Abstract
Background
We examined discharges for infective endocarditis (IE) at an academic teaching hospital for over 10 years to evaluate if an increase in hospitalizations for IE and increase in hepatitis C virus (HCV) in patients with IE could predict a new epidemic of injection drug use (IDU).
Materials and Methods
Retrospective medical record review of discharged patients with the diagnosis of IE as defined by the modified Duke criteria. Student’s t test, chi-squared test and Fisher’s exact test were used to calculate P values.
Results
There were 542 discharges among 392 unique patients with IE and 104 patients were readmitted 2–7 times. Of the total discharges, 367 (67.7%) were not screened for HCV, and of those tested, 86 (49.1%) were HCV+; 404 (74.5%) were not screened for HIV and of those tested, 28 (20.3%) were HIV+. Patients who self-identify as a person who injects drugs were more likely to be tested for HCV, 75 (69.4%) versus 12 (31.5%, P < 0.0001), and for HIV, 72 (66.6%) versus 13 (34.2%, P < 0.0001) compared with those who self-report no IDU. Those with a positive result for opiate or heroin toxicology test were more likely to be screened for HCV, 70 (66%) versus 22 (44.8%, P < 0.0001), and for HIV, 66 (62.2%) versus 25 (51%, P < 0.0001) than those with negative result for toxicology test. Over this period, there was a 2-fold increase in IE cases, a 3-fold increase in HCV antibody prevalence and a 6-fold increase in opiate toxicology screens showing positive result, but no increase in HIV.
Conclusions
Although IDU is a known risk factor for IE, the observation of a sharp increase in IE cases may signal a new epidemic of IDU and HCV.
Key Indexing Terms: Injection drug use, Human immunodeficiency virus, Hepatitis C virus, Endocarditis
BACKGROUND
It is well known that injection drug use (IDU) is associated with infective endocarditis (IE)1 because of using unsterile injection equipment and unsterile injection technique. The overall incidence of acute bacterial endocarditis is significantly higher among persons who inject drugs (PWID) compared to the non-IDU population, 150–2,000 cases per 100,000 person-years versus 1.7–6.2 cases per 100,000 person-years.2–4 Previous studies demonstrating an increase in the rate of endocarditis, differences in the causative agents, affected valves and complications have all been directly linked to the increasing numbers of IDU-associated episodes of IE.2,5,6 PWID also have an increased frequency of recurrent endocarditis.7 It is estimated that PWID at some point during their lifetime comprise 2.6% of the U.S. population; thus, a significant number of individuals are then at risk for IE, and infection with hepatitis C virus (HCV), hepatitis B virus or human immunodeficiency virus (HIV) or with all these.8 In contrast to non-IDU–associated IE, although PWID have typically been described as presenting with right-sided endocarditis, left-sided IE is not unusual.9
IDU is also a major risk factor for blood-borne viral infections, such as HIV, as well as the most common risk for HCV.10,11 The Centers for Disease Control (CDC) tracks surveillance only for acute HCV; whereas the exact prevalence of chronic HCV in the United States is unknown, it is estimated at 3.4–5.3 million. HCV accounts for approximately 60–70% of all chronic hepatitis cases and up to 50% of cases of cirrhosis in the United States, and is the most important cause of chronic liver disease, end-stage liver disease, hepato-cellular carcinoma and liver transplantation.11,12 An estimated 4.1 million Americans (1.65%), have antibody to HCV (anti-HCV), indicating either ongoing or previous infection.13 Chronic HCV causes estimated 16,627–19,659 deaths annually in the United States.13 It is estimated that at least 20% of patients with chronic HCV would develop cirrhosis approximately 20–30 years after infection and 5% would die of HCV-related liver disease.10,14 Disease progression is faster among those with both HIV and chronic HCV, with progression to cirrhosis occurring approximately 10–15 years after infection.10 Unlike HIV, HCV can remain infectious in a used syringe, filter, cooker and injection equipment surfaces for 2–9 weeks depending on factors such as temperature and humidity.15,16
The CDC estimates that more than 1 million people are living with HIV in the United States but 12.8% are unaware of their infection.17 In 2010, PWID represented 8% of new HIV infections and in 2011, 15% of those living with HIV.17,18 It is difficult to estimate the precise transmission of HIV through IDU as it is dependent on the caliber of the needle, the HIV burden of the infected individual and the blood residue in a cooker, filter or the syringe hub itself.
At the University of Cincinnati Medical Center, we began seeing an increase in the number of IE cases in the mid-2000s, including patients transferred from outlying community hospitals, many of whom reported opioid IDU, which had not previously been a problem in southwest Ohio. We decided to examine all hospitalizations for IE at a tertiary care teaching hospital over a 10-year span to evaluate whether an increase in hospitalizations for IE could be verified and whether it was associated with an evidence of opioid IDU. We looked at infection with HCV and HIV, a history of IDU and toxicology screen results to see if an increase in IE cases and blood-borne chronic viral infections in patients with IE could be used to predict a new epidemic of IDU.
METHODS
This study was conducted at the University of Cincinnati Medical Center, a 605-bed tertiary teaching hospital with more than 31,600 yearly admissions. We performed a retrospective medical record review of all discharged patients (n = 660) with an ICD-9 diagnosis of IE from January 1, 1999 to December 31, 2009. Each medical record was reviewed for notes (emergency department, admitting team, infectious diseases consultation [IDC] and discharge summary), echocardiography results and all laboratory and microbiologic results. Only cases that met the modified Duke Criteria for IE were included in the final analysis.19 We also reviewed whether screening for HCV antibody and HIV was performed during the current hospitalization for IE, or whether it was documented from prior evaluation. P values were calculated using the Student’s t test; chi-squared test and Fisher’s exact test where appropriate. We also were able to obtain the hospital charges for most cases.
We used data from the Hamilton County Coroner’s crime laboratory that serves all police departments in the greater Cincinnati area. All confiscated substances and drug-related items at a crime scene or arrest were tested for controlled and illegal substances. Specifically, “heroin confiscations” are drugs seized by law enforcement, which test positive for heroin in the crime laboratory. These data can be used as a proxy estimate of the heroin supply in greater Cincinnati from 2000–2010.20
This study received institutional review board approval from the University of Cincinnati.
RESULTS
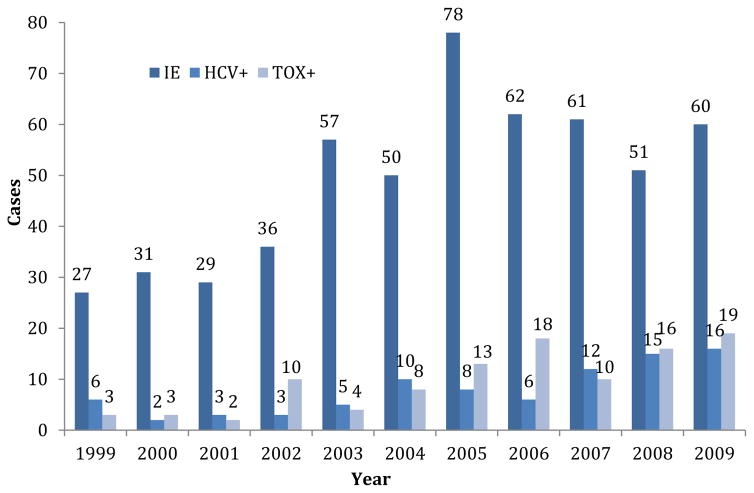
There were 542 discharges among 392 unique patients with IE and 104 patients were readmitted 2–7 times for suspected IE. The number of discharges for IE associated with HCV antibody prevalence and positive result for opioid toxicology screens increased over the 10-year period (Figure 1). Most patients were male (308, 56.8%) and white (319, 58.9%), with a mean age of 50.6 years (standard deviation [SD] = 15.8 years) and a similar median age of 50 years. Overall, there was a 20% (111 patients) in-hospital mortality rate and the mean length of stay was 14.6 days (SD = ±12 days). Most had public insurance, traditional or emergency Medicaid or Medicare (414, 76.4%), whereas 18 (3.3%) had no insurance (Table 1). However, these data do not represent the true proportion without insurance on admission, as hospital social workers aggressively pursue traditional or emergency Medicaid as soon as possible for those who may qualify. The 18 patients without insurance either died too quickly or were transferred to other hospitals before qualifying for either traditional or emergency Medicaid.
FIGURE 1.
The number of admission for infective endocarditis (IE), number testing positive for HCV, and number with a positive result for opiate or heroin toxicology test from 1999–2009. HCV+, those testing positive for hepatitis C virus antibody; IE, infected endocarditis; Tox+, those with positive result for opiate or heroin toxicology tests.
TABLE 1.
Demographics of 542 admissions for infective endocarditis.
| Variable | Number (%) |
|---|---|
| Male | 308 (56.8%) |
| Race | |
| White | 319 (58.8%) |
| Black | 203 (37.5%) |
| Other | 20 (3.7%) |
| Mean age (SD), years | 50.6 (15.8) |
| Insurance | |
| Public | 414 (76.4%) |
| Private | 110 (20.3%) |
| None | 18 (3.3%) |
| Complicationsa | 369 (68%) |
| Mean length of stay (SD), days | 14.6 (12) |
| In-hospital mortality | 111 (20%) |
SD, standard deviation.
Complications include acute kidney injury, septic pulmonary emboli, pulmonary embolism, Clostridium difficile colitis, septic renal infarct, splenic infarct, central nervous system emboli, PEG tube placement, tracheostomy, limb osteomyelitis, vertebral osteomyelitis, epidural abscess, mitral valve replacement, aortic valve replacement, psoas abscess, line infection, catheter-associated UTI, decubitus ulcer, atrial fibrillation, septic emboli of digit, purpura fulminans, septic joint, health-care-associated pneumonia, ventilator-associated pneumonia, splenic abscess, heart failure, adrenal emboli, deep vein thrombosis, heparin-associated thrombocytopenia, renal failure leading to dialysis, ileus, graft repair with replacement, hemodialysis, thrombophlebitis, pneumothorax, anoxic brain injury, liver abscess, PEA, pleural effusion, first- and third-degree heart block, below the knee amputation, above the knee amputation, pacemaker explant, limb abscess, tricuspid valve replacement, gangrene of the hand or foot, port explant, vasculitis, drug rash, GI bleed, intubation, paraplegia, septic ocular emboli, digit amputation, splenectomy and trunk abscess.
Only 138 (25.4%) discharges were screened for HIV, and 28 (20.2% of those screened) were infected with HIV. For HCV, 175 (32.2%) discharges were screened and 86 (49% of those screened) had a positive result for antibody test (Table 2). Among those with positive result versus negative result for opioid toxicology screens, 70 (66%) versus 22 (45%), P = 0.01, were screened for HCV, whereas screening for HIV was similar between these 2 groups 66 (62.2%) and 25 (51%), P = 0.22 (Table 3). Of those who self-reported IDU versus those who self-reported no IDU at their initial interview, 75 (69%) versus 12 (31.5%), P < 0.001, were screened for HCV antibody and 72 (66.6%) versus 15 (39.5%), P = 0.004, were screened for HIV.
TABLE 2.
HIV, HCV and opiate toxicology screening among 542 IE admissions.
| Variable | Number (%) |
|---|---|
| HIV status evaluated | 138 (25%) |
| Positive | 28 (5%) |
| Negative | 110 (20%) |
| Not tested | 404 (75%) |
| HCV status evaluated | 175 (32%) |
| Positive | 86 (16%) |
| Negative | 89 (16%) |
| Not tested | 367 (68%) |
| Toxicology screen performed | 155 (28%) |
| Positive | 106 (19%) |
| Negative | 49 (9%) |
| Not tested | 387 (72%) |
HCV, hepatitis C virus; HIV, human immunodeficiency virus.
TABLE 3.
Screening for HCV and HIV according to positive opiate or heroin toxicology testing and self-reported IDU.
| Tox positivea | Tox negativeb | Tox not testedc | P Valued | Self-reported IDU | Self-reported no IDU | Not asked about IDU | P Valuee | |
|---|---|---|---|---|---|---|---|---|
| Total | 106 | 49 | 387 | 108 | 38 | 396 | ||
| Screened for HCV | 70 (66%) | 22 (45%) | 83 (21%) | 0.01 | 75 (69%) | 12 (31.5%) | 88 (22.2%) | <0.001 |
| HCV positive | 50 (71%) | 6 (27%) | 30 (36%) | <0.001 | 53 (70.6%) | 3 (33.3%) | 30 (34%) | <0.001 |
| Screened for HIV | 66 (62.2%) | 25 (51%) | 47 (12%) | 0.22 | 72 (66.6%) | 15 (39.5) | 53 (13.3%) | 0.004 |
| HIV positive | 12 (18.8%) | 4 (16%) | 12 (25.5%) | 0.78 | 13 (18%) | 4 (26.6%) | 11 (20.7) | 1.0 |
HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, injection drug use; Tox, opiate or heroin toxicology test.
Discharges with a positive result for opiate or heroin toxicology test on admission.
Discharges with a negative result for opiate or heroin toxicology test on admission.
Discharges where no toxicology testing was performed.
P value between those with positive result for opiate or heroin toxicology test and those with a negative result for toxicology test.
P value between those who self-report IDU and those who report no IDU during admission.
Among the 138 discharges screened for HIV, 15 (54%) of the HIV-positive and 82 (75%) of the HIV-negative discharges were also screened for HCV (P = 0.05), and of those HIV-positive discharges screened for HCV antibody, 7 (47%) were positive (Table 4). Of the 12 (43%) HIV discharges with a positive result for opioid toxicology test, most (9, 75%) were screened for HCV. Comparing the known HIV-positive and HIV-negative discharges, HIV-negatives had more complications, 88 (80%) versus16 (57%), P = 0.03 as well as a longer hospital stay, 13.7 days versus 10.8 days, P = 0.05.
TABLE 4.
Outcomes for known HIV-positive and HIV-negative discharges.
| HIV+ | HIV− | Total | P Value | |
|---|---|---|---|---|
| Admissions | 28 | 110 | 542 | |
| Screened for HCV | 15 (54%) | 82 (75%) | 175 (32%) | 0.05 |
| HCV+ | 7 (25%) | 46 (42%) | 86 (16%) | 0.15 |
| Positive opiate or heroin tox screen | 12 (43%) | 54 (49%) | 106 (20%) | 0.53 |
| Screened for HCV | 9 (32%) | 44 (40%) | 70 (13%) | 0.76 |
| Complicationsa | 16 (57%) | 88 (80%) | 369 (68%) | 0.03 |
| Mean length of stay (days) | 10.8 | 13.7 | 14.6 | 0.05 |
| HCV+ | 9.3 | 13.4 | 12.4 | 0.08 |
| In-hospital mortality | 5 (18%) | 11 (10%) | 111 (20%) | 0.31 |
| HCV+ | 2 (7%) | 3 (3%) | 11 (10%) | 0.12 |
HCV, hepatitis C virus; HIV, human immunodeficiency virus; tox, opiate or heroin toxicology test.
Same as for Table 1.
The 338 discharges that had an IDC were more likely to be screened for both HCV, 124 (37%) versus 51 (25%) without IDC, P < 0.01, and for HIV, 105 (31%) versus 33 (19%), P < 0.01 (Table 5). Among PWID who self-reported active IDU, IDC did not affect the screening rate for HCV; however, IDC did affect the screening rate for HIV among PWID, 53 (71%) with IDC compared with 13 (42%) for those with no IDC, P < 0.01. IDC also was associated with a statistically significant difference in mean length of stay, 16 days (SD = ±2 days) compared to 12.3 days (SD = ±11.7 days) among patients without IDC, P < 0.01. Complication rates were higher among those with IDC, 242 (72%) compared to those with no IDC, 127 (61%), P = 0.03. In-hospital mortality approached statistical significance, with 60 deaths among patients receiving IDC (17.7%) versus 51 deaths (25%) in patients without IDC, P = 0.055.
TABLE 5.
Effect of infectious disease consultation (IDC) and no IDC.
| IDC | No IDC | P Value | |
|---|---|---|---|
| Total | 338 (62%) | 204 (38%) | |
| Screened for HCV | 124 (37%) | 51 (25%) | <0.01 |
| HCV+ | 66 (53%) | 20 (39%) | 0.128 |
| Screened for HIV | 105 (31%) | 33 (19%) | <0.01 |
| Positive opiate or heroin tox screen | 75 (70.7%) | 31 (63.2%) | 0.454 |
| Screened for HCV | 54 (72%) | 16 (52%) | 0.073 |
| Screened for HIV | 53 (71%) | 13 (42%) | 0.01 |
| Complicationsa | 242 (72%) | 127 (62%) | 0.03 |
| Mean length of stay, (SD) days | 16 (12) | 12.3 (11.7) | <0.01 |
| In-hospital mortality | 60 (17.7%) | 51 (25%) | 0.055 |
HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDC, infectious disease consultation; SD, standard deviation; tox, opiate or heroin toxicology test.
Same as for Table 1.
Echocardiography revealed more frequent involvement of left-sided valves overall, n = 272 (50%), compared with right-sided valves, n = 55 (10%). Left-sided involvement was also more common than right-sided disease among patients with a self-reported history of IDU and with a positive result for opioid toxicology screen (n = 106), with 45 (42%) having left-sided involvement compared to 21 (19.8%) with right-sided disease, respectively. However, tricuspid valve disease was seen more often among self-reported active IDU compared to the overall IE population, 20 (18.8%) versus 28 (6.4%).
Staphylococcus aureus was the single most common causative organism in 215 (39.7%) IE episodes. Interestingly, 64 (11.8%) episodes were polymicrobial and 71 (13.1%) were culture negative.
Hospital charges for 487 (90%) of the hospitalizations were obtained. Through an indigent tax levy that only covers hospital costs, Hamilton County, Ohio paid a mean of $55,722 (range: $5,970–$261,445) for 8 hospitalizations. Medicare or Medicaid paid a mean of $95,799 (range $971–$704,936) for a total of 368 hospitalizations, adjusted for inflation (2015). These figures underestimate the true cost of treating IE for the following reasons: (1) we were only able to receive the cost data by insurer and not by year (2009 was used as the base year), (2) these are only hospital costs and do not include physician fees and (3) patients were transferred to skilled nursing facilities as soon as they became medically appropriate, after a mean length of stay of 14.6 days (range: 1–109) to complete a typical minimum 6-week course of IV antibiotics.
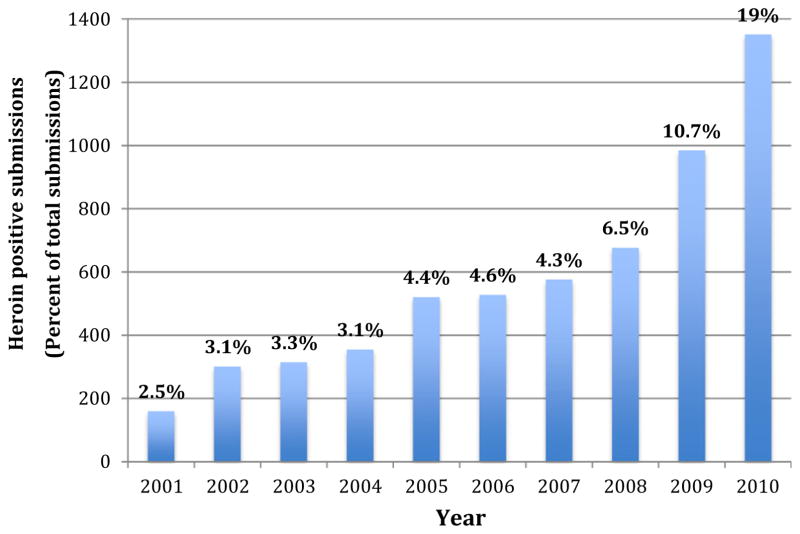
Data from the Hamilton County Coroner’s crime laboratory from 2000–2010 also identified a clear trend in the number of heroin confiscations in metropolitan Cincinnati.20 In 2000, items testing positive for heroin made up 3.7% of the total illicit drugs tested; by 2010 there was a sharp 6-fold increase to 19% of all items testing positive for heroin (Figure 2).
FIGURE 2.
Hamilton County Coroner’s Crime Laboratory Heroin Submissions 2001–2010.
CONCLUSIONS
Similar to other published studies, our IE cases over a 10-year period from 1999–2009 occurred predominantly in white men.9 The mean age of those with IE was slightly lower than other published data, which may reflect an increase in younger PWID in metropolitan Cincinnati during this time. Overall, there was a 2-fold increase in admissions for IE, a 3-fold increase in HCV antibody prevalence and a 6-fold increase in positive result for opioid toxicology screens, but no appreciable increase in HIV infections. This may reflect the greater infectiousness of hepatitis C compared with HIV.21 It may also reflect a lower HIV community viral load among young PWID in this area, or the fact that HIV has not yet entered this population. Data from the Hamilton County Coroner’s Office crime laboratory indicates a 6-fold increase in the heroin supply in greater Cincinnati during this period. Moreover, values for HCV and HIV prevalence as well as self-reporting of IDU are likely to have been significantly underestimated, as only a minority were screened for HCV (32%), HIV (25%) and opioid toxicology (20%) in this retrospective study. The lower morbidity rate seen among the HIV-infected IE cases may be because of the fact that HIV-infected patients were more likely to be seen by the IDC service, may have already had established access to healthcare or despite being HIV-infected had an overall better health status or because of all these reasons.
The increased disease severity and complexity of HIV-negative patients for whom IDC was sought is postulated to account for the failure to demonstrate decreases in either the rate of complications or length of stay. However, those with IDC did have improved survival, which suggests that infectious disease physicians should be involved in the care of patients with IE.
Traditionally, right-sided IE predominates among PWID.22 The pathogenesis is not well understood and is most likely multifactorial.23 It is thought that injection diluents can cause direct mechanical damage, causing intimal damage and thrombus formation.23–25 The increase of left-sided IE could be explained by the availability of a pure heroin that is less likely to have been adulterated with irritants such as talc, and by the availability of “black tar” heroin from Mexico, potent heroin that is considerably less refined with more foreign plant matter. It is also thought the injection of foreign antigens leads to antibody production, which in turn causes immune complex deposition on valvular surfaces.24 If PWID cannot clear deposits owing to dysfunctional immune cells, this could facilitate bacterial aggregation and adhesion on right-sided valves.23 Published data report variable involvement of right-sided valves in PWID, ranging from 30–70%. In our population, left-sided IE was more common in PWID. This has been reported in 2 other studies, and a recent study evaluating 1,234 IE episodes over 19 years also noted a substantial increase in left-sided IE.26,27 Reliance on echocardiography may be a factor in the diagnosis of the involved valve(s), as a series of PWID with IE who underwent autopsy noted that 40% of cases were left-sided and 16% had both bilateral diseases.28
It has been difficult to estimate the prevalence of IDU in a community. Most often, estimation methods rely on self-reporting of PWID in addition to the number of PWID in treatment or rehabilitation services, probation and crime data and unintentional overdose deaths.23,24 Hospitals can be helpful in reporting an increase in IE cases, especially when known to be associated with IDU, and can use this as an indirect surveillance marker for IDU in the community. An increase in IE can also act as an early warning system of new or hidden pockets of IDU. Tertiary care hospitals can help with gathering sentinel surveillance as many cases of IE in the community are transferred to these centers because of complications, the potential need for surgery or higher levels of care or need for both. Infectious disease practitioners can also contribute to sentinel surveillance as they are more likely to be involved in the care of people with IE, HCV and HIV.
This study is limited by its retrospective design and limited time frame, reliance on the accuracy of written notes, especially with patients self-reporting IDU as well as the assignment of the appropriate ICD-9 code. IDU may well have been underrepresented in our population as this element of the patient’s history may not have been asked or appropriately documented.
Access to sterile syringes and clean injection equipment through syringe access programs has been shown to save lives, are a cost-effective public health strategy to prevent HIV and HCV, and increase the number of PWID who enter drug treatment.29–32 Effective syringe access programs can also decrease cases of IDU by providing sterile syringes, clean injection equipment and alcohol swabs to clean the injection site. They can also provide education about safer injection technique including handwashing, cleaning the injection site and warning against the practice of licking the needle before injection. Our cost data demonstrate how costly IE is to the healthcare system and to taxpayers alike. A admission for IDU-related IE costs more than the initial budget of the local syringe exchange program, the Cincinnati Exchange Project that started in 2014 with an initial budget of $50,000 from a local foundation.
The observation of a sharp increase in IE cases may be useful as a sentinel marker of a new epidemic of opioid injection and as an increase in the number of PWID. This increase in IE could act as an early warning system in other areas of the country where opioid IDU has also not previously been an established problem to prevent new HCV and HIV infections. Indeed, the HIV (and HCV) outbreak recently reported in Scott County, IN might have been recognized earlier by recognizing the occurrence of local IE cases.33 Sole reliance on data from law enforcement may lead to a delay in recognizing new opioid injection epidemics—in our study, IE cases increased sharply in 2005, years before local law enforcement agencies indicated awareness of the problem. Measures such as syringe access programs and an increase in the availability of drug treatment programs could have been implemented much earlier. The increase in IE was paralleled by sharp increase in submissions testing positive for heroin by the area crime laboratory. A reporting system where hospitals, public health and law enforcement work together to share data could provide the basis for actions in the community to decrease the number of IDU-associated infections—such as IE, skin and soft tissue infection, osteomyelitis, visceral and central nervous system abscesses, HCV, HIV and hepatitis B—as well as increasing public safety by decreasing the drug supply and drug-related crime.
An increase in IE may herald a new IDU problem, and its fellow-travelers, serious bacterial and viral infection. A diagnosis of IE should prompt assessment of all patients for IDU, viral hepatitis B and C and HIV, both for optimal inpatient care and for timely linkage to appropriate outpatient follow-up care.
Footnotes
The authors have no financial or other conflicts of interest to disclose.
References
- 1.Gordon RJ, Lowy FD. Bacterial infections in drug users. N Engl J Med. 2005;353:1945–54. doi: 10.1056/NEJMra042823. [DOI] [PubMed] [Google Scholar]
- 2.Bouza E, Menasalvas A, Munoz P. Infective endocarditis—a prospective study at the end of the twentieth century. Medicine. 2001;80:298–307. doi: 10.1097/00005792-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Berlin JA, Abrutyn E, Strom BL, et al. Incidence of infective endocarditis in the Delaware valley, 1988–1990. Am J Cardiol. 1995;76:933–6. doi: 10.1016/s0002-9149(99)80264-1. [DOI] [PubMed] [Google Scholar]
- 4.Hogevik H, Olaison L, Anderson R, et al. Epidemiological aspects of infective endocarditis in an urban population: a five year prospective study. Medicine. 1995;74:324–39. doi: 10.1097/00005792-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Heiro M, Helenius H, Mäkilä S. Infective endocarditis in a Finnish teaching hospital: a study on 326 episodes treated during 1980–2004. Heart. 2006;92:1457–62. doi: 10.1136/hrt.2005.084715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreillon P, Que Y-A. Infective endocarditis. Lancet. 2004;363:139–49. doi: 10.1016/S0140-6736(03)15266-X. [DOI] [PubMed] [Google Scholar]
- 7.Cherubin CE, Sapira JD. The medical complications of drug addiction and the medical assessment of the intravenous drug user: 25 years later. Ann Intern Med. 1993;119:1017–28. doi: 10.7326/0003-4819-119-10-199311150-00009. [DOI] [PubMed] [Google Scholar]
- 8.Lansky A, Finlayson T, Johnson C, et al. Estimating the number of persons who inject drugs in the United States by meta-analysis to calculate national rates of HIV nad hepatitis C virus infections. PLoS One. 2014;9(5):e97596. doi: 10.1371/journal.pone.0097596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;69(5):463–73. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Sierra C, Arizcorreta A, Diaz F, et al. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis. 2003;36:491–8. doi: 10.1086/367643. [DOI] [PubMed] [Google Scholar]
- 11.Liang TJ, Reherman B, Seeff LB, et al. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132:296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- 12.Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20(1):17–35. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control. Viral Hepatitis Surveillance-United States. 2010–2015. [Google Scholar]
- 14.Strader DB, Wright T, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–71. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 15.Paintsil E, He H, Peters C, et al. Survival of hepatitis C virus in syringes: implication for transmission among injection drug users. J Infect Dis. 2010;202:984–90. doi: 10.1086/656212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doerrbecker J, Behrendt P, Mateu-Gelabert P, et al. Transmission of hepatitis C virus among people who inject drugs: viral stability and association with drug preparation equipment. J Infect Dis. 2013;207:281–7. doi: 10.1093/infdis/jis677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall HI, An Q, Tang T, et al. Prevalence of diagnosed and undiagnosed HIV infection-Unites States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2015;64:657–62. [PMC free article] [PubMed] [Google Scholar]
- 18.Centers of Disease Control. HIV Surveillance Reports. 2013;25 [Google Scholar]
- 19.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton County Coroner’s Office. [Accessed February 1, 2012];Drug Section. 2000–2010 Available from: http://www.hamilton-co.org/coroner/DRUGS%202011.htm.
- 21.Doerrbecker J, Friesland M, Ciesek S, et al. Inactivation and survival of hepatitis C virus on inanimate surfaces. J Infect Dis. 2011;204(12):1830–8. doi: 10.1093/infdis/jir535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moss R, Munt B. Injection drug use and right sided endocarditis. Heart. 2003;89:577–81. doi: 10.1136/heart.89.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathew J, Addai T, Anand A, et al. Clinical features, site of involvement, bacteriologic findings, and outcome of infective endocarditis in intravenous drug user. Arch Intern Med. 1995;155:1641–8. [PubMed] [Google Scholar]
- 24.Chambers HF, Morris DL, Tauber MG, et al. Cocaine use and the risk for endocarditis in intravenous drug users. Ann Intern Med. 1987;106:833–6. doi: 10.7326/0003-4819-106-6-833. [DOI] [PubMed] [Google Scholar]
- 25.Stein M. Medical complications of intravenous drug use. J Gen Intern Med. 1990;5:249–57. doi: 10.1007/BF02600544. [DOI] [PubMed] [Google Scholar]
- 26.Graves MK, Soto L. Left sided endocarditis in parental drug abusers at a large community hospital. South Med J. 1992;85(4):378–80. doi: 10.1097/00007611-199204000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Ortiz-Bautist C, Lopez J, Garcia-Granja PE, et al. Current profile of infective endocarditis in intravenous drug users: The prognostic relevance of the valves involved. J Cardiol. 2015;187:472–4. doi: 10.1016/j.ijcard.2015.03.368. [DOI] [PubMed] [Google Scholar]
- 28.Dressler FA, Roberts WC. Infective endocarditis in opiate addicts: analysis of 80 cases studied at necropsy. Am J Cardiol. 1989;63(17):1240. doi: 10.1016/0002-9149(89)90186-0. [DOI] [PubMed] [Google Scholar]
- 29.Blomé MA, Björkman P, Flamholc L, et al. Minimal transmission of HIV despite persistently high transmission of hepatitis C virus in a Swedish needle exchange program. J Viral Hepat. 2011;8:831–9. doi: 10.1111/j.1365-2893.2010.01400.x. [DOI] [PubMed] [Google Scholar]
- 30.Belani HK, Muennig PA. Cost-effectiveness of needle and syringe exchange for the prevention of HIV in New York City. J HIV AIDS Soc Serv. 2008;7:229–40. [Google Scholar]
- 31.Centers for Disease Control. Syringe exchange programs—United States, 2005. MMWR Morb Mortal Wkly Rep. 2007;56:1164–7. [PubMed] [Google Scholar]
- 32.Kwon JA, Anderson J, Kerr CC, et al. Estimating the cost-effectiveness of needle-syringe programs in Australia. AIDS. 2012;26:2201–10. doi: 10.1097/QAD.0b013e3283578b5d. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control. Community outbreak of HIV infection linked to injection drug use of oxymorphone-Indiana 2015. MMWR Morb Mortal Wkly Rep. 2015;64:443–4. [PMC free article] [PubMed] [Google Scholar]