shen J, Frye M, Lee BL, et al. Targeting VE-PTP activates TIE2 and stabilizes the ocular vasculature. J Clin Invest. 2014;124(10):4564–4576. doi: 10.1172/JCI74527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campochiaro PA, Sophie R, Tolentino M, et al. Treatment of diabetic macular edema with an inhibitor of vascular endothelial-protein tyrosine phosphatase that activates Tie2. Ophthalmology. 2015;122(9):545–554. doi: 10.1016/j.ophtha.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi K, Saishin Y, Saishin Y, et al. Intraocular expression of endostatin reduces VEGF-induced retinal vascular permeability, neovascularization, and retinal detachment. FASEB J. 2003;17(8):896–898. doi: 10.1096/fj.02-0824fje. [DOI] [PubMed] [Google Scholar]
- 26.Wu Z, Wang S, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization in transgenic mice over-expressing thrombospondin-1. Dev Dyn. 2006;235(7):1908–1920. doi: 10.1002/dvdy.20837. [DOI] [PubMed] [Google Scholar]
- 27.Sohn HJ, Han DH, Kim IT, et al. Changes in aqueous concentrations of various cytokines after intravitreal triamcinolone versus bevacizumab for diabetic macular edema. Am J Ophthalmol. 2011;152(4):686–694. doi: 10.1016/j.ajo.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 28.Funatsu H, Yamashita H, Noma H, et al. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002;133(1):70–77. doi: 10.1016/s0002-9394(01)01269-7. [DOI] [PubMed] [Google Scholar]
- 29.Funatsu H, Yamashita H, Ikeda T, et al. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2003;110(9):1690–1696. doi: 10.1016/S0161-6420(03)00568-2. [DOI] [PubMed] [Google Scholar]
- 30.Dong N, Xu B, Chu L, Tang X. Study of 27 aqueous humor cytokines in type 2 diabetic patients with or without macular edema. PLOS One. 2015;10(4):e0125329. doi: 10.1371/journal.pone.0125329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee WJ, Kang MH, Seong M, Cho HY. Comparison of aqueous concentrations of angiogenic and inflammatory cytokines in diabetic macular oedema and macular oedema due to branch vein occlusion. Br J Ophthalmol. 2012;96(11):1426–1430. doi: 10.1136/bjophthalmol-2012-301913. [DOI] [PubMed] [Google Scholar]
- 32.Funk M, Schmidinger G, Maar N, et al. Angiogenic and inflammatory markers in the intaocular fluid of eyes with diabetic macular edema and influence of therapy with bevacizumab. Retina. 2010;30(9):1412–1419. doi: 10.1097/IAE.0b013e3181e095c0. [DOI] [PubMed] [Google Scholar]
- 33.Kwon SH, Shin JP, Kim IT, Park DH. Aqueous levels of angiopoietin-like 4 and semaphorin 3E correlate with nonperfusion area and macular volume in diabetic retinopathy. Ophthalmology. 2015;122(5):968–975. doi: 10.1016/j.ophtha.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Babapoor-Farrokhran S, Jee K, Puchner B, et al. Angiopoietin-like 4 is a potent angiogenic factor and a novel therapeutic target for patients with proliferative diabetic retinopathy. Proc Natl Acad Sci USA. 2015;112(23):E3030–E3039. doi: 10.1073/pnas.1423765112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.