Abstract
Substance use disorder, especially in relation to opioids such as heroin and fentanyl, is a significant public health issue and has intensified in recent years. As a result, substantial interest exists in developing therapeutics to counteract the effects of abused drugs. A promising universal strategy for antagonizing the pharmacology of virtually any drug involves the development of a conjugate vaccine, wherein a hapten structurally similar to the target drug is conjugated to an immunogenic carrier protein. When formulated with adjuvants and immunized, the immunoconjugate should elicit serum IgG antibodies with the ability to sequester the target drug to prevent its entry to the brain, thereby acting as an immunoantagonist. Despite the failures of first-generation conjugate vaccines against cocaine and nicotine in clinical trials, second-generation vaccines have shown dramatically improved performance in preclinical models, thus renewing the potential clinical utility of conjugate vaccines in curbing substance use disorder. This review explores the critical design elements of drug conjugate vaccines such as hapten structure, adjuvant formulation, bioconjugate chemistry, and carrier protein selection. Methods for evaluating these vaccines are discussed, and recent progress in vaccine development for each drug is summarized.
I. Introduction
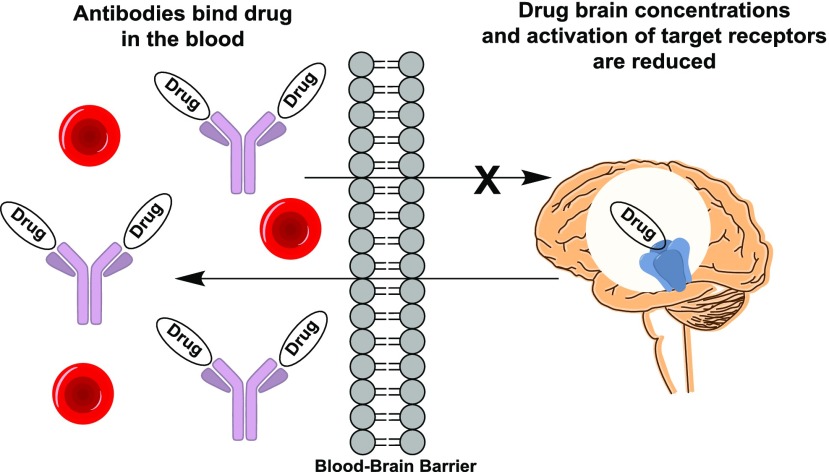
Substance use disorder is a considerable detriment to society worldwide, and, in recent years, opioid abuse has become one of the most significant public health problems in the United States. Traditional pharmacological therapies such as μ-opioid receptor (MOR) agonists methadone and buprenorphine can assist in treating opioid dependence; furthermore, MOR antagonists naltrexone and naloxone are effective at reversing opioid overdoses. As another example, the nicotinic receptor partial agonist varenicline is Food and Drug Administration (FDA) approved for smoking cessation therapy. Although these pharmacotherapies can be effective, they have fallen short at mitigating the alarming surge in recent opioid abuse and can have significant side effects. Moreover, the polypharmacology of other drugs of abuse, e.g., cocaine and methamphetamine, presents a significant challenge for the development of small-molecule addiction therapeutics. As an alternative strategy for combatting substance use disorder, conjugate vaccines hold promise due to their ability to generate high-affinity anti-drug IgG antibodies. These serum antibodies can neutralize drug doses, thus preventing the target drug from acting on receptors in the brain (Fig. 1). Moreover, conjugate vaccines function as immunoantagonists to mitigate the pharmacodynamics of the target drug. Because the anti-drug antibodies themselves do not modulate drug receptors in the brain or even in the periphery, their side effect profile is minimal, but also they cannot ameliorate drug cravings or withdrawal symptoms. Vaccine-generated IgG antibodies have long half-lives; therefore, their duration of action is considerably longer than small molecules. Lastly, conjugate vaccines are versatile and can theoretically be designed to target any drug or combination of drugs.
Fig. 1.
Mechanism of action of anti-drug antibodies. Vaccine-generated IgG antibodies bind to the target drug in the blood once it is administered. As a result, the concentrations of drug in tissue and organs (most importantly the brain) are reduced, thus diminishing the pharmacodynamic action of the drug.
The first reports of generating drug-specific antibodies were published in the early 1970s (Spector and Parker, 1970; Spector, 1971; Van Vunakis et al., 1972; Spector et al., 1973), in which anti-morphine antibodies were used in a radioimmunoassay for the detection of opioids. The therapeutic potential for anti-drug antibodies was first demonstrated in 1974, wherein a morphine–bovine serum albumin (BSA) conjugate formulated with complete Freund’s adjuvant was able to mildly attenuate heroin self-administration in a single rhesus monkey (Bonese et al., 1974). Since then, active vaccination was not further investigated as a substance abuse therapy until 20 years later when cocaine vaccines (Carrera et al., 1995; Fox et al., 1996) and nicotine vaccines (Hieda et al., 1997, 1999) were pioneered. Further investigations in clinical trials did not observe a significant effect of cocaine and nicotine vaccines on drug abstinence as a primary endpoint and did not measure patient antiserum drug affinity (a critical determinant of vaccine efficacy) (Cornuz et al., 2008; Martell et al., 2009; Hoogsteder et al., 2014; Kosten et al., 2014). Despite these failures, the trials have suggested that the vaccines could be effective if sufficient titer levels are achieved in each patient. Because efficacy is dependent on proper vaccine design to produce antibodies with adequate concentration and drug affinity, relatively recent advancements in drug conjugate vaccine design have increased the likelihood that these vaccines will one day succeed in the clinic. This review will explore these developments in relation to each aspect of vaccine design.
II. Drug Conjugate Vaccine Design
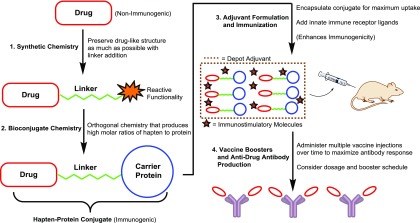
The design strategy of drug conjugate vaccines is illustrated in Fig. 2. Because small-molecule drugs inherently lack immunogenicity, they must be attached to an immunogenic entity in order for the immune system to recognize and generate antibodies against the target drug. By employing organic synthetic chemistry, a chemical linker can be attached to the drug to create what is known as a hapten. Bioconjugation of the drug hapten to a carrier protein results in the formation of a drug immunoconjugate. Formulation of this conjugate with immunostimulatory molecules, i.e., adjuvants, completes the production process of an anti-drug vaccine, and injection of this vaccine into a subject will elicit antibodies against the drug hapten.
Fig. 2.
Design and production of drug conjugate vaccines. Using synthetic chemistry, a linker is appended to the target drug containing a cross-linking functionality at the linker terminus. Activation of this functionality enables the coupling of the drug hapten to a carrier protein, yielding an immunoconjugate. Formulation of the drug immunoconjugate with depot and immunostimulatory adjuvants generates the complete vaccine. Multiple immunizations of this vaccine coax the subject’s immune system into producing drug-neutralizing IgG antibodies.
A. Drug Selection
Certain drugs are more amenable to targeting by a conjugate vaccine due to properties such as size, pharmacokinetics, and biodistribution. Size, i.e., mol. wt., and structural complexity are critical considerations because lower mol. wt. drugs do not possess a sufficient number of atoms to constitute an immune epitope. Furthermore, the presence of nitrogen and oxygen atoms is essential for forming hydrogen bonds between small-molecule drugs and antibodies. For example, ethanol is a frequently abused, small mol. wt. drug (mol. wt. = 46 g/mol) that is incompatible with the conjugate vaccine strategy. As ethanol only contains three heavy atoms, it would present as a poorly immunogenic epitope; furthermore, antibodies could not be produced with great enough affinity to ethanol because potential binding interactions are limited to only one hydrogen bond. Regardless, anti-ethanol antibodies would most likely cross-react with endogenous molecules such as serine side chains. As methamphetamine is also a relatively low molecule weight drug (mol. wt. = 149 g/mol) and contains only one hydrogen-bonding nitrogen atom, it has proven to be one of the more difficult drugs to target by means of a vaccine. Antiserum from vaccinated mice has shown methamphetamine-binding constants (Kd) in the 10−8 to 10−7 M range (Moreno et al., 2011; Miller et al., 2013; Collins et al., 2016). On the other hand, morphinan-based drugs such as heroin and oxycodone are larger (mol. wt. = 369, 315 g/mol, respectively) and contain five or more heteroatoms and a complex, three-dimensional structure. As a result, morphinan haptens can act as effective immune epitopes, and anti-hapten antibodies have shown 10−10 to 10−9 M affinity for the target drugs (Torres et al., 2016; Kimishima et al., 2017). Similarly, antiserum to a fentanyl conjugate vaccine showed affinity in the 10−9 M range for fentanyl (three heteroatoms, mol. wt. = 336 g/mol) (Bremer et al., 2016). A related reason for the success of opioid conjugate vaccines in producing high-affinity antibodies is that the structurally complex nature of opioids increases the foreignness of opioid epitopes. In contrast, methamphetamine and ethanol are structurally similar to endogenous amino acids phenylalanine and serine, respectively, possibly lending to diminished immunogenicity.
Drug pharmacodynamics and pharmacokinetics also play a major role in assessing the suitability of a drug for active vaccine targeting. The dose–effect curve of an exceptionally potent drug in behavioral assays can be shifted much more dramatically by vaccine-generated antibodies compared with a weak drug, so long as the antibodies possess sufficient affinity for the target drug. A good example of this phenomenon is a recently reported fentanyl vaccine, which shifted the fentanyl dose–effect curve by greater than 30-fold in assays for opioid-induced antinociception (Bremer et al., 2016). The ED50 of fentanyl in mice was approximately 0.1 mg/kg, but in the presence of the vaccine that ED50 increased to >3 mg/kg. In contrast, the ED50 of morphine in antinociception assays is almost 10 mg/kg; therefore, assuming anti-morphine antibodies possess equal anti-drug affinity, a significantly larger amount of antibody would be needed to shift the morphine dose–effect curve.
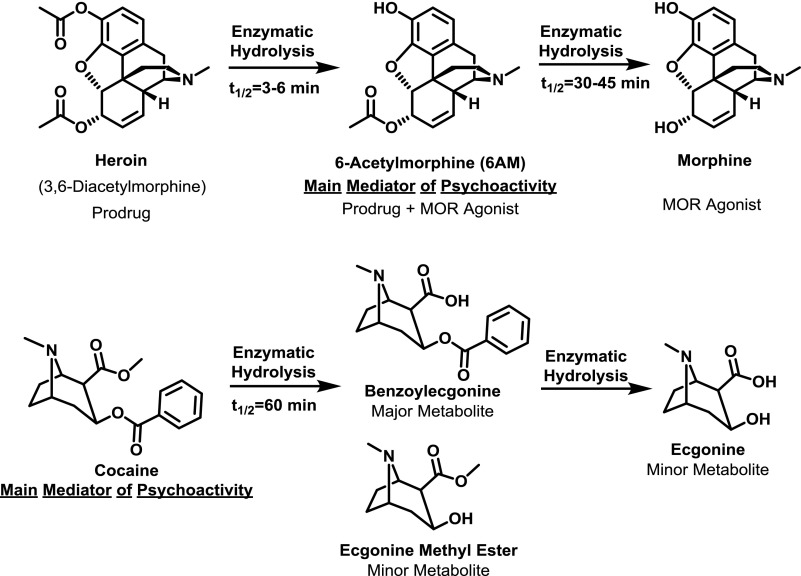
Drug pharmacokinetics is crucial for designing the hapten structure because drug metabolites often possess differing psychoactive properties than the parent drug. For instance, cocaine is hydrolyzed to the mostly inactive metabolite benzoylecgonine, whereas heroin hydrolysis produces the primary psychoactive metabolite 6 AM; therefore, effective cocaine and heroin vaccines should target cocaine and 6 AM, respectively (Fig. 4). Drug half-life should also be considered, which will influence the amount of time in which the drug will occupy the antibody. It is known that antibody–drug binding increases the half-life of the target drug (Hill et al., 1975), presumably by shielding the drug from enzyme-mediated degradation, resulting in reduced drug clearance. For example, heroin, nicotine, and oxycodone vaccines produced fold increases in drug half-life of 3–5, 7, and 68–268, respectively (McCluskie et al., 2015a; Bremer et al., 2017; Kimishima et al., 2017). A possible explanation for the lower half-life shifts of heroin, i.e., 6 AM, is that the carboxylesterase enzymes responsible for 6 AM hydrolysis are ubiquitous in the blood, whereas oxycodone is metabolized via liver-localized cytochrome p450 enzymes; therefore, 6 AM experiences a greater degree of exposure to metabolizing enzymes. Vaccine-generated antiserum that produces exceptionally long drug half-life would be anticipated to become easily saturated upon repeated drug administration.
Fig. 4.
Comparison of pharmacokinetics and metabolism of ester-containing drugs of abuse (heroin and cocaine).
A relevant pharmacokinetic parameter that serves as a good metric for vaccine efficacy is area under the curve (AUC), which is a measurement of drug plasma concentration over time. A larger AUC indicates increased antibody sequestration of drug in the blood, leading to lower distribution of drug to tissues and organs, most importantly the brain. As an example, a monoclonal cocaine antibody increased drug AUC in plasma by 26-fold, leading to a 4.5-fold decrease in brain AUC (Norman et al., 2007). The same phenomenon applies to vaccines: a Pfizer nicotine vaccine led to a 90-fold nicotine AUC increase (McCluskie et al., 2015a) and a heroin vaccine led to a 19-fold heroin AUC increase (Bremer et al., 2017). These studies in nonhuman primates did not permit quantification of drug concentrations in the brain, although it is reasonable to assume that the large serum AUC increase corresponded to a decrease in brain AUC.
Lipophilicity (LogP) of the target drug may also play a role in vaccine compatibility. For example, although a tetrahydrocannabinol (THC) vaccine was able to effectively generate anti-THC antibodies (Qi et al., 2005), the vaccine showed poor efficacy in blunting THC psychoactivity in vivo. This may be a result of the especially high LogP of THC (∼7), causing increased tissue and blood brain barrier penetration of the drug, thus shifting the equilibrium away from antibody-drug–bound state in the blood. Compared with most drugs, fentanyl is relatively lipophilic (LogP ≈ 4), yet it is still 1000 times less lipophilic than THC and has been successfully targeted using conjugate vaccines (Bremer et al., 2016).
B. Immunologic Considerations
1. Injection Route.
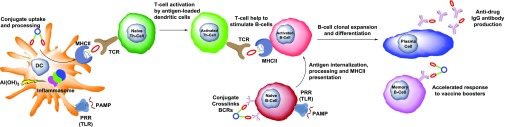
Injection of the vaccine formulation into biologic subjects causes a complex cascade of immunologic events whereby the conjugate is taken up, processed, and displayed for eventual production of antibodies with affinity for the drug hapten (Fig. 3). Drug vaccines are typically administered subcutaneously (s.c.) in rodent models, which translates well to the intramuscular (i.m.) route used in humans. Additionally, the vaccines have been introduced intradermally (i.d.) (Chen et al., 2012) and intraperitoneally (i.p.), the latter of which has been shown to elicit superior immunogenicity over other routes (Bremer et al., 2014). When vaccines are administered via the i.m. and s.c. routes, immunogens are channeled to local lymph nodes [either in free form or antigen-presenting cell (APC)–associated form], where T and B cell activation occurs. In the case of the i.p. route, antigens filter into the spleen, whereas i.d. administered immunogens encounter the skin-associated lymphoid tissue.
Fig. 3.
Immunologic mechanisms related to drug conjugate vaccines. Depot-forming adjuvants such as alum [Al(OH)3], liposomes, or oil-in-water emulsions form large particle sizes for uptake of drug conjugates by DCs, a type of APC. Immunostimulatory PAMPs activate pattern recognition receptors (PRRs) to stimulate DC and B cell maturation. For example, CpG ODNs bind to TLRs, i.e., TLR9, whereas alum stimulates NOD-like receptors, i.e., NALP3, to form a protein complex known as an inflammasome, and, in both cases, the adaptive immune response to the drug conjugate is enhanced. Important cytokines that mediate these processes are type I interferons and IL-1 family of cytokines. The function of DCs is to endocytose drug conjugates, process them intracellularly, and display them as the drug hapten linked to a carrier peptide on MHCII. Naive Th cells can be recruited to the DC via the T cell receptor for activation. B cells already exposed to the hapten conjugate via BCR cross-linking are activated by Th cells within lymph nodes. Following clonal expansion and affinity maturation in lymph node germinal centers, the activated B cells differentiate into memory B cells and plasma cells for induction of humoral (antibody-based) immunity to the target hapten.
2. Adjuvant Formulation.
Formulation of vaccines with depot adjuvants such as alum (colloidal aluminum salts), liposomes, oil-in-water, e.g., Sigma or Ribi adjuvants system, and water-in-oil emulsions, e.g., Freund’s adjuvant, is essential for vaccine efficacy due to the ability of these mixtures to form large particle sizes. Sufficient size of antigen-containing particles is crucial for holding the antigen at the site of injection and for uptake of the antigen by APCs such as dendritic cells (DCs) (Bachmann and Jennings, 2010). Our group has observed that alum, the oldest known adjuvant (Glenny et al., 1931), shows excellent performance—a fortunate result given that alum is cost-effective, widely used, and one of the only FDA-approved adjuvants. Alum is particularly well suited for use in combination with drug conjugates because it induces T-helper (Th)2-type immunity, which favors drug-neutralizing IgG antibody production in contrast to Th1-type, cell-mediated immunity (Gavin et al., 2006). Electrostatic and/or ligand exchange interactions between proteins and alum cause adsorption of the proteins onto alum, allowing for the depot effect. Moreover, antigens mixed with alum are retained at the site of injection and form large particle sizes for uptake of the particles by DCs (Morefield et al., 2005). However, many studies have found that the adjuvanting effects of alum are unrelated to antigen adsorption and retention (Romero Méndez et al., 2007), and degree of adsorption was even found to reduce antigen immunogenicity (Hansen et al., 2007, 2009; Noe et al., 2010). In all studies, coadministration of antigen and alum was necessary for antibody formation regardless of adsorption, and it has been proposed that alum aggregates can trap nonadsorbed antigen to cause immunopotentiation (Romero Méndez et al., 2007). Besides being alum-encapsulated for DC uptake, it is likely that some antigen must exist in free form to rapidly drain to lymph nodes for direct activation of follicular B cells (Bachmann and Jennings, 2010).
In addition to alum’s depot-like action, alum has been shown to act on specific immunomodulatory receptors such as the nucleotide-binding oligomerization domain NOD-like receptor NALP3, triggering the formation of a multiprotein oligomer known as an inflammasome (Fig. 3) (Eisenbarth et al., 2008; Kool et al., 2008a). Downstream inflammasome signaling results in the secretion of cytokines interleukin (IL)-1β and IL-18, which stimulate the adaptive immune response (Franchi and Nunez, 2008; Kool et al., 2008a). Additionally, alum can stimulate the release of damage-associated molecular patterns such as uric acid, which can also activate immune pathways via NALP3 (Martinon et al., 2006; Kool et al., 2008b).
Often in combination with depot adjuvants, molecules that directly target innate immune receptors are added to enhance immunogenicity of a vaccine formulation. These molecules are referred to as pathogen-associated molecular patterns (PAMPs) because they contain molecular motifs that are found in various infectious bacteria and viruses. PAMPs activate pattern recognition receptors, and a commonly targeted class of pattern recognition receptors is Toll-like receptors (TLRs) (Fig. 3). One of the most widely used PAMP-type adjuvants is monophosphoryl lipid A (MPLA). MPLA is a TLR4 ligand (the only FDA-approved TLR adjuvant), and it has been evaluated as a liposomal formulation in multiple drugs of abuse vaccines (Matyas et al., 2013, 2014; Collins et al., 2014). Direct comparisons performed in our laboratory between liposomal MPLA and alum have demonstrated superior adjuvant performance of alum for an anti-methamphetamine vaccine (K.C. Collins and K.D. Janda, unpublished data). Interestingly, MPLA alone or a combination of MPLA + alum induced lower anti-opioid antibody titers than alum alone (Pravetoni et al., 2014b). In contrast, a PAMP that has shown robust immunogenic synergy with alum is B-class CpG (ODN). These approximately 20 nucleotide oligos act on TLR9 to stimulate B cell and to a lesser extent DC maturation, ultimately resulting in enhanced antibody production (Hartmann et al., 2000; Hartmann and Krieg, 2000; Krug et al., 2001). A possible explanation for the synergy between alum and CpG ODN could be the fact that alum causes the release of host DNA due to cell death, which can also act on TLR9 to induce IL-1β and type-I interferon production (Marichal et al., 2011). The immunostimulatory nature of CpG ODN has prompted the investigation of it as an adjuvant, specifically sequence 2006, also known as 7909, in clinical trials for multiple types of vaccines (Cooper et al., 2004; Valmori et al., 2007; Mullen et al., 2008; Brody et al., 2010; Sogaard et al., 2010). For drugs of abuse vaccines, rodent-specific CpG ODN 1826 has been successfully used as an adjuvant in combination with alum (Pryde et al., 2013; Bremer et al., 2014, 2016; Collins et al., 2016; Kimishima et al., 2017). Compared with alum alone, CpG ODN 1826 + alum could boost hapten-specific titers of Th2-associated (IgG1) and especially Th1-associated (IgG2a) antibody isotypes, resulting in enhanced mitigation of heroin potency (Bremer et al., 2014). In nonhuman primates, B-class CpG ODN combined with alum has been used as a nicotine and heroin vaccine adjuvant, also with increased success compared with alum alone (McCluskie et al., 2013, 2015a,b; Bremer et al., 2017). Given the wealth of data demonstrating the safety and efficacy of the alum + CpG ODN adjuvant formulation across many species, alum + CpG ODN appears to be a promising drug vaccine adjuvant for use in clinical trials.
3. Dosing and Schedule.
Vaccines against drugs of abuse demand a relatively high titer level to achieve efficacy due to the large molar quantities of drug that must be sequestered to block drug psychoactivity. In contrast, vaccines against pathogens have a lower titer requirement to block infection. As a consequence, not only is adjuvant formulation of utmost importance but also vaccine dosing and immunization schedule. Conjugate vaccination courses usually consist of three priming injections spaced 2–4 weeks apart. Titer levels peak 2–4 weeks after a given injection, at which point they will decay until vaccine boosters are administered, typically 2–3 months after the last priming injection (Martell et al., 2005; Hatsukami et al., 2011; Maoz et al., 2013; McCluskie et al., 2015b). In most cases, subsequent booster injections could not raise titers above initial peak titer levels. Few studies have compared different vaccination schedules; however, one clinical nicotine vaccine study found that five injections versus four injections administered within the same 26-week period produced higher titers (Hatsukami et al., 2011). Although vaccines for infectious diseases can remain efficacious for years without boosters, drug vaccines would most likely require a much shorter interval between boosters of 1 year or less. A critical question that has been asked in relation to vaccination schedule is whether immunizations can occur during drug administration. Two rodent studies have compared vaccine efficacy with and without concurrent drug administration during immunization, and results have shown no impairment of vaccine efficacy due to drug exposure (Hieda et al., 2000; Byrnes-Blake et al., 2001). A clinical implication of these studies is that vaccines could be used not only to maintain drug abstinence but also to induce drug abstinence in active drug users. In terms of immunoconjugate dosing, both cocaine and nicotine clinical studies (typically using doses of 200 or 400 µg per injection) have found convincing evidence that conjugate dosing even up to 2 mg per injection correlates positively with titer levels (Hatsukami et al., 2005, 2011; Martell et al., 2005). Because no ceiling effect has been observed, these results suggest that the optimum conjugate dose is the highest possible dose at which no side effects occur, and to date no significant adverse effects to drug conjugate vaccines have been observed in clinical conjugate vaccine studies (Hatsukami et al., 2005; Martell et al., 2005).
C. Hapten Design
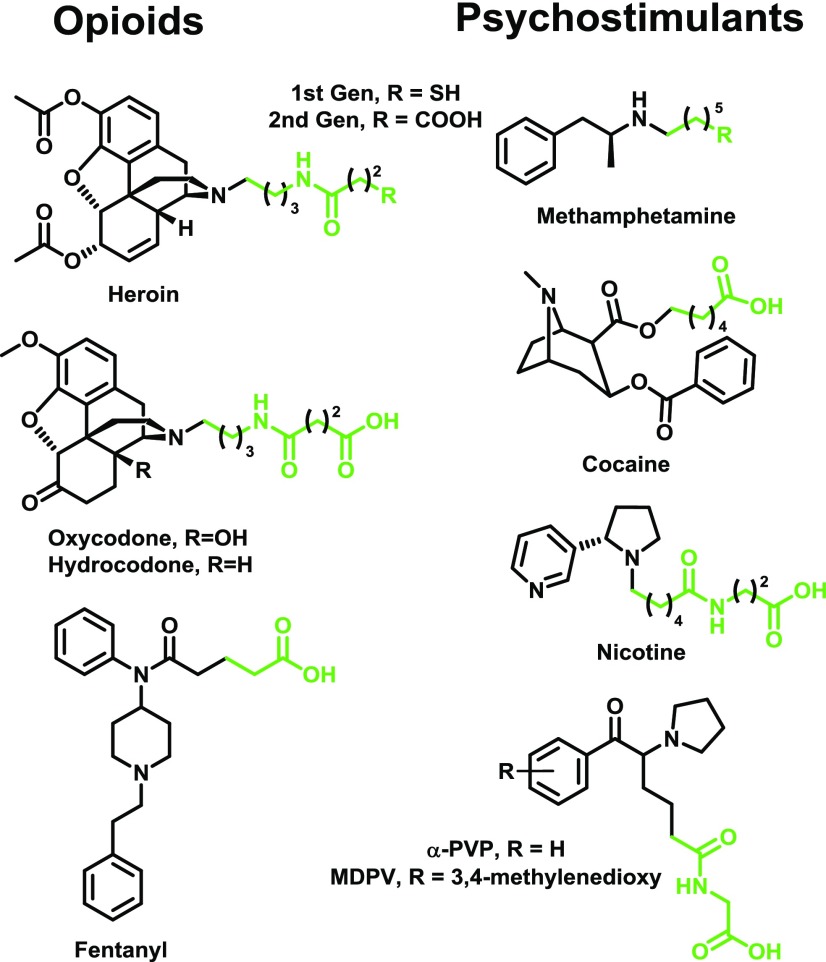
The primary goal in designing drug haptens is to preserve the native chemical structure of the drug as much as possible to generate antibodies with the highest degree of drug affinity. Obviously, addition of a linker will alter the structure, but it can be done so strategically to minimize disruption of key drug functionalities. For example, drugs containing a terminal alkyl group can simply be elongated for installment of the linker and has to date been the most successful hapten design strategy (Fig. 5) (Carrera et al., 1995; Isomura et al., 2001; Moreno et al., 2011; Stowe et al., 2011; Bremer et al., 2016; Kimishima et al., 2017). Linker installment off of an aryl ring has also been shown to be an effective hapten design strategy. For example, haptens substituted at the 3 and 4 positions of the pyridine in nicotine and the phenyl ring in methamphetamine (Figs. 9 and 10) have shown success as immunoconjugates in generating antibodies with high affinity for their target drugs (Peterson et al., 2007; Pryde et al., 2013). Although hapten design via alkyl chain elongation or aryl substitution can equally preserve the chemical functionalities present in the target drug, it is unclear which strategy produces antibodies with better affinity. However, a key influence of hapten linker position is that it will most likely permit greater structural feature flexibility at the corresponding position of antibody-recognized small molecules. For example, a fentanyl hapten linked off the propanoyl group (Fig. 5) produced antibodies that could bind fentanyls containing acetyl, propanoyl, and butanoyl groups with equally high affinity, whereas binding affinity to fentanyls with methyl groups at other positions was lower (Bremer et al., 2016).
Fig. 5.
Structures of drugs of abuse haptens designed with extended alkyl chains for linker incorporation. The target drugs are shown in black, and the linker moieties are shown in green. References for haptens depicted in figure are as follows: heroin (HerSH/COOH haptens) (Stowe et al., 2011; Bremer et al., 2017); oxy/hydrocodone (Kimishima et al., 2017); fentanyl (Bremer et al., 2016); methamphetamine (MH6/COOH haptens) (Moreno et al., 2011; Collins et al., 2016); cocaine (GNC hapten) (Carrera et al., 1995); nicotine (NIC hapten) (Isomura et al., 2001); and cathinones (Nguyen et al., 2017a).
Fig. 9.
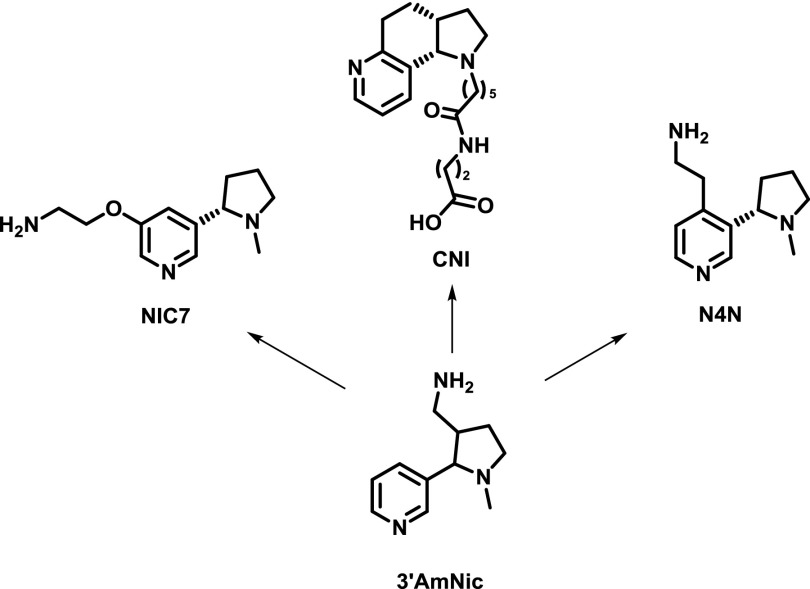
Progression of first-generation racemic 3′AmNic hapten to promising enantiopure second-generation haptens. All amine haptens (except acid hapten CNI) were conjugated to succinate groups preloaded onto the carrier (see Fig. 8) (Pentel et al., 2000; Meijler et al., 2003; Pryde et al., 2013; Jacob et al., 2016). NIC7 has also been coupled directly to surface aspartates and glutamates (McCluskie et al., 2015b).
Fig. 10.
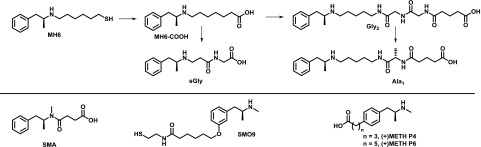
Structures of first- and second-generation methamphetamine haptens. Peptidic spacers have been added to the first-generation MH6 hapten (Moreno et al., 2011), yielding second-generation haptens (Collins et al., 2016; Gooyit et al., 2017). Other first-generation haptens are shown below (Byrnes-Blake et al., 2001; Peterson et al., 2007; Carroll et al., 2011; Shen et al., 2013).
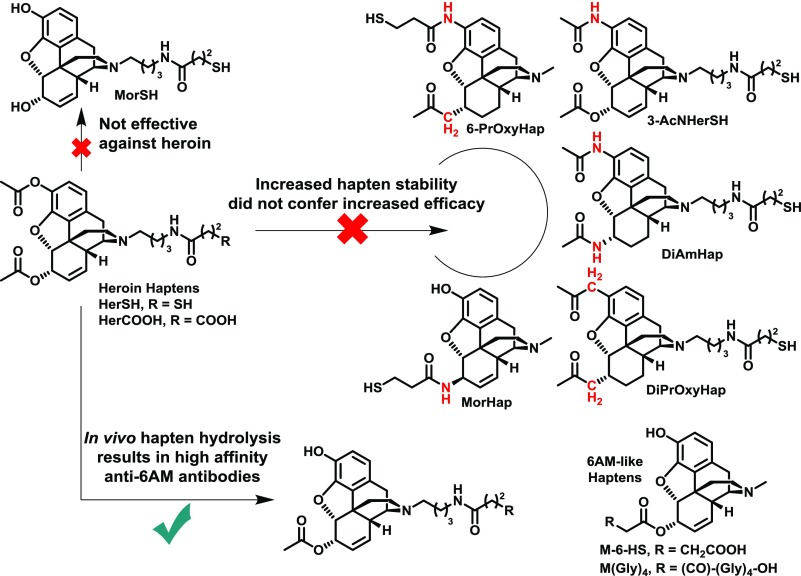
As mentioned in the previous section, drug metabolism is a critical consideration for hapten design. For example, the in vivo hydrolysis of cocaine and heroin generates less psychoactive metabolites in the former case, but more psychoactive metabolites in the latter case (Fig. 4). Attempts to stabilize heroin haptens by means of isosteric replacement of the 3- and 6-position esters to amides have failed to garner increased vaccine efficacy in blocking heroin psychoactivity (Fig. 6). This is most likely due to the failure of these haptens to properly mimic the most active heroin metabolite, 6 AM. Previously reported 3-AcN, DiAm, 6-PrOxy, and DiPrOxy haptens (Fig. 6) cannot present as a 6 AM-like epitope due to hydrolytically stable 3-position functionalities (Bremer and Janda, 2012; Li et al., 2014). The MorHap, while containing the proper 3-position phenol, possesses incorrect stereochemistry at the 6-position (Matyas et al., 2014). In contrast, M-6-HS, M(Gly)4, and hydrolyzed heroin haptens HerSH and HerCOOH (Fig. 6) can properly mimic 6 AM and have shown efficacy in heroin self-administration models (Bonese et al., 1974; Anton and Leff, 2006; Stowe et al., 2011; Schlosburg et al., 2013; Raleigh et al., 2014). In a comparison of heroin, 6 AM, and morphine haptens, the corresponding heroin and 6 AM vaccines, but not the morphine vaccine, was effective at blocking heroin psychoactivity (Stowe et al., 2011; Schlosburg et al., 2013; Bremer et al., 2017). Given these results and the poor performance of the 3-AcNHerSH hapten (Bremer and Janda, 2012), the presence of a 3-acetyl group in the hapten is most likely unnecessary for heroin vaccine potency, whereas the 6-acetyl group is critical.
Fig. 6.
Isosterism as a design strategy for heroin haptens. Replacement of labile 3- and 6-position acetyls with amide or ketone analogs did not enhance vaccine efficacy (Bremer and Janda, 2012; Li et al., 2014; Matyas et al., 2014), whereas haptens mimicking the 6 AM structure have shown success (Bonese et al., 1974; Pravetoni et al., 2012c; Bremer et al., 2017). A hapten lacking both acetyls was not effective against heroin (Schlosburg et al., 2013).
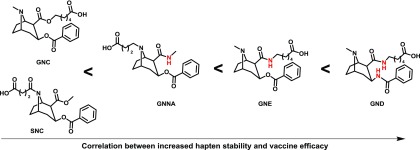
Stabilization of cocaine haptens via amide replacement has shown much greater success compared to heroin haptens (Fig. 7). A correlation was observed between cocaine hapten stability and vaccine efficacy (Carrera et al., 2001; Cai et al., 2013). The amide modification had a small effect on decreasing antibody affinity for cocaine, as one might expect; however, the small loss in affinity was greatly outweighed by an increase in antibody concentration. The success of amide cocaine haptens may also be related to the fact that the amide is positioned at the linker attachment point and thus plays less of a role in determining antibody–drug binding.
Fig. 7.
Isosterism as a design strategy for cocaine haptens. In contrast to heroin vaccine efficacy, cocaine vaccine efficacy benefitted from increased hapten stability through amide isosteric replacement (Carrera et al., 1995, 2001; Fox et al., 1996; Cai et al., 2013).
Simultaneous immunization of multiple hapten protein conjugates with distinct hapten structures has been explored (Keyler et al., 2008; Pravetoni et al., 2012a; Cornish et al., 2013; de Villiers et al., 2013). In these studies, the hapten linkers were installed at different positions on the nicotine molecule to presumably produce distinct B cell populations specific to each hapten. The resulting antiserum produced by the heterogeneous collection of B cells showed enhanced nicotine-neutralizing capacity compared with using only one immunizing hapten. Because many different permutations exist in experimental design, further research is needed to explore this approach. For example, structurally distinct haptens can be simultaneously coupled to one carrier, individually coupled to the same carrier, or each coupled to different carriers. Furthermore, the selection of haptens and adjuvants are also important factors. Combination vaccines have also been explored for producing antibodies against multiple drug targets. Coadministration of 6 AM and oxycodone conjugates did not compromise efficacy against either drug compared with the response of individual vaccines (Pravetoni et al., 2012c). These opioids are structurally similar; however, it is unclear whether this approach would work for structurally dissimilar drugs. Heroin and fentanyl are structurally dissimilar, and a combination vaccine against them would be of great value given that the two are often mixed together by drug dealers.
Another strategy for hapten design involves conformationally constraining haptens. Drugs such as methamphetamine and nicotine are fairly flexible; therefore, mimicking the most stable conformational isomers with the analogous haptens could enhance antibody binding to these drugs (Meijler et al., 2003). A study comparing a conformationally constrained nicotine hapten (CNI; Fig. 9) with a corresponding unconstrained hapten (NIC; Fig. 5) showed that the constrained CNI hapten generated higher concentrations of anti-nicotine antibodies, possibly due to enhanced immunogenicity of the bulkier CNI hapten. Additionally, the CNI hapten as a conjugate vaccine showed greater antagonism of nicotine antinociception and greater alteration of nicotine self-administration versus the unconstrained hapten (Moreno et al., 2012). In contrast, methamphetamine vaccine efficacy did not benefit from the use of conformationally constrained haptens (Moreno et al., 2011).
D. Linker Design and Bioconjugate Chemistry
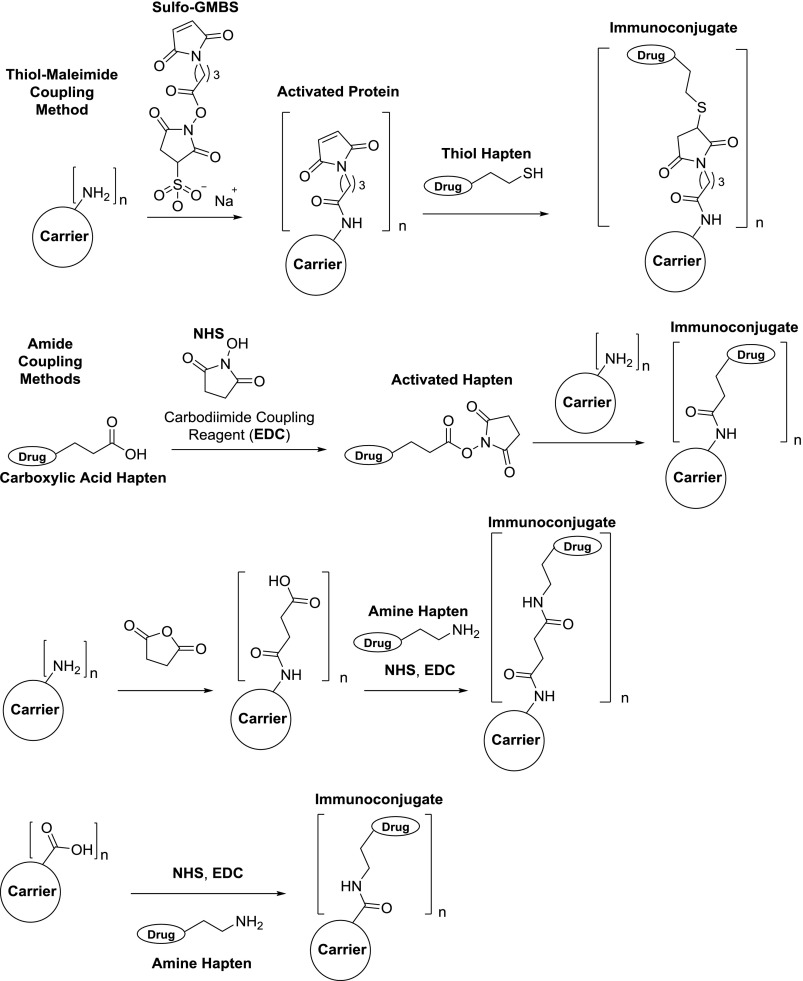
Bioconjugation of drug haptens to proteins is a critical step in forming an immunologically active conjugate that will stimulate the production of anti-drug antibodies. Studies have shown that higher hapten loading onto the protein, i.e., epitope density, results in greater anti-hapten antibody titers and enhanced vaccine efficacy, both in the context of drugs of abuse vaccines (Jalah et al., 2015; McCluskie et al., 2015b) and vaccines for infectious diseases (Clarke et al., 1987; Anderson et al., 1989; Liu et al., 2004; Liu and Chen, 2005; Zhou et al., 2012). As a possible explanation of this phenomenon, higher epitope density most likely causes more effective cross-linking of B cell receptors (BCRs) to activate B cells for a stronger humoral response (Fig. 3). It should be noted that hapten to protein ratios on CRM [diphtheria toxoid (DT) mutant] of >18 reduced conjugate efficacy (11–18 was ideal), whereas no hapten-loading ceiling effect was observed with tetanus toxoid (TT) (Jalah et al., 2015; McCluskie et al., 2015b). To enable conjugation, the hapten linker must contain a functional group typically at the hapten terminus that is compatible with coupling chemistry. The two most commonly used methods for conjugation are thiol-maleimide and amide-coupling reactions (Fig. 8). The former requires the presence of a sulfhydryl group on the hapten as well as maleimide-loaded carrier protein for Michael addition of the sulfhydryl to the maleimide. The latter requires a carboxylic acid-containing hapten or carrier that can be converted into an activated N-hydroxysuccinimide ester for amine substitution to form an amide. Although the thiol-maleimide coupling is more bio-orthogonal compared with amide coupling, we have shown that amide coupling produces an immunoconjugate with superior anti-drug efficacy (Collins et al., 2016; Bremer et al., 2017). We attribute this result to the fact that the thiol-maleimide–coupling procedure ultimately leaves unconjugated maleimides, which could present as an immunologically distracting epitope, thus reducing response to the drug-like epitope.
Fig. 8.
Commonly used bioconjugation methods for generating drug hapten–protein immunoconjugates. (Top) Surface lysine residues on the carrier protein are used for loading of maleimides. Thiol haptens can perform Michael addition to the maleimides to generate the conjugate. (Upper middle) N-hydroxysuccinimide (NHS) esters are formed from carboxylic acid haptens using a carbodiimide-coupling reagent, e.g., 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC). The resulting esters can then be reacted with surface protein lysines to form the conjugate. (Bottom middle) Surface protein lysines are modified with succinic anhydride, which allows for amide coupling to an amino-terminal hapten. (Bottom) Instead of lysines, carboxyl-containing amino acids on the carrier (aspartic and glutamic acids) can be coupled directly to amino-terminal haptens to generate immunoconjugates. Typical hapten loading onto carrier proteins: n = 5–30.
Design of the hapten terminus is determined by the desired bioconjugation method; however, the structure of the remainder of the linker is unrestricted. As mentioned before, minimizing the potential for the linker to present as an epitope is a critical design strategy, which can be accomplished by utilizing nonsterically bulky alkyl chains. In a comprehensive survey of linkers in nicotine haptens, neither the polarity nor the length of the linker significantly affected vaccine efficacy, and the only linker feature that markedly reduced vaccine efficacy was the presence of bulky squaramide or cyclohexyl groups (Pryde et al., 2013). These two groups as well as maleimide are large enough to interfere with the drug-like epitope. Although length and lipophilicity do not appear to be an important aspect of the linker, recent studies have suggested that incorporation of peptidic spacers can enhance vaccine responses. For example, the addition of a diglycine unit into the linker of a nicotine vaccine improved the antibody response (Collins and Janda, 2014), and recent studies have shown a similar result with a methamphetamine hapten (Collins et al., 2016). Addition of a tetraglycine spacer was found to enhance the immunogenicity of an oxycodone hapten, giving higher titers than a similar vaccine without the tetraglycine (Pravetoni et al., 2012b, 2013). In preliminary experiments, we investigated the use of a di-β-alanine linker; however, this showed inferior results compared to a shorter linker without the di-β-alanine (Bremer et al., 2017). We postulate that the failure of the β-alanine linker could be attributed to the unnatural nature of the linker. The major histocompatibility complex (MHC)II is likely to favor the loading of peptidic fragments containing natural amino acids (Rudensky et al., 1991; Brown et al., 1993). MHCII loading is an essential process in recruiting T cell help for the generation of anti-hapten antibodies (Fig. 3), and, although nonpeptidic fragments of MHC ligands are permitted in some cases, these elements have to be designed carefully so as not to substantially disrupt the MHC–ligand interaction (Weiss et al., 1995; Bianco et al., 1998). Furthermore, antigen processing of hapten–protein conjugates results in hapten–peptide fragments, and the peptidic portion of these fragments is essential for anchoring the hapten to the MHCII (Avci et al., 2011), which may explain why peptidic linkers can influence hapten immunogenicity. Interestingly, in a recent study of methamphetamine vaccines, addition of a mono-L-alanine spacer to the linker was superior to other peptidic spacers, including di-glycine, di-L-alanine, L-proline-glycine, and L-proline-L-alanine (Gooyit et al., 2017) (Fig. 10). The introduction of amino acids into hapten linkers has to date proven promising; however, future studies should seek to rationalize this strategy and establish whether it can translate to other drug haptens.
E. Carrier Protein Selection
The carrier protein is an essential component of drug immunoconjugates because it serves as a source of T cell epitopes. These epitopes are necessary for activation of T cells via APCs, and activated T cells can then stimulate B cells, i.e., T cell help (Fig. 3). The cognate interactions between these cells occur via the MHCII loaded with the hapten linked to the T cell epitope (∼15 amino acids derived from the carrier protein). MHCII loading occurs first through uptake and intracellular proteolytic digestion of the immunoconjugate. An essential part of immunoconjugate processing by B cells is the cross-linking of multiple BCRs by the hapten antigen (Fig. 3). For this reason, an adequate degree of carrier protein haptenation, i.e., epitope density, is desired to induce efficient BCR cross-linking for downstream recruitment of T cell help.
Few studies have compared the efficacy of various proteins as carriers for small-molecule haptens. In nicotine and heroin vaccine studies from our group, we have uncovered a trend in immunogenicity in the following order: keyhole limpet hemocyanin (KLH) < DT < TT (Moreno et al., 2010; Bremer et al., 2017). Proteins BSA and ovalbumin are usually considered to be less immunogenic carriers than KLH. In many cases, DT is used as a detoxified mutant of diphtheria toxin (G52E) known as CRM197. The typical detoxifying procedure for diphtheria and tetanus toxins to produce the corresponding toxoids involves formaldehyde treatment; in contrast, CRM can simply be expressed in bacteria and used directly following purification without further treatment. Of the aforementioned carriers, CRM, DT, and TT have been approved for clinical use in polysaccharide conjugate vaccines (Pichichero, 2013). However, KLH has yet to be approved despite extensive use as a carrier. Native KLH exists as two distinct ∼400-kDa subunits that form multidecameric quaternary structures, the size and complexity of which hamper characterization of KLH immunoconjugates. Drug conjugate vaccine studies have mostly used native KLH, although monomeric KLH has been successfully used (Ruedi-Bettschen et al., 2013; Stevens et al., 2016).
In nicotine vaccine studies, other carriers such as the outer protein capsid of bacteriophage Q-β and recombinant Pseudomonas aeruginosa exoprotein A (rEPA) have been explored. Q-β has been directly compared with CRM and has been found to be slightly less effective (McCluskie et al., 2015a). An assessment of KLH and rEPA as carriers has revealed higher anti-nicotine antibody concentrations when vaccinating with the KLH conjugate, although different haptens on each carrier were used (Cornish et al., 2013). Another protein derived from the cholera B toxin (rCTB) has been used in a cocaine vaccine, which has been tested with limited success in clinical trials (phases I–III) (Kosten et al., 2002, 2014; Martell et al., 2005).
Liposomes have been used as carriers for heroin and nicotine haptens, in which the haptens are directly linked to lipids (Lockner et al., 2013; Matyas et al., 2013); however, a lack of evaluation in animal behavioral models has tempered enthusiasm for these vaccines. In a related strategy, a nicotine nanoparticle vaccine (SEL-068) has been developed containing a fully synthetic polymer matrix containing a TLR agonist and Th peptide, and nicotine haptens are covalently linked to this matrix (Pittet et al., 2012). Results from monkey studies evaluating vaccine immunogenicity and effects on nicotine discrimination have proved promising (Fraser et al., 2014; Desai and Bergman, 2015), although key structural information regarding the vaccine components has not been disclosed.
III. Methods for Evaluating Drug Conjugate Vaccines
A. Immunochemical Methods
Characterizing vaccine-generated antibodies via immunochemical methods is essential for judging vaccine efficacy. Both antibody concentration and drug affinity are key determinants of how well antiserum can neutralize drug doses. Enzyme-linked immunosorbent assay (ELISA) is an important tool for measuring antibody responses to any vaccine, whereas radioimmunoassay (RIA) can accurately quantify small-molecule affinity and concentration of antibodies. In the context of drugs of abuse vaccines, ELISA plates are typically coated with the immunizing hapten linked to a nonimmunizing carrier protein (often BSA). Serum collected from immunized animals is then serially diluted across the ELISA plates, and the serum antibodies bound to the plate are detected with an enzyme-conjugated secondary antibody (horseradish peroxidase secondary antibodies are often used). The antibody dilution that gives either 50% signal (midpoint) or no signal (endpoint) is reported as the antibody titer. Typically, the greater the affinity and concentration of polyclonal antibodies for the coating antigen, the higher this titer value will be (Friguet et al., 1985). For drug vaccines, our group prefers determining the 50% titer by means of a nonlinear fit of titer data because increased objectivity is obtained by interpolating the 50% value via curve fitting, in contrast to “eye-balling” the final well that produces signal for endpoint titer determination. ELISA has many limitations that include lack of consistency (repeat assays may give different results) and the fact that results will only show relative differences between samples run at the same time. Many different factors can influence output signal, such as incubation times and secondary antibody quality. A particularly important factor is the structure of the hapten used in the coating antigen. Binding of antiserum to immobilized hapten does not necessarily translate to binding to actual drug; therefore, the hapten should be highly congruent in structure to the target drug. Assuming that the appropriate hapten is used for ELISA, the titer results should correlate to the degree of antibody-mediated drug sequestration and potency attenuation in vivo, as observed in previous studies (de Villiers et al., 2010; Pravetoni et al., 2012c; Miller et al., 2013; Bremer et al., 2017).
To assess actual drug affinity of antiserum produced from vaccination, competitive ELISA can be used. In this variant of the ELISA, the limiting dilution of antiserum is first determined, which is the dilution that gives ∼70% signal, and increasing concentrations of free drug are added to antiserum at the limiting dilution in the presence of immobilized hapten. Free drug will compete with the hapten for antibody binding and will decrease ELISA signal as a function of drug concentration. The resulting IC50 values from this assay will represent relative antiserum affinity for the specific drug. This competition experiment has been adapted to a surface plasmon resonance platform for increased ease and reproducibility (Bremer et al., 2016; Kimishima et al., 2017). Instead of competition with immobilized hapten, RIA can be employed, involving the use of isotopically labeled drug tracers in an equilibrium dialysis setup for determination of antibody Kd. In RIA, a scintillation counter must be used for detection of the tritium-labeled tracer. As a nonradioactive alternative, deuterated drug tracer can be used and detected by liquid chromatography–mass spectrometry (LCMS) (Torres et al., 2016). Using the Kd value discerned from the assay as well as the tracer concentration, the antibody concentration can be calculated using Müller’s method (Muller, 1983). Assays directly measuring antibody interactions with drug tracers (RIA) compared with immobilized drug haptens (ELISA) are considerably more accurate and represent antibody binding to the actual drug. However, caution should be used in assigning absolute affinity and concentration to a polyclonal antibody mixture as the values represent an averaged result; vaccination produces a distribution of B cell populations with varying antigen affinities.
B. In Vivo Evaluation of Drug Vaccines
Because the level of antibody titer, concentration, and affinity needed for an efficacious vaccine is not well known and depends on the target drug, methods for measuring the pharmacokinetics and pharmacodynamics of the drug in vaccinated subjects are essential. As mentioned before, drug-sequestering antibodies will increase half-life and AUC in the blood of the target drug. These parameters can be measured by administering a drug dose to vaccinated animals and extracting blood at various time points. Despite being a terminal procedure, brain tissue can be harvested and analyzed for determination of central nervous system drug exposure. Following sample preparation, LCMS is typically used to quantify both free and antibody-bound drug concentrations. Higher blood concentrations of drug as a result of antibody sequestration in vaccinated animals have consistently correlated with lowered distribution of drug to the brain (Fox et al., 1996; Pentel et al., 2000; Pravetoni et al., 2012c; Miller et al., 2013). Although alteration of drug pharmacokinetics and biodistribution is predictive of vaccine efficacy, an assessment of pharmacodynamics is arguably more important. Behavioral models are an important tool in measuring drug pharmacodynamic effects. It is known that most addictive drugs (opioids or psychostimulants) elicit their rewarding effects by increasing dopamine levels in a brain region known as the nucleus accumbens (Di Chiara and Imperato, 1988). In vivo voltammetry has been used to observe vaccine-mediated blockade of nicotine-induced dopamine release in this brain region (de Villiers et al., 2002, 2010). With the exception of producing downstream dopamine increases, opioids and psychostimulants have different pharmacodynamic profiles: opioids activate MORs, whereas psychostimulants target the dopamine transport system. In considering these differing mechanisms, behavioral models can be specifically tailored to measure the effects of either drug type. For example, opioids like heroin are potent analgesics, and their effects can be readily quantified by nociception (acute pain) assays such as the tail-flick and hot-plate tests (Bannon and Malmberg, 2007). In these assays, the animals’ response time to nociception induced by noxious stimuli (in this case heat) is measured as a function of increasing doses of opioid to generate a dose–response curve. In the presence of the vaccine, the dose–response curve will be right shifted, and the degree to which it is shifted serves as an excellent quantitative metric for assessing vaccine efficacy (Schlosburg et al., 2013; Bremer et al., 2014). Psychostimulants, in contrast, do not necessarily possess analgesic activity but produce high levels of locomotor activity that can be readily measured with infrared photobeam or video-tracking systems (Di Chiara and Imperato, 1988; Riviere et al., 1999). Vaccination against psychostimulants such as cocaine, methamphetamine, and cathinones can attenuate drug effects on locomotor activity, and the degree to which this occurs is indicative of vaccine efficacy (Carrera et al., 2001; Byrnes-Blake et al., 2003; Miller et al., 2013; Nguyen et al., 2017a,b). The locomotor and antinociception assays are the most basic and easy to perform behavioral tests for evaluating the immunoantagonistic action of drug vaccines and are predictive of efficacy in more sophisticated models for drug abuse such as conditioned-place preference, drug discrimination, and self-administration (Carrera et al., 2001; LeSage et al., 2006; Miller et al., 2013; Schlosburg et al., 2013; Desai and Bergman, 2015; Nguyen et al., 2017a,b). Most vaccine studies have used mice [BALB/c (inbred) or Swiss Webster (oubred) strains] or rats (Wistar or Sprague Dawley strains). Immune and drug response variation as a result of strain and species differences are likely to impact apparent vaccine efficacy, although no studies to our knowledge have investigated this directly. Male rodents have been more commonly used over female rodents, and it is unclear whether sex differences can affect vaccine responses as well. However, nicotine vaccine studies in pregnant female rats have shown that vaccine-generated antibodies can protect fetuses from nicotine (Keyler et al., 2003, 2005). Furthermore, a methamphetamine vaccine (MH6-KLH) was recently shown to be equally as effective in female rats, as was previously shown in male rats (Nguyen et al., 2017b).
IV. Recent Progress in Drug Conjugate Vaccine Research
A. Psychostimulants
1. Nicotine.
Nicotine is an addictive natural product found in tobacco and acts as a nicotinic acetylcholine receptor agonist. Because the metabolites of nicotine (half-life of nicotine = 1–2 hours) are less physiologically active than the parent compound, drug vaccine haptens have sought to replicate the structure of nicotine (Fig. 5) (Hieda et al., 1997; Isomura et al., 2001; Pryde et al., 2013). The 3′AmNic hapten as a rEPA conjugate, also known as NicVax, has been tested in clinical trials, and results have demonstrated only mild efficacy (Hatsukami et al., 2005, 2011; Wagena et al., 2008; Hoogsteder et al., 2014). Improvements have been made to this vaccine that include the use of DT carrier, addition of CpG ODN (McCluskie et al., 2013), and an enantiopure version of the hapten mimicking the naturally occurring (-)-nicotine enantiomer (Lockner et al., 2015). The second-generation nicotine hapten known as NIC7 has shown promise in generating anti-nicotine antibodies in nonhuman primates, which could reduce brain concentrations of the drug (Fig. 9) (Pryde et al., 2013; McCluskie et al., 2015a,b). Another second-generation nicotine known as N4N (Fig. 9) has displayed superior performance when compared with 3′AmNic in attenuating nicotine-induced antinociception and hypothermia (Jacob et al., 2016). A fully synthetic nicotine vaccine (SEL-068) containing an unknown hapten has been shown to block nicotine discrimination in nonhuman primates (Fraser et al., 2014; Desai and Bergman, 2015).
2. Cocaine.
Cocaine is a relatively short-acting (half-life = 30 minutes) drug and has complex inhibitory effects on the uptake of monoamine neurotransmitters in the central nervous system. Importantly, it modulates the dopamine transporter, increasing synaptic dopamine—a key pharmacodynamic property that lends to the rewarding and reinforcing effects of cocaine (Ritz et al., 1987). Considering the metabolism of cocaine (Fig. 4), hapten design strategies have involved the stabilization of labile cocaine esters via isosteric replacement of the esters with amides (Fig. 7). Although the amide haptens generated antibodies with slightly less affinity for cocaine, a large boost in antibody concentration was supposedly responsible for a greater attenuation of cocaine-induced hyperlocomotion (Carrera et al., 2001; Cai et al., 2013). In terms of vaccine formulation, GNE hapten (Fig. 7) conjugated to carrier proteins flagellin and TT coadministered with alum and CpG ODN has proven effective in the mouse locomotor assay (Kimishima et al., 2016). The GNC hapten but also the more promising GNE hapten conjugated to a disrupted adenovirus formulated with Adjuplex (proprietary combination of lecithin and carbomer homopolymer) has also shown therapeutic potential (Hicks et al., 2011; Wee et al., 2012). In nonhuman primate studies, the GNE vaccine reduced cocaine occupancy of the dopamine transporter (Maoz et al., 2013), mitigated biodistribution of cocaine to the brain among other organs through serum antibody-mediated drug sequestration (Hicks et al., 2014), and attenuated cocaine self-administration and reacquisition (Evans et al., 2016). Another cocaine vaccine consisting of N-succinyl norcocaine (SNC)-conjugated rCTB formulated with alum has been tested in clinical trials through phase III, and results have shown a lack of efficacy (Kosten et al., 2002, 2014; Martell et al., 2005). These trials may have benefitted from the inclusion of CpG ODN in the adjuvant formulation, but more importantly the redesign of the SNC hapten to exclude the non-native amide at the cocaine nitrogen (Fig. 7). Efforts to convert the amide to an alkyl chain have been reported; however, the newer haptens contain labile esters that are unlikely to be stable in vivo (Ramakrishnan et al., 2014).
3. Methamphetamine.
Methamphetamine, also known as meth, and its related parent compound amphetamine are in a large class of drugs known as substituted phenethylamines. These drugs produce their effects by modulating monoamine neurotransmitter systems. Meth potently activates trace amino-associated receptor 1, thus inhibiting dopamine uptake to increase extracellular dopamine levels (Harris and Baldessarini, 1973; Borowsky et al., 2001; Xie and Miller, 2009). This pharmacodynamic action of meth lends to its psychoactive and addictive effects, and, of the two isomers, the dextrorotatory isomer [(+)-methamphetamine] has been shown to be at least four times more potent than (-)-meth (Harris and Baldessarini, 1973). Initial immunotherapeutic approaches to meth abuse have focused on the creation of anti-meth monoclonal antibodies (mAbs), and phenyl-substituted haptens such as (+)METH P4 and P6 (Fig. 10) could generate mAbs with low nanomolar Kd affinity for meth (Peterson et al., 2007). Passive immunization of these mAbs in rodents reduced meth distribution to the brain and attenuated the effects of meth in self-administration and locomotor assays (Byrnes-Blake et al., 2003; McMillan et al., 2004). A first-generation vaccine containing a (+)-meth hapten known as MH6 (Figs. 5 and 10) conjugated to KLH and formulated with alum mitigated meth-induced thermoregulation, hyperlocomotion, and self-administration in rats (Moreno et al., 2011; Miller et al., 2013, 2015). Second-generation haptens have focused on terminal sufhydryl to carboxyl conversion (MH6-COOH; Fig. 10) (Collins et al., 2016). As mentioned previously, peptidic spacers have been incorporated into meth haptens for increased anti-hapten antibody responses (Collins et al., 2016; Gooyit et al., 2017). A shortened glycine-terminal hapten (sGly; Fig. 10) conjugated to TT and formulated with alum + CpG ODN attenuated meth-induced hyperlocomotion in mice (Collins et al., 2016). Because a diglycine-containing hapten (Gly2) showed promise, a survey of peptidic spacers was performed and identified a monoalanine (Ala1) hapten (Fig. 10) that significantly reduced brain concentrations of meth in vaccinated mice (Gooyit et al., 2017). Other efforts in meth vaccine development include a succinyl methamphetamine hapten (SMA; Fig. 10)–KLH conjugate formulated with MPLA, which reduced meth-induced locomotor effects in mice (Shen et al., 2013). A SMA-TT conjugate formulated with alum also showed efficacy in the methamphetamine conditioned place preference model (Haile et al., 2015). However, the SMA hapten has been found to elicit antibodies with poor binding affinity for methamphetamine, presumably due to the substitution of the secondary amine in methamphetamine for a secondary amide in the SMA hapten (Fig. 10) (Collins et al., 2016). Phenyl-substituted meth haptens have also been reported for active vaccination such as SMO9 and (+)METH P4 (Fig. 10). A SMO9-KLH conjugate formulated with alum elicited antibodies with high affinity for meth and ameliorated meth-induced impairment of behavioral responding for food (Carroll et al., 2011; Ruedi-Bettschen et al., 2013). The same conjugate formulated with TLR4 agonist glucopyranosyl lipid A/oil-in-water emulsion also elicited high-affinity anti-meth antibodies (Stevens et al., 2016). Lastly, a fully synthetic peptide construct linked to the (+)METH P4 hapten (Byrnes-Blake et al., 2001) generated meth-binding antibodies that could cause alteration of meth self-administration in rats (Duryee et al., 2009).
4. Cathinones.
Cathinones are a class of psychostimulant drugs similar in structure and pharmacology to amphetamines but contain an aryl ketone (Fig. 5). Two cathinones, in particular 3,4-methylenedioxypyrovalerone and α-pyrrolidinopentiophenone (αPVP), have shown significant potential for abuse because of the observed rewarding and reinforcing effects of these drugs in rodent models ( Watterson et al., 2014; Aarde et al., 2015a,b). These drugs were relatively unknown until reports of their recreational use first appeared in the 2000s, and a decade later they have been designated as schedule I drugs. Recently, vaccines against both 3,4-methylenedioxypyrovalerone and αPVP have been developed (Fig. 5), which consisted of the corresponding hapten–KLH conjugates formulated with alum and CpG ODN. In rats these vaccines could attenuate cathinone-induced hyperlocomotor activity, and the αPVP vaccine reduced self-administration of αPVP (Nguyen et al., 2017a).
B. Opioids
1. Heroin.
Heroin is a highly addictive opioid that causes more overdose deaths in the United States than any other drug (Warner et al., 2016). As shown in Fig. 4, heroin is a prodrug that rapidly deacetylates to metabolites 6 AM and morphine, both of which are MOR agonists (Selley et al., 2001; Andersen et al., 2009). Since the early report of a 6 AM-like hapten known as M-6-HS (Fig. 6) (Bonese et al., 1974), a similar hapten containing a longer linker conjugated to TT formulated with alum showed efficacy in a rat self-administration model (Anton and Leff, 2006). Further development of 6-position–linked haptens resulted in the inclusion of a tetraglycine spacer to yield M(Gly)4 (Fig. 6) (Pravetoni et al., 2012c). This hapten was conjugated to KLH and formulated with complete and incomplete Freund’s adjuvants (complete Freund’s is forbidden for human use), and the vaccine formulations mitigated heroin biodistribution, antinociception, and self-administration (Raleigh et al., 2013, 2014). A hapten known as MorHap (Fig. 6), which is the opposite epimer of heroin at the 6-position (Matyas et al., 2013, 2014), has been shown to reduce heroin antinociception as a TT or CRM conjugate formulated with MPLA-containing liposomes (Jalah et al., 2015). In a different hapten design strategy, the linker has been attached to the heroin nitrogen (HerSH and HerCOOH) (Figs. 5 and 6). When HerSH was conjugated to KLH and formulated with alum, the resulting vaccine could attenuate heroin reinstatement, self-administration, conditioned place preference, and antinociception (Stowe et al., 2011; Schlosburg et al., 2013; Bremer et al., 2014). In contrast to other reported vaccines, the HerSH conjugate vaccine containing alum and CpG ODN could reduce heroin antinociception at a dose of >5 mg/kg (Bremer et al., 2014) compared with the single dose of 1 mg/kg used in other studies (Raleigh et al., 2013; Jalah et al., 2015). Furthermore, a second generation HerCOOH-TT conjugate formulated with the same adjuvants reduced heroin antinociception at a doses of >10 mg/kg in mice and mitigated heroin-induced decreases in operant responding in nonhuman primates (Bremer et al., 2017).
2. Fentanyl.
Fentanyl is a powerful MOR agonist (50–100 times more potent than morphine) and is responsible for a recent surge in overdose deaths (Frank and Pollack, 2017). Fentanyl or various analogs of fentanyl such as acetylfentanyl are often mixed with heroin, thus enhancing the potency and danger of presumed heroin use (Centers for Disease Control and Prevention (CDC), 2013; Stogner, 2014). To date, one report of a fentanyl-TT conjugate formulated with alum + CpG ODN has been disclosed. This vaccine shifted the fentanyl dose–response curve by 30-fold in mouse antinociceptive testing and showed significant cross-reactivity with fentanyl derivatives that differed by one methyl group (Bremer et al., 2016). Furthermore, mouse serum antibodies bound fentanyl with single-digit nanomolar affinity.
3. Oxycodone and Hydrocodone.
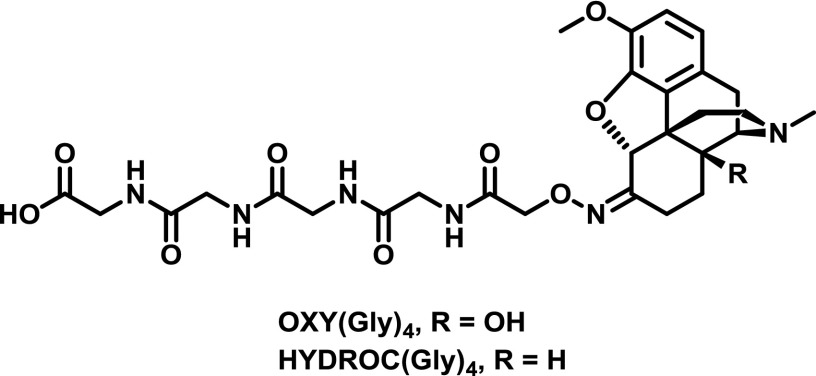
Oxycodone, also known as OxyContin, and hydrocodone, also known as Vicodin, are some of the most commonly prescribed opioids for pain relief, yet they are in the top 10 drugs involved in overdose deaths in the United States (Warner et al., 2016). The first oxy and hydrocodone haptens were reported relatively recently and consisted of the tetraglycine spacer found in M(Gly)4 linked to oxycodone via an oxime [OXY and HYDROC(Gly)4; Fig. 11]. Immunization of the corresponding KLH conjugates formulated with Freund’s adjuvant could block oxycodone and hydrocodone biodistribution and antinociception (Pravetoni et al., 2012b,c, 2013). Interestingly, the OXY(Gly)4 hapten showed much greater efficacy, even against hydrocodone, compared to the HYDROC(Gly)4 hapten (Pravetoni et al., 2013). Further investigation of OXY(Gly)4 revealed that TT and alum could be used with similar efficacy in place of KLH and Freund’s, respectively, whereas MPLA addition did not increase efficacy (Pravetoni et al., 2014b). The OXY(Gly)4-KLH conjugate immunized with Freund’s adjuvant blocked acquisition of i.v. oxycodone in a rat self-administration procedure and mitigated oxycodone-induced modulation of brain gene expression (Pravetoni et al., 2014a). Investigation of oxy and hydrocodone haptens linked through the nitrogen similar to heroin hapten HerSH (Fig. 6) showed significant right shifting (5- to 10-fold) of opioid dose–effect curves in aninociceptive testing of vaccinated mice (Kimishima et al., 2017). Additionally, the vaccinated mice showed resistance to opioid overdose mortality and reduced opioid biodistribution. Isolated serum IgG antibodies possessed subnanomolar affinity for both oxycodone and hydrocodone regardless of immunizing hapten, with minimal cross-reactivity to other opioids (Kimishima et al., 2017).
Fig. 11.
Oxime-linked oxycodone and hydrocodone haptens (Pravetoni et al., 2013).
V. Conclusions and Outlook
In the last few decades, a wealth of studies has been published disclosing efforts to develop conjugate vaccines against a variety of harmful opioids and psychostimulants. First-generation cocaine and nicotine vaccines have failed in clinical trials, which may have dampened enthusiasm for the therapeutic viability of conjugate vaccines to ameliorate substance use disorder. We remain optimistic that these vaccines will one day meet clinical endpoints because of the major scientific advances that have been made in the design and development of the vaccines. Arguably, the most critical design element is the necessity of the hapten to possess maximal structural congruence to the target drug. A lack of adherence to this principle has most likely played a key role in the failure of the aforementioned vaccines in clinical trials. Specifically, stereochemistry should be preserved: racemic nicotine haptens will not generate antibodies with optimal specificity to (-)-nicotine found in tobacco. Furthermore, hydrolytically labile esters in cocaine haptens and inappropriately placed amides in both cocaine and methamphetamine haptens in place of native amines will reduce antibody affinity to the target drugs, thus hampering vaccine performance. Second-generation haptens have mostly addressed these shortcomings and will likely experience greater success in the clinic. Improvements to adjuvant formulation are also anticipated to boost efficacy of future vaccines entering clinical trials. For example, addition of TLR9 agonist CpG ODN to virtually any immunoconjugate–alum vaccine formulation has enhanced anti-hapten antibody responses.
An emerging trend in vaccine evaluation is the generation of drug dose–response curves in an appropriate behavioral assay. The degree to which a vaccine shifts these dose–response curves gives a quantitative measurement of the vaccine’s immunoantagonistic capacity as a relevant representation of efficacy. However, it is currently not clear what minimum fold-shift benchmark is required for efficacy against substance use disorder, and the benchmark will most likely depend on the type of drug and the severity of an individual’s substance use disorder. First-generation vaccines were not subjected to rigorous preclinical testing and were most likely prematurely entered into clinical trials. Before pursuing clinical evaluation of second-generation vaccines, they should first show robust production of antibodies with high titer and drug affinity and should markedly shift drug dose–effect curves; preferably, this should be demonstrated in non-human primates to maximize the potential for translational efficacy in humans. Despite the failure of the first-generation vaccines in clinical trials, the vaccines proved to be safe and mitigated drug intake in the highest responding individuals. These studies provide theoretical grounds that redesigned vaccines, which meet the aforementioned preclinical requirements could perform well in clinical trials.
Because no vaccines against drug abuse have been FDA-approved, the specific clinical applications of the vaccines have not been determined. One could imagine conjugate vaccines being used to prevent first-time drug use, drug overdose, or drug dependence. Furthermore, it could be used to assist active drug users in halting drug intake or to help former drug users in maintaining abstinence. A key limitation of immunotherapies for substance use disorder is that antibodies cannot ameliorate drug cravings and may precipitate symptoms of withdrawal from blunting drug potency. Fortunately, the exquisite selectivity of antibodies enables coadministration of pharmacotherapies that would quell cravings; in the case of opioid abuse, methadone and/or buprenorphine could be administered while vaccinating against heroin and/or fentanyl. Because substance use disorder occurs on a spectrum, vaccine therapy would most likely require case-specific tailoring of vaccine dosage, immunization schedule, and administration of adjunct pharmacotherapies.
Abbreviations
- APC
antigen-presenting cell
- AUC
area under the curve
- BCR
B cell receptor
- BSA
bovine serum albumin
- DC
dendritic cell
- DT
diphtheria toxoid
- ELISA
enzyme-linked immunosorbent assay
- FDA
Food and Drug Administration
- HerCOOH
heroin carboxylic acid hapten
- HerSH
heroin thiol hapten
- IL
interleukin
- KLH
keyhole limpet hemocyanin
- LogP
partition coefficient
- mAb
monoclonal antibody
- MDPV
methylenedioxypyrovalerone
- MHC
major histocompatibility complex
- MOR
μ-opioid receptor
- MPLA
monophosphoryl lipid A
- ODN
oligodeoxynucleotide
- PAMP
pathogen-associated molecular pattern
- αPVP
α-pyrrolidinopentiophenone
- rEPA
recombinant Pseudomonas aeruginosa exoprotein A
- RIA
radioimmunoassay
- SMA
N-succinyl methamphetamine hapten
- SNC
N-succinyl norcocaine
- Th
T-helper
- THC
tetrahydrocannabinol
- TLR
Toll-like receptor
- TT
tetanus toxoid
Footnotes
This work was supported by National Institutes of Health National Institute on Drug Abuse [Grants R01DA008590, R01DA026625, R21DA039634, and UH2DA041146].
References
- Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA. (2015a) In vivo potency and efficacy of the novel cathinone α-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology (Berl) 232:3045–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Dickerson TJ, Taffe MA. (2015b) Binge-like acquisition of 3,4-methylenedioxypyrovalerone (MDPV) self-administration and wheel activity in rats. Psychopharmacology (Berl) 232:1867–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JM, Ripel A, Boix F, Normann PT, Mørland J. (2009) Increased locomotor activity induced by heroin in mice: pharmacokinetic demonstration of heroin acting as a prodrug for the mediator 6-monoacetylmorphine in vivo. J Pharmacol Exp Ther 331:153–161. [DOI] [PubMed] [Google Scholar]
- Anderson PW, Pichichero ME, Stein EC, Porcelli S, Betts RF, Connuck DM, Korones D, Insel RA, Zahradnik JM, Eby R. (1989) Effect of oligosaccharide chain length, exposed terminal group, and hapten loading on the antibody response of human adults and infants to vaccines consisting of Haemophilus influenzae type b capsular antigen unterminally coupled to the diphtheria protein CRM197. J Immunol 142:2464–2468. [PubMed] [Google Scholar]
- Anton B, Leff P. (2006) A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents. Vaccine 24:3232–3240. [DOI] [PubMed] [Google Scholar]
- Avci FY, Li X, Tsuji M, Kasper DL. (2011) A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med 17:1602–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MF, Jennings GT. (2010) Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol 10:787–796. [DOI] [PubMed] [Google Scholar]
- Bannon AW and Malmberg AB (2007) Models of nociception: hot-plate, tail-flick, and formalin tests in rodents. Curr Protoc Neurosci Chapter 8:Unit 8.9. [DOI] [PubMed] [Google Scholar]
- Bianco A, Brock C, Zabel C, Walk T, Walden P, Jung G. (1998) New synthetic non-peptide ligands for classical major histocompatibility complex class I molecules. J Biol Chem 273:28759–28765. [DOI] [PubMed] [Google Scholar]
- Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. (1974) Changes in heroin self-administration by a rhesus monkey after morphine immunization. Nature 252:708–710. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, et al. (2001) Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci USA 98:8966–8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer PT, Janda KD. (2012) Investigating the effects of a hydrolytically stable hapten and a Th1 adjuvant on heroin vaccine performance. J Med Chem 55:10776–10780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer PT, Kimishima A, Schlosburg JE, Zhou B, Collins KC, Janda KD. (2016) Combatting synthetic designer opioids: a conjugate vaccine ablates lethal doses of fentanyl class drugs. Angew Chem Int Ed Engl 55:3772–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer PT, Schlosburg JE, Banks ML, Steele FF, Zhou B, Poklis JL, Janda KD. (2017) Development of a clinically-viable heroin vaccine. J Amer Chem Soc, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer PT, Schlosburg JE, Lively JM, Janda KD. (2014) Injection route and TLR9 agonist addition significantly impact heroin vaccine efficacy. Mol Pharm 11:1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, Kim YH, Hoppe RT, Knox SJ, Shin LK, et al. (2010) In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol 28:4324–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JH, Jardetzky TS, Gorga JC, Stern LJ, Urban RG, Strominger JL, Wiley DC. (1993) Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature 364:33–39. [DOI] [PubMed] [Google Scholar]
- Byrnes-Blake KA, Carroll FI, Abraham P, Owens SM. (2001) Generation of anti-(+)methamphetamine antibodies is not impeded by (+)methamphetamine administration during active immunization of rats. Int Immunopharmacol 1:329–338. [DOI] [PubMed] [Google Scholar]
- Byrnes-Blake KA, Laurenzana EM, Carroll FI, Abraham P, Gentry WB, Landes RD, Owens SM. (2003) Pharmacodynamic mechanisms of monoclonal antibody-based antagonism of (+)-methamphetamine in rats. Eur J Pharmacol 461:119–128. [DOI] [PubMed] [Google Scholar]
- Cai X, Whitfield T, Moreno AY, Grant Y, Hixon MS, Koob GF, Janda KD. (2013) Probing the effects of hapten stability on cocaine vaccine immunogenicity. Mol Pharm 10:4176–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera MR, Ashley JA, Parsons LH, Wirsching P, Koob GF, Janda KD. (1995) Suppression of psychoactive effects of cocaine by active immunization. Nature 378:727–730. [DOI] [PubMed] [Google Scholar]
- Carrera MR, Ashley JA, Wirsching P, Koob GF, Janda KD. (2001) A second-generation vaccine protects against the psychoactive effects of cocaine. Proc Natl Acad Sci USA 98:1988–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FI, Blough BE, Pidaparthi RR, Abraham P, Gong PK, Deng L, Huang X, Gunnell M, Lay JO, Jr, Peterson EC, et al. (2011) Synthesis of mercapto-(+)-methamphetamine haptens and their use for obtaining improved epitope density on (+)-methamphetamine conjugate vaccines. J Med Chem 54:5221–5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2013) Acetyl fentanyl overdose fatalities: Rhode Island, March-May 2013. MMWR Morb Mortal Wkly Rep 62:703–704. [PMC free article] [PubMed] [Google Scholar]
- Chen X, Pravetoni M, Bhayana B, Pentel PR, Wu MX. (2012) High immunogenicity of nicotine vaccines obtained by intradermal delivery with safe adjuvants. Vaccine 31:159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke BE, Newton SE, Carroll AR, Francis MJ, Appleyard G, Syred AD, Highfield PE, Rowlands DJ, Brown F. (1987) Improved immunogenicity of a peptide epitope after fusion to hepatitis B core protein. Nature 330:381–384. [DOI] [PubMed] [Google Scholar]
- Collins KC, Janda KD. (2014) Investigating hapten clustering as a strategy to enhance vaccines against drugs of abuse. Bioconjug Chem 25:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins KC, Schlosburg JE, Bremer PT, Janda KD. (2016) Methamphetamine vaccines: improvement through hapten design. J Med Chem 59:3878–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins KC, Schlosburg JE, Lockner JW, Bremer PT, Ellis BA, Janda KD. (2014) Lipid tucaresol as an adjuvant for methamphetamine vaccine development. Chem Commun (Camb) 50:4079–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CL, Davis HL, Morris ML, Efler SM, Adhami MA, Krieg AM, Cameron DW, Heathcote J. (2004) CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol 24:693–701. [DOI] [PubMed] [Google Scholar]
- Cornish KE, de Villiers SH, Pravetoni M, Pentel PR. (2013) Immunogenicity of individual vaccine components in a bivalent nicotine vaccine differ according to vaccine formulation and administration conditions. PLoS One 8:e82557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, van Melle G, Bangala Y, Guessous I, Müller P, Willers J, et al. (2008) A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS One 3:e2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers SH, Lindblom N, Kalayanov G, Gordon S, Baraznenok I, Malmerfelt A, Marcus MM, Johansson AM, Svensson TH. (2010) Nicotine hapten structure, antibody selectivity and effect relationships: results from a nicotine vaccine screening procedure. Vaccine 28:2161–2168. [DOI] [PubMed] [Google Scholar]
- de Villiers SHL, Cornish KE, Troska AJ, Pravetoni M, Pentel PR. (2013) Increased efficacy of a trivalent nicotine vaccine compared to a dose-matched monovalent vaccine when formulated with alum. Vaccine 31:6185–6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers SHL, Lindblom N, Kalayanov G, Gordon S, Malmerfelt A, Johansson AM, Svensson TH. (2002) Active immunization against nicotine suppresses nicotine-induced dopamine release in the rat nucleus accumbens shell. Respiration 69:247–253. [DOI] [PubMed] [Google Scholar]
- Desai RI, Bergman J. (2015) Effects of the nanoparticle-based vaccine, SEL-068, on nicotine discrimination in squirrel monkeys. Neuropsychopharmacology 40:2207–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85:5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duryee MJ, Bevins RA, Reichel CM, Murray JE, Dong Y, Thiele GM, Sanderson SD. (2009) Immune responses to methamphetamine by active immunization with peptide-based, molecular adjuvant-containing vaccines. Vaccine 27:2981–2988. [DOI] [PubMed] [Google Scholar]
- Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. (2008) Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminum adjuvants. Nature 453:1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Foltin RW, Hicks MJ, Rosenberg JB, De BP, Janda KD, Kaminsky SM, Crystal RG. (2016) Efficacy of an adenovirus-based anti-cocaine vaccine to reduce cocaine self-administration and reacqusition using a choice procedure in rhesus macaques. Pharmacol Biochem Behav 150-151:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox BS, Kantak KM, Edwards MA, Black KM, Bollinger BK, Botka AJ, French TL, Thompson TL, Schad VC, Greenstein JL, et al. (1996) Efficacy of a therapeutic cocaine vaccine in rodent models. Nat Med 2:1129–1132. [DOI] [PubMed] [Google Scholar]
- Franchi L, Núñez G. (2008) The Nlrp3 inflammasome is critical for aluminum hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol 38:2085–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RG, Pollack HA. (2017) Addressing the fentanyl threat to public health. N Engl J Med 376:605–607. [DOI] [PubMed] [Google Scholar]
- Fraser CC, Altreuter DH, Ilyinskii P, Pittet L, LaMothe RA, Keegan M, Johnston L, Kishimoto TK. (2014) Generation of a universal CD4 memory T cell recall peptide effective in humans, mice and non-human primates. Vaccine 32:2896–2903. [DOI] [PubMed] [Google Scholar]
- Friguet B, Chaffotte AF, Djavadi-Ohaniance L, Goldberg ME. (1985) Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J Immunol Methods 77:305–319. [DOI] [PubMed] [Google Scholar]
- Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. (2006) Adjuvant-enhanced antibody responses in the absence of Toll-like receptor signaling. Science 314:1936–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenny A, Buttle A, Stevens M. (1931) Rate of disappearance of diphtheria toxoid injected into rabbits and guinea pigs: toxoid precipitated with alum. J Pathol Bacteriol 34:267–275. [Google Scholar]
- Gooyit M, Miranda PO, Wenthur CJ, Ducime A, Janda KD. (2017) Influencing antibody-mediated attenuation of methamphetamine CNS distribution through vaccine linker design. ACS Chem Neurosci 8:468–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, Kosten TA, Shen XY, O’Malley PW, Winoske KJ, Kinsey BM, Wu Y, Huang Z, Lykissa ED, Naidu N, et al. (2015) Altered methamphetamine place conditioning in mice vaccinated with a succinyl-methamphetamine-tetanus-toxoid vaccine. Am J Addict 24:748–755. [DOI] [PubMed] [Google Scholar]
- Hansen B, Belfast M, Soung G, Song L, Egan PM, Capen R, Hogenesch H, Mancinelli R, Hem SL. (2009) Effect of the strength of adsorption of hepatitis B surface antigen to aluminum hydroxide adjuvant on the immune response. Vaccine 27:888–892. [DOI] [PubMed] [Google Scholar]
- Hansen B, Sokolovska A, HogenEsch H, Hem SL. (2007) Relationship between the strength of antigen adsorption to an aluminum-containing adjuvant and the immune response. Vaccine 25:6618–6624. [DOI] [PubMed] [Google Scholar]
- Harris JE, Baldessarini RJ. (1973) Uptake of (3H)-catecholamines by homogenates of rat corpus striatum and cerebral cortex: effects of amphetamine analogues. Neuropharmacology 12:669–679. [DOI] [PubMed] [Google Scholar]
- Hartmann G, Krieg AM. (2000) Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol 164:944–953. [DOI] [PubMed] [Google Scholar]
- Hartmann G, Weeratna RD, Ballas ZK, Payette P, Blackwell S, Suparto I, Rasmussen WL, Waldschmidt M, Sajuthi D, Purcell RH, et al. (2000) Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J Immunol 164:1617–1624. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, Tashkin DP, Reus VI, Akhavain RC, Fahim REF, et al. (2011) Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther 89:392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Rennard S, Jorenby D, Fiore M, Koopmeiners J, de Vos A, Horwith G, Pentel PR. (2005) Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin Pharmacol Ther 78:456–467. [DOI] [PubMed] [Google Scholar]
- Hicks MJ, De BP, Rosenberg JB, Davidson JT, Moreno AY, Janda KD, Wee S, Koob GF, Hackett NR, Kaminsky SM, et al. (2011) Cocaine analog coupled to disrupted adenovirus: a vaccine strategy to evoke high-titer immunity against addictive drugs. Mol Ther 19:612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks MJ, Kaminsky SM, De BP, Rosenberg JB, Evans SM, Foltin RW, Andrenyak DM, Moody DE, Koob GF, Janda KD, et al. (2014) Fate of systemically administered cocaine in nonhuman primates treated with the dAd5GNE anticocaine vaccine. Hum Gene Ther Clin Dev 25:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieda Y, Keyler DE, Ennifar S, Fattom A, Pentel PR. (2000) Vaccination against nicotine during continued nicotine administration in rats: immunogenicity of the vaccine and effects on nicotine distribution to brain. Int J Immunopharmacol 22:809–819. [DOI] [PubMed] [Google Scholar]
- Hieda Y, Keyler DE, Vandevoort JT, Kane JK, Ross CA, Raphael DE, Niedbalas RS, Pentel PR. (1997) Active immunization alters the plasma nicotine concentration in rats. J Pharmacol Exp Ther 283:1076–1081. [PubMed] [Google Scholar]
- Hieda Y, Keyler DE, Vandevoort JT, Niedbala RS, Raphael DE, Ross CA, Pentel PR. (1999) Immunization of rats reduces nicotine distribution to brain. Psychopharmacology (Berl) 143:150–157. [DOI] [PubMed] [Google Scholar]
- Hill JH, Wainer BH, Fitch FW, Rothberg RM. (1975) Delayed clearance of morphine from the circulation of rabbits immunized with morphine-6-hemisuccinate bovine serum albumin. J Immunol 114:1363–1368. [PubMed] [Google Scholar]
- Hoogsteder PHJ, Kotz D, van Spiegel PI, Viechtbauer W, van Schayck OCP. (2014) Efficacy of the nicotine vaccine 3′-AmNic-rEPA (NicVAX) co-administered with varenicline and counselling for smoking cessation: a randomized placebo-controlled trial. Addiction 109:1252–1259. [DOI] [PubMed] [Google Scholar]
- Isomura S, Wirsching P, Janda KD. (2001) An immunotherapeutic program for the treatment of nicotine addiction: hapten design and synthesis. J Org Chem 66:4115–4121. [DOI] [PubMed] [Google Scholar]
- Jacob NT, Lockner JW, Schlosburg JE, Ellis BA, Eubanks LM, Janda KD. (2016) Investigations of enantiopure nicotine haptens using an adjuvanting carrier in anti-nicotine vaccine development. J Med Chem 59:2523–2529. [DOI] [PubMed] [Google Scholar]
- Jalah R, Torres OB, Mayorov AV, Li F, Antoline JFG, Jacobson AE, Rice KC, Deschamps JR, Beck Z, Alving CR, et al. (2015) Efficacy, but not antibody titer or affinity, of a heroin hapten conjugate vaccine correlates with increasing hapten densities on tetanus toxoid, but not on CRM197 carriers. Bioconjug Chem 26:1041–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyler DE, Dufek MB, Calvin AD, Bramwell TJ, LeSage MG, Raphael DE, Ross CA, Le CT, Pentel PR. (2005) Reduced nicotine distribution from mother to fetal brain in rats vaccinated against nicotine: time course and influence of nicotine dosing regimen. Biochem Pharmacol 69:1385–1395. [DOI] [PubMed] [Google Scholar]
- Keyler DE, Roiko SA, Earley CA, Murtaugh MP, Pentel PR. (2008) Enhanced immunogenicity of a bivalent nicotine vaccine. Int Immunopharmacol 8:1589–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyler DE, Shoeman D, LeSage MG, Calvin AD, Pentel PR. (2003) Maternal vaccination against nicotine reduces nicotine distribution to fetal brain in rats. J Pharmacol Exp Ther 305:587–592. [DOI] [PubMed] [Google Scholar]
- Kimishima A, Wenthur CJ, Eubanks LM, Sato S, Janda KD. (2016) Cocaine vaccine development: evaluation of carrier and adjuvant combinations that activate multiple Toll-like receptors. Mol Pharm 13:3884–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimishima A, Wenthur CJ, Zhou B, Janda KD. (2017) An advance in prescription opioid vaccines: overdose mortality reduction and extraordinary alteration of drug half-life. ACS Chem Biol 12:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool M, Pétrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J. (2008a) Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol 181:3755–3759. [DOI] [PubMed] [Google Scholar]
- Kool M, Soullié T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. (2008b) Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med 205:869–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Domingo CB, Shorter D, Orson F, Green C, Somoza E, Sekerka R, Levin FR, Mariani JJ, Stitzer M, et al. (2014) Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend 140:42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Rosen M, Bond J, Settles M, Roberts JSC, Shields J, Jack L, Fox B. (2002) Human therapeutic cocaine vaccine: safety and immunogenicity. Vaccine 20:1196–1204. [DOI] [PubMed] [Google Scholar]
- Krug A, Rothenfusser S, Hornung V, Jahrsdörfer B, Blackwell S, Ballas ZK, Endres S, Krieg AM, Hartmann G. (2001) Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol 31:2154–2163. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Hieda Y, Collins G, Burroughs D, Le C, Pentel PR. (2006) Effects of a nicotine conjugate vaccine on the acquisition and maintenance of nicotine self-administration in rats. Psychopharmacology (Berl) 184:409–416. [DOI] [PubMed] [Google Scholar]
- Li F, Cheng K, Antoline JFG, Iyer MR, Matyas GR, Torres OB, Jalah R, Beck Z, Alving CR, Parrish DA, et al. (2014) Synthesis and immunological effects of heroin vaccines. Org Biomol Chem 12:7211–7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Chen YH. (2005) High epitope density in a single protein molecule significantly enhances antigenicity as well as immunogenicity: a novel strategy for modern vaccine development and a preliminary investigation about B cell discrimination of monomeric proteins. Eur J Immunol 35:505–514. [DOI] [PubMed] [Google Scholar]
- Liu W, Peng Z, Liu Z, Lu Y, Ding J, Chen YH. (2004) High epitope density in a single recombinant protein molecule of the extracellular domain of influenza A virus M2 protein significantly enhances protective immunity. Vaccine 23:366–371. [DOI] [PubMed] [Google Scholar]
- Lockner JW, Ho SO, McCague KC, Chiang SM, Do TQ, Fujii G, Janda KD. (2013) Enhancing nicotine vaccine immunogenicity with liposomes. Bioorg Med Chem Lett 23:975–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockner JW, Lively JM, Collins KC, Vendruscolo JC, Azar MR, Janda KD. (2015) A conjugate vaccine using enantiopure hapten imparts superior nicotine-binding capacity. J Med Chem 58:1005–1011. [DOI] [PubMed] [Google Scholar]
- Maoz A, Hicks MJ, Vallabhjosula S, Synan M, Kothari PJ, Dyke JP, Ballon DJ, Kaminsky SM, De BP, Rosenberg JB, et al. (2013) Adenovirus capsid-based anti-cocaine vaccine prevents cocaine from binding to the nonhuman primate CNS dopamine transporter. Neuropsychopharmacology 38:2170–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marichal T, Ohata K, Bedoret D, Mesnil C, Sabatel C, Kobiyama K, Lekeux P, Coban C, Akira S, Ishii KJ, et al. (2011) DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med 17:996–1002. [DOI] [PubMed] [Google Scholar]
- Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. (2005) Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry 58:158–164. [DOI] [PubMed] [Google Scholar]
- Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. (2009) Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry 66:1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440:237–241. [DOI] [PubMed] [Google Scholar]
- Matyas GR, Mayorov AV, Rice KC, Jacobson AE, Cheng K, Iyer MR, Li F, Beck Z, Janda KD, Alving CR. (2013) Liposomes containing monophosphoryl lipid A: a potent adjuvant system for inducing antibodies to heroin hapten analogs. Vaccine 31:2804–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyas GR, Rice KC, Cheng K, Li F, Antoline JFG, Iyer MR, Jacobson AE, Mayorov AV, Beck Z, Torres OB, et al. (2014) Facial recognition of heroin vaccine opiates: type 1 cross-reactivities of antibodies induced by hydrolytically stable haptenic surrogates of heroin, 6-acetylmorphine, and morphine. Vaccine 32:1473–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskie MJ, Pryde DC, Gervais DP, Stead DR, Zhang N, Benoit M, Robertson K, Kim IJ, Tharmanathan T, Merson JR, et al. (2013) Enhancing immunogenicity of a 3'aminomethylnicotine-DT-conjugate anti-nicotine vaccine with CpG adjuvant in mice and non-human primates. Int Immunopharmacol 16:50–56. [DOI] [PubMed] [Google Scholar]
- McCluskie MJ, Thorn J, Gervais DP, Stead DR, Zhang N, Benoit M, Cartier J, Kim IJ, Bhattacharya K, Finneman JI, et al. (2015a) Anti-nicotine vaccines: comparison of adjuvanted CRM197 and Qb-VLP conjugate formulations for immunogenicity and function in non-human primates. Int Immunopharmacol 29:663–671. [DOI] [PubMed] [Google Scholar]
- McCluskie MJ, Thorn J, Mehelic PR, Kolhe P, Bhattacharya K, Finneman JI, Stead DR, Piatchek MB, Zhang N, Chikh G, et al. (2015b) Molecular attributes of conjugate antigen influence function of antibodies induced by anti-nicotine vaccine in mice and non-human primates. Int Immunopharmacol 25:518–527. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Hardwick WC, Li M, Gunnell MG, Carroll FI, Abraham P, Owens SM. (2004) Effects of murine-derived anti-methamphetamine monoclonal antibodies on (+)-methamphetamine self-administration in the rat. J Pharmacol Exp Ther 309:1248–1255. [DOI] [PubMed] [Google Scholar]
- Meijler MM, Matsushita M, Altobell LJ, 3rd, Wirsching P, Janda KD. (2003) A new strategy for improved nicotine vaccines using conformationally constrained haptens. J Am Chem Soc 125:7164–7165. [DOI] [PubMed] [Google Scholar]
- Miller ML, Aarde SM, Moreno AY, Creehan KM, Janda KD, Taffe MA. (2015) Effects of active anti-methamphetamine vaccination on intravenous self-administration in rats. Drug Alcohol Depend 153:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Moreno AY, Aarde SM, Creehan KM, Vandewater SA, Vaillancourt BD, Wright MJ, Jr, Janda KD, Taffe MA. (2013) A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol Psychiatry 73:721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morefield GL, Sokolovska A, Jiang D, HogenEsch H, Robinson JP, Hem SL. (2005) Role of aluminum-containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine 23:1588–1595. [DOI] [PubMed] [Google Scholar]
- Moreno AY, Azar MR, Koob GF, Janda KD. (2012) Probing the protective effects of a conformationally constrained nicotine vaccine. Vaccine 30:6665–6670. [DOI] [PubMed] [Google Scholar]
- Moreno AY, Azar MR, Warren NA, Dickerson TJ, Koob GF, Janda KD. (2010) A critical evaluation of a nicotine vaccine within a self-administration behavioral model. Mol Pharm 7:431–441. [DOI] [PubMed] [Google Scholar]
- Moreno AY, Mayorov AV, Janda KD. (2011) Impact of distinct chemical structures for the development of a methamphetamine vaccine. J Am Chem Soc 133:6587–6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen GE, Ellis RD, Miura K, Malkin E, Nolan C, Hay M, Fay MP, Saul A, Zhu D, Rausch K, et al. (2008) Phase 1 trial of AMA1-C1/Alhydrogel plus CPG 7909: an asexual blood-stage vaccine for Plasmodium falciparum malaria. PLoS One 3:e2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R. (1983) Determination of affinity and specificity of anti-hapten antibodies by competitive radioimmunoassay. Methods Enzymol 92:589–601. [DOI] [PubMed] [Google Scholar]
- Nguyen JD, Bremer PT, Ducime A, Creehan KM, Kisby BR, Taffe MA, Janda KD. (2017a) Active vaccination attenuates the psychostimulant effects of α-PVP and MDPV in rats. Neuropharmacology 116:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Bremer PT, Hwang CS, Vandewater SA, Collins KC, Creehan KM, Janda KD, Taffe MA. (2017b) Effective active vaccination against methamphetamine in female rats. Drug Alcohol Depend 175:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe SM, Green MA, HogenEsch H, Hem SL. (2010) Mechanism of immunopotentiation by aluminum-containing adjuvants elucidated by the relationship between antigen retention at the inoculation site and the immune response. Vaccine 28:3588–3594. [DOI] [PubMed] [Google Scholar]
- Norman AB, Tabet MR, Norman MK, Buesing WR, Pesce AJ, Ball WJ. (2007) A chimeric human/murine anticocaine monoclonal antibody inhibits the distribution of cocaine to the brain in mice. J Pharmacol Exp Ther 320:145–153. [DOI] [PubMed] [Google Scholar]
- Pentel PR, Malin DH, Ennifar S, Hieda Y, Keyler DE, Lake JR, Milstein JR, Basham LE, Coy RT, Moon JW, et al. (2000) A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacol Biochem Behav 65:191–198. [DOI] [PubMed] [Google Scholar]
- Peterson EC, Gunnell M, Che Y, Goforth RL, Carroll FI, Henry R, Liu H, Owens SM. (2007) Using hapten design to discover therapeutic monoclonal antibodies for treating methamphetamine abuse. J Pharmacol Exp Ther 322:30–39. [DOI] [PubMed] [Google Scholar]
- Pichichero ME. (2013) Protein carriers of conjugate vaccines: characteristics, development, and clinical trials. Hum Vaccin Immunother 9:2505–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet L, Altreuter D, Ilyinskii P, Fraser C, Gao Y, Baldwin S, Keegan M, Johnston L, Kishimoto T. (2012) Development and preclinical evaluation of SEL-068, a novel targeted synthetic vaccine particle (tSVP (TM)) for smoking cessation and relapse prevention that generates high titers of antibodies against nicotine. J Immunol 188 (Suppl):75.11. [Google Scholar]
- Pravetoni M, Keyler DE, Pidaparthi RR, Carroll FI, Runyon SP, Murtaugh MP, Earley CA, Pentel PR. (2012a) Structurally distinct nicotine immunogens elicit antibodies with non-overlapping specificities. Biochem Pharmacol 83:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Le Naour M, Harmon TM, Tucker AM, Portoghese PS, Pentel PR. (2012b) An oxycodone conjugate vaccine elicits drug-specific antibodies that reduce oxycodone distribution to brain and hot-plate analgesia. J Pharmacol Exp Ther 341:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Le Naour M, Tucker AM, Harmon TM, Hawley TM, Portoghese PS, Pentel PR. (2013) Reduced antinociception of opioids in rats and mice by vaccination with immunogens containing oxycodone and hydrocodone haptens. J Med Chem 56:915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Pentel PR, Potter DN, Chartoff EH, Tally L, LeSage MG. (2014a) Effects of an oxycodone conjugate vaccine on oxycodone self-administration and oxycodone-induced brain gene expression in rats. PLoS One 9:e101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Raleigh MD, Le Naour M, Tucker AM, Harmon TM, Jones JM, Birnbaum AK, Portoghese PS, Pentel PR. (2012c) Co-administration of morphine and oxycodone vaccines reduces the distribution of 6-monoacetylmorphine and oxycodone to brain in rats. Vaccine 30:4617–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Vervacke JS, Distefano MD, Tucker AM, Laudenbach M, Pentel PR. (2014b) Effect of currently approved carriers and adjuvants on the pre-clinical efficacy of a conjugate vaccine against oxycodone in mice and rats. PLoS One 9:e96547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryde DC, Jones LH, Gervais DP, Stead DR, Blakemore DC, Selby MD, Brown AD, Coe JW, Badland M, Beal DM, et al. (2013) Selection of a novel anti-nicotine vaccine: influence of antigen design on antibody function in mice. PLoS One 8:e76557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Yamamoto N, Meijler MM, Altobell LJ, 3rd, Koob GF, Wirsching P, Janda KD. (2005) Delta9-tetrahydrocannabinol immunochemical studies: haptens, monoclonal antibodies, and a convenient synthesis of radiolabeled delta9-tetrahydrocannabinol. J Med Chem 48:7389–7399. [DOI] [PubMed] [Google Scholar]
- Raleigh MD, Pentel PR, LeSage MG. (2014) Pharmacokinetic correlates of the effects of a heroin vaccine on heroin self-administration in rats. PLoS One 9:e115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh MD, Pravetoni M, Harris AC, Birnbaum AK, Pentel PR. (2013) Selective effects of a morphine conjugate vaccine on heroin and metabolite distribution and heroin-induced behaviors in rats. J Pharmacol Exp Ther 344:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan M, Kinsey BM, Singh RA, Kosten TR, Orson FM. (2014) Hapten optimization for cocaine vaccine with improved cocaine recognition. Chem Biol Drug Des 84:354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237:1219–1223. [DOI] [PubMed] [Google Scholar]
- Rivière GJ, Byrnes KA, Gentry WB, Owens SM. (1999) Spontaneous locomotor activity and pharmacokinetics of intravenous methamphetamine and its metabolite amphetamine in the rat. J Pharmacol Exp Ther 291:1220–1226. [PubMed] [Google Scholar]
- Romero Méndez IZ, Shi Y, HogenEsch H, Hem SL. (2007) Potentiation of the immune response to non-adsorbed antigens by aluminum-containing adjuvants. Vaccine 25:825–833. [DOI] [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P, Hong SC, Barlow A, Janeway CA., Jr (1991) Sequence analysis of peptides bound to MHC class II molecules. Nature 353:622–627. [DOI] [PubMed] [Google Scholar]
- Rüedi-Bettschen D, Wood SL, Gunnell MG, West CM, Pidaparthi RR, Carroll FI, Blough BE, Owens SM. (2013) Vaccination protects rats from methamphetamine-induced impairment of behavioral responding for food. Vaccine 31:4596–4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Vendruscolo LF, Bremer PT, Lockner JW, Wade CL, Nunes AA, Stowe GN, Edwards S, Janda KD, Koob GF. (2013) Dynamic vaccine blocks relapse to compulsive intake of heroin. Proc Natl Acad Sci USA 110:9036–9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley DE, Cao CC, Sexton T, Schwegel JA, Martin TJ, Childers SR. (2001) mu Opioid receptor-mediated G-protein activation by heroin metabolites: evidence for greater efficacy of 6-monoacetylmorphine compared with morphine. Biochem Pharmacol 62:447–455. [DOI] [PubMed] [Google Scholar]
- Shen XY, Kosten TA, Lopez AY, Kinsey BM, Kosten TR, Orson FM. (2013) A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug Alcohol Depend 129:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard OS, Lohse N, Harboe ZB, Offersen R, Bukh AR, Davis HL, Schønheyder HC, Østergaard L. (2010) Improving the immunogenicity of pneumococcal conjugate vaccine in HIV-infected adults with a Toll-like receptor 9 agonist adjuvant: a randomized, controlled trial. Clin Infect Dis 51:42–50. [DOI] [PubMed] [Google Scholar]
- Spector S. (1971) Quantitative determination of morphine in serum by radioimmunoassay. J Pharmacol Exp Ther 178:253–258. [PubMed] [Google Scholar]
- Spector S, Berkowitz B, Flynn EJ, Peskar B. (1973) Antibodies to morphine, barbiturates, and serotonin. Pharmacol Rev 25:281–291. [PubMed] [Google Scholar]
- Spector S, Parker CW. (1970) Morphine: radioimmunoassay. Science 168:1347–1348. [DOI] [PubMed] [Google Scholar]
- Stevens MW, Gunnell MG, Tawney R, Owens SM. (2016) Optimization of a methamphetamine conjugate vaccine for antibody production in mice. Int Immunopharmacol 35:137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stogner JM. (2014) The potential threat of acetyl fentanyl: legal issues, contaminated heroin, and acetyl fentanyl “disguised” as other opioids. Ann Emerg Med 64:637–639. [DOI] [PubMed] [Google Scholar]
- Stowe GN, Vendruscolo LF, Edwards S, Schlosburg JE, Misra KK, Schulteis G, Mayorov AV, Zakhari JS, Koob GF, Janda KD. (2011) A vaccine strategy that induces protective immunity against heroin. J Med Chem 54:5195–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OB, Antoline JFG, Li F, Jalah R, Jacobson AE, Rice KC, Alving CR, Matyas GR. (2016) A simple nonradioactive method for the determination of the binding affinities of antibodies induced by hapten bioconjugates for drugs of abuse. Anal Bioanal Chem 408:1191–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O’Neill D, Pavlick A, Escalon JB, Cruz CM, Angiulli A, et al. (2007) Vaccination with NY-ESO-1 protein and CpG in montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci USA 104:8947–8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vunakis H, Wasserman E, Levine L. (1972) Specificities of antibodies to morphine. J Pharmacol Exp Ther 180:514–521. [PubMed] [Google Scholar]
- Wagena EJ, de Vos A, Horwith G, van Schayck CP. (2008) The immunogenicity and safety of a nicotine vaccine in smokers and nonsmokers: results of a randomized, placebo-controlled phase 1/2 trial. Nicotine Tob Res 10:213–218. [DOI] [PubMed] [Google Scholar]
- Warner M, Trinidad JP, Bastian BA, Minino AM, Hedegaard H. (2016) Drugs most frequently involved in drug overdose deaths: United States, 2010-2014. Natl Vital Stat Rep 65:1–15. [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. (2014) Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Addict Biol 19:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Hicks MJ, De BP, Rosenberg JB, Moreno AY, Kaminsky SM, Janda KD, Crystal RG, Koob GF. (2012) Novel cocaine vaccine linked to a disrupted adenovirus gene transfer vector blocks cocaine psychostimulant and reinforcing effects. Neuropsychopharmacology 37:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss GA, Collins EJ, Garboczi DN, Wiley DC, Schreiber SL. (1995) A tricyclic ring system replaces the variable regions of peptides presented by three alleles of human MHC class I molecules. Chem Biol 2:401–407. [DOI] [PubMed] [Google Scholar]
- Xie Z, Miller GM. (2009) A receptor mechanism for methamphetamine action in dopamine transporter regulation in brain. J Pharmacol Exp Ther 330:316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Zhou L, Chen YH. (2012) Immunization with high epitope density of M2e derived from 2009 pandemic H1N1 elicits protective immunity in mice. Vaccine 30:3463–3469. [DOI] [PubMed] [Google Scholar]