Abstract
Background
The Centers for Disease Control and Prevention (CDC) monitors the occurrence of respiratory syncytial virus (RSV) in the United States and has historically reported on activity at the regional level. Prior to the 2007–2008 RSV season, the CDC did not report seasonal RSV data for cities within North Carolina or for the state. The purpose of the present study is to characterize RSV seasonal activity within North Carolina and to determine the appropriate months in which at-risk children should receive prophylaxis.
Methods
We prospectively collected RSV test data monthly over three seasons (fall through spring), from September 2003 through July 2006, from a diverse group of hospitals and a community pediatric practice located within five regions throughout North Carolina.
Results
Approximately 14,000 laboratory tests, including 23.7% that were RSV positive, were evaluated over the three seasons, and RSV was detected within the state during all but three months of the study. Seasonal variation in the onset (October–November) of RSV activity and duration (6–7 months) of the RSV season according to the specified definition of seasonality was noted yearly within individual regions and among regions. On average over the study period, the greatest percentage of positive tests (33.8%) statewide occurred during January.
Conclusion
Our data suggest the RSV season in North Carolina is longer than the national average, and RSV epidemics persist during months that fall outside of those in which RSV prophylaxis is given to high-risk children. Guidelines on the administration of RSV prophylaxis should ideally be based on results of local RSV test data.
Keywords: respiratory syncytial virus (RSV), surveillance, seasonality, palivizumab, North Carolina
Respiratory syncytial virus (RSV) is a major cause of lower respiratory tract infections among young children1 and is responsible for more hospitalizations—approximately 2.4 per 100 births yearly—than any other disease during infancy.2,3 RSV affects nearly all children at least once by age 2.4 Those born prematurely or with either a history of chronic lung disease or significant congenital heart disease are at highest risk for severe disease.5 Potential consequences of RSV-related infection include recurrent wheezing and asthma through later life6 and deaths.7 This underscores the need for adequate preventive measures. There are no vaccines currently available against RSV, but the severity of infection and likelihood for hospitalization are reduced in at-risk children who are administered palivizumab prior to and during the RSV season.5,8,9
Recommendations on the use of palivizumab are issued periodically by the Committee on Infectious Diseases and published in the Redbook.5 The most current guideline states that palivizumab should be administered as a series of five monthly injections beginning in November and ending in March. However, these recommendations do not take into consideration the substantial seasonal variability in the onset and duration of RSV epidemics noted over time within a given geographic area or among different regions of the country.10 The Centers for Disease Control and Prevention (CDC) has monitored temporal and geographic trends for RSV activity through a passive surveillance system (i.e., National Respiratory and Enteric Virus Surveillance System [NREVSS]) that included approximately 70 laboratories located throughout the country. Results of RSV test data from participating sites for the 2003 through 2006 RSV seasons were grouped into one of four regions, and seasonal data were reported by region and not state, with the exception of Florida.11 There has been increased interest by individual state departments of health12,13 and independent investigators14–17 to gain a better understanding of the seasonality of RSV at the local level and to place less reliance on regional data. As a result, more than 20 state health departments have some form of RSV surveillance program. Recognizing the need for better reporting of local data, the number of reporting laboratories within the NREVSS has been greatly expanded and state data are currently available (i.e., as of the 2007–2008 RSV season) through the network. At the time of our study, North Carolina did not have any formal coordinated RSV monitoring program, and to our knowledge there were no available published data on the seasonality of RSV within the state. The purpose of this study was to identify when RSV was present in epidemic levels within different regions of the state over time and to determine the appropriate timing and duration of RSV prophylaxis specific to North Carolina.
METHODS
We prospectively collected RSV test data monthly from children over three seasons (fall through spring), beginning September 2003 and ending July 2006 from a diverse group of academic and community hospitals and a group of pediatric primary care offices. The purpose of the study was to determine the onset, peak, duration, and conclusion of each RSV season within various regions of the state and in the state overall. A further objective was to determine appropriate timing and duration of palivizumab prophylaxis specific for North Carolina.
Laboratory tests from patients were reported monthly and the number of positive tests was divided by the total number of tests to determine the percent of positive tests by region and for the state. The onset of the RSV season was defined as the first of consecutive months in which 10% or more of RSV tests were positive and at least 10 tests were reported. If fewer than 10 tests were reported, RSV was considered not to be present during the month. The conclusion of the RSV season was defined as the first month following successive epidemic months in which <10% of tests were positive or fewer than 10 tests were noted. To simulate real-life conditions, each site used its own collection and testing methods and made its own determination as to which patients would be tested. Presence of RSV was confirmed through antigen detection methods such as virus direct fluorescent assay (VDFA) and enzyme immunoassay (EIA) and/or viral culture. Antigen detection assays rather than viral cultures were used to determine the RSV season, as viral culture results were only available from one site. Data were submitted and analyzed at the Duke Clinical Research Institute.
The participating institutions included academic and community hospitals and a group of pediatric primary care offices, which represented five regions within the state. Sites included Wake Medical Center (now known as WakeMed) in Raleigh (i.e., north central North Carolina); four sites in Greensboro/Winston-Salem (i.e., northwestern North Carolina), which included Baptist Medical Center, Forsyth Medical Center, High Point Regional Hospital, and Moses H. Cone Memorial Hospital; Pitt County Memorial Hospital in Greenville (i.e., eastern North Carolina); Carolinas Medical Center in Charlotte (i.e., southwestern North Carolina); and three sites of Hendersonville Pediatrics located in Brevard, Fletcher, and Hendersonville (i.e., western North Carolina). The populations of the study areas are quite variable, representing urban (200,000–650,000 people; Winston-Salem, Greensboro, Raleigh, Charlotte), suburban (72,000 people; Greenville), and rural locales (5,000–15,000 people; Fletcher, Brevard, Hendersonville). The farthest distance spanning any two sites exceeds 300 miles, between Brevard and Greenville; the closest distance, between Baptist Medical Center and Forsyth Medical Center, is approximately three miles.
Data from Forsyth Medical Center, High Point Regional Hospital, Moses H. Cone Memorial Hospital, and Baptist Medical Center were pooled as one regional site (i.e., Greensboro/Winston-Salem), as were data from all sites of Hendersonville Pediatrics. Wake Medical Center and the 4 sites in Greensboro/Winston-Salem reported data throughout each of the 3 study seasons: 2003–2004, 2004–2005, and 2005–2006. Pitt County Memorial Hospital collected data through 2 seasons (i.e., 2003–2004 and 2004–2005), whereas Carolinas Medical Center and Hendersonville Pediatrics reported data for a lone season each (i.e., 2003–2004 and 2005–2006, respectively).
RESULTS
Approximately 14,000 RSV test results were reported during the evaluation period and almost 80% of all data were contributed from the Greensboro/Winston-Salem and Wake Medical Center sites. All patients were children, but further demographic data (i.e., ages, gender, race) were not available. Seasonal variation in the onset of RSV activity, month of peak activity, and duration of the RSV season was noted yearly within individual regions and among regions. Collectively, RSV was detected during all but three months, seasons began in the months of October or November, activity peaked between November and February (most often January), and seasons concluded in March, April, or May. Seasons lasted from two to seven months in individual regions and six months statewide during the 2003–2004 and 2004–2005 seasons and seven months statewide during the 2005–2006 season.
Statewide Results
During the three seasons, 13,920 samples were collected overall from five regions across the state, and of these, 3,297 (23.7%) were positive. (See Table 1.) The month with the most laboratory test reports was December (3,501) although the greatest percentage of positive tests (33.8%) occurred during January. The highest percentage of positive RSV tests occurred during the 2005–2006 season (29.2%), with data for the other two seasons being fairly similar around 21% to 22%.
Table 1.
Percent Positive RSV Tests From All Sites Each RSV Seasona
| Month | 2003 –2004 | 2004 – 2005 | 2005 – 2006 | Total |
|---|---|---|---|---|
| September | 7.2 (9/125) | 1.9 (2/104) | 3.4 (2/58) | 4.5 (13/287) |
| October | 17.7 (72/407)b | 6.1 (16/263) | 9.6 (22/228) | 12.2 (110/898) |
| November | 26.3 (187/712) | 15.4 (68/441) | 27.2 (115/423) | 23.5 (370/1576) |
| December | 17.7 (304/1722) | 32.8 (248/757) | 40.8 (417/1022) | 27.7 (969/3501) |
| January | 29.9 (272/910) | 34.3 (309/902) | 36.9 (384/1042) | 33.8 (965/2854) |
| February | 31.7 (214/675) | 20.9 (147/703) | 26.6 (174/654) | 26.3 (535/2032) |
| March | 14.6 (88/603) | 11.4 (58/507) | 20 (90/449) | 15.1 (236/1559) |
| April | 4.3 (12/281) | 10.5 (18/171) | 15 (30/200) | 9.2 (60/652) |
| May | 5.3 (5/95) | 6.6 (8/122) | 11.9 (13/109) | 8.0 (26/326) |
| June | 0 (0/37) | 0 (0/9) | 5.3 (3/57) | 2.9 (3/103) |
| July | 10 (2/20) | 7.1 (1/14) | 10.5 (6/57) | 9.9 (9/91) |
| August | 0 (0/32) | 11 (1/9)c | ----- | 2.4 (1/41) |
| Total | 20.7 (1165/5619) | 21.9 (876/4002) | 29.2 (1256/4299) | 23.7 (3297/13920) |
Numbers in parentheses represent the number of positive samples/total number of tests.
Numbers in bold represent months in which >10% positive tests and more than 10 samples were reported.
Fewer than ten samples reported in this month.
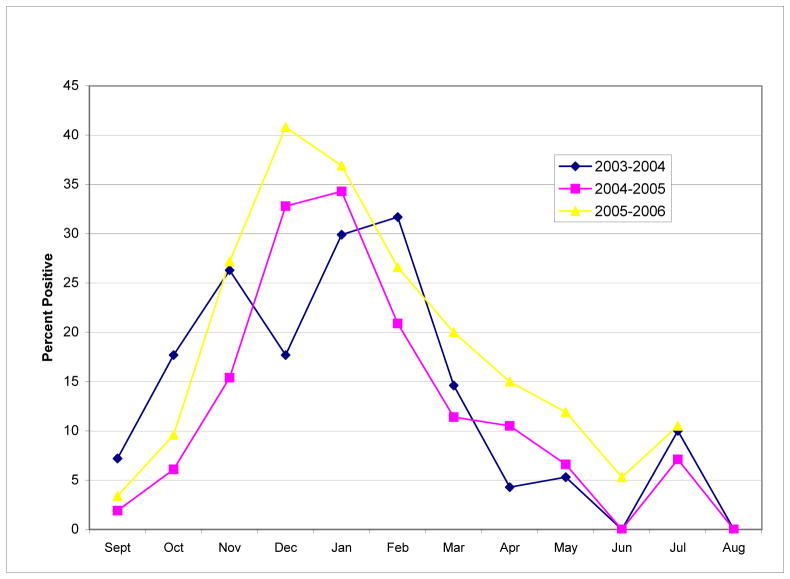
Monthly data tabulated from all regions monitored within the state revealed that the onset of the RSV season occurred either in October or November and ended in March, April, or May. During 2003–2004 and 2004–2005, the RSV season lasted six months and during 2005–2006 was seven months long, beginning in November and ending in May. (See Table 2, Figure 1.) Peak months for RSV activity differed annually and included February in the 2003–2004 season, January in the 2004–2005 season, and December in the 2005–2006 season. Overall statewide data yielded a six month RSV season from October through March with peak activity in January. (See Table 2.) Of interest, in all three time periods there was a noticeable increase in the percentage of positive RSV tests from June to July including two seasons in which threshold limits were exceeded. It is unclear whether this result is a function of small sample size or high circulating levels of RSV in local communities. Nevertheless, it indicates that RSV is present in North Carolina during months outside of the traditionally-reported season.
Figure 1.
RSV Test Data in North Carolina for Years 2003–2006
Greensboro/Winston-Salem
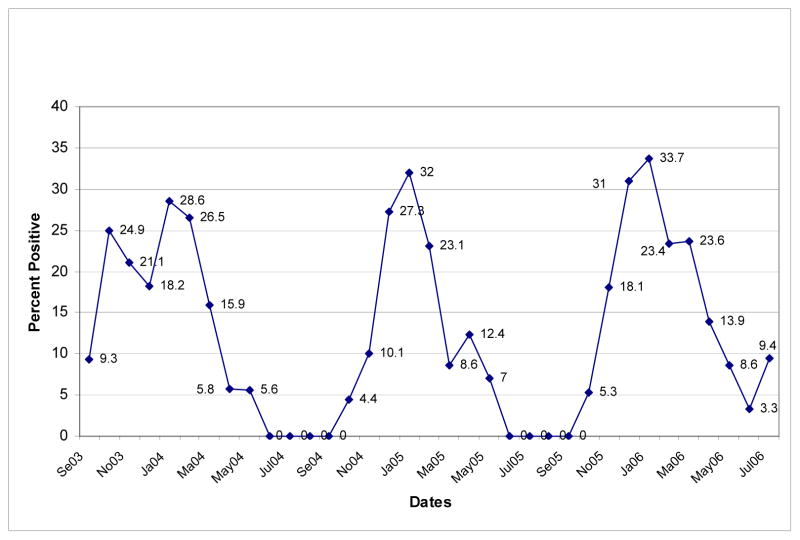
The four hospitals within this region—Forsyth Medical Center, High Point Regional Hospital, Moses H. Cone Memorial Hospital, and Baptist Medical Center—tested 6,438 samples from inpatients and outpatients, contributing 46.3% of the total. Combined results from all hospitals in this region revealed two RSV seasons lasting six months, beginning in either October or November and concluding in March or April with a peak in January and a third RSV season of four months with peak activity in January. (See Figure 2.)
Figure 2.
RSV Test Data for Greensboro/Winston-Salem Study Sites for Years 2003–2006
Wake Medical Center
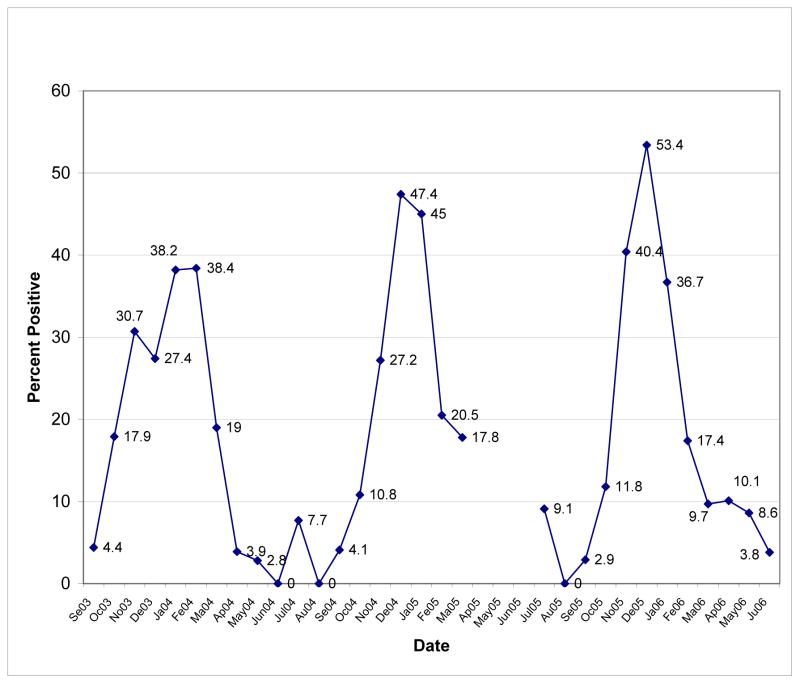
This facility tested 4,565 samples or 32.8% of the total. Results showed six month RSV seasons the first two years, starting in October and concluding in March. (See Figure 3.) Unfortunately, data for 2004–2005 were not reported for the months April through June; it is possible that this RSV season could have been longer than that reported. As with the first two seasons, the 2005–2006 season started in October but was of five months duration as the percent of positive RSV tests dipped to 9.7% in March. In the first season, the peak lasted essentially for two months, spanning January and February. In seasons two and three, peak activity occurred in December. (See Figure 3.)
Figure 3.
RSV Test Data for Wake Medical Center for Years 2003–2006
Pitt County Memorial Hospital
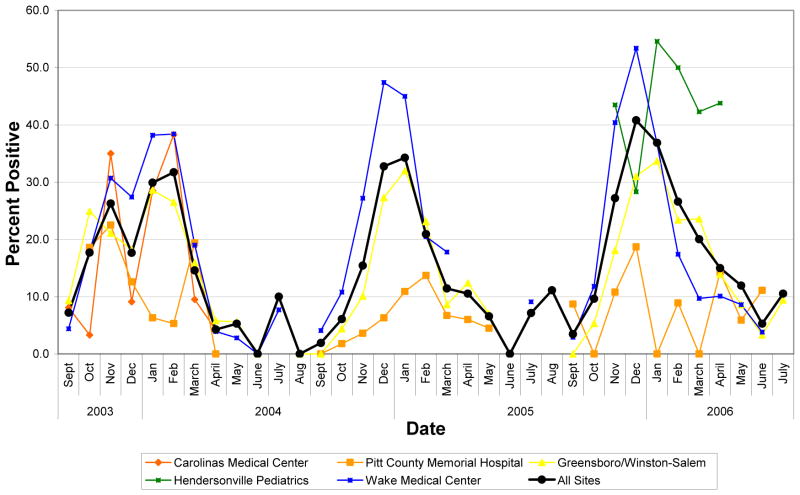
This hospital contributed 1,139 samples, 8.2% of the total, over two RSV seasons. Data from this site differs from the others in that in both time periods the RSV season was short, lasting three months in 2003–2004, starting in October, and only two months in 2004–2005, beginning in January. (See Figure 4.)
Figure 4.
RSV Test Data From Each Study Site in North Carolina for Years 2003–2006
Carolinas Medical Center and Hendersonville Pediatrics
Sites in these two regions each participated for one season and provided 1,778 or 12.8% of the samples. The Hendersonville practice contributed data for a full year from three locations and reported a seven-month RSV season during 2005–2006 including three months during which 50% or more of RSV tests were positive, including January, the peak month. (See Figure 4.) These findings should be interpreted cautiously as the high positivity rates are based on the fewest number of tests (i.e., 367) per all regions and could also reflect a very strict test screening process. It is difficult to draw conclusions for Carolinas Medical Center as there is no general pattern to their data, which was reported over a period of only eight months.
DISCUSSION
RSV epidemics occur yearly throughout the United States, and a select group of infants and young children are at increased risk for severe disease and hospitalization. Results of recent studies provide strong evidence that hospitalizations for RSV-related illness parallel RSV virology data reported in the community.14,15 At the time of our study, the CDC monitored RSV outbreaks through the NREVSS, which consisted of a limited number of laboratories in North Carolina. Data from participating laboratories in individual states that were part of the NREVSS were arbitrarily grouped into four distinct regions; North Carolina was part of the south reporting region. Analyses of regional data reported by the CDC indicated that the onset and duration of the RSV season varied substantially by year and location.10,14 Data were not available at the state level from the CDC.
Given the variability in the timing of RSV outbreaks, there has been an increase in RSV surveillance monitoring at the local level.15,17 At least 20 state health departments presently have some form of RSV surveillance program.12 Two states—Florida and Georgia—monitor RSV in several regions within their states and report results on a state-supported website.12,13 The RSV season in both of these states, and in Hawaii18,19 and Alaska,15 is longer than that reported for other parts of the country. The National Respiratory and Enteric Virus Surveillance System (NREVSS) has expanded the network of reporting laboratories, and data for the 2007–2008 RSV season is available for North Carolina (16 reporting laboratories) and other states on the CDC website. Several of the laboratories that are currently within the NREVSS network were included in our study. In contrast to the data now available on the CDC website with NREVSS-reported data for the entire state, our data is regional within North Carolina and statewide. Without knowing the total number of laboratory tests ordered and the number positive per site, it is possible that there could be inherent biases in the statewide data reported by NREVSS.
In North Carolina, RSV prophylaxis is typically given between November and March and longer if deemed medically necessary by the CDC or a local health department. For the 2007–2008 RSV season, North Carolina Medicaid, the main provider of palivizumab in the state, has approved palivizumab for no more than five monthly doses, while some insurance carriers will reimburse for six doses if that is supported by virology data. Our results suggest that the RSV season in North Carolina during the time of our study was at least six months long, including periods that extended outside of the months recommended by the American Academy of Pediatrics (AAP) for palivizumab administration.
There are inherent strengths and weaknesses to our study. We reported results of almost 14,000 RSV tests, which is a considerably larger study sample than that reported by other investigators.17 RSV activity was monitored from 5 regions within the state over a period of 3 seasons and included cities with the largest populations within the state. Thus, we were able to determine trends in RSV activity within some regions and for the state over time. We acknowledge the following limitations, which could have biased study outcomes. Results of antigen testing were used to determine the presence of RSV in communities as only 1 site reported results of viral cultures; nevertheless, numerous state health departments and the NREVSS use similar testing methodology. The decision to test individual patients for RSV was left to the discretion of each clinician involved in the study, and data were reported by month rather than by week. As can be expected, the overwhelming majority of data came from large metropolitan areas. The imbalance of data from centers could limit generalizations about statewide results.
Our data suggest that the RSV season in regions of North Carolina may be longer than the national average reported by the CDC. Additionally, RSV epidemics can persist during months that fall outside of those in which RSV prophylaxis is recommended by AAP for high-risk children. Guidelines on the administration of RSV prophylaxis should ideally be based on results of local RSV test data.
Acknowledgments
The authors and NC RSV Study Team acknowledge the efforts of Dr. Jay H. Bauman, Scientific and Technical Evaluation, of Pharmaceuticals, Inc., for his analysis and assistance with manuscript preparation.
Footnotes
Financial Disclosure: MedImmune, Inc., provided funding for data management. None of the participating principal investigators received any monetary compensation.
Contributor Information
David A. Wilfret, Research Fellow in the Department of Pediatrics at Duke University. He can be contacted at Duke University Medical Center, Department of Pediatrics, Division of Pediatric Blood and Marrow Transplantation, Durham, North Carolina 27710.
Brent T. Baker, Pediatrician for the North Carolina Respiratory Syncytial Virus Study Team.
Elizabeth Palavecino, Medical Microbiologist for the North Carolina Respiratory Syncytial Virus Study Team.
Cassandra Moran, Instructor in the Department of Pediatrics at Duke University Medical Center.
Daniel K. Benjamin, Jr., Associate Professor in the Department of Pediatrics at Duke University Medical Center.
References
- 1.Shay DK, Holman RC, Newman RD, et al. Bronchiolitis-associated hospitalizations among US children, 1980–1986. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 2.Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143:S127–S132. doi: 10.1067/s0022-3476(03)00510-9. [DOI] [PubMed] [Google Scholar]
- 3.McLaurin K, Leader S. Growing impact of RSV hospitalizations among infants in the US, 1997–2002. Paper presented at Pediatric Academic Societies’ Meeting; May 2005; Washington, DC. [Google Scholar]
- 4.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics. Respiratory Syncytial Virus. In: Pickering KL, editor. Redbook: 2006 Report of the Committee on Infectious Diseases. 27. Elk Grove Village: American Academy of Pediatrics; 2006. pp. 560–566. [Google Scholar]
- 6.Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 7.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 8.Feltes TF, Cabalka AK, Meissner C, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant heart disease. J Pediatr. 2003;143:532–540. doi: 10.1067/s0022-3476(03)00454-2. [DOI] [PubMed] [Google Scholar]
- 9.Connor EM Mpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 10.Mullins JA, Lamonte AC, Bresee JS, Anderson LJ. Substantial variability in community respiratory syncytial virus season timing. Pediatr Infect Dis J. 2003;22:857–862. doi: 10.1097/01.inf.0000090921.21313.d3. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Brief report: respiratory syncytial virus activity—United States, 2005–2006. MMWR Morb Mortal Wkly Rep. 2006;55:1277–1279. [PubMed] [Google Scholar]
- 12.Bauman J, Eggleston M, Oquist N, Malinoski F. Respiratory syncytial virus: seasonal data for regions of Florida and implications for palivizumab. South Med. 2007;100:669–676. doi: 10.1097/SMJ.0b013e318048589e. [DOI] [PubMed] [Google Scholar]
- 13.Eggleston M, Bauman J. Regional respiratory syncytial virus surveillance data for Georgia and implications for prophylactic administration of palivizumab. [Abstract.]. Paper presented at American Academy of Pediatrics National Conference and Exhibition; October 2007; San Francisco, CA. [Google Scholar]
- 14.Fergie J, Purcell K. Respiratory syncytial virus laboratory surveillance and hospitalization. Pediatr Infect Dis J. 2007;26(suppl):S51–S54. doi: 10.1097/INF.0b013e318157daae. [DOI] [PubMed] [Google Scholar]
- 15.Singleton RJ, Bruden D, Bulkow LR, Varney G, Butler JC. Decline in respiratory syncytial virus hospitalizations in a region with high hospitalization rates and prolonged season. Pediatr Infect Dis J. 2006;25:1116–1122. doi: 10.1097/01.inf.0000245104.26996.57. [DOI] [PubMed] [Google Scholar]
- 16.Ellis SE, Coffey CS, Mitchel EF, Dittus RS, Griffin MR. Influenza—and respiratory syncytial virus—associated morbidity and mortality in the nursing home population. J Am Geriatr Soc. 2003;51:761–767. doi: 10.1046/j.1365-2389.2003.51254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halstead DC, Jenkins SG. Continuous non-seasonal epidemic of respiratory syncytial virus infection in the southeast United States. South Med J. 1998;91:433–436. doi: 10.1097/00007611-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Reese PE, Marchette NJ. Respiratory syncytial virus infection and prevalence of subgroups A and B in Hawaii. J Clin Microbiol. 1991;29:2614–2615. doi: 10.1128/jcm.29.11.2614-2615.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Consensus Committee. Guidelines for prophylaxis for RSV infections in high-risk infants in Hawaii. [Accessed August 31, 2007];Hawaii Academy of American Pediatric Organization website. http://www.hawaiiaap.org/pdfs/ConsensusStatement31AUG2007.pdf.