Abstract
HIV-1 viral protein R (Vpr) is a multifunctional viral protein that plays important role at multiple stages of the HIV-1 viral life cycle. Although the molecular mechanisms underlying these activities are subject of ongoing investigations, overall, these activities have been linked to promotion of viral replication and impairment of anti-HIV immunity. Importantly, functional defects of Vpr have been correlated with slow disease progression of HIV-infected patients. Vpr is required for efficient viral replication in non-dividing cells such as macrophages, and it promotes, to some extent, viral replication in proliferating CD4+ T cells. The specific activities of Vpr include modulation of fidelity of viral reverse transcription, nuclear import of the HIV-1 pre-integration complex (PIC), transactivation of the HIV-1 LTR promoter, induction of cell cycle G2 arrest and cell death via apoptosis. In this review, we focus on description of the cellular proteins that specifically interact with Vpr and discuss their significance with regard to the known Vpr activities at each step of the viral life cycle in proliferating and non-proliferating cells.
Keywords: HIV-1, Viral protein R (Vpr), Macrophages, Reverse Transcription, Nuclear transport, Transcriptional Activation of LTR promoter, G2 Arrest, Apoptosis
Introduction
Human immunodeficiency virus type 1 (HIV-1) Vpr is a virion-associated accessory protein with an average length of 96 amino acids and a calculated molecular weight of 12.7 kDa. Vpr is highly conserved among HIV, simian immunodeficiency viruses (SIV) and other lentiviruses (Tristem et al., 1992; Tristem et al., 1998). Besides lentiviruses, the Vpr protein sequence shares no strong homology with any other known protein. A tertiary structure of Vpr proposed based on the NMR analysis (Fig. 1) consists of an α-helix-turn-α-helix domain in the amino-terminal half from amino acids 17 to 46, and a long α-helix from aa 53 to 78 in the carboxy-terminal half (Schuler et al., 1999; Wecker and Roques, 1999). These three α-helices are folded around a hydrophobic core in a structure which allows interaction of Vpr with different cellular proteins (Morellet et al., 2003). These interactions underlie the specific roles of Vpr during the HIV-1 viral life cycle.
Fig. 1.
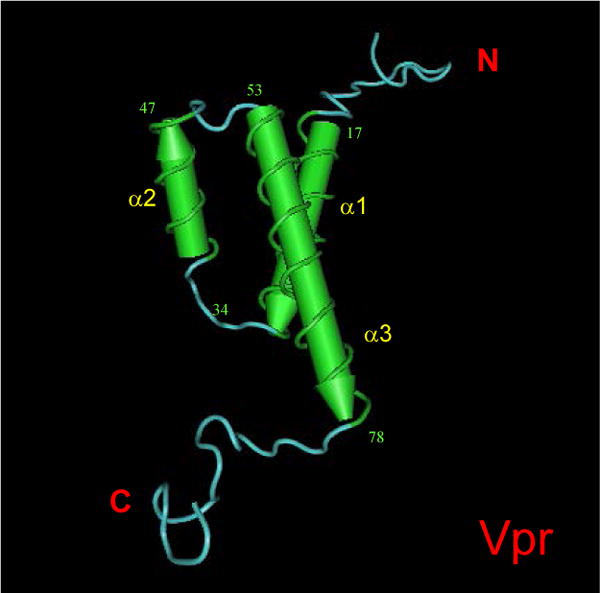
Putative tertiary structure of Vpr.
Increasing evidence suggest that HIV-1 Vpr plays a significant role in viral pathogenesis. For example, some of the earlier studies in SIV-infected Rhesus monkeys suggested that depletion of the SIV vpr and vpx genes, two homologous counterparts of the HIV-1 Vpr, severely sabotaged the ability of SIV to cause AIDS (Gibbs et al., 1995; Lang et al., 1993). The requirement of Vpr in viral survival and pathogenesis was further supported by the finding that a mutated vpr gene reverted to the wild type in both infected chimpanzees and a human subject (Goh et al., 1998). Conversely, viruses in some patients with slow disease progression were shown to carry functionally defective Vpr (Caly et al., 2008; Goh et al., 1998; Somasundaran et al., 2002; Zhao et al., 2002).
Vpr displays a number of unique activities in host cells during the HIV-1 viral life cycle (Fig. 2). These include nuclear transport of PIC (Heinzinger et al., 1994b), activation of the HIV-1 LTR promoter (Felzien et al., 1998; Kino et al., 2002), induction of cell cycle G2 arrest (He et al., 1995; Li et al., 2007; Re et al., 1995), and induction of apoptosis (Stewart et al., 1997).
Fig. 2.
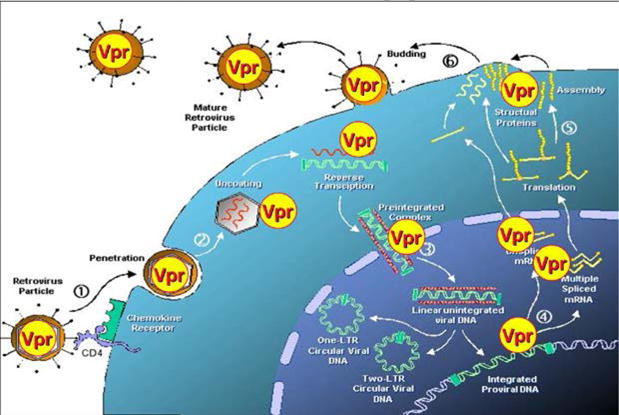
Roles of Vpr at various stages of HIV-1 viral life cycle. Vpr incorporated into the virions is released during uncoating and contributes to reverse transcription and nuclear transport of the PIC. Transactivation of HIV-1 LTR promoter, induction of cell cycle G2 arrest, and induction of apoptosis are accomplished by de novo produced Vpr. The specific Vpr activity at each step of the HIV-1 viral life cycle is described in the text. The figure is modified from a picture of HIV-1 life cycle provided by Bruce Patterson.
In this review, we summarize our current understanding of each of these Vpr activities by focusing specifically on the cellular proteins that interact with Vpr. The functional relevance of these Vpr-protein interactions and their impact on viral replication in proliferating and non-proliferating cells is discussed.
Role of Vpr in HIV-1 reverse transcription
Upon penetration of HIV into the host cell, the nucleocapsid of the virus is released into the cytoplasm of the host cell, where reverse transcription takes place to convert viral RNA to proviral DNA. In HIV-1, initiation of reverse transcription requires the tRNALys3-mediated priming (Huang et al., 1994). Importantly, to function as a primer for reverse transcription, tRNALys3 must have a free 3′ end, i.e. be deacetylated. Vpr appears to promote incorporation of deacetylated tRNALys3 into assembling viral particles because it specifically binds to the Lys-tRNA synthetase, which acetylates tRNALys3, and inhibits its activity (Stark and Hay, 1998).
HIV-mediated reverse transcription is an error-prone process, which generates on average one mutation out of 2,000 to 5,000 polymerized nucleotides it transcribes (Li et al., 1997; Romani and Engelbrecht, 2009). These mutations are generated during reverse transcription to a large extent by accidental incorporation of dUTP or deamination of cytosine that yields uracil (Chen et al., 2004; Mansky et al., 2000). If the uracil is not properly removed from the viral DNA by uracil-N-glycosylase (UNG; also known as UDG) or dUTPase, it will lead to transitional mutations that convert Cytosine (C) to Thymine (T) on one strand, and Guanine (G) to Adenine (A) on the other strand.
One of the advantages for the virus to have such an error-prone reverse transcriptional process is to generate a pool of highly diversified viral genomes that can quickly adapt to an adverse host environment such as that in patients who are receiving highly active antiretroviral therapies (HAART). This is one of the reasons why drug resistant HIV can emerge rapidly in HAART-treated patients. On the other hand, too many viral mutations can also be detrimental to the virus. For instance, mis-incorporation of uracil into viral DNA could result in viral DNA degradation or inhibition of initiation of viral DNA synthesis (Klarmann et al., 2003). Therefore, a fine balance between generation of viral genomic diversity and production of functionally defective viral DNA could potentially be a critical factor for viral survival.
Vpr appears to play a specific role in viral reverse transcription via a strong interaction with the nuclear form of uracil-N-glycosylase (UNG2) (Bouhamdan et al., 1996). UNG2 is a DNA excision DNA repair enzyme that specifically removes uracil from nuclear DNA (Parikh et al., 2000). However, it is not totally clear at the moment what the role of Vpr is in this process. One school of thought is that Vpr augments the UNG2 activity, i.e., it promotes increased fidelity of the viral reverse transcription by recruiting UNG2 to the viral particles in order to proof-read or remove uracil from viral DNA. Evidence to support this notion includes Vpr-mediated incorporation of UNG2 into the HIV-1 viral particles (Chen et al., 2004). Consistent with this notion, in the absence of Vpr, viral mutational rates increase at least 4- or 18-fold in proliferating T-cells and non-dividing macrophages, respectively (Chen et al., 2004; Chen et al., 2002; Mansky et al., 2000). In contrast to a positive role of Vpr in supporting the UNG2 activity, others suggest that Vpr may antagonize the UNG2 activity, i.e., UNG2 is a host antiviral factor and the Vpr-UNG2 interaction is intended to reduce the number of abasic sites in the viral DNA genome that are generated after the removal of uracil by UNG2 (Fenard et al., 2009; Schrofelbauer et al., 2005). Thus, Vpr may actually counteract the UNG2 effect by promoting viral survival or genomic diversity by preventing virion incorporation of UNG2 through proteasome-mediated degradation (Ahn et al., 2010; Schrofelbauer et al., 2005). This notion is also consistentwith a recent report showing that overexpression of UNG2 inhibits HIV-1 replication through reduced viral transcription (Fenard et al., 2009). Interestingly, the function of Vpr-UNG2 interaction seems to duplicate the function of the Vif-APOBEC3G interaction. The cellular antiviral factor APOBEC3G, a cellular deaminase, destroys viral transcripts by creating uracil in viral DNA genome through deamination of cytosine residues during reverse transcription; Vif counteracts this antiviral effect by preventing virion incorporation of APOBEC3G through proteasome-mediated proteolysis (Mariani et al., 2003; Sheehy et al., 2002). Similarly, assuming that UNG2 is indeed an antiviral factor, by removing uracil UNG2 could generate sufficient numbers of abasic sites in the viral transcripts to cause destruction of the viral genome; Vpr counteracts the effect of UNG2 by preventing virion incorporation of UNG2 via proteasome-mediated protein destruction. Moreover, uracil is the common theme and is manipulated in the viral particles by both APOBEC3G and UNG2. There might be even an interrelationship between the Vpr-UNG2 and the Vif-APOBEC3G effects, as suggested by a report showing that Vpr enhances protein production of Vif (Li et al., 2008).
Although it is clear that Vpr plays an important role in viral reverse transcription, the specific function of Vpr in the Vpr-UNG interaction during this viral infection step awaits resolution.
Nuclear transport of HIV-1 preintegration complex (PIC)
One of the unique Vpr activities is its ability to shuttle between the cytoplasm and the nucleus (Heinzinger et al., 1994a), which is believed to contribute to nuclear import of the viral preintegration complex (PIC) (de Noronha et al., 2001; Heinzinger et al., 1994a; Popov et al., 1998b). To infect a host cell, HIV-1 needs to transport its genomic DNA in the context of the viral PIC from the cytoplasm into the nucleus of a target cell. Vpr is believed to be among the main regulators of HIV-1 nuclear import (Connor et al., 1995; Heinzinger et al., 1994a). Other viral proteins involved in the nuclear transport process of PIC include Matrix Antigen (MA) (Bukrinsky et al., 1993) and integrase (IN) (Gallay et al., 1997). The involvement of Vpr in nuclear transport of PIC was further supported by co-localization of Vpr and PIC within 4–16 hrs after onset of viral infection (Fassati and Goff, 2001).
In normal cells, nuclear transport of a particular protein involves a 2-step process, which includes an energy-independent docking of the cargo protein to the nuclear envelope and the subsequent energy-dependent translocation and release of the cargo protein from the nuclear envelope. The imported protein usually carries a nuclear localization sequence (NLS) domain that consists of a short region of basic amino acids (lysines and arginines) or 2 such regions spaced about 10 amino acids apart (Nakielny and Dreyfuss, 1999; Wente, 2000). Typically, the importin-α binds the NLS-containing cargo and serves as a bridge between the cargo and the receptor importin β through the importin β-binding domain (IBB) on importin α. The transporting process involves docking of the ternary protein complex to the nuclear envelope, translocation through the nuclear pore, and release of the cargo protein into the nucleoplasm (Herold et al., 1998; Kobe, 1999).
Currently, there are three hypotheses, which are not necessarily mutually exclusive, that could potentially explain the mode of action of Vpr in nuclear transport of the HIV-1 PIC. The first model hypothesizes that Vpr targets the HIV-1 PIC to the nucleus via a distinct, importin-independent pathway (Gallay et al., 1996; Jenkins et al., 1998); the second suggests that Vpr modifies cellular importin-dependent import machinery (Popov et al., 1998a; Popov et al., 1998b); and the third implies that importin α alone is sufficient for Vpr-mediated nuclear import activity.
The first model was based on the observation that in the in vitro nuclear import assay Vpr can enter nuclei in the absence of soluble import factors (Jenkins et al., 1998). Consistent with this concept, Vpr was shown to induce dynamic disruptions in the nuclear envelope (de Noronha et al., 2001) which may serve as entry points for isolated Vpr and for the PICs. HIV-1 Vpr has been shown to co-precipitate with fission yeast nucleoporin Nup124p and its human homolog, NUP153, and nuclear import of Vpr was impaired in nup124 null mutant strain (Varadarajan et al., 2005). Vpr also interacts with human nucleoporin CG1, which contributes to Vpr docking to the nuclear envelope (Le Rouzic et al., 2002). Therefore, Vpr may function as a substitute for importin β, which also interacts with nucleoporins to mediate nuclear translocation of its cargo (Pemberton and Paschal, 2005). Consistent with this notion, Vpr was shown to interact specifically with nucleoporin phenylalanine-glycine (FG)-repeat regions, critical for importin-mediated nuclear import (Fouchier et al., 1998).
The second model postulates that Vpr uses a modification of the importin α,β-dependent pathway to enter the nucleus (Bukrinsky, 2004). Vpr was shown to bind to importin α both from human and budding yeast cells, but the binding site is different from the binding site for NLS (Agostini et al., 2000; Popov et al., 1998a; Popov et al., 1998b; Vodicka et al., 1998). This binding of Vpr to importin α appears to stimulate subsequent nuclear import of the cargo (Popov et al., 1998b), likely by increasing the affinity of NLS-importin α interaction (Agostini et al., 2000). The effect of Ran-GTP binding to importin β on the ternary complex has not been reported and it is not understood why Vpr is frequently observed to localize at the nuclear envelope, although this may be related to the binding of Vpr to nucleoporins described above. Another possibility is that Vpr may localize on the nuclear envelope via interaction with the 26S proteasome (Li et al., 2010a). In fission yeast, the 26S proteasome complex attaches to the inner side of the nuclear membrane (Wilkinson et al., 1998). Consistent with this idea, Vpr fell off the nuclear membrane when the 26S proteasome was released from the nuclear membrane in a temperature-sensitive cut8 mutant (Li et al., 2010a). Even though it is unclear where the 26S proteasome localizes in mammalian cells, one study found Vpr to be at the inside of the nuclear envelope (Vodicka et al., 1998). This suggests that Vpr may be transported through the pore but may not be released into the nucleoplasm.
The third model suggests that importin α alone may be sufficient for the nuclear transport activity of Vpr, without utilizing the classical importin β-dependent transport pathway (Nitahara-Kasahara et al., 2007). In support of this hypothesis, depletion of importin α from HeLa cells by using siRNAs markedly decreased the nuclear import of Vpr in an in vitro nuclear transport assay, whereas no effect was seen when importin β was depleted from the cell extracts (Nitahara-Kasahara et al., 2007). A similar importin α-driven mechanism was also observed in macrophages. The requirement of importin α in Vpr-mediated nuclear transport of PIC and viral replication in macrophages was further demonstrated by a significant defect in replication of the virus carrying mutations in the first α-helix region of Vpr critical for interaction with importin α (Nitahara-Kasahara et al., 2007). Thus, expression of importin α and importin α-driven nuclear import of Vpr are essential for efficient viral replication in macrophages.
The ability of HIV-1 to infect and replicate in non-dividing cells (terminally differentiated macrophages and incompletely activated CD4+ T lymphocytes) is the characteristic feature of lentiviruses that determines to a large extent their high replicative capacity and pathogenesis. Moreover, the ability of Vpr to promote nuclear transport of PIC is generally accepted as the reason why Vpr is required for efficient viral replication in non-dividing cells such as macrophages (Connor et al., 1995; Di Marzio et al., 1995; Heinzinger et al., 1994b; Subbramanian et al., 1998). However, a recent paper argued against this explanation by showing that infection of growth-arrested T-cells by Vpr(−) HIV-1 was reduced by 2-fold compared to the wild-type virus (Yamashita et al., 2007), which was essentially the same level of reduction observed in proliferating cells. In addition, Vpr likely participates in nuclear import of PIC in T cells in a similar manner as it does in macrophages, and nuclear import through the nuclear pore is essential for HIV replication in both cell types (BouHamdan et al., 1998; Bukrinsky and Haffar, 1997; Riviere et al., 2010). Therefore, the differential contribution of Vpr to viral replication in macrophages and T-cells can be attributed neither to the proliferation status of the target cell nor to the ability of Vpr to promote nuclear import in non-dividing cells.
Other evidence suggests that the differences in Vpr’s contribution to viral replication in different cell types could be due to the presence of different cellular factors that influence Vpr’s activity. For example, HSP70 present in T-cells can substitute for the activity of Vpr thus diminishing dependence of viral replication on Vpr in T-cells. This is in sharp contrast to macrophages where HSP70 is less abundant (Agostini et al., 2000) and Vpr is required for efficient viral replication (de Noronha et al., 2001; Heinzinger et al., 1994a; Popov et al., 1998b). Vpr co-precipitates with HSP70 in both proliferating T-cells and macrophages (Iordanskiy et al., 2004a; Iordanskiy et al., 2004b). While HSP70 minimizes the Vpr dependence in proliferating T-cells, the direct Vpr-HSP70 interaction creates functional competition between these two proteins. For example, HSP70 inhibits viral replication in macrophages in the presence of Vpr but stimulates nuclear transport and viral replication in the absence of Vpr (Iordanskiy et al., 2004a).
In summary, at least three different models can potentially explain the possible role of Vpr in nuclear transport of PIC. Interactions of Vpr with different cellular proteins such as importin α, NUP153, CG1 and HSP70 may represent different underlying molecular mechanisms occurring in different cell types under different circumstances.
Activation of HIV-1 LTR-mediated transcription
Once the HIV-1 proviral DNA is integrated into the human chromosomes, Vpr promotes HIV-1 viral gene transcription by direct interaction with the long terminal repeat (LTR) promoter. The Vpr-mediated LTR gene transcription is achieved by association of Vpr with various transcriptional factors or co-factors on the LTR promoter (Table 1). The electrophoretic mobility shift analysis (EMSA) and other DNA-protein binding assays suggested direct binding of Vpr to the LTR DNA sequences that span a number of transcriptional binding sites on LTR including the NF-kB, SP1, p300/CBP binding sites and the adjacent C/EBP sites (Hogan et al., 2003). Because Vpr interacts with these highly conserved transcription factor binding sites, besides LTR it also enhances gene transcription of other related promoters, such as mouse mammary tumor virus (MMTV) promoter (Kino et al., 2002) and CMV or SV40 promoters (Roux et al., 2000).
Table 1.
Vpr-interacting proteins and their potential functional relevance in HIV-1 viral life cycle.
| Vpr-binding protein | Protein function | Reference |
|---|---|---|
| Modulation of Fidelity of HIV-1 Reverse Transcription | ||
| UNG2 | Uracil DNA glycosylase | (Bouhamdan et al., 1996) |
| SMUG | SMUG uracil-DNA glycosylase | (Schrofelbauer et al., 2005) |
| Lys-tRNA synthetase | Lys tRNA synthetase | (Stark and Hay, 1998) |
| Nuclear Transport of Preintegration Complex | ||
| Importin-α | Karyopherin | (Kamata et al., 2005; Popov et al., 1998b; Vodicka et al., 1998) |
| NUP153/Nup124 | Nuclear pore proteins | (Fouchier et al., 1998; Varadarajan et al., 2005) |
| hCG1 | Nucleoporin | (Le Rouzic et al., 2002) |
| HSPs (HSP27, HSP70)/Hsp16 | Heat shock proteins; chaperone protein | (Benko et al., 2004; Iordanskiy et al., 2004a; Iordanskiy et al., 2004b; Liang et al., 2007) |
| Activation of HIV-1 LTR-mediated Transcription | ||
| SP1 | Transcription factor | (Wang et al., 1995) |
| p300/CBP | Nuclear receptor and transcriptional co-activator; CBP, CREB binding protein | (Felzien et al., 1998; Kino et al., 2002) |
| NFĸB | Transcriptional factor nuclear factor kappa B | (Roux et al., 2000) |
| TFII B | Transcription factor | (Agostini et al., 1996) |
| GR | Glucocorticoid receptor | (Refaeli et al., 1995) |
| C/EBP | CCAAT/enhancer binding protein | (Hogan et al., 2003) |
| Induction of Cell Cycle G2 Arrest | ||
| CDC25C | Protein phosphatase | (Goh et al., 2004) |
| WEE1 | Protein kinase | (Kamata et al., 2008) |
| 14-3-3 | Adaptor protein | (Kino et al., 2005) |
| PP2A | Protein phosphatase 2A | (Godet et al.) |
| VprBP | Receptor of E3 ligase | (Zhang et al., 2001) |
| HHR23A/Rhp23 | Excision DNA repair protein | (Gragerov et al., 1998; Li et al., 2010a; Withers-Ward et al., 1997) |
| Induction of Apoptosis | ||
| ANT | Adenine nucleotide translocator | (Jacotot et al., 2001a) |
| PTPC | Permeability transition pore complex | (Jacotot et al., 2000) |
| HAX-1 | Antiapoptotic mitochondrial protein | (Yedavalli et al., 2005) |
One of the Vpr trans-activating activities on LTR is through promoting phosphorylation of IkB, which leads to binding of NF-kB to Vpr via its LxxLL motif (Northrop et al., 1992), nuclear translocation of NF-kB, and subsequent binding of NF-kB to the LTR response element resulting in NF-kB and SP1-mediated increase of gene transcription (Varin et al., 2005). Another Vpr trans-activating activity on LTR includes the interaction of Vpr with the transcriptional factor IIB (TFIIB) (Agostini et al., 1996). Follow-up studies demonstrated that Vpr may activate transcription by promoting conformational changes of TFIIB (Agostini et al., 1999). Vpr also promotes LTR gene transcription by forming a stable complex with p300/CBP (a CREB-binding protein) and the ligand-bound GR through the glucocorticoid response element (GRE) (Felzien et al., 1998; Kino et al., 2002). Here Vpr acts as an adapter that links p300/CBP and GR coactivator for LTR gene transcription (Kino, 2002). Interestingly, mutation analysis showed that p300/CBP binds to the C-terminal end of Vpr, which is a critical region for induction of cell cycle G2 arrest. This finding suggests a possible association of Vpr-mediated cell cycle G2 arrest and viral gene transcription. Consistently, Vpr is in the same protein complex with p300 and cellular protein p21 (WAF1), which alleviates p21-mediated inhibition of cell departure from G1 phase (Cui et al., 2006). A recent study showed that the entry of Vpr-producing cells from G1 to the S phase triggers an S-phase dependent induction of cell cycle G2 arrest by Vpr (Li et al., 2010b). It is conceivable, therefore, that the regulation of p300 by Vpr ensures transition of HIV-1 infected cells from G1 to S and ultimately results in growth arrest in the G2 phase of the cell cycle. This scenario may also provide a potential mechanism to explain enhanced viral replication in proliferating cells after growth arrest in the G2 phase (Felzien et al., 1998; Goh et al., 1998).
In monocytes and macrophages, binding of Vpr to the LTR C/EBP binding region and its adjacent regions including the NF-kB site is required for subtype B HIV-1 gene expression (Liu et al., 1999). Vpr promotes C/EBP binding to LTR either indirectly or jointly to form a complex (Hogan et al., 2003; Kilareski et al., 2009). Interestingly, there is a sequence variation in the HIV-1 C/EBP binding site. It appears that Vpr has stronger binding to the C/EBP binding site I variants, which correlates with the late stage of HIV-1 associated disease. Consistently, increased binding of Vpr to the C/EBP binding site I variants within LTR was found to correlate with HIV-1 associated dementia (Burdo et al., 2004).
Altogether, Vpr promotes HIV-1 LTR gene transcription through interactions with various transcriptional factors and co-factors in both proliferating T-cells and non-dividing macrophages. In addition, this transacting effect on LTR is synergistic with the effect of HIV-1 Tat that also promotes LTR gene transcription (Sawaya et al., 2000).
Induction of cell cycle G2 arrest
Another unique activity of HIV-1 Vpr is its ability to inhibit host cell proliferation by blocking infected cells in the G2/M phase of the cell cycle, which is commonly known as the G2 arrest (He et al., 1995; Jowett et al., 1995; Re et al., 1995). The cell cycle G2 arrest induced by Vpr is thought to suppress human immune function by preventing T-cell clonal expansion (Poon et al., 1998) and to provide an optimized cellular environment for maximal levels of viral replication (Goh et al., 1998). However, contribution of Vpr to viral replication in proliferating T-cells is relatively small (Goh et al., 1998; Iordanskiy et al., 2004a); no direct evidence has been provided to demonstrate the role of Vpr in preventing T-cell clonal expansion. Thus the virological role of Vpr-induced G2 arrest remains unclear.
Induction of cell cycle G2 arrest by Vpr is a highly conserved activity as the same effect has been observed also in other eukaryotes such as fission yeast (Schizosaccharomyces pombe) (Masuda et al., 2000; Zhao and Elder, 2005; Zhao et al., 1996; Zhao and Elder, 2000). For example, Vpr induces cell cycle G2 arrest specifically through Tyr15 hyperphosphorylation of Cdc2/CDK1, which is the cyclin-dependent kinase that determines onset of mitosis in all eukaryotic cells (He et al., 1995; Re et al., 1995; Zhao et al., 1996). Consistently, Vpr induces G2 arrest by inhibiting the Cdc25 phosphatase through direct interaction and activation of the Wee1 kinase; both Cdc25 and Wee1 enzymes directly regulate Cdc2/CDK1 (de Noronha et al., 2001; Elder et al., 2001; Goh et al., 2004; Kino et al., 2005; Masuda et al., 2000).
One of the recent developments in understanding Vpr-induced G2 arrest is the involvement of ubiquitin-proteasome system (UPS). In particular, a specific Cullin ubiquitin E3 ligase known as Cul4A-DDB1-DCAF1/VprBP was found to be associated specifically with this G2 induction process (Belzile et al., 2007; DeHart et al., 2007; Hrecka et al., 2007; Le Rouzic et al., 2007; Schrofelbauer et al., 2007; Tan et al., 2007; Wen et al., 2007). This E3 ligase association is mediated through direct interaction of VprBP with Vpr on chromatin (Belzile et al., 2010a; Zhang et al., 2001). The identification of VprBP-associated E3 as a contributor to Vpr-induced G2 arrest implicates a role of Vpr in protein polyubiquitination (poly-Ub) and proteasome-mediated proteolysis. Indeed, Vpr promotes the E3 ligase activity (Hrecka et al., 2007) and promotes protein polyubiquitination through the K48 linkages (Belzile et al., 2010b). Inhibition of polyubiquitination by Ub(K48R) or suppression of proteasome activity by epoxomicin or MG132 reversed Vpr-induced G2 arrest (DeHart et al., 2007; Tan et al., 2007). Moreover, Vpr is now known to associate directly with the 26S proteasome through an hHR23A-mediated mechanism (Li et al., 2010a). However, the downstream cellular protein(s) that are targeted by Vpr-mediated UPS for the G2 induction are still unknown Recently, CDC25C was shown to be one of this downstream effectors during Vpr-induced cell cycle G2 arrest (Li et al., 2010b).
It is worthwhile to note that the recent finding on hHR23A-mediated interaction of Vpr with 26S proteasome may shed new light on an old unresolved story of the specific role that HHR23A plays in the Vpr activities. hHR23A is a member of a highly conserved excision DNA repair Rad23 protein family that contains an N-terminal ubiquitin-like (UbL) and two C-terminal ubiquitin-associated (UbA) domains (Elder et al., 2002b; Gragerov et al., 1998; Withers-Ward et al., 1997). Earlier studies showed that Vpr binds to hHR23A through its C-terminal UbA domain (Dieckmann et al., 1998). Interaction of Vpr with hHR23A was thought to play a role in the induction of G2 arrest (Gragerov et al., 1998; Withers-Ward et al., 1997). However, subsequent mutational analysis could not relate this interaction to any known biological function of Vpr (Mansky et al., 2001). A recent report showed that hHR23A serves as a bridging protein to associate Vpr with proteasome and this function is essential for the stimulatory effect of Vpr on HIV-1 replication in growth-arrested HeLa cells and macrophages (Li et al., 2010a). Since new evidence suggests that Vpr induces G2 arrest through UPS, it is possible that hHR23A may also be involved in induction of cell cycle G2 arrest through Vpr-mediated proteolysis of downstream cellular proteins via UPS. This possibility remains to be tested.
A major question in studying Vpr-induced G2 arrest is the actual cause of this arrest. Is it a consequence of host cell cycle checkpoints modified by Vpr? Or is it the result of Vpr’s active actions in modulating host cell cycle regulation? The main confusion came from the fact that some of the host cell cycle checkpoint proteins are involved in this process. For example, the eukaryotic cell cycle DNA damage or replication checkpoint controls, as well as Vpr, all induce G2 arrest through inhibitory phosphorylation of CDK1 regulated by CDC25 or WEE1. Thus, it is logic to think that Vpr might induce G2 arrest through one of these two checkpoint pathways (for detailed reviews, see (Amini et al., 2004; Andersen et al., 2008; Elder et al., 2002a; Zhao and Elder, 2005; Zhao and Elder, 2000)). Consistent with this notion, Vpr induces DNA double-strand breaks (DSBs), which supports the idea that Vpr induces G2 arrest through DNA damage checkpoint (Tachiwana et al., 2006). However, expression of vpr does not change the radiosensitivity of the checkpoint defective mutants (Elder et al., 2000) or increase gene mutation frequency (Mansky, 1996), which argues against the possibility that Vpr actually causes DNA damage. Similarly, another report showed that Vpr does not induce DNA DSBs (Lai et al., 2005). Moreover, down-regulation of H2AX, the hallmark of DSBs, had little or no effect on Vpr-induced G2 arrest suggesting that this process is a late event and the G2 induction is most likely independent of DNA damage checkpoint (Li et al., 2007). In addition, the ATR kinase, rather than the ATM kinase involved in cell cycle arrest in response to double-strand DNA breaks, was found to play a major role in Vpr-induced G2 arrest through activation of Chk1 via S345 phosphorylation (Li et al., 2007; Li et al., 2010b; Roshal et al., 2003; Zimmerman et al., 2004). These studies suggested that Vpr-induced G2 arrest may in fact resemble more the activation of DNA replication checkpoint than the DNA damage checkpoint control. Further studies have shown numerous similarities between the ATR pathway activated by Vpr and by hidroxyurea/UV light. These similarities include the requirement for Rad17 and Hus1, the induction of phosphorylation on Chk1 and the formation of nuclear foci by RPA, 53BP1, BRCA1 and γH2AX (Lai et al., 2005; Roshal et al., 2003; Zimmerman et al., 2004), all of which indicate activation of DNA replication checkpoint control. However, these conclusions remain unsatisfactory because activation of DNA replication checkpoint generally leads to S phase arrest but not G2 arrest.
One of the possible attributing factors for the reported controversies in examining this molecular event is that most of those studies on Vpr-induced G2 arrest measured the Vpr effect 48–72 hrs after introduction of Vpr in an asynchronized cell population. With this single late time point, it is not possible to establish the sequence of events to determine which events cause the G2 arrest, and which events occur after the initiation of G2 arrest, such as DSBs, and therefore are not responsible for the G2 arrest. Therefore, defining the temporal order of events is critical for identifying the cause of the G2 arrest. Characterization of the initiating event(s) for Vpr-induced G2 arrest would benefit from a system that uses synchronized cells and minimizes the time between initiation of Vpr expression and measurement of the G2 arrest. For this purpose, we have adapted an approach that allows us to monitor the cellular signaling for Vpr-induced G2 arrest within eleven hours of a single cell cycle (Li et al., 2010b). Surprisingly, results of this study suggested that Vpr induces cell cycle G2 arrest through an S phase-dependent mechanism (Li et al., 2010b), i.e., even though Vpr stops the cell cycle at the G2/M phase, the initiation event, such as Chk1-Ser345 phosphorylation, actually occurs in the S phase of the cell cycle. Subsequent mechanistic characterization suggested that the triggering cellular signal(s) in the S phase of the cell cycle include, at least in part, a DNA licensing factor Cdt1 (Li et al., 2010b).
Overall, accumulating evidence suggest that Vpr-induced G2 arrest is unique in many aspects and different from activation of the DNA damage or DNA replication checkpoint. This notion is supported by several findings. First, DNA damage and replication checkpoint control mechanisms are highly conserved among eukaryotes. However, Vpr is still able to induce G2 arrest in mutant fission yeast strains that are defective in the DNA damage and/or replication checkpoints, arguing that these cell cycle checkpoint machineries were not activated in Vpr-induced cell cycle arrest in fission yeast (Elder et al., 2000; Matsuda et al., 2006). Second, both fission yeast and mammalian protein phosphatase 2A (PP2A) are uniquely required for induction of Chk1-Ser345 phosphorylation and Vpr-induced G2 arrest (Elder et al., 2001; Li et al., 2007; Masuda et al., 2000). Significantly, this PP2A-dependent Chk1-Ser345 phosphorylation is not required for hydroxyurea (HU) or ultraviolet light (UV) induced cell cycle arrest, even though both of them cause the same ATR-dependent Chk1-Ser345 phosphorylation through activation of DNA replication checkpoint control. Third, the cellular signal(s) that lead to G2 arrest is initiated through Chk1-Ser345 phosphorylation at the S-phase of the cell cycle. Even though HU and UV also induce Chk1-Ser345 phosphorylation in the S phase under the same conditions, neither HU nor UV-treated cells were able to pass through the S phase, whereas vpr-expressing cells completed the S phase and stopped at the G2/M boundary. Fourth, unlike HU/UV, Vpr promotes Chk1- and proteasome-mediated protein degradations of Cdc25B/C for G2 induction, and Vpr had little or no effect on Cdc25A protein degradation normally mediated by HU/UV (Li et al., 2010b).
Altogether, these data suggest that Vpr induces cell cycle G2 arrest through a unique molecular mechanism that regulates host cell cycle regulation in an S-phase dependent fashion. It is quite likely that Vpr-induced G2 arrest is an active viral action, in which Vpr utilizes the cell cycle G/M checkpoint proteins, such as ATR and Chk1, ito induce G2 arrest.
Induction of apoptosis
Vpr also causes cell death, primarily through apoptosis. It is unclear at present what the biological significance of Vpr-induced apoptosis to HIV-1 infection is. However, this cytotoxic effect may contribute to depletion of CD4+ T cells, and associates with the clinical symptoms such as dementia and the painful peripheral neuropathy of HIV-infected patients (Acharjee et al., 2010; Lum et al., 2003; Pomerantz, 2004). Molecular mechanisms underlying Vpr-induced cell death and apoptosis are complex. For example, the mitochondria-mediated apoptosis, i.e., the intrinsic apoptotic pathway, is believed to play a major role in Vpr-induced apoptosis. However, the extrinsic pathway, i.e., the receptor-mediated apoptosis, has also been reported. Thus far, there have been a number of genes and pathways proposed to have a role in Vpr-induced apoptosis, e.g., direct permeabilization of the mitochondrial membranes through interaction with an adenine nucleotide translocator (ANT) or with HAX-1, the ATR-GADD45α pathway with a downstream mitochondrial role through the BAX protein, depletion of Wee1, activation of the JNK pathway or activation of the NF-κB pathway (Andersen et al., 2005; Green and Kroemer, 2004; Jacotot et al., 2000). It is certainly possible that more than one pathway are required for full induction of apoptosis by Vpr since apoptosis pathways are often found to be redundant.
A major pathway for the induction of apoptosis by Vpr is through the mitochondria. This intrinsic pathway for apoptosis is initiated by mitochondrial membrane permeabilization (MMP) (Green and Kroemer, 2004). The release of proteins from the space between the inner and outer mitochondrial membranes ultimately leads to apoptosis. Cytochrome C is particularly important in this process since it co-operates in the cytoplasm with Apaf-1 to activate procaspase 9, the initiating caspase for the intrinsic pathway. Activated caspase 9 in turn activates the downstream caspases, such as caspase 3, which carry out many of the apoptotic events (Green and Kroemer, 2004). Vpr is thought to lead to MMP by virtue of binding to ANT protein of the inner mitochondrial membrane through its C-terminal aa71–82 domain (Brenner and Kroemer, 2003; Jacotot et al., 2001b; Jacotot et al., 2000). This binding occurs after Vpr crossing of the outer mitochondrial membrane, possibly through VDA (Voltage Dependent Anion Channel), and leads to depolarization of the inner mitochondrial membrane, swelling of the inner mitochondria and ultimately to MMP with release of the apoptosis factors. Among the considerable evidence supporting this model are depolarization of the inner mitochondrial membrane by Vpr in both fission yeast and mammalian cells (Huard et al., 2008), depolarization of isolated mitochondria by purified Vpr, strong binding between Vpr and ANT shown by several methods, reduced cell killing when ANT levels are decreased (Brenner and Kroemer, 2003; Huard et al., 2008; Jacotot et al., 2001a; Jacotot et al., 2000) and activation of caspase 9 and caspase 3 by Vpr (Muthumani et al., 2002a; Muthumani et al., 2002b; Zelivianski et al., 2006). The mitochondria-mediated apoptosis by Vpr was further supported by the anti-apoptotic activities of several cellular suppressors identified in fission yeast and mammalian cells (Huard et al., 2008; Li et al., 2009; Zelivianski et al., 2006). For example, the elongation factor 2 (EF2), a newly identified anti-apoptotic cellular factor, specifically suppresses caspase 9 and caspase 3-mediated apoptosis induced by HIV-1 Vpr (Zelivianski et al., 2006). Overexpression of fission yeast Hsp16 and Skp1 also restores mitochondria morphology in fission yeast and prevents apoptosis in mammalian cells (Benko et al., 2004; Huard et al., 2008). Interestingly, the human homologue of fission yeast Skp1, glycogen synthase kinase-3 (GSK3), appears to play both pro- and anti-apoptotic regulatory roles. GSK3 promotes mitochondria-dependent apoptosis, but it inhibits apoptosis induced by the death receptor-mediated signaling pathway (Beurel and Jope, 2006). Since GSK3 has two isoforms (α and β), it is currently unclear whether both isoforms have the same regulatory activities on apoptosis or each of the isoforms has its unique regulatory role in apoptosis. Further characterization of fission yeast Skpl, its mammalian counterparts, and their role in Vpr-induced apoptosis should provide additional insights into the important regulatory mechanisms of Skpl/GSK3 in Vpr-mediated apoptosis.
While activation of caspase-9 with no activation of caspase-8 supports the role of mitochondria-dependent induction of apoptosis by Vpr (Muthumani et al., 2002a), there have been other reports showing that Vpr activates capase-8 (Lum et al., 2003; Patel et al., 2000; Snyder et al., 2010). Caspase-8 activation is thought to be a hallmark of the extrinsic pathway for apoptosis induction by death receptors, such as FAS and TNFR1 (Barnhart and Peter, 2003). The mitochondria- and receptor-mediated apoptotic pathways were originally considered to be separate, but later work showed that in type II cells, the apoptotic pathway initiated at death receptors by ligands such as TNFα requires amplification through the mitochondria for apoptosis to occur (Scaffidi et al., 1998; Scaffidi et al., 1999). Interestingly, a recent report showed that the C-terminal end of Vpr (aa77–92) binds to PP2A1 inducing apoptosis (Godet et al., 2010). This Vpr fragment overlaps with its mitochondrial ANT binding domain (aa71–82) that is known to induce apoptosis through mitochondria-dependent pathway.
While there is ample support for involvement of mitochondria in Vpr-induced apoptosis, there are some reports that do not readily fit into this model and which raise the possibility that Vpr may kill cells through other pathways. For instance, the localization of Vpr raises one question about the mitochondria model since Vpr has been consistently reported to be in the nucleus or at the nuclear membrane (Chen et al., 1999; Di Marzio et al., 1995; Lu et al., 1993; Mahalingam et al., 1995; Vodicka et al., 1998; Waldhuber et al., 2003) rather than in the mitochondria (Jacotot et al., 2000). It may be that only a small fraction of Vpr molecules localizes to the mitochondria, which is sufficient to induce apoptosis, and methods used to visualize Vpr may have overlooked this small amount. However, the predominant nuclear localization of Vpr and the association of nuclear localization with cell killing in Vpr mutants (Chen et al., 1999; Waldhuber et al., 2003) suggest that Vpr located in the nucleus may have some role in initiating cell killing. Consistent with this idea, Anderson et al. have presented evidence that ATR is not only responsible for the G2 arrest but has an essential role in Vpr-induced apoptosis of human cells (Andersen et al., 2005). It has also been reported that a fragment of Vpr induces cell death without caspase activation (Roumier et al., 2002), and even that Vpr induces a necrotic type of cell death in neurons (Huang et al., 2000).
Thus, the actual apoptosis inducing effect of Vpr may depend upon localization of Vpr within the cell or cellular compartments.
Summary
The activities of HIV-1 Vpr link to various steps during the HIV-1 viral life cycle, and include modulation of viral reverse transcription, nuclear transport of the PIC, transcriptional activation of the LTR promoter, induction of cell cycle G2 arrest and apoptosis. Although the molecular details of these Vpr actions are not fully understood and require further investigation, these Vpr activities are clearly executed through interactions with various cellular proteins. For example, Vpr modulates fidelity of the viral reverse transcription by direct interaction with UNG2 and SMUG. It may promote initiation of viral reverse transcription by binding to the Lys-tRNA synthetase. Even though it is debatable whether the requirement of Vpr for HIV-1 infection of non-dividing cells is due to its ability to transport the PIC into the nucleus, it is clear that the cytoplasmic-nuclear shuttling process involves associations of Vpr with numerous proteins including importin α, NUP153 and hCG1. Human heat shock proteins, such as HSP70, may in part substitute for Vpr in proliferating cells. Trans-activation of HIV-1 LTR promoter by Vpr is non-specific because Vpr promotes gene transcription of LTR through associations with a group of highly conserved transcriptional factors including SP1, p300/CBP, NF-kB, TFIIB, GR and C/EBP. All of these transcriptional factors are also involved in activation of other cellular or viral promoters. It is evident that Vpr is a very potent cytotoxin that induces cell death and apoptosis through a rather complex mechanism but its impact depends upon where it resides in the cell and is mediated primarily through mitochondria by direct associations between Vpr and ANT, PTPC and HAX-1. Most interestingly, the interactions of Vpr with the UPS through hHR23A and the VprBP-associated E3 ligase link it both to cell cycle G2 arrest and viral replication in macrophages. However, the current challenge is to identify the cellular substrates that are specifically targeted by Vpr-induced UPS for G2 induction and viral replication. Thus, identification of these relevant substrates for Vpr mediated ubiquitination and proteolysis will help us clarify the molecular mechanisms of Vpr’s activities and will provide important hints for revealing how and why Vpr is important for HIV infection in macrophages. Altogether, Vpr plays a pivotal role in viral life cycle and pathogenesis. Since Vpr activities are linked to promotion of viral infection in non-dividing macrophages and monocytes, prevention of T-cell clonal expansion, and depletion of CD4+ T-lymphocytes, future strategies to inhibit these adverse Vpr effects could potentially alleviate the impact of the viral infection and thus benefit HIV-infected patients.
Acknowledgments
The work was supported in part by grants from the National Institute of Health NS063880, EB009509 and an intramural funding from the University of Maryland Medical Center (RYZ).
References
- Acharjee S, Noorbakhsh F, Stemkowski PL, Olechowski C, Cohen EA, Ballanyi K, Kerr B, Pardo C, Smith PA, Power C. HIV-1 viral protein R causes peripheral nervous system injury associated with in vivo neuropathic pain. Faseb J. 2010;24:4343–4353. doi: 10.1096/fj.10-162313. [DOI] [PubMed] [Google Scholar]
- Agostini I, Navarro JM, Bouhamdan M, Willetts K, Rey F, Spire B, Vigne R, Pomerantz R, Sire J. The HIV-1 Vpr co-activator induces a conformational change in TFIIB. FEBS Lett. 1999;450:235–239. doi: 10.1016/s0014-5793(99)00501-3. [DOI] [PubMed] [Google Scholar]
- Agostini I, Navarro JM, Rey F, Bouhamdan M, Spire B, Vigne R, Sire J. The human immunodeficiency virus type 1 Vpr transactivator: cooperation with promoter-bound activator domains and binding to TFIIB. J Mol Biol. 1996;261:599–606. doi: 10.1006/jmbi.1996.0485. [DOI] [PubMed] [Google Scholar]
- Agostini I, Popov S, Li J, Dubrovsky L, Hao T, Bukrinsky M. Heat-shock protein 70 can replace viral protein R of HIV-1 during nuclear import of the viral preintegration complex. Exp Cell Res. 2000;259:398–403. doi: 10.1006/excr.2000.4992. [DOI] [PubMed] [Google Scholar]
- Ahn J, Vu T, Novince Z, Guerrero-Santoro J, Rapic-Otrin V, Gronenborn AM. HIV-1 Vpr loads uracil DNA glycosylase-2 onto DCAF1, a substrate recognition subunit of a cullin 4A-ring E3 ubiquitin ligase for proteasome-dependent degradation. J Biol Chem. 2010;285:37333–37341. doi: 10.1074/jbc.M110.133181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini S, Saunders M, Kelley K, Khalili K, Sawaya BE. Interplay between HIV-1 Vpr and Sp1 modulates p21(WAF1) gene expression in human astrocytes. J Biol Chem. 2004;279:46046–46056. doi: 10.1074/jbc.M403792200. [DOI] [PubMed] [Google Scholar]
- Andersen JL, Le Rouzic E, Planelles V. HIV-1 Vpr: mechanisms of G2 arrest and apoptosis. Exp Mol Pathol. 2008;85:2–10. doi: 10.1016/j.yexmp.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JL, Zimmerman ES, DeHart JL, Murala S, Ardon O, Blackett J, Chen J, Planelles V. ATR and GADD45alpha mediate HIV-1 Vpr-induced apoptosis. Cell Death Differ. 2005;12:326–334. doi: 10.1038/sj.cdd.4401565. [DOI] [PubMed] [Google Scholar]
- Barnhart BC, Peter ME. The TNF receptor 1: a split personality complex. Cell. 2003;114:148–150. doi: 10.1016/s0092-8674(03)00561-0. [DOI] [PubMed] [Google Scholar]
- Belzile JP, Abrahamyan LG, Gerard FC, Rougeau N, Cohen EA. Formation of Mobile Chromatin-Associated Nuclear Foci Containing HIV-1 Vpr and VPRBP Is Critical for the Induction of G2 Cell Cycle Arrest. PLoS Pathog. 2010a;6 doi: 10.1371/journal.ppat.1001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzile JP, Duisit G, Rougeau N, Mercier J, Finzi A, Cohen EA. HIV-1 Vpr-Mediated G2 Arrest Involves the DDB1-CUL4A(VPRBP) E3 Ubiquitin Ligase. PLoS Pathog. 2007;3:e85. doi: 10.1371/journal.ppat.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzile JP, Richard J, Rougeau N, Xiao Y, Cohen EA. HIV-1 Vpr induces the K48-linked polyubiquitination and proteasomal degradation of target cellular proteins to activate ATR and promote G2 arrest. J Virol. 2010b;84:3320–3330. doi: 10.1128/JVI.02590-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benko Z, Liang D, Agbottah E, Hou J, Chiu K, Yu M, Innis S, Reed P, Kabat W, Elder RT, Di Marzio P, Taricani L, Ratner L, Young PG, Bukrinsky M, Zhao RY. Anti-Vpr activity of a yeast chaperone protein. J Virol. 2004;78:11016–11029. doi: 10.1128/JVI.78.20.11016-11029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhamdan M, Benichou S, Rey F, Navarro JM, Agostini I, Spire B, Camonis J, Slupphaug G, Vigne R, Benarous R, Sire J. Human immunodeficiency virus type 1 Vpr protein binds to the uracil DNA glycosylase DNA repair enzyme. J Virol. 1996;70:697–704. doi: 10.1128/jvi.70.2.697-704.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BouHamdan M, Xue Y, Baudat Y, Hu B, Sire J, Pomerantz RJ, Duan LX. Diversity of HIV-1 Vpr interactions involves usage of the WXXF motif of host cell proteins. J Biol Chem. 1998;273:8009–8016. doi: 10.1074/jbc.273.14.8009. [DOI] [PubMed] [Google Scholar]
- Brenner C, Kroemer G. The mitochondriotoxic domain of Vpr determines HIV-1 virulence. J Clin Invest. 2003;111:1455–1457. doi: 10.1172/JCI18609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky MI, Haggerty S, Dempsey MP, Sharova N, Adzhubel A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky M. A hard way to the nucleus. Mol Med. 2004;10:1–5. [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky MI, Haffar OK. HIV-1 nuclear import: in search of a leader. Front Biosci. 1997;2:d578–587. doi: 10.2741/a213. [DOI] [PubMed] [Google Scholar]
- Burdo TH, Nonnemacher M, Irish BP, Choi CH, Krebs FC, Gartner S, Wigdahl B. High-affinity interaction between HIV-1 Vpr and specific sequences that span the C/EBP and adjacent NF-kappaB sites within the HIV-1 LTR correlate with HIV-1-associated dementia. DNA Cell Biol. 2004;23:261–269. doi: 10.1089/104454904773819842. [DOI] [PubMed] [Google Scholar]
- Caly L, Saksena NK, Piller SC, Jans DA. Impaired nuclear import and viral incorporation of Vpr derived from a HIV long-term non-progressor. Retrovirology. 2008;5:67. doi: 10.1186/1742-4690-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Elder RT, Yu M, O’Gorman MG, Selig L, Benarous R, Yamamoto A, Zhao Y. Mutational analysis of Vpr-induced G2 arrest, nuclear localization, and cell death in fission yeast. J Virol. 1999;73:3236–3245. doi: 10.1128/jvi.73.4.3236-3245.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Le Rouzic E, Kearney JA, Mansky LM, Benichou S. Vpr-mediated incorporation of UNG2 into HIV-1 particles is required to modulate the virus mutation rate and for replication in macrophages. J Biol Chem. 2004;279:28419–28425. doi: 10.1074/jbc.M403875200. [DOI] [PubMed] [Google Scholar]
- Chen R, Wang H, Mansky LM. Roles of uracil-DNA glycosylase and dUTPase in virus replication. J Gen Virol. 2002;83:2339–2345. doi: 10.1099/0022-1317-83-10-2339. [DOI] [PubMed] [Google Scholar]
- Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- Cui J, Tungaturthi PK, Ayyavoo V, Ghafouri M, Ariga H, Khalili K, Srinivasan A, Amini S, Sawaya BE. The role of Vpr in the regulation of HIV-1 gene expression. Cell Cycle. 2006;5:2626–2638. doi: 10.4161/cc.5.22.3442. [DOI] [PubMed] [Google Scholar]
- de Noronha CM, Sherman MP, Lin HW, Cavrois MV, Moir RD, Goldman RD, Greene WC. Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science. 2001;294:1105–1108. doi: 10.1126/science.1063957. [DOI] [PubMed] [Google Scholar]
- DeHart JL, Zimmerman ES, Ardon O, Monteiro-Filho CM, Arganaraz ER, Planelles V. HIV-1 Vpr activates the G2 checkpoint through manipulation of the ubiquitin proteasome system. Virol J. 2007;4:57. doi: 10.1186/1743-422X-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzio P, Choe S, Ebright M, Knoblauch R, Landau NR. Mutational analysis of cell cycle arrest, nuclear localization and virion packaging of human immunodeficiency virus type 1 Vpr. J Virol. 1995;69:7909–7916. doi: 10.1128/jvi.69.12.7909-7916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann T, Withers-Ward ES, Jarosinski MA, Liu CF, Chen IS, Feigon J. Structure of a human DNA repair protein UBA domain that interacts with HIV-1 Vpr. Nat Struct Biol. 1998;5:1042–1047. doi: 10.1038/4220. [DOI] [PubMed] [Google Scholar]
- Elder RT, Benko Z, Zhao Y. HIV-1 VPR modulates cell cycle G2/M transition through an alternative cellular mechanism other than the classic mitotic checkpoints. Front Biosci. 2002a;7:d349–357. doi: 10.2741/elder. [DOI] [PubMed] [Google Scholar]
- Elder RT, Song XQ, Chen M, Hopkins KM, Lieberman HB, Zhao Y. Involvement of rhp23, a Schizosaccharomyces pombe homolog of the human HHR23A and Saccharomyces cerevisiae RAD23 nucleotide excision repair genes, in cell cycle control and protein ubiquitination. Nucleic Acids Res. 2002b;30:581–591. doi: 10.1093/nar/30.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder RT, Yu M, Chen M, Edelson S, Zhao Y. Cell cycle G2 arrest induced by HIV-1 Vpr in fission yeast (Schizosaccharomyces pombe) is independent of cell death and early genes in the DNA damage checkpoint. Virus Res. 2000;68:161–173. doi: 10.1016/s0168-1702(00)00167-2. [DOI] [PubMed] [Google Scholar]
- Elder RT, Yu M, Chen M, Zhu X, Yanagida M, Zhao Y. HIV-1 Vpr induces cell cycle G2 arrest in fission yeast (Schizosaccharomyces pombe) through a pathway involving regulatory and catalytic subunits of PP2A and acting on both Wee1 and Cdc25. Virology. 2001;287:359–370. doi: 10.1006/viro.2001.1007. [DOI] [PubMed] [Google Scholar]
- Fassati A, Goff SP. Characterization of intracellular reverse transcription complexes of human immunodeficiency virus type 1. J Virol. 2001;75:3626–3635. doi: 10.1128/JVI.75.8.3626-3635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felzien LK, Woffendin C, Hottiger MO, Subbramanian RA, Cohen EA, Nabel GJ. HIV transcriptional activation by the accessory protein, VPR, is mediated by the p300 co-activator. Proc Natl Acad Sci U S A. 1998;95:5281–5286. doi: 10.1073/pnas.95.9.5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenard D, Houzet L, Bernard E, Tupin A, Brun S, Mougel M, Devaux C, Chazal N, Briant L. Uracil DNA Glycosylase 2 negatively regulates HIV-1 LTR transcription. Nucleic Acids Res. 2009;37:6008–6018. doi: 10.1093/nar/gkp673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier RA, Meyer BE, Simon JH, Fischer U, Albright AV, Gonzalez-Scarano F, Malim MH. Interaction of the human immunodeficiency virus type 1 Vpr protein with the nuclear pore complex. J Virol. 1998;72:6004–6013. doi: 10.1128/jvi.72.7.6004-6013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallay P, Hope T, Chin D, Trono D. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci U S A. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallay P, Stitt V, Mundy C, Oettinger M, Trono D. Role of the karyopherin pathway in human immunodeficiency virus type 1 nuclear import. J Virol. 1996;70:1027–1032. doi: 10.1128/jvi.70.2.1027-1032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JS, Lackner AA, Lang SM, Simon MA, Sehgal PK, Daniel MD, Desrosiers RC. Progression to AIDS in the absence of a gene for vpr or vpx. J Virol. 1995;69:2378–2383. doi: 10.1128/jvi.69.4.2378-2383.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godet AN, Guergnon J, Croset A, Cayla X, Falanga PB, Colle JH, Garcia A. PP2A1 binding, cell transducing and apoptotic properties of Vpr(77-92): a new functional domain of HIV-1 Vpr proteins. PLoS One. 5:e13760. doi: 10.1371/journal.pone.0013760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godet AN, Guergnon J, Croset A, Cayla X, Falanga PB, Colle JH, Garcia A. PP2A1 binding, cell transducing and apoptotic properties of Vpr(77–92): a new functional domain of HIV-1 Vpr proteins. PLoS One. 2010;5:e13760. doi: 10.1371/journal.pone.0013760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh WC, Manel N, Emerman M. The human immunodeficiency virus Vpr protein binds Cdc25C: implications for G2 arrest. Virology. 2004;318:337–349. doi: 10.1016/j.virol.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Goh WC, Rogel ME, Kinsey CM, Michael SF, Fultz PN, Nowak MA, Hahn BH, Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat Med. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- Gragerov A, Kino T, Ilyina-Gragerova G, Chrousos GP, Pavlakis GN. HHR23A, the human homologue of the yeast repair protein RAD23, interacts specifically with Vpr protein and prevents cell cycle arrest but not the transcriptional effects of Vpr. Virology. 1998;245:323–330. doi: 10.1006/viro.1998.9138. [DOI] [PubMed] [Google Scholar]
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- He J, Choe S, Walker R, Di Marzio PD, Morgan DO, Landau NR. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzinger N, Bukinsky M, Haggerty S, Ragland A, Kewalramani V, Lee M, Gendelman H, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Nat Acad Sci USA. 1994a;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzinger NK, Bukinsky MI, Haggerty SA, Ragland AM, Kewalramani V, Lee MA, Gendelman HE, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci U S A. 1994b;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold A, Truant R, Wiegand H, Cullen BR. Determination of the functional domain organization of the importin alpha nuclear import factor. J Cell Biol. 1998;143:309–318. doi: 10.1083/jcb.143.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan TH, Nonnemacher MR, Krebs FC, Henderson A, Wigdahl B. HIV-1 Vpr binding to HIV-1 LTR C/EBP cis-acting elements and adjacent regions is sequence-specific. Biomed Pharmacother. 2003;57:41–48. doi: 10.1016/s0753-3322(02)00333-5. [DOI] [PubMed] [Google Scholar]
- Hrecka K, Gierszewska M, Srivastava S, Kozaczkiewicz L, Swanson SK, Florens L, Washburn MP, Skowronski J. Lentiviral Vpr usurps Cul4-DDB1[VprBP] E3 ubiquitin ligase to modulate cell cycle. Proc Natl Acad Sci U S A. 2007;104:11778–11783. doi: 10.1073/pnas.0702102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MB, Weeks O, Zhao LJ, Saltarelli M, Bond VC. Effects of extracellular human immunodeficiency virus type 1 vpr protein in primary rat cortical cell cultures. J Neurovirol. 2000;6:202–220. doi: 10.3109/13550280009015823. [DOI] [PubMed] [Google Scholar]
- Huang Y, Mak J, Cao Q, Li Z, Wainberg MA, Kleiman L. Incorporation of excess wild-type and mutant tRNA(3Lys) into human immunodeficiency virus type 1. J Virol. 1994;68:7676–7683. doi: 10.1128/jvi.68.12.7676-7683.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard S, Chen M, Burdette KE, Fenyvuesvolgyi C, Yu M, Elder RT, Zhao RY. HIV-1 Vpr-induced cell death in Schizosaccharomyces pombe is reminiscent of apoptosis. Cell Res. 2008 doi: 10.1038/cr.2008.272. [DOI] [PubMed] [Google Scholar]
- Iordanskiy S, Zhao Y, DiMarzio P, Agostini I, Dubrovsky L, Bukrinsky M. Heat-shock protein 70 exerts opposing effects on Vpr-dependent and Vpr-independent HIV-1 replication in macrophages. Blood. 2004a;104:1867–1872. doi: 10.1182/blood-2004-01-0081. [DOI] [PubMed] [Google Scholar]
- Iordanskiy S, Zhao Y, Dubrovsky L, Iordanskaya T, Chen M, Liang D, Bukrinsky M. Heat shock protein 70 protects cells from cell cycle arrest and apoptosis induced by human immunodeficiency virus type 1 viral protein R. J Virol. 2004b;78:9697–9704. doi: 10.1128/JVI.78.18.9697-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacotot E, Ferri K, El Hamel C, Brenner C, Druillennec S, Hoebeke J, Rustin P, Metivier D, Lenoir C, Geuskens M, Vieira H, Loeffler M, Belzacq A, Briand J, Zamzami N, Edelman L, Xie Z, Reed J, Roques B, Kroemer G. Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 viral protein r and bcl-2. J Exp Med. 2001a;193:509–520. doi: 10.1084/jem.193.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacotot E, Ferri KF, El Hamel C, Brenner C, Druillennec S, Hoebeke J, Rustin P, Metivier D, Lenoir C, Geuskens M, Vieira HL, Loeffler M, Belzacq AS, Briand JP, Zamzami N, Edelman L, Xie ZH, Reed JC, Roques BP, Kroemer G. Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 viral protein rR and Bcl-2. J Exp Med. 2001b;193:509–519. doi: 10.1084/jem.193.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacotot E, Ravagnan L, Loeffler M, Ferri KF, Vieira HL, Zamzami N, Costantini P, Druillennec S, Hoebeke J, Briand JP, Irinopoulou T, Daugas E, Susin SA, Cointe D, Xie ZH, Reed JC, Roques BP, Kroemer G. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J Exp Med. 2000;191:33–46. doi: 10.1084/jem.191.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins Y, McEntee M, Weis K, Greene WC. Characterization of HIV-1 vpr nuclear import: analysis of signals and pathways. J Cell Biol. 1998;143:875–885. doi: 10.1083/jcb.143.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jowett JB, Planelles V, Poon B, Shah NP, Chen M, Chen ISY. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata M, Nitahara-Kasahara Y, Miyamoto Y, Yoneda Y, Aida Y. Importin-alpha promotes passage through the nuclear pore complex of human immunodeficiency virus type 1 Vpr. J Virol. 2005;79:3557–3564. doi: 10.1128/JVI.79.6.3557-3564.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata M, Watanabe N, Nagaoka Y, Chen IS. Human immunodeficiency virus type 1 Vpr binds to the N lobe of the Wee1 kinase domain and enhances kinase activity for CDC2. J Virol. 2008;82:5672–5682. doi: 10.1128/JVI.01330-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilareski EM, Shah S, Nonnemacher MR, Wigdahl B. Regulation of HIV-1 transcription in cells of the monocyte-macrophage lineage. Retrovirology. 2009;6:118. doi: 10.1186/1742-4690-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T, Gragerov A, Slobodskaya O, Tsopanomichalou M, Chrousos GP, Pavlakis GN. Human immunodeficiency virus type 1 (HIV-1) accessory protein Vpr induces transcription of the HIV-1 and glucocorticoid-responsive promoters by binding directly to p300/CBP coactivators. J Virol. 2002;76:9724–9734. doi: 10.1128/JVI.76.19.9724-9734.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T, Gragerov A, Valentin A, Tsopanomihalou M, Ilyina-Gragerova G, Erwin-Cohen R, Chrousos GP, Pavlakis GN. Vpr protein of human immunodeficiency virus type 1 binds to 14-3-3 proteins and facilitates complex formation with Cdc25C: implications for cell cycle arrest. J Virol. 2005;79:2780–2787. doi: 10.1128/JVI.79.5.2780-2787.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarmann GJ, Chen X, North TW, Preston BD. Incorporation of uracil into minus strand DNA affects the specificity of plus strand synthesis initiation during lentiviral reverse transcription. J Biol Chem. 2003;278:7902–7909. doi: 10.1074/jbc.M207223200. [DOI] [PubMed] [Google Scholar]
- Kobe B. Autoinhibition by an internal nuclear localization signal revealed by the crystal structure of mammalian importin alpha. Nat Struct Biol. 1999;6:388–397. doi: 10.1038/7625. [DOI] [PubMed] [Google Scholar]
- Lai M, Zimmerman ES, Planelles V, Chen J. Activation of the ATR pathway by human immunodeficiency virus type 1 Vpr involves its direct binding to chromatin in vivo. J Virol. 2005;79:15443–15451. doi: 10.1128/JVI.79.24.15443-15451.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang SM, Weeger M, Stahl-Hennig C, Coulibaly C, Hunsmann G, Muller J, Muller-Hermelink H, Fuchs D, Wachter H, Daniel MM, Desrosiers RC, Fleckenstein B. Importance of vpr for infection of rhesus monkeys with simian immunodeficiency virus. J Virol. 1993;67:902–912. doi: 10.1128/jvi.67.2.902-912.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rouzic E, Belaidouni N, Estrabaud E, Morel M, Rain JC, Transy C, Margottin-Goguet F. HIV1 Vpr arrests the cell cycle by recruiting DCAF1/VprBP, a receptor of the Cul4-DDB1 ubiquitin ligase. Cell Cycle. 2007;6:182–188. doi: 10.4161/cc.6.2.3732. [DOI] [PubMed] [Google Scholar]
- Le Rouzic E, Mousnier A, Rustum C, Stutz F, Hallberg E, Dargemont C, Benichou S. Docking of HIV-1 Vpr to the nuclear envelope is mediated by the interaction with the nucleoporin hCG1. J Biol Chem. 2002;277:45091–45098. doi: 10.1074/jbc.M207439200. [DOI] [PubMed] [Google Scholar]
- Li G, Bukrinsky M, Zhao RY. HIV-1 viral protein R (Vpr) and its interactions with host cell. Curr HIV Res. 2009;7:178–183. doi: 10.2174/157016209787581436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Elder RT, Dubrovsky L, Liang D, Pushkarsky T, Chiu K, Fan T, Sire J, Bukrinsky M, Zhao RY. HIV-1 replication through hHR23A-mediated interaction of Vpr with 26S proteasome. PLoS One. 2010a;5:e11371. doi: 10.1371/journal.pone.0011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Elder RT, Qin K, Park HU, Liang D, Zhao RY. Phosphatase type 2A-dependent and -independent pathways for ATR phosphorylation of Chk1. J Biol Chem. 2007;282:7287–7298. doi: 10.1074/jbc.M607951200. [DOI] [PubMed] [Google Scholar]
- Li G, Park HU, Liang D, Zhao RY. Cell cycle G2/M arrest through an S phase-dependent mechanism by HIV-1 viral protein R. Retrovirology. 2010b;7:59. doi: 10.1186/1742-4690-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Liang D, Li JY, Zhao YQ. Enhanced protein production of Vif and APOBEC3G by HIV-1 Vpr. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2008;22:39–41. [PubMed] [Google Scholar]
- Li Y, Zhang Z, Wakefield JK, Kang SM, Morrow CD. Nucleotide substitutions within U5 are critical for efficient reverse transcription of human immunodeficiency virus type 1 with a primer binding site complementary to tRNA(His) J Virol. 1997;71:6315–6322. doi: 10.1128/jvi.71.9.6315-6322.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Benko Z, Agbottah E, Bukrinsky M, Zhao RY. Anti-vpr activities of heat shock protein 27. Mol Med. 2007;13:229–239. doi: 10.2119/2007-00004.Liang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wu X, Xiao H, Kappes JC. Targeting human immunodeficiency virus (HIV) type 2 integrase protein into HIV type 1. J Virol. 1999;73:8831–8836. doi: 10.1128/jvi.73.10.8831-8836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YL, Spearman P, Ratner L. Human immunodeficiency virus type 1 viral protein R localization in infected cells and virions. J Virol. 1993;67:6542–6550. doi: 10.1128/jvi.67.11.6542-6550.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum JJ, Cohen OJ, Nie Z, Weaver JG, Gomez TS, Yao XJ, Lynch D, Pilon AA, Hawley N, Kim JE, Chen Z, Montpetit M, Sanchez-Dardon J, Cohen EA, Badley AD. Vpr R77Q is associated with long-term nonprogressive HIV infection and impaired induction of apoptosis. J Clin Invest. 2003;111:1547–1554. doi: 10.1172/JCI16233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam S, Collman RG, Patel M, Monken CE, Srinivasan A. Functional analysis of HIV-1 Vpr: identification of determinants essential for subcellular localization. Virol. 1995;212:331–339. doi: 10.1006/viro.1995.1490. [DOI] [PubMed] [Google Scholar]
- Mak J, Jiang M, Wainberg MA, Hammarskjold ML, Rekosh D, Kleiman L. Role of Pr160gag-pol in mediating the selective incorporation of tRNA(Lys) into human immunodeficiency virus type 1 particles. J Virol. 1994;68:2065–2072. doi: 10.1128/jvi.68.4.2065-2072.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansky LM. The mutation rate of human immunodeficiency virus type 1 is influenced by the vpr gene. Virology. 1996;222:391–400. doi: 10.1006/viro.1996.0436. [DOI] [PubMed] [Google Scholar]
- Mansky LM, Preveral S, Le Rouzic E, Bernard LC, Selig L, Depienne C, Benarous R, Benichou S. Interaction of human immunodeficiency virus type 1 Vpr with the HHR23A DNA repair protein does not correlate with multiple biological functions of Vpr. Virology. 2001;282:176–185. doi: 10.1006/viro.2000.0791. [DOI] [PubMed] [Google Scholar]
- Mansky LM, Preveral S, Selig L, Benarous R, Benichou S. The interaction of vpr with uracil DNA glycosylase modulates the human immunodeficiency virus type 1 In vivo mutation rate. J Virol. 2000;74:7039–7047. doi: 10.1128/jvi.74.15.7039-7047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani R, Chen D, Schrofelbauer B, Navarro F, Konig R, Bollman B, Munk C, Nymark-McMahon H, Landau NR. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell. 2003;114:21–31. doi: 10.1016/s0092-8674(03)00515-4. [DOI] [PubMed] [Google Scholar]
- Masuda M, Nagai Y, Oshima N, Tanaka K, Murakami H, Igarashi H, Okayama H. Genetic studies with the fission yeast Schizosaccharomyces pombe suggest involvement of wee1, ppa2, and rad24 in induction of cell cycle arrest by human immunodeficiency virus type 1 Vpr. J Virol. 2000;74:2636–2646. doi: 10.1128/jvi.74.6.2636-2646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Tanaka H, Yamazaki S, Suzuki J, Tanaka K, Yamada T, Masuda M. HIV-1 Vpr induces G2 cell cycle arrest in fission yeast associated with Rad24/14-3-3-dependent, Chk1/Cds1-independent Wee1 upregulation. Microbes Infect. 2006;8:2736–2744. doi: 10.1016/j.micinf.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Morellet N, Bouaziz S, Petitjean P, Roques BP. NMR structure of the HIV-1 regulatory protein VPR. J Mol Biol. 2003;327:215–227. doi: 10.1016/s0022-2836(03)00060-3. [DOI] [PubMed] [Google Scholar]
- Muthumani K, Hwang DS, Desai BM, Zhang D, Dayes N, Green DR, Weiner DB. HIV-1 Vpr induces apoptosis through caspase 9 in T cells and peripheral blood mononuclear cells. J Biol Chem. 2002a;277:37820–37831. doi: 10.1074/jbc.M205313200. [DOI] [PubMed] [Google Scholar]
- Muthumani K, Zhang D, Hwang DS, Kudchodkar S, Dayes NS, Desai BM, Malik AS, Yang JS, Chattergoon MA, Maguire HC, Jr, Weiner DB. Adenovirus encoding HIV-1 Vpr activates caspase 9 and induces apoptotic cell death in both p53 positive and negative human tumor cell lines. Oncogene. 2002b;21:4613–4625. doi: 10.1038/sj.onc.1205549. [DOI] [PubMed] [Google Scholar]
- Nakielny S, Dreyfuss G. Transport of proteins and RNAs in and out of the nucleus. Cell. 1999;99:677–690. doi: 10.1016/s0092-8674(00)81666-9. [DOI] [PubMed] [Google Scholar]
- Nitahara-Kasahara Y, Kamata M, Yamamoto T, Zhang X, Miyamoto Y, Muneta K, Iijima S, Yoneda Y, Tsunetsugu-Yokota Y, Aida Y. Novel nuclear import of Vpr promoted by importin alpha is crucial for human immunodeficiency virus type 1 replication in macrophages. J Virol. 2007;81:5284–5293. doi: 10.1128/JVI.01928-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop JP, Crabtree GR, Mattila PS. Negative regulation of interleukin 2 transcription by the glucocorticoid receptor. J Exp Med. 1992;175:1235–1245. doi: 10.1084/jem.175.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh SS, Walcher G, Jones GD, Slupphaug G, Krokan HE, Blackburn GM, Tainer JA. Uracil-DNA glycosylase-DNA substrate and product structures: conformational strain promotes catalytic efficiency by coupled stereoelectronic effects. Proc Natl Acad Sci U S A. 2000;97:5083–5088. doi: 10.1073/pnas.97.10.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel CA, Mukhtar M, Pomerantz RJ. Human immunodeficiency virus type 1 Vpr induces apoptosis in human neuronal cells. J Virol. 2000;74:9717–9726. doi: 10.1128/jvi.74.20.9717-9726.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton LF, Paschal BM. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic. 2005;6:187–198. doi: 10.1111/j.1600-0854.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Pomerantz RJ. Effects of HIV-1 Vpr on neuroinvasion and neuropathogenesis. DNA Cell Biol. 2004;23:227–238. doi: 10.1089/104454904773819815. [DOI] [PubMed] [Google Scholar]
- Poon B, Grovit-Ferbas K, Stewart SA, Chen IS. Cell cycle arrest by Vpr in HIV-1 virions and insensitivity to antiretroviral agents. Science. 1998;281:266–269. doi: 10.1126/science.281.5374.266. [DOI] [PubMed] [Google Scholar]
- Popov S, Rexach M, Ratner L, Blobel G, Bukrinsky M. Viral protein R regulates docking of the HIV-1 preintegration complex to the nuclear pore complex. J Biol Chem. 1998a;273:13347–13352. doi: 10.1074/jbc.273.21.13347. [DOI] [PubMed] [Google Scholar]
- Popov S, Rexach M, Zybarth G, Reiling N, Lee MA, Ratner L, Lane CM, Moore MS, Blobel G, Bukrinsky M. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 1998b;17:909–917. doi: 10.1093/emboj/17.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re F, Braaten D, Franke EK, Luban J. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refaeli Y, Levy DN, Weiner DB. The glucocorticoid receptor type II complex is a target of the HIV-1 vpr gene product. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3621–3625. doi: 10.1073/pnas.92.8.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere L, Darlix JL, Cimarelli A. Analysis of the viral elements required in the nuclear import of HIV-1 DNA. J Virol. 2010;84:729–739. doi: 10.1128/JVI.01952-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani B, Engelbrecht S. Human immunodeficiency virus type 1 Vpr: functions and molecular interactions. J Gen Virol. 2009;90:1795–1805. doi: 10.1099/vir.0.011726-0. [DOI] [PubMed] [Google Scholar]
- Roshal M, Kim B, Zhu Y, Nghiem P, Planelles V. Activation of the ATR-mediated DNA damage response by the HIV-1 viral protein R. J Biol Chem. 2003;278:25879–25886. doi: 10.1074/jbc.M303948200. [DOI] [PubMed] [Google Scholar]
- Roumier T, Vieira HL, Castedo M, Ferri KF, Boya P, Andreau K, Druillennec S, Joza N, Penninger JM, Roques B, Kroemer G. The C-terminal moiety of HIV-1 Vpr induces cell death via a caspase-independent mitochondrial pathway. Cell Death Differ. 2002;9:1212–1219. doi: 10.1038/sj.cdd.4401089. [DOI] [PubMed] [Google Scholar]
- Roux P, Alfieri C, Hrimech M, Cohen EA, Tanner JE. Activation of transcription factors NF-kappaB and NF-IL-6 by human immunodeficiency virus type 1 protein R (Vpr) induces interleukin-8 expression. J Virol. 2000;74:4658–4665. doi: 10.1128/jvi.74.10.4658-4665.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaya BE, Khalili K, Gordon J, Taube R, Amini S. Cooperative interaction between HIV-1 regulatory proteins Tat and Vpr modulates transcription of the viral genome. J Biol Chem. 2000;275:35209–35214. doi: 10.1074/jbc.M005197200. [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. Embo J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi C, Schmitz I, Zha J, Korsmeyer SJ, Krammer PH, Peter ME. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem. 1999;274:22532–22538. doi: 10.1074/jbc.274.32.22532. [DOI] [PubMed] [Google Scholar]
- Schrofelbauer B, Hakata Y, Landau NR. HIV-1 Vpr function is mediated by interaction with the damage-specific DNA-binding protein DDB1. Proc Natl Acad Sci U S A. 2007;104:4130–4135. doi: 10.1073/pnas.0610167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrofelbauer B, Yu Q, Zeitlin SG, Landau NR. Human immunodeficiency virus type 1 Vpr induces the degradation of the UNG and SMUG uracil-DNA glycosylases. J Virol. 2005;79:10978–10987. doi: 10.1128/JVI.79.17.10978-10987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler W, Wecker K, de Rocquigny H, Baudat Y, Sire J, Roques BP. NMR structure of the (52–96) C-terminal domain of the HIV-1 regulatory protein Vpr: molecular insights into its biological functions. J Mol Biol. 1999;285:2105–2117. doi: 10.1006/jmbi.1998.2381. [DOI] [PubMed] [Google Scholar]
- Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- Snyder A, Alsauskas ZC, Leventhal JS, Rosenstiel PE, Gong P, Chan JJ, Barley K, He JC, Klotman ME, Ross MJ, Klotman PE. HIV-1 viral protein r induces ERK and caspase-8-dependent apoptosis in renal tubular epithelial cells. Aids. 2010;24:1107–1119. doi: 10.1097/QAD.0b013e328337b0ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaran M, Sharkey M, Brichacek B, Luzuriaga K, Emerman M, Sullivan JL, Stevenson M. Evidence for a cytopathogenicity determinant in HIV-1 Vpr. Proc Natl Acad Sci U S A. 2002;99:9503–9508. doi: 10.1073/pnas.142313699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark LA, Hay RT. Human immunodeficiency virus type 1 (HIV-1) viral protein R (Vpr) interacts with Lys-tRNA synthetase: implications for priming of HIV-1 reverse transcription. J Virol. 1998;72:3037–3044. doi: 10.1128/jvi.72.4.3037-3044.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SA, Poon B, Jowett JB, Chen IS. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbramanian RA, Kessous-Elbaz A, Lodge R, Forget J, Yao XJ, Bergeron D, Cohen EA. Human immunodeficiency virus type 1 Vpr is a positive regulator of viral transcription and infectivity in primary human macrophages. J Exp Med. 1998;187:1103–1111. doi: 10.1084/jem.187.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachiwana H, Shimura M, Nakai-Murakami C, Tokunaga K, Takizawa Y, Sata T, Kurumizaka H, Ishizaka Y. HIV-1 Vpr induces DNA double-strand breaks. Cancer Res. 2006;66:627–631. doi: 10.1158/0008-5472.CAN-05-3144. [DOI] [PubMed] [Google Scholar]
- Tan L, Ehrlich E, Yu XF. DDB1 and Cul4A are required for HIV-1 Vpr-induced G2 arrest. J Virol. 2007 doi: 10.1128/JVI.01380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristem M, Marshall C, Karpas A, Hill F. Evolution of the primate lentiviruses: evidence from vpx and vpr. EMBO Journal. 1992;11:3405–3412. doi: 10.1002/j.1460-2075.1992.tb05419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristem M, Purvis A, Quicke DL. Complex evolutionary history of primate lentiviral vpr genes. Virology. 1998;240:232–237. doi: 10.1006/viro.1997.8929. [DOI] [PubMed] [Google Scholar]
- Varadarajan P, Mahalingam S, Liu P, Ng SB, Gandotra S, Dorairajoo DS, Balasundaram D. The functionally conserved nucleoporins Nup124p from fission yeast and the human Nup153 mediate nuclear import and activity of the Tf1 retrotransposon and HIV-1 Vpr. Mol Biol Cell. 2005;16:1823–1838. doi: 10.1091/mbc.E04-07-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varin A, Decrion AZ, Sabbah E, Quivy V, Sire J, Van Lint C, Roques BP, Aggarwal BB, Herbein G. Synthetic Vpr protein activates activator protein-1, c-Jun N-terminal kinase, and NF-kappaB and stimulates HIV-1 transcription in promonocytic cells and primary macrophages. J Biol Chem. 2005;280:42557–42567. doi: 10.1074/jbc.M502211200. [DOI] [PubMed] [Google Scholar]
- Vodicka MA, Koepp DM, Silver PA, Emerman M. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 1998;12:175–185. doi: 10.1101/gad.12.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhuber MG, Bateson M, Tan J, Greenway AL, McPhee DA. Studies with GFP-Vpr fusion proteins: induction of apoptosis but ablation of cell-cycle arrest despite nuclear membrane or nuclear localization. Virology. 2003;313:91–104. doi: 10.1016/s0042-6822(03)00258-7. [DOI] [PubMed] [Google Scholar]
- Wang L, Mukherjee S, Jia F, Narayan O, Zhao LJ. Interaction of virion protein Vpr of human immunodeficiency virus type 1 with cellular transcription factor Sp1 and trans-activation of viral long terminal repeat. J Biol Chem. 1995;270:25564–25569. doi: 10.1074/jbc.270.43.25564. [DOI] [PubMed] [Google Scholar]
- Wecker K, Roques BP. NMR structure of the (1–51) N-terminal domain of the HIV-1 regulatory protein Vpr. Eur J Biochem. 1999;266:359–369. doi: 10.1046/j.1432-1327.1999.00858.x. [DOI] [PubMed] [Google Scholar]
- Wen X, Duus KM, Friedrich TD, de Noronha CM. The HIV1 protein VPR acts to promote G2 cell cycle arrest by engaging a DDB1 and cullin4A containing ubiquitin ligase complex using VPRBP/DCAF1 as an adaptor. J Biol Chem. 2007 doi: 10.1074/jbc.M703955200. [DOI] [PubMed] [Google Scholar]
- Wente SR. Gatekeepers of the nucleus. Science. 2000;288:1374–1377. doi: 10.1126/science.288.5470.1374. [DOI] [PubMed] [Google Scholar]
- Wilkinson CR, Wallace M, Morphew M, Perry P, Allshire R, Javerzat JP, McIntosh JR, Gordon C. Localization of the 26S proteasome during mitosis and meiosis in fission yeast. EMBO J. 1998;17:6465–6476. doi: 10.1093/emboj/17.22.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers-Ward ES, Jowett JB, Stewart SA, Xie YM, Garfinkel A, Shibagaki Y, Chow SA, Shah N, Hanaoka F, Sawitz DG, Armstrong RW, Souza LM, Chen IS. Human immunodeficiency virus type 1 Vpr interacts with HHR23A, a cellular protein implicated in nucleotide excision DNA repair. J Virol. 1997;71:9732–9742. doi: 10.1128/jvi.71.12.9732-9742.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Perez O, Hope TJ, Emerman M. Evidence for direct involvement of the capsid protein in HIV infection of nondividing cells. PLoS Pathog. 2007;3:1502–1510. doi: 10.1371/journal.ppat.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yedavalli VS, Shih HM, Chiang YP, Lu CY, Chang LY, Chen MY, Chuang CY, Dayton AI, Jeang KT, Huang LM. Human immunodeficiency virus type 1 Vpr interacts with antiapoptotic mitochondrial protein HAX-1. J Virol. 2005;79:13735–13746. doi: 10.1128/JVI.79.21.13735-13746.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelivianski S, Liang D, Chen M, Mirkin BL, Zhao RY. Suppressive effect of elongation factor 2 on apoptosis induced by HIV-1 viral protein R. Apoptosis. 2006;11:377–388. doi: 10.1007/s10495-006-4030-9. [DOI] [PubMed] [Google Scholar]
- Zhang S, Feng Y, Narayan O, Zhao LJ. Cytoplasmic retention of HIV-1 regulatory protein Vpr by protein-protein interaction with a novel human cytoplasmic protein VprBP. Gene. 2001;263:131–140. doi: 10.1016/s0378-1119(00)00583-7. [DOI] [PubMed] [Google Scholar]
- Zhao RY, Elder RT. Viral infections and cell cycle G2/M regulation. Cell Res. 2005;15:143–149. doi: 10.1038/sj.cr.7290279. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Cao J, O’Gorman MR, Yu M, Yogev R. Effect of human immunodeficiency virus type 1 protein R (vpr) gene expression on basic cellular function of fission yeast Schizosaccharomyces pombe. J Virol. 1996;70:5821–5826. doi: 10.1128/jvi.70.9.5821-5826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Chen M, Wang B, Yang J, Elder RT, Song XQ, Yu M, Saksena NK. Functional conservation of HIV-1 Vpr and variability in a mother-child pair of long-term non-progressors. Virus Res. 2002;89:103–121. doi: 10.1016/s0168-1702(02)00127-2. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Elder RT. Yeast perspectives on HIV-1 VPR. Front Biosci. 2000;5:905–916. doi: 10.2741/zhao. [DOI] [PubMed] [Google Scholar]
- Zimmerman ES, Chen J, Andersen JL, Ardon O, Dehart JL, Blackett J, Choudhary SK, Camerini D, Nghiem P, Planelles V. Human immunodeficiency virus type 1 Vpr-mediated G2 arrest requires Rad17 and Hus1 and induces nuclear BRCA1 and gamma-H2AX focus formation. Mol Cell Biol. 2004;24:9286–9294. doi: 10.1128/MCB.24.21.9286-9294.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


