Abstract
Background
Dabigatran, a direct thrombin inhibitor, has been shown to be more effective than warfarin in the prevention of stroke in patients with atrial fibrillation. Until recently, it lacked a reversal agent, and its contribution to the risk of transfusion in injured patients is unknown.
Objective
We sought to determine whether patients who sustain traumatic injuries while taking dabigatran receive more blood transfusions than matched patients taking warfarin, aspirin, clopidogrel, or controls.
Methods
This retrospective, single-center cohort consisted of injured patients who were taking dabigatran before admission to a major trauma center (January 2010–December 2013) who were compared with cohorts of patients taking warfarin, clopidogrel, or aspirin and a control group. The outcome was bleeding risk as measured by the use of blood products, with mortality as a secondary outcome. Outcomes were controlled for by age, sex, injury severity, and blunt mechanism.
Results
Thirty-eight patients were taking dabigatran. Compared with the general trauma population, patients taking dabigatran were more likely to be male, older, and to have higher injury severity. Patients taking dabigatran received transfusions (odds ratio [OR] 1.31 [95% confidence interval {CI} 0.56–3.04]), packed red blood cells (OR 1.43 [95% CI 0.54–3.77]), frozen plasma (OR 1.20 [95% CI 0.42–3.49]), and platelets (OR 2.01 [95% CI 0.63–6.37]) as often as matched controls. The mortality rate among patients on dabigatran was 12.5% (OR 1.51 [95% CI 0.39–5.89]) compared with 9.1% in matched controls. None of these results was statistically significant.
Conclusions
In this small study, injured patients taking dabigatran were transfused as often and had similar in-hospital mortality as matched controls who were not taking anticoagulants.
Keywords: epidemiology, dabigatran, hematology, mortality, transfusion, trauma
INTRODUCTION
Anticoagulants and antiplatelet agents are prescribed for a number of acute and chronic conditions related to thrombosis: the prevention and treatment of deep venous thrombosis (DVT) and pulmonaryembolism (PE), the prevention of myocardial infarction (MI), the prevention of thrombosis in patients with artificial heart valves, and long-term anticoagulation for the prevention of embolic stroke in patients with nonvalvular atrial fibrillation (AF) (1–4). Warfarin has been recommended as the outpatient anticoagulant of choice, yet bleeding complications and medication dosing errors are the cause of an estimated 43,000 emergency department (ED) visits annually, second only to insulin (5). Together with antiplatelet agents, such as aspirin and clopidogrel, warfarin accounts for 10.3% of all adverse drug events treated in EDs (5).
Several studies have compared dabigatran, a new direct thrombin inhibitor, with the standard of care (i.e., warfarin). Importantly, the Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) trial showed a lower incidence of embolic stroke among patients taking dabigatran than among those taking warfarin in the setting of nonvalvular AF (6–12). The 2013 guidelines from the American College of Chest Physicians and the American Heart Association state that dabigatran is preferred over dose-adjusted warfarin (grade 2B evidence) for patients with AF requiring oral anticoagulation (i.e., those with a CHADS2 score ≥2) (4).
Despite the current recommendations, dabigatran has some significant drawbacks. Unlike warfarin, it has no readily available test to measure its efficacy and no reversal agent during the study period. Under those conditions, combined with a 12- to 17-hour half-life, this drug poses a significant problem for specialists working in trauma centers and EDs, given the potential for massive, uncontrolled, and irreversible hemorrhage. Treatment protocols for bleeding patients taking dabigatran have been developed by the drug’s manufacturer (Boehringer Ingelheim, Ingelheim am Rhein, Germany), experts, and universities, yet none of these interventions is supported by studies (13–16). Case reports have described atraumatic subdural hemorrhage and increased or uncontrolled bleeding in patients taking dabigatran (17,18). Warnings about the use of dabigatran have been issued in Australia, Japan, and by the US Food and Drug Administration after a series of poor patient outcomes (19–22).
As more physicians follow the current guidelines, more patients will be placed on dabigatran. To date, few data regarding the potential side effects and bleeding complications of this new drug are available outside of industry-sponsored trials. A high-volume trauma center provides an opportunity to gauge the safety of dabigatran in a more clinically relevant context. The purpose of this study is to determine whether patients who sustain a traumatic injury while taking dabigatran receive more blood transfusions or are at greater risk for death than their peers taking warfarin, aspirin, or clopidogrel and those taking none of these drugs.
METHODS
Approval for human subject research was obtained through the institutional review board at the university with which the authors are affiliated. In this retrospective cohort study, subjects were selected from patients admitted to the level 1 trauma center affiliated with that university between January 1, 2010 and December 31, 2013 (the last year for which data are available). We extracted deidentified data from the trauma center’s patient registry, which contains prospectively collected data for patients transported and transferred to the trauma center and their laboratory values, demographic information, and outcome data. The list of medications patients were taking before admission was obtained from the medical records. Personnel at an affiliated research group provided the technical assistance for querying the registry.
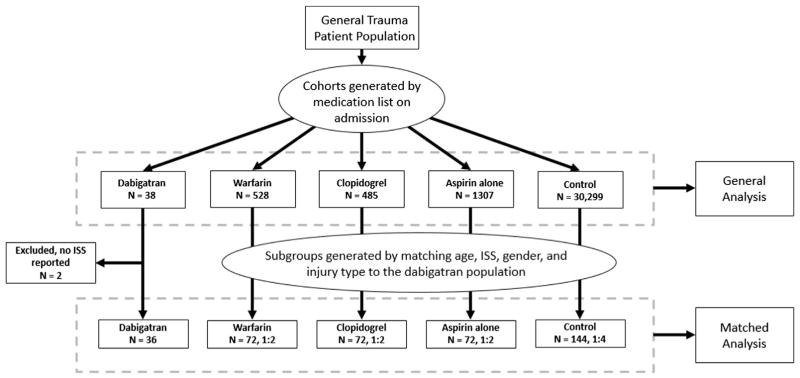
We generated 5 patient cohorts using deidentified medication records (Figure 1). All patients taking dabigatran with or without other drugs before admission were considered cases. Patients taking only aspirin, warfarin, or clopidogrel were considered members of their respective cohorts. Patients taking warfarin and aspirin, or clopidogrel and aspirin, were considered part of the warfarin and clopidogrel cohorts, respectively. Patients taking clopidogrel and warfarin were excluded from the analysis (32 subjects) because we felt the combined effect of the drugs would create a bias. A control group, made up of those reporting they did not take any of the study medications before injury, was also created. We collected data about these cohorts so that we could analyze their demographic information (i.e., age, sex, and race), injury patterns (i.e., Injury Severity Score [ISS], Abbreviated Injury Score [AIS], mechanism of injury [e.g., fall vs. motor vehicle crash]), initial laboratory data (i.e., prothrombin time [PT], partial thromboplastin time [PTT], international standardized ratio [INR], hemoglobin, platelets, creatinine, and lactate), and disposition (i.e., discharge to home, transfer to another institution, or death). Comparisons were made using t-tests for continuous data and chi-squared tests for categorical data, with an a priori alpha of 0.05. Subjects without a recorded ISS were excluded.
Figure 1.
Consort diagram showing the excluded groups and the number of subjects in each cohort. ISS = Injury Severity Score.
A matched case control analysis was then conducted using subgroups from the warfarin, clopidogrel, aspirin, and general cohorts. Matching groups were generated by reference to the dabigatran cases in regard to the following groups: 1) age ± 3 years; 2) sex; 3) blunt injury mechanism; and 4) ISS category. If multiple cases fulfilled the matching criteria, 1 was randomly selected to be matched with dabigatran in the final analysis. ISS categories were defined as mild (1–8), moderate (9–15), severe (16–24), and very severe (≥25). We matched 2 warfarin, clopidogrel, and aspirin cases to each dabigatran case (2:1). We matched 4 controls not taking any anticoagulant to each dabigatran case (4:1). These were the maximum matching ratios that could be achieved with our population and matching criteria. We then generated odds ratios (ORs) for the risks of transfusion and death for each cohort compared with those of the control population using conditional logistic regression. All statistics were calculated using SAS software (v 9.2; SAS Inc, Cary, NC).
RESULTS
Demographics
We retrieved 32,657 patient records that were generated during the study period (Table 1). The lack of documentation of the ISS was the reason for exclusion of 1010 subjects (3% of the total population) from the matched analysis, including 2 taking dabigatran. Our subject population was primarily male and young (mean age 41.4 ± 19.5 years). All of the groups taking anticoagulants or antiplatelet agents, especially the dabigatran group, were predominantly male, older, and white.
Table 1.
Demographics of All Trauma Patients Admitted Between 2010 and 2013
| Drug | n | Male (%) | Mean Age, y (SD) | White (%) | Black (%) | Hispanic (%) | Other/Unknown (%) |
|---|---|---|---|---|---|---|---|
| Dabigatran | 38 | 81.6 | 75.3 (8.8) | 92.1 | 2.6 | 0 | 5.3 |
| Warfarin | 528 | 54.0 | 74.5 (14.4) | 87.9 | 9.5 | 0.2 | 2.5 |
| Clopidogrel | 485 | 54.2 | 72.9 (13.2) | 84.1 | 12.2 | 0.2 | 3.5 |
| Aspirin | 1307 | 56.5 | 71.0 (14.0) | 80.6 | 14.6 | 0.2 | 4.6 |
| General | 30299 | 68.8 | 41.4 (19.5) | 57.6 | 34.3 | 2.3 | 5.8 |
SD = standard deviation.
The injury patterns are summarized in Table 2. The dabigatran cohort had relatively more patients with severe injuries (30.6%) than the other cohorts. The warfarin, clopidogrel, and aspirin groups had similar distributions of injuries. Minor injuries predominated in the control group (48.8%).
Table 2.
Abbreviated Injury Scores for All Trauma Patients Admitted between 2010 and 2013
| Dabigatran | Warfarin | Clopidogrel | Aspirin | Control | |
|---|---|---|---|---|---|
| AIS, n (%) | 36 (94.7) | 517 (97.9) | 479 (98.8) | 1279 (97.9) | 29,304 (96.7) |
| 1–8 | 9 (25.0) | 149 (28.8) | 127 (26.5) | 327 (25.6) | 14,254 (48.6) |
| 9–15 | 10 (27.8) | 169 (32.7) | 147 (30.7) | 435 (34.0) | 7429 (25.4) |
| 16–24 | 11 (30.6) | 115 (22.2) | 129 (26.9) | 313 (24.5) | 4081 (13.9) |
| ≥25 | 6 (16.7) | 84 (16.3) | 76 (15.9) | 204 (16.0) | 3540 (12.1) |
| Body region for maximum AIS, n (%) | |||||
| Brain | 14 (38.9) | 204 (40.2) | 199 (41.9) | 449 (35.4) | 7029 (25.0) |
| Face | 1 (2.8) | 42 (8.3) | 31 (6.5) | 86 (6.8) | 3923 (13.9) |
| Neck | 0 | 3 (0.6) | 4 (0.8) | 16 (1.3) | 402 (1.4) |
| Thorax | 9 (25.0) | 69 (13.6) | 63 (13.3) | 182 (14.4) | 4596 (16.3) |
| Abdomen | 0 | 16 (3.2) | 18 (3.8) | 24 (1.9) | 1704 (6.0) |
| Upper extremity | 3 (8.3) | 37 (7.3) | 28 (5.9) | 108 (8.5) | 2585 (9.2) |
| Lower extremity | 5 (13.9) | 67 (13.2) | 75 (15.8) | 210 (16.6) | 3696 (13.1) |
| Spine | 4 (11.1) | 59 (11.6) | 46 (9.7) | 165 (13.0) | 2802 (9.9) |
| Unspecified | 0 | 10 (2.0) | 11 (2.3) | 28 (2.2) | 1434 (5.1) |
| Injury type, n (%) | |||||
| Blunt | 38 (100) | 520 (98.5) | 469 (96.7) | 1254 (96.2) | 25,626 (84.6) |
| Fall | 29 | 383 | 343 | 836 | 7924 |
| Motor vehicle crash | 6 | 103 | 93 | 321 | 12,477 |
| Penetrating | 0 | 6 (1.1) | 11 (2.3) | 29 (2.2) | 3818 (12.6) |
| Gunshot wound | 3 | 6 | 9 | 1748 | |
| Stabbing | 2 | 4 | 15 | 1610 | |
| Other | 0 | 1 (0.3) | 4 (1.1) | 13 (1.5) | 640 (2.7) |
AIS = Abbreviated Injury Score.
In regard to the worst injury sustained (body region for maximum AIS), the patients taking dabigatran had mostly head injuries (38.9%), similar to the warfarin (40.2%), clopidogrel (41.9%), and aspirin (35.4%) groups; the percentages were not significantly different. Similarly, the majority of patients taking warfarin, clopidogrel, and aspirin sustained blunt injuries (from falls) with similar anatomic distributions of injuries. In contrast, the control patients contained larger portions of patients who were African American (34.3%) and who had sustained penetrating injuries (12.6%).
Matched Analysis
Patients who were taking dabigatran had significantly elevated PT (mean 17.3 ± 3.3 seconds), PTT (mean 43.0 ± 17.7 seconds), and INR (mean 1.4 ± 0.3) values compared with controls (Table 3). Patients taking warfarin, clopidogrel, and aspirin had similar alterations in their coagulation profiles. Those taking warfarin had a slightly higher creatinine concentration (mean 1.4 ± 0.9 mg/dL) and median duration of stay (mean 3.8 days; interquartile range 1.9–8.4 days). The cohorts had no significant differences in regard to disposition (i.e., roughly the same proportions of patients were discharged, transferred to rehabilitation, or transferred to a medical service after their injuries were treated).
Table 3.
Initial Laboratory Values and Final Disposition of Study Cohorts
| Initial Laboratory Values | Dabigatran | Warfarin | Clopidogrel | Aspirin | Control |
|---|---|---|---|---|---|
| PT, seconds (SD) | 17.3 (3.3)* | 25.6 (8.7)* | 13.9 (1.6)* | 14.1 (1.7)* | 15.1 (5.4) |
| PTT, seconds (SD) | 43.0 (17.7)* | 30.3 (10.8)* | 28.4 (5.5) | 29.6 (7.2) | 30.3 (10.8) |
| INR (SD) | 1.4 (0.3)* | 2.3 (1.1)* | 1.1 (0.2)* | 1.1 (0.2)* | 1.2 (0.6) |
| Hemoglobin, g/dL (SD) | 12.5 (2.3) | 11.8 (2.0) | 12.0 (2.2) | 12.5 (2.0) | 12.2 (2.1) |
| Platelets (x103/mm3) (SD) | 191.8 (79.3) | 199.3 (57.5) | 219.2 (95.0) | 219.7 (78.1) | 206.0 (66.0) |
| Creatinine, mg/dL (SD) | 1.1 (0.5) | 1.4 (0.9)* | 1.4 (0.9) | 1.3 (0.9) | 1.1 (0.4) |
| Lactate, mmol/L (SD) | 2.1 (1.0) | 2.2 (1.4) | 2.2 (1.4) | 2.3 (1.6) | 2.3 (1.4) |
| Disposition | 36 | 72 | 72 | 72 | 144 |
| Outpatient services/no services (%) | 20 (50.0) | 26 (36.1) | 37 (51.4) | 29 (40.3) | 70 (48.6) |
| Transferred to medical service† | 10 (27.8) | 34 (47.2) | 30 (41.7) | 36 (50.0) | 57 (39.6) |
| Other | 1 (2.8) | 0 | 0 | 0 | 0 |
| Rehab | 0 | 2 (2.8) | 1 (1.4) | 1 (1.4) | 5 (3.5) |
| Died | 5 (13.9) | 10 (13.9) | 4 (5.6) | 6 (8.3) | 12 (8.3) |
| Median duration of stay, days (IQR) | 2.2 (1.0–5.1) | 3.8 (1.9–8.4)* | 3.02 (1.1–5.2) | 4.0 (1.7–8.1)* | 2.2 (0.5–4.9) |
INR = international normalized ratio; IQR = interquartile range; PT = prothrombin time; PTT = partial thromboplastin time; SD = standard deviation.
Statistically significant (p < 0.05).
Patients transferred to a medical service after their acute traumatic injury was addressed.
The relative transfusion risk and mortality rates of trauma patients taking dabigatran are shown in Table 4. In our cohort, the transfusion rate in the dabigatran population (OR 1.3 [95% confidence interval {CI} 0.6–3.0]) was similar to matched controls, and less than those patients taking warfarin (OR 1.6 [95% CI 1.3–1.9]; p < 0.05), clopidogrel (OR 1.6 [95% CI 1.2–2.3]; p < 0.05), or aspirin (OR 1.3 [95% CI 1.0–1.6]; p < 0.05). Specifically, the dabigatran cohort received the same amount of packed red blood cells (PRBCs; OR 1.4 [95% CI 0.5–3.8]), fresh frozen plasma (FFP; OR 1.2 [95% CI 0.4–3.5]), and platelets (OR 2.0 [95% CI 0.6–6.4]) as controls (the 95% CI for all ORs included 1). There was no difference in the in-hospital mortality of those patients injured while taking dabigatran compared to matched controls (OR 1.5 [95% CI 0.4–5.9]).
Table 4.
Odds of Death and Transfusion within the Matched Analysis
| Dabigatran | Warfarin | Clopidogrel | Aspirin | Control | |
|---|---|---|---|---|---|
| Transfused | 9 | 40 | 28 | 24 | 29 |
| Not transfused | 27 | 32 | 44 | 48 | 115 |
| OR (95% CI) | 1.3 (0.6–3.0) | 1.6 (1.3–1.9)* | 1.6 (1.2–2.3)* | 1.3 (1.0–1.6)* | 1.0 |
| PRBC | 7 | 21 | 19 | 19 | 21 |
| OR (95% CI) | 1.4 (0.5–3.8) | 1.3 (1.1–1.5)* | 1.5 (1.0–2.2)* | 1.3 (1.0–1.6)* | 1.0 |
| FFP | 5 | 39 | 11 | 12 | 17 |
| OR (95% CI) | 1.2 (0.4–3.5) | 1.9 (1.5–2.3)* | 1.2 (0.8–1.8) | 1.1 (0.9–1.5) | 1.0 |
| PLT | 5 | 7 | 19 | 9 | 11 |
| OR (95% CI) | 2.0 (0.6–6.4) | 1.1 (0.8–1.4) | 2.1 (1.4–3.3)* | 1.2 (0.9–1.7) | 1.0 |
| Survived | 32 | 62 | 68 | 66 | 132 |
| Died | 4 | 10 | 4 | 6 | 12 |
| OR (95% CI) | 1.5 (0.4–5.9) | 1.2 (0.9–1.5) | 0.8 (0.4–1.5) | 1.0 (0.7–1.5) | 1.0 |
CI = confidence interval; FFP = fresh frozen plasma; OR = odds ratio; PLT = platelets; PRBC = packed red blood cells.
Statistically significant (p < 0.05).
DISCUSSION
In this retrospective study, we calculated the risk of bleeding and death in a cohort of trauma patients who were taking dabigatran before admission to a level 1 trauma center. Despite the increasing use of the newer anticoagulants in the community, the vast majority of patients admitted to our trauma center were not taking dabigatran. During the 4-year study period, medical records indicated that only 38 patients (0.1% of the initial study group) were taking this drug. Patients taking dabigatran were compared with 4 cohorts: 3 consisting of patients taking clopidogrel, aspirin, or warfarin and a control group not taking any of these medications. Surveillance of bleeding patterns in the RE-LY trial showed a decreased overall incidence of intracranial hemorrhage among patients taking dabigatran compared with those taking therapeutic dose-adjusted warfarin (0.3% vs. 0.74% per year, respectively) (23). However, the RE-LY trial did not focus on what trauma surgeons and emergency physicians worry about: uncontrolled hemorrhage after traumatic injury. Our study sought to examine patients with a spectrum of injuries similar to what is encountered at modern major trauma centers.
In the general analysis, we found that patients taking dabigatran were rare in our patient population. They were more often male, white, older, and had more serious injuries than the average trauma patient. One explanation for this finding could be that nonvalvular AF, the only United States Food and Drug Administration (FDA)–approved indication for dabigatran, predominantly affects older white patients. Another is that white patients might have greater access to health care and therefore can acquire expensive medications (a 1-month supply of dabigatran costs $256, while warfarin costs $6.84) (24). It is not clear why the vast majority of dabigatran patients were male. The patients taking dabigatran and the controls were similar in regard to disposition after treatment in the trauma center.
The initial laboratory values did not differ greatly among the cohorts, except that the dabigatran cohort had a prolonged PTT. Dabigatran has been shown to prolong the PTT 1.5 to 2 times the upper limit of normal, similar to our study. This finding suggests that the patients taking dabigatran were compliant with their medications and had therapeutic levels of the drug when they were admitted to the trauma center. Kidney injury leading to a toxic accumulation of dabigatran is a known cause of bleeding when taking dabigatran, yet patients taking dabigatran in our study were not found to have renal insufficiency (Cr 1.1 ± 0.5 mg/dL, not statistically different from controls; Table 3) (20–22). Lactate levels at admission did not differ significantly between groups, further indicating that the groups were evenly matched in terms of illness severity (lactate 2.1 ± 1.0 mmol/L, not statistically different from any other cohort) (Table 3).
Falls become an increasingly common mechanism of injury as we age. Patients with head injuries while taking anticoagulants or antiplatelet medications are at a higher risk for intracranial bleeds like subdural hematomas. Our statewide emergency medical services protocols funnel these patients into regional trauma centers with neurosurgery coverage, such as the institution where this study took place. Our institution has a standard transfusion protocol that is automatically initiated in patients with continuing hypotension and serious injuries or ongoing blood loss, but intracranial bleeds generally do not involve significant blood loss. Looking at the other patients in this study, those taking warfarin and clopidogrel were more likely to receive FFP and platelets (respectively) than matched controls. These patterns of blood product use are consistent with current guidelines for treating medication-induced coagulopathy in patients with traumatic intracranial bleeds. Our surgeons may have also transfused the patients taking dabigatran in an attempt to reverse the effects of the drug. Therefore, our findings may not translate other types of injuries, such as penetrating torso trauma, where massive blood loss is a possibility.
In August 2015, the results of the Study of the REVERSal Effects of Idarucizumab on Active Dabigatran were published, showing that idarucizumab was able to decrease the ecarin clotting time, dilute thrombin time, and free dabigatran levels in patients with serious bleeding or who required an urgent procedure (25). The FDA shortly thereafter approved idarucizumab for the reversal of dabigatran in patients with life-threatening bleeding or procedures (26). The current study is therefore one of the few reports describing the risk of uncontrolled hemorrhage and mortality in injured patients taking dabigatran before a reversal agent was available.
Future studies of the implications of dabigatran for trauma care should be prospective. Dabigatran was a rare medication in our cohort from a single medical facility; a multi-institutional study would provide greater statistical power and greater generalizability. A multisite study would help to diminish the effect of being a referral center, giving a broader range of patient injuries and further increasing study generalizability. These questions are of critical importance because the prevalence of AF is increasing, and these medications might supplant warfarin as the outpatient oral anticoagulant of choice.
Limitations
This retrospective study is subject to selection bias, and although we tried to control for as many variables as possible, unknown biases might be present in the data. Our investigation is a single-center review and represents only our local patient population. We relied on hospital records to tell us which medication(s) a patient was taking before injury, because testing for dabigatran level or activity is not available at our center. However, patients recorded as taking dabigatran in our study had elevations in their PT and PTT, consistent with dabigatran use. Despite a large annual patient census, our study sample is small, which limits the power of our analysis (post-hoc β 0.122 for transfusion risk and β 0.159 for death) and the generalizability of our results. As more information comes to light about the efficacy and risks of dabigatran, it could be prescribed more often and therefore will be encountered in seriously injured patients brought to trauma centers for stabilization. Trauma surgeons and emergency physicians need to know the risks that accompany this drug so that they can make informed decisions about treatment strategies.
SUMMARY
In our population, patients taking dabigatran were predominantly white, male, and older, and had more severe injuries than general trauma patients. In this small study, patients taking dabigatran were given transfusions of blood products at a similar rate and had a similar mortality when compared to controls matched on age, sex, injury severity, and mechanism of injury.
ARTICLE SUMMARY.
1. Why is this topic important?
Physicians treating patients with acute injures and primary care physicians need to know the risks posed by novel anticoagulants, such as dabigatran.
2. What does this study attempt to show?
This study attempts to show how dabigatran, a novel oral anticoagulant, alters the risk of transfusion and mortality in acutely injured patients, compared to patients matched on age, sex, and injury severity.
3. What are the key findings?
In this small matched analysis, injured patients on dabigatran had a similar mortality and transfusion rate when compared to peers who were not taking dabigatran.
4. How is patient care impacted?
This study attempts to quantify the risks posed to injured patients who were taking dabigatran before a reversal agent was available.
Acknowledgments
Supported by a departmental resident research grant. This work did not receive any funding commercial funding.
Footnotes
Reprints are not available from the authors.
References
- 1.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition) Chest. 2008;133(6 suppl):381S–453. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 2.Goldhaber SZ, Fanikos J. Prevention of deep vein thrombosis and pulmonary embolism. Circulation. 2004;110:e445–7. doi: 10.1161/01.CIR.0000145141.70264.C5. [DOI] [PubMed] [Google Scholar]
- 3.Kushner FG, Hand M, Smith SC, Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 Guildine and 2007 Focused Update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:2271–306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 4.Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:1144–50. doi: 10.1161/CIR.0b013e31820f14c0. [DOI] [PubMed] [Google Scholar]
- 5.Budnitz DS, Pollock DA, Weidenbach KN, et al. National Surveillance of emergency department visits for outpatient adverse drug events. JAMA. 2006;296:1858–66. doi: 10.1001/jama.296.15.1858. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949–56. doi: 10.1016/S0140-6736(07)61445-7. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson BI, Dahl OE, Büller HR, et al. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost. 2005;3:103–11. doi: 10.1111/j.1538-7836.2004.01100.x. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost. 2007;5:2178–85. doi: 10.1111/j.1538-7836.2007.02748.x. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson BI, Dahl OE, Huo MH, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*): a randomised, double-blind, non-inferiority trial. Thromb Haemost. 2011;105:721–9. doi: 10.1160/TH10-10-0679. [DOI] [PubMed] [Google Scholar]
- 10.Ezekowitz MD, Reilly PA, Nehmiz G, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (ETRO Study) Am J Cardiol. 2007;100:1419–26. doi: 10.1016/j.amjcard.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 11.Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–52. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 12.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 13.Dabigatran (pradaxa) [package insert] Ingelheim am Rhein, Germany: Boehringer-Ingelheim; 2015. [Google Scholar]
- 14.Crowther MA, Warkentin TE. Managing bleeding in anticoagulated patients with a focus on novel therapeutic agents. J Thromb Haemost. 2009;7(suppl 1):107–10. doi: 10.1111/j.1538-7836.2009.03429.x. [DOI] [PubMed] [Google Scholar]
- 15.University of Washington website. [Accessed November 5, 2015];Guidelines for reversal of anticoagulants. Available at: http://depts.washington.edu/anticoag/home/
- 16.University of North Carolina website. [Accessed November 5, 2015];Healthcare anticoagulation reversal guidelines. Available at: https://www.med.unc.edu/emergmed/files/emergent-anticoagulation-reversal-in-the-ed.
- 17.Garber ST, Sivakumar W, Schmidt RH. Neurosurgical complications of direct thrombin inhibitors—catastrophic hemorrhage after mild traumatic brain injury in a patient receiving dabigatran. J Neurosurg. 2012;116:1093–6. doi: 10.3171/2012.2.JNS112132. [DOI] [PubMed] [Google Scholar]
- 18.Cotton BA, McCarthy JJ, Holcomb JB. Acutely injured patients on dabigatran. N Engl J Med. 2011;365:2039–40. doi: 10.1056/NEJMc1111095. [DOI] [PubMed] [Google Scholar]
- 19.NPS Medicinewise website. [Accessed November 5, 2015];Dabigatran (pradaxa) safety update: assess and monintor kidney function. Available at: http://www.nps.org.au/publications/health-professional/nps-radar/2011/december-2011/brief-item-dabigatran.
- 20.Australian Department of Health and Aging, Therpeutic Goods Administration website. [Accessed November 5, 2015];Dabigatran (pradaxa) and risk of bleeding: information for health professionals. Available at: www.tga.gov.au/alert/dabigatran-pradaxa-and-risk-bleeding-information-healthprofessionals.
- 21.Japanese Pharmaceutical and Food Safety Bureau, Pharmaceuticals and Medical Devices Agency website. [Accessed November 16, 2015];Severe hemorrhages in patients treated with an anticoagulant ‘‘prazaxa capsules’’. Available at: www.pmda.go.jp/english/service/pdf/mhlw/Prazaxa_capsules.pdf.
- 22.US Food and Drug Administration website. [Accessed March 6, 2015];Pradaxa (dabigatran etexilate mesylate): drug safety communication - safety review of post market reports of serious bleeding events. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm250657.htm.
- 23.Hart RG, Diener HC, Yang S, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke. 2012;43:1511–7. doi: 10.1161/STROKEAHA.112.650614. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Medicare and Medicaid Services website. [Accessed November 5, 2015];National average drug retail price. Available at: http://www.medicaid.gov/medicaid-chip-program-information/by-topics/benefits/prescription-drugs/survey-of-retail-prices.html.
- 25.Pollack CV, Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373:511–20. doi: 10.1056/NEJMoa1502000. [DOI] [PubMed] [Google Scholar]
- 26.US Food and Drug Administration website. [Accessed March 29, 2016];Idarucizumab (prax-bind) Available at: http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm467396.htm.