Abstract
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in the elderly. The pathogenesis of dry AMD remains indistinct and the mechanism of retinal pigment epithelium (RPE) cells death in dry AMD is controversial. The aim of the present study was to investigate the functions of Notch signaling in ultraviolet B (UVB)-induced damage of RPE cells. It was identified that, in RPE cells, UVB increased intracellular reactive oxygen species (ROS) and induced cell apoptosis. In addition, UVB activated Notch signaling in a dose dependent manner. Surprisingly, NOTCH2, but not NOTCH1, was demonstrated to be the major Notch receptor in RPE cells. Under normal conditions, the inhibition of NOTCH2 reduced cell growth and cell migration, but had no impact on intracellular ROS and cell apoptosis. However, in the presence of UVB, the inhibition of NOTCH2, but not NOTCH1, attenuated intracellular ROS and cell apoptosis. The function of Notch signaling involved in UVB damage of RPE cells may not only be significant to understanding the pathogenesis of AMD (especially dry AMD), but also useful for designing effective therapeutic agents for dry AMD.
Keywords: age-related macular degeneration, retinal pigment epithelium, ultraviolet B, Notch signaling
Introduction
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness among the aged in advanced countries. AMD causes ~20% of legal blindness AMD, and it is estimated that ~80 million people worldwide will suffer from AMD by the year 2020 (1). Late AMD has two forms: One is the ‘dry’ form defined by degeneration of the retinal pigment epithelium (RPE) cells and photoreceptor cells, and the other is ‘wet’ form, associated with choroidal neovascularization (CNV). Anti-angiogenic therapies have been successful in managing wet AMD. However, dry AMD, which accounts for 90% of AMD cases, currently lacks an effective treatment to stop or even slow down disease progression.
AMD is a multi-factorial complex condition with poorly understood molecular mechanisms. Age, smoking, specific genetic polymorphisms, oxidative stress, the complement pathway, inflammation and pathogenic RNA species (Alu) are significant contributors to AMD pathogenesis (2–8). There is much evidence that suggests that cumulative long-term exposure to ultraviolet B (UVB) may lead to AMD irrespective of age, because of the direct DNA damage and reactive oxygen species (ROS) production in RPE cells (9). Although most UVB is mostly absorbed by the cornea and lens, as the depletion of the ozone layer increases, there is a considerable growth in the accumulated lifetime exposure of the retina to UVB, especially following cataract removal (10). Visual impairment in dry AMD is associated with the degeneration of RPE cells and photoreceptor cells (11). RPE, a polarized monolayer epithelium cell layer, locates between the neural retina and choroid, acting as the guardians of the photoreceptor (12). Moreover, RPE cells selectively absorb the lower wavelength light particles (13). Therefore, it is believed that RPE cells may be the main target of UVB reaching the retina.
Notch signaling is a conserved adjacent cell signaling mechanism. In mammals, there are four Notch receptors (Notch1-4) and each has a cytoplasmic domain implicated in signal transduction (14,15). Notch activation is initiated by the binding between Notch receptors and ligands on adjacent cells, resulting in multiple steps of proteolytic cleavages of the receptors, as well as the release of the Notch intracellular domain (NICD) from the membrane, which translocates into the nucleus. In the nucleus, NICD binds to the transcription factor CSL and the co-activator mastermind-like proteins (MAML1-3), initiating transcriptional activation of Notch target genes, such as those in the Hes, Hey family (14,15).
Notch signaling serves an important role in many cellular processes: Cell proliferation, differentiation, apoptosis, migration and angiogenesis in many tissues, including pigmented and non-pigmented cells in the eyes (14,16,17). To date, the role of Notch signaling in dry AMD has been explored to a very limited extent. In the present study, the effects of UVB and the function of Notch signaling in RPE cells were investigated. Surprisingly, the authors identified that NOTCH2 was the major Notch receptor in RPE cells. More interestingly, the inhibition of NOTCH2, but not NOTCH1, attenuated intracellular ROS and cell apoptosis induced by UVB.
Materials and methods
Plasmids, small hairpin (sh)RNAs and reagents
The following shRNA lentiviral constructs targeting the human NOTCH1 and NOTCH2 were obtained from Thermo Fisher Scientific, Inc. (Waltham, MA, USA) and were used in a previous study (18). The hairpin sequence numbers are TRCN0000003359, TRCN0000003362 (targeting NOTCH1) and TRCN0000004895 and TRCN0000004896 (targeting NOTCH2).
The following antibodies were used in western blotting: Notch1 (cat. no. sc-6014; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), Notch2 (cat. no. D76A6; Cell Signaling Technology, Inc., Danvers, MA, USA) and α-tubulin (cat. no. AF7010; Affinity Biosciences, Inc., Cincinnati, OH, USA).
Cell culture and UV light apparatus
Human RPE cells (ARPE19 cell line) were obtained from the American Type Culture Collection (Manassas, VA, USA). RPE cells were cultured in Dulbecco's Modified Eagle's medium (DMEM; HyClone, GE Healthcare Life Sciences, Chalfont, UK), supplemented with 10% heat-inactivated fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 ng/ml streptomycin in a humidified incubator at 37°C and 5% CO2. When cells reached ~90% confluence, they were detached with 0.3% trypsin solution (Gibco; Thermo Fisher Scientific, Inc.) and collected for the subsequent experiments.
UV radiation apparatus used in this study is 3UV™-34UV Lamp (UVP, Inc., Upland, CA, USA), containing three wavelengths: 254 nm (UVA), 302 nm (UVB), and 365 nm (UVC). RPE cells (8×105) were seeded in 60 mm plates at ~50% confluence and subjected to UVB radiation 8 h later. Prior to UV radiation, cells were washed once with 2 ml pre-warmed PBS, and 1 ml PBS was left in the dish. Cells were radiated by UVB at various doses (0, 25, 50, 100 mJ/cm2) without the lid of petri dish, in the dark. Following UVB radiation, cells were cultured in fresh culture medium for 36 h.
Flow cytometry analysis of ROS and apoptosis
RPE cells were trypsinized and washed twice in pre-warmed PBS prior to the analyses of ROS and apoptosis. The harvested cells (2×105) were incubated for 30 min in a humidified incubator with dihydroethidium (EMD Millipore, Billerica, MA, USA), a well characterized reagent that has been extensively used for the detection of reactive oxidative species, according to the manufacturer's instructions. Following incubation, the fluorescence intensity was measured using a Muse™ Cell Analyzer (EMD Millipore). For apoptosis, the harvested cells (2×105) were incubated for 30 min in a humidified incubator with MultiCaspase (EMD Millipore), according to the manufacturer's protocol, and incubated for 5 min at room temperature with 7-AAD (EMD Millipore) and analyzed with a Muse™ Cell Analyzer.
Lentiviral transduction
Lentiviral transduction was performed as previously described (19). In brief, lentiviral vectors targeting NOTCH1, NOTCH2, along with the packing plasmid PSPAX2 and pseudotyped envelope pMD2.G (provided by Professor Lizi Wu, UF Health Shands Hospital, University of Florida, Gainesville, FL, USA) were transfected into 293T cells (American Type Culture Collection, Manassas, VA, USA) using the Effectene Transfection Reagent (Qiagen GmbH, Hilden, Germany). RPE cells were plated at 40–50% confluence in 60 mm plates and subsequently infected three times with 2 ml viruses plus 1 ml fresh complete medium containing 2 µg/ml polybrene (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Finally, the RPE cells were screened with puromycin (1.5 µg/ml; Sigma-Aldrich; Merck KGaA) for one week.
Cell viability assays and scratch assay
Cell viability was determined by MTT assay. Briefly, RPE cells were plated at a density of 5,000 cells/well in 96-well plates. Following culturing for the desired time (0, 24, 48 and 72 h), 20 µl MTT (5 mg/ml; Sigma-Aldrich; Merck KGaA) was added in each well, and then incubated for 4 h at 37°C. Then, all fluid was removed, and the crystallized dyes were dissolved in 150 µl dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) per well and shaken on a shaking table bed for 10 min. The absorbance at 490 nm wavelength was detected with a micro-plate reader (BioTek Instruments, Inc., Winooski, VT, USA).
For the scratch assay, RPE cells (8×105) were seeded into a 6-well plate in growth medium at 80–90% confluence. The scratch was drawn using a white tip and the floating cells were removed. Then the cells were cultured in DMEM medium with 2% fetal bovine serum. Migration of the cells into the scratch area was observed 48 h after the scratch had been drawn.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blotting
Real-time RT-PCR was performed as described previously (20). Total RNA was extracted from RPE cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's instructions. Then, cDNA was reverse transcribed using the PrimeScript II 1st Strand cDNA Synthesis kit (Takara Bio, Inc., Japan). RT-qPCR analysis was performed on LightCycler 96 (Roche Diagnostics, Basel, Switzerland) using the SYBR Premix Ex TaqTM kit (Takara Bio, Inc.). The human GAPDH gene was used as an endogenous control for sample normalization. The sequences of the 17 pairs of primers used in this study are not presented.
Western blot analysis was performed as described previously (21). In brief, 50 mg proteins were separated by using 8% SDS-PAGE, electrotransferred to pure nitrocellulose blotting membranes, and probed with the indicated antibodies as recommended by the manufacturer. Prior to incubation with primary antibodies, membranes were blocked with 5% fat-free milk for 1 h at room temperature. This was followed by incubation with Notch1 (1:200), Notch2 (1:1,000) and α-tubulin (1:1,000) primary antibodies overnight at 4°C. The membranes were subsequently washed three times with TBS-Tween-20 for 10 min and incubated with goat anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5,000; cat. no. 14708; Cell Signaling Technology, Inc.) for 1 h at 37°C. Antibody binding was visualized using Immobilon™ Western Chemiluminescent HRP Substrate (EMD Millipore) and detected by a Tanon5200 Chemiluminescent Imaging System (Tanon Science and Technology Co., Ltd., Shanghai, China). Loading was normalized with α-tubulin.
Statistical analysis
Each experiment was conducted in triplicate. The values were expressed as the mean ± standard deviation. Statistical analyses were performed by using Student's t-test assuming equal variances for all data (comparison of two groups) by SPSS software (version, 13.0; SPSS, Inc., Chicago, IL, USA). P<0.05 and P<0.01 were determined to indicate a statistically significant difference.
Results
UVB radiation increased intracellular ROS and induced apoptosis in RPE cells
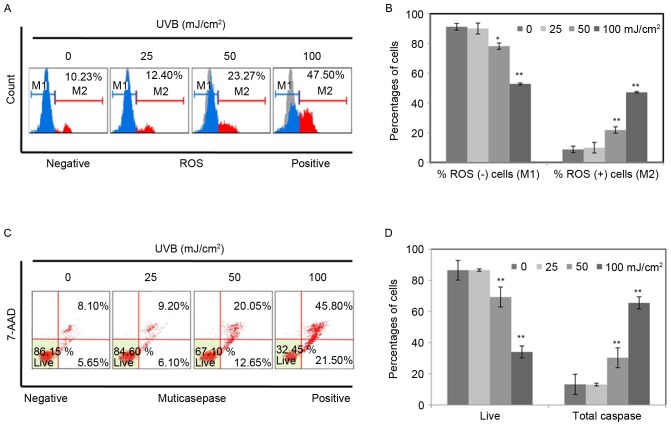
To determine UVB-induced damage, RPE cells were exposed to UVB (0, 25, 50, 100 mJ/cm2), and then cultured for 36 h. Intracellular ROS and apoptotic cells were measured by flow cytometry. As presented in Fig. 1A, the percentages of ROS positive cells were identified to increase from 10.23 to 47.50% with an increasing intensity of UVB, indicating that UVB significantly increased ROS production in RPE cells. In addition, the percentage of apoptotic cells increased notably following UVB treatment in a dose dependent manner (Fig. 1C). The percentage of total caspase increased from 13.75 to 67.3% along with the increase of intensity of UVB. The results are represented as the means ± standard deviation determined from three independent experiments (Fig. 1B and D). The results in Fig. 1 indicated that UVB induced damage in RPE cells.
Figure 1.
UVB increased intracellular ROS and induced cell apoptosis in retinal pigment epithelium cells. The cells were exposed to UVB (0, 25, 50 and 100 mJ/cm2), and then incubated for 36 h. Intracellular ROS and cell apoptosis were determined by flow cytometry. (A) The numbers represent the percentages of ROS positive cells. The shadow indicated the control group without UVB. (C) The numbers represent early- and late-stage apoptotic cell percentages. Therefore, the total caspase is the sum of them. (B and D) The results are expressed as the means ± standard deviation determined from three independent experiments. Statistical analysis was performed using Student's t-test. *P<0.05, **P<0.01 vs. control. UVB, ultraviolet B; ROS, reactive oxygen species.
UVB activated the Notch signaling in RPE cells
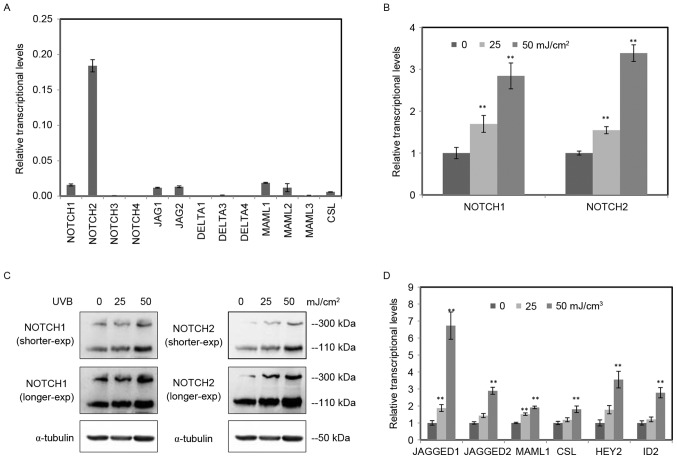
To understand the function of Notch signaling in RPE cells, RT-qPCR was conducted to confirm the expression levels of all 13 key components of Notch signaling, including four receptors (NOTCH1-4), five NOTCH ligands (JAGGED1 and 2, DELTA-LIKE-1, 3 and 4), three transcriptional co-activators (MAML1, 2 and 3) and the transcription factor (CSL) (Fig. 2A). Notably, NOTCH2 was the major Notch receptor in RPE cells. Among them, the expression levels of NOTCH3, 4, DELTA-LIKE 1, 3, 4 and MAML 3 are too low to display.
Figure 2.
UVB activated the Notch signaling in RPE cells. (A) The relative expression levels of Notch signaling components in RPE cells were determined by RT-qPCR. The detected components included four receptors (NOTCH1-4), five ligands (JAGGED 1, 2 and DELTA-LIKE1, 3 and 4), three transcriptional coactivators (MAML1, 2 and 3) and the transcription factor CSL. Among them, the expression of NOTCH3, 4, DELTA-LIKE 1, 3 and 4 and MAML 3 are too low to display. (B) The relative expression levels of NOTCH1 and NOTCH2 in RPE cells radiated by UVB (0, 25, 50 mJ/cm2) were tested by RT-qPCR. (C) The protein level of NOTCH1 and NOTCH2 in RPE cells radiated by UVB (0, 25, 50 mJ/cm2) were tested by western blotting using α-tubulin as an internal control. The full length of NOTCH is 300 kDa, and the cleaved Notch intracellular domain was 110 kDa. (D) The other genes of Notch signaling (JAGGED1, JAGGED2, MAML1, CSL, HEY2 and ID2) in RPE cells treated by UVB were determined by RT-qPCR. The data are expressed as the mean ± standard deviation of three independent experiments. Statistical analysis was performed using Student's t-test. **P<0.01 vs. control. UVB, ultraviolet B; RPE, retinal pigment epithelium; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
Among the four receptors, the expression levels of NOTCH1 and NOTCH2 were obviously higher compared with NOTCH3 and NOTCH4 receptors (Fig. 2A), indicating they were main receptors of Notch signaling in RPE cells. To study the function of Notch signaling in UVB-induced damage of RPE cells, RT-qPCR was performed to investigate the mRNA expression of NOTCH1 and NOTCH2 in RPE cells treated with various doses of UVB (0, 25, 50 mJ/cm2; Fig. 2B). The exposure of UVB enhanced the mRNA expression of NOTCH1 and NOTCH2 in a dose dependent manner. The protein levels of NOTCH1 and NOTCH2 were tested by western blot analysis using α-tubulin as an internal control (Fig. 2C). Consistent with the RT-qPCR data, both the full length and the cleaved NICD increased in RPE cells radiated by UVB.
Moreover, expression of other important Notch signaling components was detected by RT-qPCR. All six important components increased following UVB radiation, including NOTCH ligands (JAGGED 1 and 2), transcriptional co-activator (MAML1), transcription factor (CSL) and target genes (HEY2 and ID2) (Fig. 2D). These data suggested that the exposure of UVB activated the Notch signaling in RPE cells.
The inhibition of NOTCH2 reduced cell growth and cell migration, but had no impacts on intracellular ROS and cell apoptosis
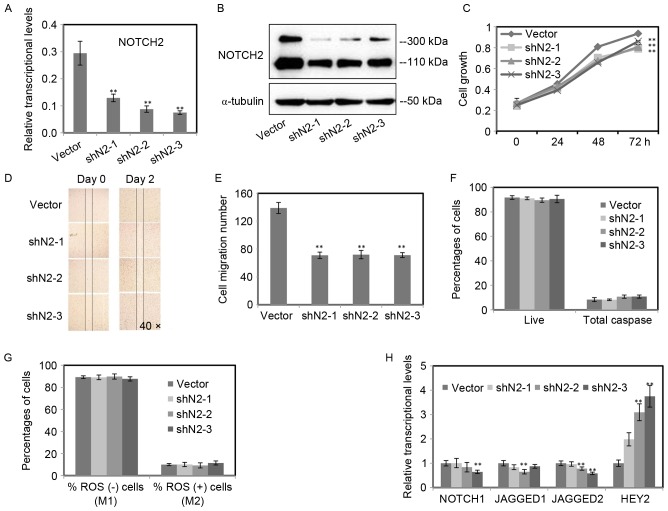
As presented in Fig. 2, NOTCH2 was the major Notch receptor and increased following UVB radiation in RPE cells. To gain an insight into the role of NOTCH2 in RPE cells, the expression of NOTCH2 was inhibited by lentiviral-based small hairpin RNA (shRNA). Three shRNAs (shN2-1, 2, 3) targeting NOTCH2 were selected. Then, the efficiency of NOTCH2 knockdown was determined by RT-qPCR (Fig. 3A) and western blot analysis (Fig. 3B). Both mRNA and protein levels of NOTCH2 were inhibited in the three stable RPE cell lines.
Figure 3.
The inhibition of NOTCH2 reduced the cell growth and cell migration, but had no impacts in intracellular ROS and cell apoptosis. (A) NOTCH2 was blocked by lentiviral-based shRNA. The knockdown efficiency of NOTCH2 was determined by RT-qPCR. (B) The downregulation of NOTCH2 proteins was detected by western blot analysis using α-tubulin as an internal control. (C) Cell growth was assayed by MTT assay (n=4). (D and E) The migratory ability of RPE cells was analyzed using the scratch assay. The number of migration cells was counted in the scratch assay. (F and G) Intracellular ROS and cell apoptosis were determined by flow cytometry. (H) The other components (NOTCH1, JAG1, JAG2 and HEY2) of Notch signaling were determined by RT-qPCR after NOTCH2 was inhibited. The data are expressed as the mean ± standard deviation of three independent experiments. Statistical analysis was performed using Student's t-test. **P<0.01. ROS, reactive oxygen species; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; shRNA, short hairpin RNA.
To determine whether the inhibition of NOTCH2 has an effect on RPE cell, the cell growth, migratory capacity, intracellular ROS and cell apoptosis of three stable RPE cell lines were monitored. An obvious reduction of cell growth was detected in RPE cells with NOTCH2 inhibited, when compared with the control (Fig. 3C). The migratory capacity of RPE cells was confirmed by scratch assay, and the migration of the three NOTCH2-knockdown RPE cell lines were significantly inhibited, while the control scratch wound almost recovered following two days incubation (Fig. 3D). The number of migration cells significantly decreased in three NOTCH2 knockdown RPE cell lines (Fig. 3E).
Surprisingly, there were no statistical changes for the percentage of ROS positive cells and apoptotic cells between RPE cells with NOTCH2 inhibited and the control (P>0.05; Fig. 3F and G). To investigate the mechanism of the effects on the migration and proliferation of RPE cells, RT-qPCR was performed to confirm the expression levels of other genes of the Notch signaling. The target gene HEY2 increased notably. Moreover, JAGGED1 and 2 and NOTCH1 decreased (Fig. 3H).
The inhibition of NOTCH2 attenuated the intracellular ROS and cell apoptosis induced by UVB
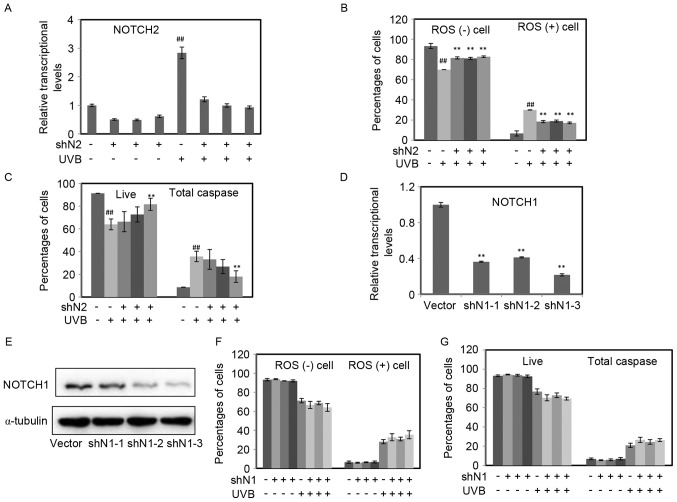
To investigate the role of Notch signaling on the damage induced by UVB, RPE cells with NOTCH1 or NOTCH2 inhibited were exposed to UVB (50 mJ/cm2), and then incubated for 36 h. The expression of NOTCH2 in each group was measured by RT-qPCR. As demonstrated in Fig. 4A, UVB increased NOTCH2, whereas the inhibition of NOTCH2 was kept at a similar level to the blank control. The percentage of ROS positive cells was significantly increased following UVB treatment, nevertheless, the enhanced effect of UVB was decreased in RPE cells with the inhibition of NOTCH2 (P<0.01; Fig. 4B). Similar to the above results, cell apoptosis increased following UVB radiation, and then decreased because of the inhibition of NOTCH2 (Fig. 4C).
Figure 4.
The inhibition of NOTCH2 attenuated the intracellular ROS and cell apoptosis induced by UVB. (A) The mRNA relative expression level of NOTCH2 in RPE cells with NOTCH2 inhibited radiated by UVB were determined by RT-qPCR. (B and C) Intracellular ROS and cell apoptosis were determined by flow cytometry in RPE cells with NOTCH2 inhibited by UVB. (D and E) The efficiency of NOTCH1 knockdown was determined by RT-qPCR and western blot analysis. (F and G) Cell apoptosis and intracellular ROS were determined by flow cytometry. The data are expressed as the mean ± standard deviation of three independent experiments. Statistical analysis was performed using Student's t-test. ##P<0.01 vs. control without UVB radiation. **P<0.01 vs. control radiated by UVB without NOTCH2 inhibition. UVB, ultraviolet B; RPE, retinal pigment epithelium; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
In addition, the authors constructed three stable cell lines with NOTCH1 inhibited. The efficiency of NOTCH1 knockdown was determined by RT-qPCR (Fig. 4D) and western blot analysis (Fig. 4E). The inhibition of NOTCH1 had no effects on intracellular ROS and cell apoptosis, moreover, it could not protect RPE cells from UVB induced damage (P>0.05; Fig. 4F and G).
Discussion
The present study provided evidence that UVB induced damage and activated Notch signaling in RPE cells, and the inhibition of NOTCH2, which presented the highest expression among various Notch receptors, reduced the damage induced by UVB. To the best of the authors' knowledge, the effect of Notch signaling on the protection of RPE cells against UVB damage has not yet been reported.
It was previously demonstrated that UVB exposure increased (22) or downregulated (23) the Notch signaling in keratinocytes. These contrary results urged us to investigate the change of Notch signaling and the mechanism upon UVB stimulation in RPE cells. Interestingly, in the current study, the exposure of UVB activated the Notch signaling in RPE cells. But the mechanism requires further research. To investigate the function of Notch signaling in RPE cells, the expression of all of 13 key components of Notch signaling were determined. It was discovered that in RPE cells NOTCH2 demonstrated the highest expression among various Notch receptors. NOTCH2 is strongly expressed in the pigmented epithelium of eye, including the RPE, but the investigation of NOTCH2 only focused on the roles in development and morphogenesis (24–26). In the present study, the inhibition of NOTCH2 had no impacts on intracellular ROS and cell apoptosis under normal conditions. However, in the presence of UVB, the inhibition of NOTCH2 attenuated intracellular ROS and cell apoptosis.
More interestingly, the inhibition of NOTCH2, but not NOTCH1, attenuated intracellular ROS and cell apoptosis in RPE cells stimulated with UVB. However, in keratinocytes, the apoptotic response is increased by deletion of the NOTCH1 gene (22). The results indicated above could be explained by the fact that although NOTCH1 and NOTCH2 are closely related paralogs and function through the same canonical signaling, they contribute to different outcomes in cell and disease contexts.
In the current study, the inhibition of NOTCH2 reduced the cell growth and cell migration. Based on previous work of the authors, blockage of NOTCH1 also inhibited the migration and proliferation of RPE cells (27). The increase of cell migration is linked to epithelial to mesenchymal transition leading to wet AMD and proliferative vitreoretinopathy (PVR). The downregulation of cleaved NOTCH1 blocked the activation of migration-related signaling molecules (28). It revealed that the blockage of Notch signaling may contribute to the treatment of wet AMD and PVR.
As shown in the present study and in a previous report (29), the exposure of UVB increased intracellular ROS and induced cell apoptosis in RPE cells. It was reported that RPE cells exposed to UVB exhibit several cellular pathological features, such as the reduction of cell viability, loss of phagocytotic activity and activation of inflammatory signaling, which are features of dry AMD (30,31). Some of these effects may be mediated through the production of ROS. UVB usually produces ROS and DNA damage. Beyond the repair capacity, both them may activate signaling pathways which determine the death or survival of a cell (32,33).
Notch signaling was key regulator of CNV and a molecular target for therapy in wet AMD (28). However, there are no explicit evidences studying the function of Notch signaling in the pathogenesis and therapies of dry AMD. To date, the therapy available for dry AMD is an intake of antioxidant formulations, which presents limited efficacy. It is hoped that, through these efforts, understanding the therapies of AMD will sooner be elucidated.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant nos. 81270914, 81670874 and 81170891 to Professor Huangxuan Shen). The authors would like to thank Professor Lizi Wu (UF Health Shands Hospital, University of Florida, Gainesville, FL, USA), who provided the plasmid and helped to review the paper.
References
- 1.Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL., III Age-Related Eye Disease Study Research Group: Risk factors for the incidence of advanced age-related macular degeneration in the age-related eye disease study (AREDS) AREDS report no. 19. Ophthalmology. 2005;112:533–539. doi: 10.1016/j.ophtha.2004.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biarnés M, Monés J, Alonso J, Arias L. Update on geographic atrophy in age-related macular degeneration. Optom Vis Sci. 2011;88:881–889. doi: 10.1097/OPX.0b013e31821988c1. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: The beaver dam eye study. Ophthalmology. 2007;114:253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Knudtson MD, Wong TY, Cotch MF, Liu K, Burke G, Saad MF, Jacobs DR., Jr Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology. 2006;113:373–380. doi: 10.1016/j.ophtha.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Thornton J, Edwards R, Mitchell P, Harrison RA, Buchan I, Kelly SP. Smoking and age-related macular degeneration: A review of association. Eye (Lond) 2005;19:935–944. doi: 10.1038/sj.eye.6701978. [DOI] [PubMed] [Google Scholar]
- 6.Yates JR, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H, Clayton DG, Hayward C, Morgan J, Wright AF, et al. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357:553–561. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- 7.Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013;13:438–451. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarallo V, Hirano Y, Gelfand BD, Dridi S, Kerur N, Kim Y, Cho WG, Kaneko H, Fowler BJ, Bogdanovich S, et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149:847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraljević Pavelić S, Klobučar M, Sedić M, Micek V, Gehrig P, Grossman J, Pavelić K, Vojniković B. UV-induced retinal proteome changes in the rat model of age-related macular degeneration. Biochim Biophys Acta. 2015;1852:1833–1845. doi: 10.1016/j.bbadis.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Kerr JB, McElroy CT. Evidence for large upward trends of ultraviolet-B radiation linked to ozone depletion. Science. 1993;262:1032–1034. doi: 10.1126/science.262.5136.1032. [DOI] [PubMed] [Google Scholar]
- 11.Forest DL, Johnson LV, Clegg DO. Cellular models and therapies for age-related macular degeneration. Dis Model Mech. 2015;8:421–427. doi: 10.1242/dmm.017236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 13.Cruickshanks KJ, Klein R, Klein BE, Nondahl DM. Sunlight and the 5-year incidence of early age-related maculopathy: The beaver dam eye study. Arch Ophthalmol. 2001;119:246–250. [PubMed] [Google Scholar]
- 14.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 15.Kopan R, Ilagan MX. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schouwey K, Aydin IT, Radtke F, Beermann F. RBP- Jk-dependent Notch signaling enhances retinal pigment epithelial cell proliferation in transgenic mice. Oncogene. 2010;30:313–322. doi: 10.1038/onc.2010.428. [DOI] [PubMed] [Google Scholar]
- 17.Bolós V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. 2007;28:339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Kesari S, Rooney C, Strack PR, Chen J, Shen H, Wu L, Griffin JD. Inhibition of notch signaling blocks growth of glioblastoma cell lines and tumor neurospheres. Genes Cancer. 2010;1:822–835. doi: 10.1177/1947601910383564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin S, Tian L, Shen H, Gu Y, Li JL, Chen Z, Sun X, You MJ, Wu L. DDX5 is a positive regulator of oncogenic NOTCH1 signaling in T cell acute lymphoblastic leukemia. Oncogene. 2013;32:4845–4853. doi: 10.1038/onc.2012.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, Jin G, Lin S, Lin X, Gu Y, Zhu Y, Hu C, Zhang Q, Wu L, Shen H. DNA methyltransferase inhibitor CDA-II inhibits myogenic differentiation. Biochem Biophys Res Commun. 2012;422:522–526. doi: 10.1016/j.bbrc.2012.05.068. [DOI] [PubMed] [Google Scholar]
- 21.Vatsyayan R, Lelsani PC, Chaudhary P, Kumar S, Awasthi S, Awasthi YC. The expression and function of vascular endothelial growth factor in retinal pigment epithelial (RPE) cells is regulated by 4-hydroxynonenal (HNE) and glutathione S-transferaseA4-4. Biochem Biophys Res Commun. 2012;417:346–351. doi: 10.1016/j.bbrc.2011.11.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandinova A, Lefort K, di Vignano Tommasi A, Stonely W, Ostano P, Chiorino G, Iwaki H, Nakanishi J, Dotto GP. The FoxO3a gene is a key negative target of canonical Notch signalling in the keratinocyte UVB response. EMBO J. 2008;27:1243–1254. doi: 10.1038/emboj.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukunaga-Kalabis M, Hristova DM, Wang JX, Li L, Heppt MV, Wei Z, Gyurdieva A, Webster MR, Oka M, Weeraratna AT, Herlyn M. UV-induced Wnt7a in the human skin microenvironment specifies the fate of neural crest-like cells via suppression of Notch. J Invest Dermatol. 2015;135:1521–1532. doi: 10.1038/jid.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bao ZZ, Cepko CL. The expression and function of Notch pathway genes in the developing rat eye. J Neurosci. 1997;17:1425–1434. doi: 10.1523/JNEUROSCI.17-04-01425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, Tanzie C, Yan Z, Chen S, Duncan M, Gaudenz K, Li H, Seidel C, Lewis B, Moran A, et al. Notch2 regulates BMP signaling and epithelial morphogenesis in the ciliary body of the mouse eye. Proc Natl Acad Sci USA. 2013;110:8966–8971. doi: 10.1073/pnas.1218145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCright B, Gao X, Shen L, Lozier J, Lan Y, Maguire M, Herzlinger D, Weinmaster G, Jiang R, Gridley T. Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development. 2001;128:491–502. doi: 10.1242/dev.128.4.491. [DOI] [PubMed] [Google Scholar]
- 27.Liu W, Jin G, Long C, Zhou X, Tang Y, Huang S, Kuang X, Wu L, Zhang Q, Shen H. Blockage of Notch signaling inhibits the migration and proliferation of retinal pigment epithelial cells. ScientificWorldJournal. 2013;2013:178708. doi: 10.1155/2013/178708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park GB, Kim D, Kim YS, Kim JW, Sun H, Roh KH, Yang JW, Hur DY. Regulation of ADAM10 and ADAM17 by sorafenib inhibits epithelial-to-mesenchymal transition in Epstein-Barr virus-infected retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2015;56:5162–5173. doi: 10.1167/iovs.14-16058. [DOI] [PubMed] [Google Scholar]
- 29.Balaiya S, Murthy RK, Brar VS, Chalam KV. Evaluation of ultraviolet light toxicity on cultured retinal pigment epithelial and retinal ganglion cells. Clin Ophthalmol. 2010;4:33–39. doi: 10.2147/opth.s7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roduit R, Schorderet DF. MAP kinase pathways in UV-induced apoptosis of retinal pigment epithelium ARPE19 cells. Apoptosis. 2008;13:343–353. doi: 10.1007/s10495-008-0179-8. [DOI] [PubMed] [Google Scholar]
- 31.Roehlecke C, Schaller A, Knels L, Funk RH. The influence of sublethal blue light exposure on human RPE cells. Mol Vis. 2009;15:1929–1938. [PMC free article] [PubMed] [Google Scholar]
- 32.Kulms D, Schwarz T. Independent contribution of three different pathways to ultraviolet-B-induced apoptosis. Biochem Pharmacol. 2002;64:837–841. doi: 10.1016/S0006-2952(02)01146-2. [DOI] [PubMed] [Google Scholar]
- 33.Valerie K, Yacoub A, Hagan MP, Curiel DT, Fisher PB, Grant S, Dent P. Radiation-induced cell signaling: Inside-out and outside-in. Mol Cancer Ther. 2007;6:789–801. doi: 10.1158/1535-7163.MCT-06-0596. [DOI] [PubMed] [Google Scholar]