Abstract
Purpose
This study aims (1) to investigate the feasibility of robot-assisted penetrating keratoplasty (PK) using the new Da Vinci Xi Surgical System and (2) to report what we believe to be the first use of this system in experimental eye surgery.
Methods
Robot-assisted PK procedures were performed on human corneal transplants using the Da Vinci Xi Surgical System. After an 8-mm corneal trephination, four interrupted sutures and one 10.0 monofilament running suture were made. For each procedure, duration and successful completion of the surgery as well as any unexpected events were assessed. The depth of the corneal sutures was checked postoperatively using spectral-domain optical coherence tomography (SD-OCT).
Results
Robot-assisted PK was successfully performed on 12 corneas. The Da Vinci Xi Surgical System provided the necessary dexterity to perform the different steps of surgery. The mean duration of the procedures was 43.4 ± 8.9 minutes (range: 28.5–61.1 minutes). There were no unexpected intraoperative events. SD-OCT confirmed that the sutures were placed at the appropriate depth.
Conclusions
We confirm the feasibility of robot-assisted PK with the new Da Vinci Surgical System and report the first use of the Xi model in experimental eye surgery. Operative time of robot-assisted PK surgery is now close to that of conventional manual surgery due to both improvement of the optical system and the presence of microsurgical instruments.
Translational Relevance
Experimentations will allow the advantages of robot-assisted microsurgery to be identified while underlining the improvements and innovations necessary for clinical use.
Keywords: cornea, graft, keratoplasty, robot, surgery
Introduction
Corneal transplantation has been performed for over a century. Since its inception, surgical techniques have constantly improved, especially over the two past decades with the use of lamellar anterior and posterior surgeries. Penetrating keratoplasty (PK) is an important technique because it remains the only way to repair a cornea in corneal perforations, some cases of full-thickness traumatic injuries, and in patients presenting deep infections or needing a graft replacement. Furthermore, a recent study reported that there is no noticeable improvement with the new lamellar technique in terms of graft survival in comparison to PK.1
The Da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, CA) is by far the most widespread robotic surgical system commercially available and is FDA approved. Its introduction in the operating room has revolutionized a number of specialties such as urology, digestive surgery, gynecology, plastic reconstructive surgery, throat surgery, neurosurgery, thoracic surgery, vascular surgery, hand surgery, and peripheral nerve surgery. Robots are currently used in many situations, and the list of appropriate indications is growing rapidly as the technology improves. There have been four different versions of the Da Vinci Surgical System since the first model was launched in 2001: S, Si, Si HD, and Xi. The latest version, the Da Vinci Xi, has an improved optical system that allows higher visual definition and clarity than the previous model. Its camera is equipped with an autofocus system and can be attached to any of the four arms as needed. Additionally, the arms have been redesigned. Consequently, the ports can be placed closer together and still avoid collision. There is also a new overhead instrument arm architecture designed to facilitate anatomical access from virtually any position.
The utilization of robotic techniques in microsurgical specialties, including ophthalmology, has progressed neither to the same extent nor at the same rhythm as have other specialties. Different experimental anterior and posterior segment procedures were performed on pig and cadaver eyes using the Da Vinci System,2–5 but the first cases of robot-assisted ocular surgery in patients were only recently reported at the Strasbourg University Hospital. The Si HD system was used to perform robot-assisted amniotic membrane transplantations and pterygium surgeries (Clinicaltrials.gov, NCT02116062).6,7 The decision to perform robot-assisted PK is the next logical step as it is technically more difficult and the globe is widely opened after trephination. This procedure requires delicate tissue manipulation, 360-degree suturing with excellent visualization, and fast placement of corneal sutures. The proof of concept of robot-assisted PK was provided on porcine and cadaver eyes by Bourges et al.2 in 2009 using a Da Vinci Si version. However, several technical limitations were considered to be major hurdles to further investigations.
Considering the recent technological improvements in the Da Vinci Surgical System, the aim of the present study is to investigate the improved efficacy of robot-assisted PK surgery using the new Da Vinci Xi Surgical System and to report the first use, to our knowledge, of the Xi in experimental eye surgery.
Material and Methods
The Robot
The DaVinci Xi Surgical System consists of three components: a mobile instrument cart with four articulated arms, a vision cart, and a surgeon console used to control the robotic arms (Fig. 1).8 The mobile cart contains the articulated robotic arms, three of which carry surgical instruments, and a fourth arm that manipulates the digital stereoscopic camera that allows the surgeon to visualize the surgical field. The camera provides three-dimensional vision with progressive magnification up to 15 times. It can be placed on any of the arms, and it autofocuses. Each of the arms has multiple joints that allow for three-dimensional movement of the surgical instruments. The surgeon's console is equipped with an optical viewing system, two telemanipulation handles, and five pedals. The optical viewing system offers a three-dimensional, high-definition view of the operating field and displays text messages and icons that reflect the status of the system in real time. The two telemanipulation handles allow the remote manipulation of the four articulated robotic arms. Master-slave controls replicate the surgeon's hand motions, filtering tremor and offering the possibility of using an adjustable motion-scaling ratio. The following robotic tools were used for the surgical procedures: two Black Diamond micro forceps and one Potts scissors. A 30-degree surgical blade (Alcon, Fort Worth, TX) was prepared by removing the metallic extremity from the plastic shaft.
Figure 1.
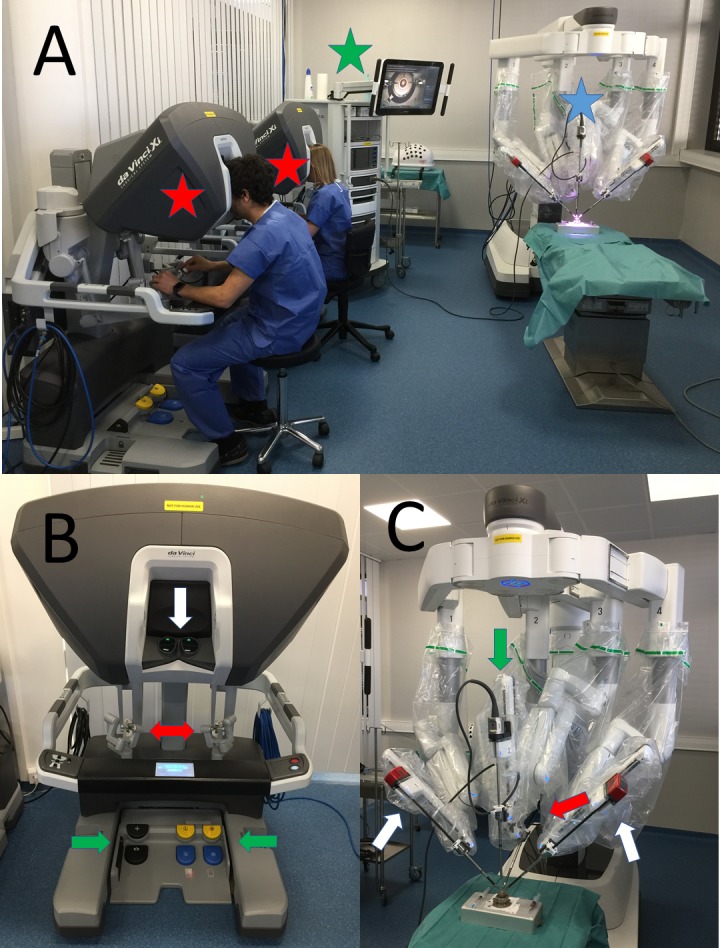
The DaVinci Xi Surgical System. General view (A) with the mobile instrument cart (white star), the two surgeon's consoles (red stars), and the vision cart (green star). The surgeon's console (B) is equipped with an optical viewing system (white arrow), two telemanipulation handles (red arrow), and five pedals (green arrows) and the mobile instrument cart (C) with four articulated arms, of which two carry the Black Diamond micro forceps (white arrows), one carries the Potts scissors (red arrow), and the fourth the digital stereoscopic camera (green arrow).
The Corneas
Human corneal transplants with large scleral collarets and low endothelial cell densities were provided by the EFS Bourgogne Franche-Comté Cornea Bank (Besancon, France). The corneas were placed on an artificial chamber (Moria SA, Antony, France) (Fig. 2). A viscoelastic substance (Viscoat; Alcon) was injected posteriorly into the transplant to achieve an estimated intraocular pressure ranging between 10 and 20 mm Hg measured by digital palpation.
Figure 2.
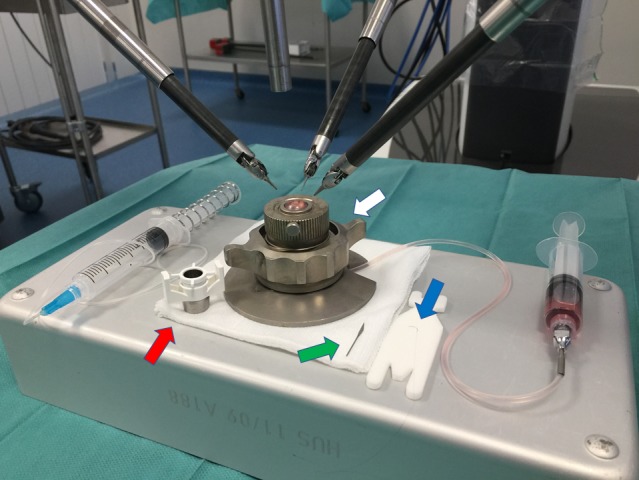
Installation. Standard corneal surgery conditions were reproduced by placing the camera vertically above the cornea installed on the artificial chamber (white arrow). The robotic arms were placed on either side of the globe at about 45-degree angles from the axis created by the midline position of the camera. The Hessburg-Barron vacuum trephine (red arrow), the 30-degree blade (green arrow) used to cut the cornea, and the 10.0 monofilament (blue arrow) are beside the artificial chamber.
The Surgical Procedure
The camera was installed vertically above the cornea, reproducing standard ocular surgery conditions (Fig. 2). An ophthalmic surgeon (TB) with prior experience in keratoplasty and robotic microsurgery certified by the Robotic Assisted Microsurgical and Endoscopic Society (RAMSES) performed the surgical procedures. Surgical movements were scaled to 1.5:1. An 8-mm Hessburg-Barron vacuum trephine (BPI, Grand Blanc, MI) was used to cut the cornea. The trephination was completed using the 30-degree blade. The arms of the robot equipped with the Black Diamond micro forceps held the transplant (Fig. 3). The two Black Diamond micro forceps were then used to perform the three suture loops. After four sutures at the cardinal points (Fig. 4), the corneal button was reattached with a running suture using 10.0 monofilament (Fig. 5). The knots of the stitches were cut using Potts scissors held by the fourth arm. For each procedure, operative time of the different steps and successful completion of surgery as well as any complications or unexpected events were assessed. The first and the last procedures were recorded with still photography and video (Movie 1).
Figure 3.

Trephination. The trephination is performed using an 8-mm Hessburg-Barron vacuum trephine (white arrow) held by one Black Diamond micro forceps (red arrow) and moved by the tip of a second one (green arrow) (A). This step is completed using a 30-degree blade (blue arrow) held by one robotic arm (B).
Figure 4.
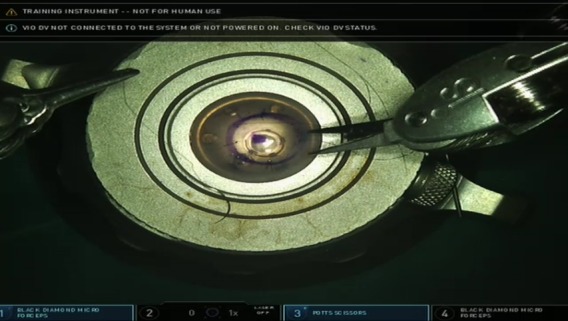
The cardinal stitches. The two Black Diamond micro forceps were used to perform four stitches at the cardinal points with three suture loops. The stitches were cut using an arm equipped with the Potts scissors.
Figure 5.
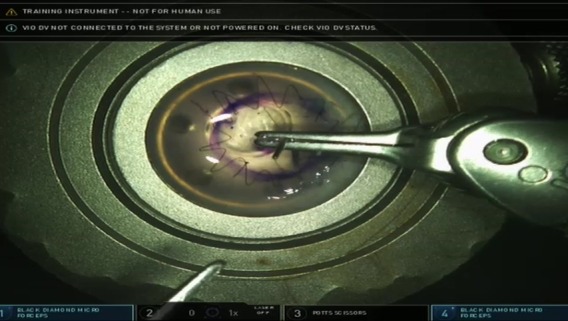
The running suture. Using two Black Diamond micro forceps, the corneal button was reattached with a running suture using 10.0 monofilament cut by the Potts scissors.
Movie 1.
Spectral-Domain Optical Coherence Tomography (SD-OCT) Examination
After completion of the procedures, the anterior chamber with the sutured cornea was fixed on a plate and placed in front of the SD-OCT device. The HRA Spectralis corneal module (Heidelberg Engineering, Heidelberg, Germany) was used.
The study was conducted following the guidelines of the Declaration of Helsinki.
Results
Twelve procedures were performed at the IRCAD center (Strasbourg, France) between January and August 2016. The feasibility of robot-assisted PK surgery using the Da Vinci Xi Surgical System is confirmed. The Da Vinci Xi robotic surgical system provided the dexterity and operative field visualization necessary to perform the main steps of the PK procedure: corneal trephination, corneal button removal, and reattachment. In all cases, the entire procedure could be completed using the robot. The mean duration of the procedures was 43.4 ± 8.9 minutes (range: 28.5–61.1 minutes). The mean duration for one interrupted suture was 102.1 ± 28.4 seconds (range: 63–187 seconds). The mean duration for completion of the running suture was 19.3 ± 4.8 minutes (range: 13.0–30.2 minutes). There were no intraoperative complications and no unexpected events such as thread or needle breakage or conflict between the arms of the robot. Postoperative SD-OCT examination of the cornea confirmed placement of the sutures at the accurate depth (Fig. 6).
Figure 6.
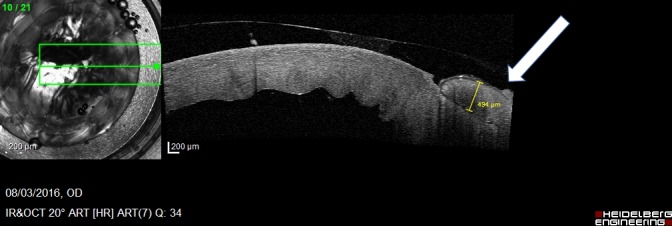
SD-OCT examination of the cornea. The hyporeflective line (white arrow) starting from the epithelium layer and continuing through the stroma layer coincides with route of the thread through the cornea.
Discussion
The Da Vinci Surgical System is a master-slave surgical robot initially designed for endoscopic macrosurgeries. However, some microsurgical specialties, such as urology or head and neck surgery, have explored its possibilities with success.9,10 The surgical robot enables restitution of surgeons' movements with improved accuracy through motion scaling and tremor filtering. Although not specifically designed for eye surgery, the commercial availability of the Da Vinci robotic surgical system has led to many investigations of its applicability in both anterior and posterior segment eye surgery since 2006.2,3,5–7,11,12 In the field of corneal surgery, Bourges et al.2 demonstrated the feasibility of robot-assisted PK surgery in 2009 using the Si model on enucleated porcine and cadaver eyes. However, poor visualization of the operative field, limited maneuverability of the instruments, and the absence of microsurgical instruments were considered by the authors to be hurdles to further investigations with the Da Vinci system.2–4,12–14
Given the reservations expressed concerning poor visualization, we decided to perform experimental robot-assisted PK using the Xi version because it provides both magnification and three-dimensional image quality close to that found in modern surgical microscopes. The resolution of the image projected on the surgeon's console is 1280 × 1024 (SXGA standard). Thus, visualization of the operative field was perfectly adequate for PK surgery. It was easy to position the instruments at the appropriate depth in the operative field. Furthermore, the camera lens is designed to offer an automatic focus on the operative field when the distance between the arm and the tissue is 34 ± 5 mm. This autofocus on the image is very practical and time saving. This is a real advantage over conventional operating microscopes and the previous Da Vinci models.
The second major improvement of the Xi version compared to the Si and Si HD versions is the new design of the robot's arms. Even if paired with the same instrument (Black Diamond micro forceps and Potts scissors), there is improved maneuverability. Additionally, even if robotic tools are not as perfectly adapted to corneal surgery as conventional instruments, they were safe for ocular surface tissues as we noticed neither tissue lesions nor conflict between the instruments.
Several factors, including these two improvements, led to an important decrease in operative time (43.4 minutes) when compared to a previous publication6,7 (Bourges performed PK on cadaver eyes in 115 and 80 minutes), bringing it close to conventional manual PK duration (around 20 minutes). The use of fresh human corneas from the tissue bank that are thinner and less rigid than porcine corneas was an additional factor. Finally, we can underline a learning curve effect, as for any other new procedure, confirming the necessity of specific training. Despite this learning curve confirmed by other robotic microsurgeons from the RAMSES group, the changeover from conventional microsurgery to robotic microsurgery was fairly simple as its main characteristics such as indirect visualization and ergonomic positioning are similar.
The absence of thread or needle breakage during the suture phase confirms that the absence of haptic feedback was compensated by the visual control provided while tying knots.15 However, because of a potential benefit of haptic feedback in retinal surgery, there is a need for research on that topic.16
Another difficulty encountered when using the Da Vinci Surgical System is its inability to handle a remote center of motion, which is characteristic of intraocular surgery. To our knowledge, except for some prototypes developed to overcome this limitation,11,16–18 this remains a problem in endocular surgery, causing motion-related stress at the site of instrument insertion.3 This reservation is of low relevance in our study since PK is not endoscopic surgery.
Despite this drawback, we appreciated the extreme mobility of the distal articulation of the arm supporting the robotic instrument, motion scaling, and the suppression of physiological tremor that provided high-quality surgical movements. The millimetric precision provided by the system is acceptable for corneal surgeries. The robot also allows the surgeon to change the orientation of the instruments in the operative field during surgery. This could also be of help for all surgeons–left- or right-handed. They can perform surgery on either side, freed from constraints related to patient anatomy (prominent noses or supraciliary arches).
We also appreciated the ergonomic position during surgery and the possibility of leaving the instruments in position. This allows the surgeon to rest or to check information in the patient file. The possibility of using a second surgeon's console with the Xi version is also of great interest when considering the robot as a pertinent pedagogic tool for telementoring. Each console can take control of any instrument, and the mentor can immediately correct students' movements. This possibility has already been investigated in other specialties.19,20 The specificity of robotic surgery, and more specifically robotic microsurgery, created the need to develop basic skill courses for robotically assisted microsurgery.8
Finally, a unique feature of the robot is telesurgery, as demonstrated by Marescaux et al.21 during a transatlantic robotic-assisted procedure. This possibility is of great interest for the ophthalmologic field as it could bring state-of-the-art surgical expertise to virtually anywhere in the world. In the current context of high demand for ocular surgery,22 this could be beneficial. Telesurgery takes on even greater meaning in remote places or hazardous regions such as battlefields. The idea of robotic surgery was intensively explored for military purposes to “bring the surgeon to the wounded soldier—through telepresence.”23 The fact that in this experiment the whole operation was carried out using the robot alone does not mitigate the need for a dedicated surgical team to assist the surgeon, whether it is to deploy the robot, perform the initial installation, or change one of the robot's arms. Some ophthalmologic procedures would also need an assistant for fluid injection, device connection, or the preparation of the intraocular lens in cataract surgery, for instance. Furthermore, for security reasons, a surgeon must remain close to the patient in case it is necessary to convert to a manual procedure.
The main hurdle for more widespread research into and the use of the robot in ophthalmic surgery might be its current high cost. Indeed, ophthalmic surgery is already very efficient, and recent innovations such as femtosecond laser devices for cataract surgery already provide very good results with limited costs. The robot needs to bring better results in terms of safety or the reduction of adverse effects to justify the extra cost. Another important obstacle is patient confidence, which we believe will improve over time as new studies show a better final outcome in comparison with manual procedures.
Despite these hurdles, the perspectives of surgical robotics in corneal surgery are numerous. The forthcoming possibility to integrate femtosecond laser and/or imaging devices, such as anterior segment OCT or corneal topography, allows us to imagine a near future with the automation of more and more important parts of surgery, such as suturing with real-time adjustment of suture depth and corneal astigmatism correction. The recent development in artificial intelligence and progress in augmented-reality imaging will benefit this technology. Specific ophthalmic instruments should also be designed to improve the robot's efficacy, and further research should be done to confirm the clinical value of this new version of the Da Vinci robot.
Acknowledgments
The authors thank Richard Bastier, Janel Hooven, Julien Lacaux, and Eric Leplat for their advice, proofreading, and technical support.
This study was funded by les Hôpitaux Universitaires de Strasbourg, Strasbourg, France (API2012#5540).
Disclosure: J. Chammas, None; A. Sauer, None; J. Pizzuto, None; F. Pouthier, None; D. Gaucher, None; J. Marescaux, None; D. Mutter, None; T. Bourcier, None
References
- 1. Bidaut-Garnier M,, Monnet E,, Prongue A,, et al. Evolution of corneal graft survival over a 30-year period and comparison of surgical techniques: a cohort study. Am J Ophthalmol. 2016; 163: 59–69. [DOI] [PubMed] [Google Scholar]
- 2. Bourges JL,, Hubschman JP,, Burt B,, Culjat M,, Schwartz SD. Robotic microsurgery: corneal transplantation. Br J Ophthalmol. 2009; 93: 1672–1675. [DOI] [PubMed] [Google Scholar]
- 3. Bourla DH,, Hubschman JP,, Culjat M,, Tsirbas A,, Gupta A,, Schwartz SD. Feasibility study of intraocular robotic surgery with the da Vinci Surgical System. Retina. 2008; 28: 154–158. [DOI] [PubMed] [Google Scholar]
- 4. Tsirbas A,, Mango C,, Dutson E. Robotic ocular surgery. Br J Ophthalmol. 2007; 91 1: 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bourcier T,, Nardin M,, Sauer A,, et al. Robot-assisted pterygium surgery: feasibility study in a nonliving porcine model. Trans Vis Sci Technol. 2015; 4 1: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bourcier T,, Becmeur PH,, Mutter D. Robotically assisted amniotic membrane transplant surgery. JAMA Ophthalmol. 2015; 133: 213–214. [DOI] [PubMed] [Google Scholar]
- 7. Bourcier T,, Chammas J,, Becmeur PH,, et al. Robotically assisted pterygium surgery: first human case. Cornea. 2015; 34: 1329–1330. [DOI] [PubMed] [Google Scholar]
- 8. Liverneaux PA,, Hendriks S,, Selber JC,, Parekattil SJ. Robotically assisted microsurgery: development of basic skills course. Arch Plast Surg. 2013; 40: 320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gudeloglu A,, Brahmbhatt JV,, Parekattil SJ. Robotic microsurgery in male infertility and urology-taking robotics to the next level. Transl Androl Urol. 2014; 3: 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weinstein GS,, O'Malley BW, Jr.,, Hockstein NG. Transoral robotic surgery: supraglottic laryngectomy in a canine model. Laryngoscope. 2005; 115: 1315–1319. [DOI] [PubMed] [Google Scholar]
- 11. Bourges JL,, Hubschman JP,, Wilson J,, Prince S,, Tsao TC,, Schwartz S. Assessment of a hexapod surgical system for robotic micro-macro manipulations in ocular surgery. Ophthalmic Res. 2011; 46: 25–30. [DOI] [PubMed] [Google Scholar]
- 12. Hubschman JP,, Wilson J,, Tsao TC,, Schwartz S. Robotic eye surgery. Ophthalmology. 2010; 117: 857. [DOI] [PubMed] [Google Scholar]
- 13. Douglas R. Robotic surgery in ophthalmology: reality or fantasy? Br J Ophthalmol. 2007; 91: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fine HF,, Wei W,, Goldman R,, Simaan N. Robot-assisted ophthalmic surgery. Can J Ophthalmol. 2010; 45: 581–584. [DOI] [PubMed] [Google Scholar]
- 15. Panchulidze I,, Berner S,, Mantovani G,, Liverneaux P. Is haptic feedback necessary to microsurgical suturing? Comparative study of 9/0 and 10/0 knot tying operated by 24 surgeons. Hand Surg. 2011; 16: 1–3. [DOI] [PubMed] [Google Scholar]
- 16. Maclachlan RA,, Becker BC,, Tabares JC,, Podnar GW,, Lobes LA, Jr.,, Riviere CN. Micron: an actively stabilized handheld tool for microsurgery. IEEE Trans Robot. 2012; 28: 195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hubschman JP,, Bourges JL,, Choi W,, et al. ‘The Microhand': a new concept of micro-forceps for ocular robotic surgery. Eye (Lond). 2010; 24: 364–367. [DOI] [PubMed] [Google Scholar]
- 18. Rahimy E,, Wilson J,, Tsao TC,, Schwartz S,, Hubschman JP. Robot-assisted intraocular surgery: development of the IRISS and feasibility studies in an animal model. Eye (Lond). 2013; 27: 972–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chitwood WR, Jr.,, Nifong LW,, Chapman WH,, et al. Robotic surgical training in an academic institution. Ann Surg. 2001; 234: 475–484; discussion 84–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith AL,, Scott EM,, Krivak TC,, Olawaiye AB,, Chu T,, Richard SD. Dual-console robotic surgery: a new teaching paradigm. J Robot Surg. 2013; 7: 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marescaux J,, Leroy J,, Gagner M,, et al. Transatlantic robot-assisted telesurgery. Nature. 2001; 413: 379–380. [DOI] [PubMed] [Google Scholar]
- 22. Pascolini D,, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012; 96: 614–618. [DOI] [PubMed] [Google Scholar]
- 23. Satava RM. Surgical robotics: the early chronicles: a personal historical perspective. Surg Laparosc Endosc Percutan Tech. 2002; 12: 6–16. [DOI] [PubMed] [Google Scholar]


