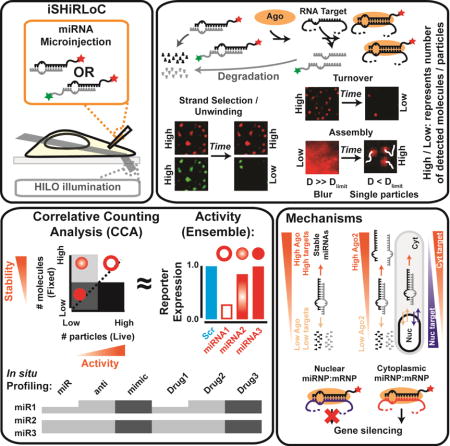
SUMMARY
Regulation of miRNA localization and stability is critical for their extensive cytoplasmic RNA silencing activity and emerging nuclear functions. Here, we have developed single-molecule fluorescence-based tools to assess the sub-cellular trafficking, integrity and activity of miRNAs. We find that seed-matched RNA targets protect miRNAs against degradation and enhance their nuclear retention. While target-stabilized, functional, cytoplasmic miRNAs reside in high molecular weight complexes, nuclear miRNAs as well as cytoplasmic miRNAs targeted by complementary anti-miRNAs are sequestered stably within significantly lower molecular weight complexes and rendered repression-incompetent. miRNA stability and activity depend on Argonaute protein abundance, whereas miRNA strand selection, unwinding and nuclear retention depend on Argonaute identity. Taken together, our results show that miRNA degradation competes with Argonaute loading and target binding to control sub-cellular miRNA abundance for gene silencing surveillance. Probing single cells for miRNA activity, trafficking and metabolism promises to facilitate screening for effective miRNA mimics and anti-miRNA drugs.
Keywords: microRNA, Argonaute, mRNA targets, anti-miRs, correlative counting analysis, single-molecule microscopy
eTOC
Pitchiaya et al. describe tools to interrogate gene-regulatory microRNAs inside living cells at single-molecule resolution. They find that the RNA silencing machinery and RNA targets mediate gene silencing surveillance by modulating abundance and sub-cellular location of microRNAs. These findings and tools promise to facilitate single-cell screening of microRNA activity.
INTRODUCTION
MicroRNA(miRNA)-mediated gene silencing is a pervasive, evolutionarily conserved cellular pathway wherein endogenous small non-coding RNAs (ncRNAs) guide the RNA induced silencing complex (RISC) to translationally repress and eventually degrade complementary sequence-matched messenger RNA (mRNA) targets (Pasquinelli, 2012). Biosynthesis of functional miRNAs follows a tightly regulated multi-step pathway, encompassing both the nuclear and cytoplasmic compartments of the cell (Ha and Kim, 2014; Lin and Gregory, 2015). Following the transcription of a miRNA gene, a primary miRNA transcript (pri-miRNA) is processed in the nucleus to generate a corresponding precursor-miRNA (pre-miRNA), which is then exported to the cytoplasm and further processed to yield mature, ~22-base pair miRNA duplexes. One strand of the miRNA duplex (the guide strand, G) is preferentially retained within the Argonaute (Ago) protein containing RISC, whereas the other strand (passenger, P or “*”) is released and possibly degraded. Activated miRISC then engages mRNAs by hybridizing the G strand with complementary seed sequences, leading to RNA silencing (Pasquinelli, 2012). Recent reports suggest that many RISC factors and mature miRNAs are also present in the nucleus, allowing for potentially widespread nuclear functions (Gagnon et al., 2014; Khudayberdiev et al., 2013; Liao et al., 2010; Weinmann et al., 2009; Zisoulis et al., 2012). Collectively, miRNAs regulate many developmental and physiological processes, and their dysregulation is known to lead to pathologies including cancer (Lin and Gregory, 2015). The cellular abundance and sub-cellular localization of miRNAs is thus central to maintaining physiological homeostasis.
miRNA levels are regulated via both transcriptional and post-transcriptional mechanisms. Post-transcriptional regulation of miRNAs can occur via protein- or target RNA-mediated pathways. One protein-mediated pathway involves the regulation of the levels or activities of key role-players in the miRNA biosynthesis pathway (Ha and Kim, 2014; Pasquinelli, 2012). Another pathway encompasses proteins, including the XRN1/2 5′-to-3′ (Chatterjee and Grosshans, 2009) and SDN 3′-to-5′ exonuclease complexes (Ramachandran and Chen, 2008), that directly mediate miRNA turnover (Kai and Pasquinelli, 2010). There is conflicting evidence, however, whether targets of miRNAs promote or rather inhibit turnover of mature miRNA. Brown and coworkers (Baccarini et al., 2011) reported that extensive complementarity between mRNA targets and miRNAs promotes miRNA degradation in human cells, suggesting that miRNA decay through 3′ uridylation is promoted by targets. Moreover, Zamore and coworkers have found evidence that targets promote 3′ adenylation and/or trimming of cognate miRNA 3′ ends to initiate degradation in Drosophila (Ameres et al., 2010). In contrast, mRNA targets have been shown to protect miRNAs in C. elegans (Chatterjee et al., 2011).
Recent reports suggest that sub-cellular localization is critical to miRNA function (Leung, 2015). In particular, the discoveries of mature miRNAs in the nucleus (Gagnon et al., 2014; Khudayberdiev et al., 2013; Liao et al., 2010) and of the ability of small RNAs to guide RNA target cleavage in the nucleus (Gagnon et al., 2014) were unexpected. Several groups have suggested that engineered and exogenously added small RNAs and Ago proteins can mediate nuclear gene regulation, in the forms of inhibition (Castanotto et al., 2005; Janowski et al., 2005; Janowski et al., 2006; Kim et al., 2006; Morris et al., 2004; Napoli et al., 2009; Ting et al., 2005) or activation of transcription (Janowski et al., 2007; Li et al., 2006; Matsui et al., 2013), and of control over alternative splicing (Allo et al., 2009; Liu et al., 2012), but mechanisms mediating these processes largely remain unresolved. While import and export factors mediating the nucleo-cytoplasmic trafficking of mature miRNAs have been identified (Castanotto et al., 2009; Ohrt et al., 2006; Yi et al., 2003), factors that retain mammalian miRNAs in the nucleus and the kinetics associated with trafficking are largely unknown.
Consequently, there is an urgent need for approaches that can dissect miRNA localization, function and turnover within the cellular environment. To this end, we previously reported the ability of intracellular single-molecule, high-resolution localization and counting (iSHiRLoC) to dissect two kinetically sequential miRNA assembly pathways in single HeLa cervical cancer cells (Pitchiaya et al., 2012; Pitchiaya et al., 2013). Here, we have extended iSHiRLoC to exploit single, microinjected, mature miRNA reporters for a comprehensive survey of the sub-cellular localization, maturation, turnover and function of miRNAs. Our current technology interrogates RISC loading and subsequent steps of the miRNA-mediated RNA silencing pathway, but is potentially extendable to investigate upstream pathway steps, simultaneously visualize small RNAs and RISC complexes, and probe target search mechanisms of RISC inside cells. Introducing a novel correlative counting analysis of live- and fixed-cell images, we find that Ago proteins and seed-matched targets play a critical role in mediating nuclear retention and stabilization of mature miRNAs. In cells lacking Ago2, passenger strand discard is diminished and nuclear localization enhanced compared to cells either overexpressing Ago2 or lacking Ago1, suggesting additional non-redundant functions of nuclear Ago proteins. We further find that nuclear miRNAs are not as effective in silencing their cognate targets as their cytoplasmic counterparts. Our work reveals under-appreciated roles of both cognate RNA targets and Ago2 in controlling sub-cellular miRNA abundance for surveillance of gene regulation.
RESULTS
iSHiRLoC resolves miRNA localization, unwinding and passenger strand removal
To dissect the RNA silencing pathway in mammalian cells, we expanded our previously established iSHiRLoC assay (Figure 1A). We used the highly conserved tumor suppressor miRNA let-7-a1 (henceforth, l7/l7*, where l7 refers to the guide or G strand and l7* refers to the passenger or P strand, known to have ~1200 mRNA targets in human cells per targetscan 7.1) (Roush and Slack, 2008) as a reporter and the flat, microscopically amenable human U2OS osteosarcoma cells as our model system. Based on prior reports that fluorophore labels on miRNAs do not affect the activity and binding kinetics of eukaryotic Ago in vitro (Chandradoss et al., 2015), we further confirmed that the conjugation of a fluorescent dye to the 3′ end of individual or both strands of a mature, double-stranded (ds) miRNA affects their RNA silencing activity in U2OS cells (Figure S1). To avoid photobleaching of the miRNA-conjugated Cy3 or Cy5 dye during initial visualization of cells, microinjection samples were supplemented with a spectrally distinct, fluorescein- or Alexa 405-labeled dextran. Hydrophilic dextrans are biologically inert, stable and cannot permeate through the cell membrane so that they are retained within cells (Ludtke et al., 2002). Moreover, high-molecular weight (>70 kDa) dextrans are refractory to nucleo-cytoplasmic transport so that they remain within the intracellular compartment of microinjection (Kee et al., 2012). In addition to serving as a visual aid to identify cellular and nuclear boundaries, the co-injected dextrans were used henceforth as a loading control to normalize for the amount of material microinjected (Figures 1B, 1C and S2).
Figure 1. Outline of iSHiRLoC Data Acquisition and Analysis.
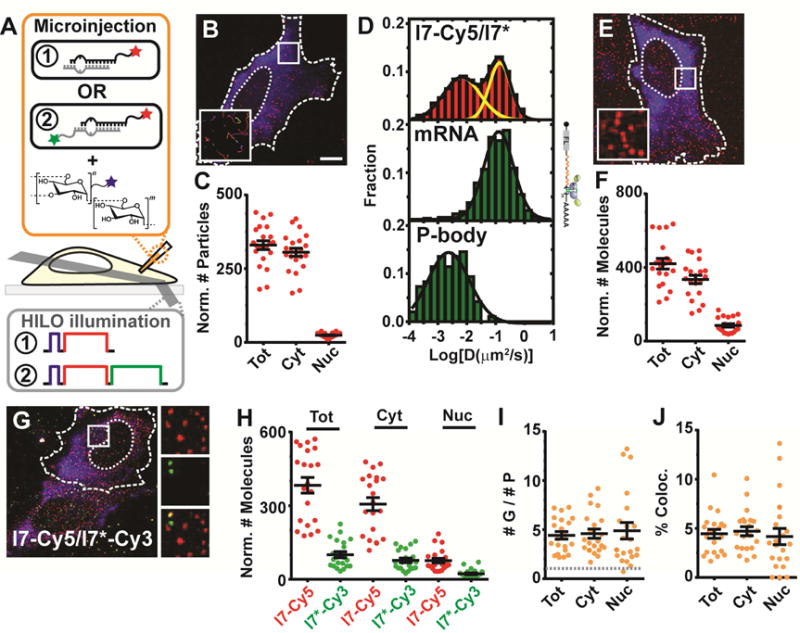
(A) Schematic of iSHiRLoC assays. Singly (red only, “1”) or doubly (red and green, “2”), 3′end labeled miRNAs and fluorophore labeled dextran (blue) are co-microinjected into cells and imaged by HILO microscopy. Dextran (blue, 1 s), Cy5 (red, 30 s) and Cy3 (green, 30 s) were excited one after another, as represented in the schematic of excitation pulses. (B) Representative image of a live U2OS cell containing stably assembled Cy5-labeled miRNA complexes as diffusing particles (red) and fluorescein-labeled, 500 kDa dextran (blue), 2 h after microinjection into the cytosol. Dotted and dashed lines represent nuclear and cellular boundaries respectively. Scale bar, 10 μm. Inset, 5.3 × 5.3 μm2 represents zoomed-in tracks of particles within white box. (C) Scatter plot depicting the total number of miRNA particles in the cell (“Tot”) or within the cytoplasmic (“Cyt”) or nuclear (“Nuc”) compartments. Error bars, SEM (n ≥ 3, # cells ≥ 18). Scale bars, labeling scheme and error bars are the same for other panels in this figure. (D) Diffusion coefficient distribution of l7-Cy5/l7* miRNAs, MCP-GFP labeled firefly luciferase (FL) mRNA with the mH3UM 3′UTR and GFP-Dcp1a punctate structures in U2OS cells. (E) Representative image of a formaldehyde-fixed U2OS cell containing individual Cy5-labeled miRNA particles (red), a majority of which are individual molecules, and fluorescein-labeled, 500 kDa dextran (blue). Inset represents zoomed-in view of particles within white box. (F) Scatter plot depicting the number of miRNA molecules. (G) Representative image of formaldehyde-fixed U2OS cells containing individual particles l7-Cy5 and l7*-Cy3, and Alexa-405-labeled, 500 kDa dextran (blue). Insets represent zoomed-in view of red (top), green (middle) and co-localized (bottom) particles within white box. (H) Scatter plot depicting the number of guide (G) and passenger (P) strand molecules. (I) Scatter plot representing the ratio of guide to passenger strand. Grey line represents a 1:1 ratio of G:P. Scale bars, labeling scheme and error bars as in (C). (J) Scatter plot representing the percentage of co-localized molecules, as normalized to the more abundant guide strand molecules.
Upon microinjection of just ~10,000 miRNA molecules, or less than 5% of the total cellular miRNA count, l7-Cy5/l7* assembled into spatially resolved, diffusing particles in living U2OS cells 2 h after microinjection (Figure 1B), akin to our previous observation in HeLa cells (Pitchiaya et al., 2012; Pitchiaya et al., 2013). Using an optimized counting algorithm (Figure S2 and Supplemental Experimental Procedures), we quantified the normalized sub-cellular abundance of diffusing particles and found that l7 predominantly remained in the cytoplasm, with a small yet significant fraction of 9 ± 3 % found in the nucleus (Figure 1C). Consistent with our observations in HeLa cells (Pitchiaya et al., 2012; Pitchiaya et al., 2013), diffusion coefficients of cytosolic l7 in U2OS cells distributed within two Gaussian populations that resembled MS2-MCP labeled mRNAs and GFP labeled P-bodies respectively (Figure 1D), as expected for a functional miRNA (Pitchiaya et al., 2012). To assess sub-cellular miRNA abundance more quantitatively, we performed stepwise photobleaching counting of similarly microinjected cells after fixation (Figure 1E). We found that >85% of all labeled particles contained only a single miRNA reporter molecule (Figure S2), and that 17 ± 4% of these molecules localized to the cytoplasm (Figure 1E and F). Considering that U2OS cells are only ~2.5–5 μm deep (Macdonald et al., 2013) and the depth of our highly inclined laminar optical sheet (HILO) illumination is ~3 μm (Liu et al., 2015), miRNAs present within the focal-plane of illumination represent ~50% of all miRNAs in the cell (Figure S2).
In the cytosol, ds-miRNAs are unwound to retain the single-stranded guide strand within RISC, and to eject and possibly degrade the passenger strand. To further test for full functionality of our miRNA reporters, we performed two-color iSHiRLoC on fixed cells with the G and P strands of l7 miRNA labeled with spectrally distinct Cy5 and Cy3 dyes (l7-Cy5/l7*-Cy3), respectively (Figure 1A,G). Strikingly, we found that cells contained a large (>5-fold) excess of l7 over l7* in both cytoplasm and nucleus (Figure 1H,I), independent of dye identity (Figure S3), ruling out differential fluorescence detection sensitivity as a cause. These observations are consistent with the known asymmetric loading of l7 over l7* into RISC (Chatterjee et al., 2011), combined with loss of the ejected passenger strand due to RNA degradation and the expected expulsion of the resulting free dye from the cell (Homolya et al., 1993) or other forms of exocytic pathways. 2 h after microinjection, less than 5% of guide strand molecules still co-localized with passenger strand (Figure 1J), indicating that very few miRNA molecules are still double-stranded and attesting to efficient loading of the guide. Taken together, our data establish iSHiRLoC as a quantitative single molecule tool for detecting the sub-cellular localization, unwinding and degradation of miRNAs in individual cells. Conversely, each cell in effect becomes an isolated reaction vessel for probing miRNA processing pathways.
Correlative live- and fixed-cell counting probes miRNA integrity and activity
To verify that our microinjected ds-miRNAs functionally engage mRNA targets in the form of guide-strand loaded RISC, as opposed to merely hybridizing to complementary targets in the absence of a protein complex, we performed a series of iSHiRLoC assays with several RNA and DNA variants of l7/l7* miRNA. 2 h after microinjection, l7-Cy5/l7* in live cells had again assembled into slowly diffusing RNPs (Figures 2A and 2B), which together with corresponding reporter gene assays (Figure 2C) indicated that l7 loaded RISC binds to and actively represses target mRNAs (Pitchiaya et al., 2012; Pitchiaya et al., 2013; Shankar et al., 2016). Strikingly, microinjecting an equivalent quantity of single-stranded (ss) l7 (l7-Cy5) resulted in similarly diffusing complexes, however, the (normalized) number of such particles was significantly (~3-fold) less than for l7-Cy5/l7* (Figures 2A and 2B). This observation suggests that a majority of l7-Cy5 either resides in rapidly diffusing particles (with a diffusion constant >10 μm2/s and a molecular weight <0.5 MDa) that blur out at 100-ms time resolution (Liu et al., 2015; Pitchiaya et al., 2012), or is degraded and/or lost from the cell. To distinguish these possibilities, we performed iSHiRLoC’s stepwise photobleaching counting in fixed cell (Pitchiaya et al., 2013) and found that the number of l7-Cy5 molecules was diminished by ~3-fold compared to l7-Cy5/l7*, very similar to our observations in live cells (Figures 2A, 2B and 2C). These results suggest that ssRNAs are actively degraded and the dye then expelled from the cell, similar to our observation for the unused passenger strand (Figure 1). Accordingly, the ssRNA does not downregulate a potential target in a gene reporter assay, unlike the corresponding dsRNA (Figure 2D). Further supporting our interpretation, guide-strand labeled dsDNA or ssDNA versions of l7/l7*, expected to be more stable intracellularly but incapable of RNA silencing, failed entirely to assemble into slowly diffusing RNPs (Figures 2A and 2B), even though their intracellular abundance in fixed cells remained high (Figures 2A and 2C), yet without regulatory potential (Figure 2D).
Figure 2. Validation of the correlative counting analysis (CCA).
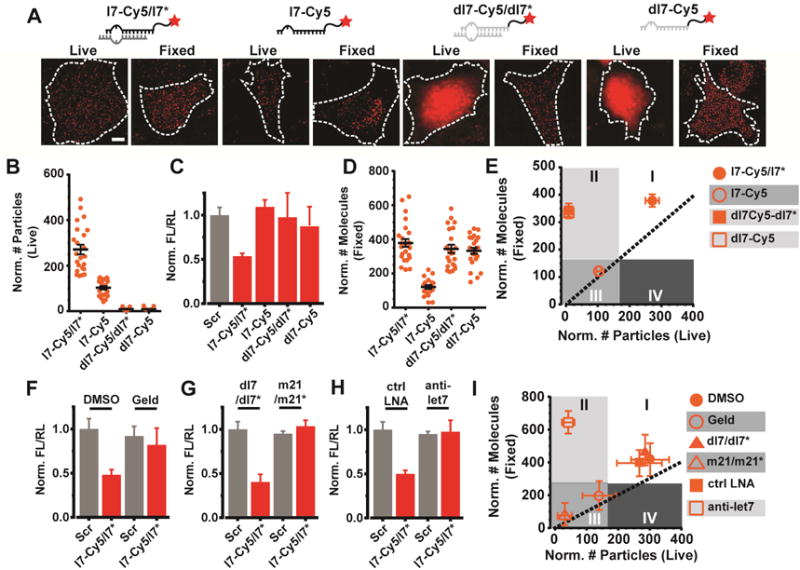
(A) Representative images of live and fixed U2OS cells injected with various Cy5-labeled oligonucleotides (red) bearing the sequence of let-7 miRNA. Scale bar, 10 μm. Images were acquired 2 h after microinjection. (B) Scatter plot depicting the total number of particles for each “Live” sample in (A). Error bars, SEM (n ≥ 2, # cells ≥ 20). (C) Luciferase reporter assays of U2OS cells transfected with the pmG-mH3U plasmid and the appropriate oligonucleotide. Error bars, SEM (n = 3, with 4 technical replicates per trail). (C) Scatter plot depicting the total number of molecules for each “Fixed” sample in (A). Error bars, SEM (n ≥ 2, # cells ≥ 20). (E) Plot correlating the abundance of particles in live and fixed cells. Dotted line represents a perfect correlation. Each data point depicts the mean value and error bars depict SEM. Shaded regions were arbitrarily chosen. (F–H) Luciferase reporter assays of U2OS cells transfected with the pmG-mH3U plasmid and either a scrambled double stranded oligonucleotide (Scr) or l7-Cy5/l7* under various treatment conditions. Error bars, SEM (n = 3, with 4 technical replicates per trail). For inhibiting RISC loading (F), cells were treated with Geld and compared to control cells treated with DMSO (ctrl). For competitive inhibition experiments (G), cells were treated with a 5-fold excess of unlabeled miR21 (m21/m21*) or equivalent amount of dl7/dl7*. For blocking target binding (H), cells were treated with a 3-fold excess of anti-let7 anti-miR (LNA) or control anti-miR (ctrl LNA) bearing no complementarity to let7. (I) Plot correlating the abundance of particles in live and fixed cells, as plotted in (E).
Since live-cell particle counting provided complementary information to our fixed-cell analysis, we correlated the two data sets within a comprehensive plot that we refer to here as ‘correlative counting analysis’, or CCA (Figure 2E). Plotting the live-cell imaging data on the x-axis with the fixed-cell data on the y-axis, we used the standard error of our data to empirically determine 35:65 and 30:70 splits of the x- and y-axes, respectively, to delineate the plot into four practically useful quadrants. Samples such as l7-Cy5/l7* that efficiently assemble into complexes diffusing like mRNPs in live cells and are stably retained as observed in fixed cells, will occupy the top right quadrant (Figure 2E, “I”). Samples prone to degradation, such as l7-Cy5, lead to both low live- and low fixed-cell counts and will occupy the bottom-left quadrant (Figure 2E, “III”). By contrast, stable samples that fail to assemble into slowly diffusing complexes but are retained in the cell, such as ss- or ds-let-7 DNA (dl7-Cy5 or dl7-Cy5/dl7*), will occupy the top-left quadrant of the plot (Figure 2E, “II”). Quadrant IV is populated when the particles identified in live cells exceed those identified in fixed cells – a scenario that typically manifests due to fixation induced dye photobleaching or loss from cell permeation. We find that Quadrant IV is never populated in our assays. This correlative plot thus delineates the differences in intracellular behavior between species, and further supports the notion that microinjected miRNAs act functionally through RNP assembly and not simple hybridization to mRNA targets.
To further test the robustness of our interpretation, we blocked two distinct steps of the RNA silencing pathway and performed iSHiRLoC-based correlative live/fixed-cell counting (Figures 2F–I). First, we inhibited the loading of miRNA into RISC by treating cells with geldanamycin (Geld), an inhibitor of the Hsp90/Hsc70 chaperone, a known RISC loading factor (Iwasaki et al., 2010). Alternatively, we co-microinjected a 5-fold excess of unlabeled miR21 (m21/m21*) miRNA as a direct competitor for RISC loading. Finally, we co-microinjected an anti-miR complementary to l7 strand (anti-let7) to block target binding by the miRNA. All three strategies relieved miRNA mediated silencing in our luciferase gene reporter assay (Figures 2F–H), in contrast to controls in which cells were treated with only DMSO (the geldanamycin solvent), with dark dl7/dl7*, or with a scrambled anti-miR (Figures 2F–H), as expected. Concordantly, all three controls occupied quadrant I of the CCA plot, suggesting that the microinjected l7-Cy5/l7* stably assembled into functional RNPs (Figure 2I). In contrast, both strategies for blocking RISC loading shifted l7-Cy5 into quadrant III (Figure 2I), consistent with the miRNA no longer efficiently loading into RISC and consequently being degraded. Strikingly, anti-let7 treated cells occupied quadrant II of the plot (Figure 2I), indicative instead of intracellular stabilization of the miRNA within low molecular weight complexes of limited efficacy in engaging mRNAs. Our data thus suggest that, 2 h after microinjection, ~50–80% of all observed miRNA molecules are stably retained inside cells as functional RNPs, while the rest are still undergoing RISC assembly or are involved in other cellular pathways. Our results also establish iSHiRLoC as a quantitative approach to dissecting miRNA integrity and activity.
Seed-matched targets enhance intracellular miRNA stability
Based on the calibration of our microinjector (Pitchiaya et al., 2013), an intra-needle concentration of 1 μM should introduces ~10,000 molecules of miRNA per cell. 2 h after microinjection we typically observed only ~200–600 miRNA molecules per cell, suggesting that signal from a large fraction of molecules is lost due to degradation, expulsion from the cell, and/or other processes. To test for miRNA turnover and fluorophore expulsion from U2OS cells, we performed time courses of fixed- and live-cell counting of microinjected l7-Cy5/l7*-Cy3 and l7-Cy5/l7* respectively. Observing fixed cells, loss of l7-Cy5 signal occurred in two distinct phases, fitted well with a double-exponential decay function (Figure 3A and Table S1). Signal loss was rapid during the first phase, i.e., within an hour after microinjection (t1 ~20 min), with a second miRNA population observed to be lost 7-fold more slowly over the next several hours (t2 ~8 – 24 h) so that at 16 h no miRNAs were left (Figure 3A and Table S1). The half-life of l7-Cy5 measured using iSHiRLoC is similar to that of endogenous l7 in other cell lines (Ruegger and Grosshans, 2012). Strikingly, at zero time the fit intersects the y-axis at ~9,700 molecules, consistent with the microinjection of ~10,000 molecules per cell (Table S1). Importantly, loss of l7* passenger strand was also biphasic and considerably more rapid throughout the time course (Figure 3A and Table S1), wherein a significant fraction of the molecules disappeared with a t1 of ~7 min with the remaining molecules disappearing from the cells with a t2 of 84 min. These data further corroborate our finding of asymmetric RISC loading of the guide strand (Figure 1), and suggest that, while a large fraction of the microinjected miRNA guide strand is degraded, a significant fraction is stabilized over the passenger strand. Further supporting this view, in live cells the appearance of slowly diffusing l7-Cy5 particles also occurred in two phases (Figure 3A), with the number of observable particles rapidly increasing within the first hour (t1 ~6 min), and slowly disappearing from view (t2 ~6 h) until no observable signal was detected 16 h after microinjection. Taken together, these results suggest that a large fraction of l7/l7* molecules assemble as guide strand enriched miRISC (Figures 1, 2 and 3A), with a small-yet-significant fraction rapidly assembling into quite stable miRISC-mRNP complexes.
Figure 3. Seed-matched targets protect miRNAs in cellulo.
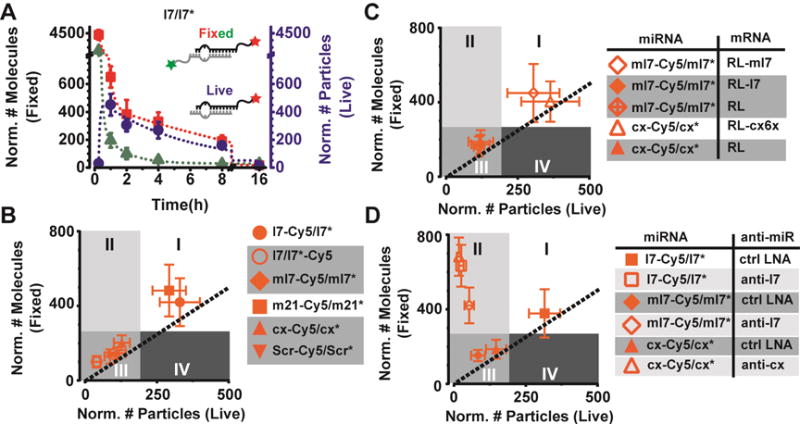
(A) Time course analysis of microinjected let-7 abundance in live (blue) and fixed cells (green and red). Assays were done over a time course of 0.3 – 16 h. Number of l7-Cy5 and l7*-Cy3 molecules in 2-color iSHiRLoC assays are depicted in red (squares) and green (triangles) respectively and their corresponding double-exponential fit are represented as appropriately colored dotted lines. iSHiRLoC live cell assays using l7-Cy5/l7* miRNA were done on a complementary set of samples. Particles abundances are depicted in blue (circles) and the corresponding double-exponential fit is represented as a blue dotted line. (B) CCA plot of various miRNAs, 2 h after microinjection. (C) CCA plot of various miRNAs co-injected with target mRNAs, 2 h after microinjection. All miRNAs were labeled with Cy5 on the guide strand and were co-injected with the appropriate target. RL-ml7 and RL-cx6x are seed matched targets of ml7 and cx respectively. RL-ml7 has two MREs for ml7, whereas the RL-cx6x has six MREs for cx. RL-l7 bears a seed mismatch for ml7 at each of the MREs and is a target of l7, whereas RL mRNA does not have any 3′UTR. (C) CCA plot of various miRNAs co-injected with anti-miRs, 2 h after microinjection.
We next asked how the intracellular assembly and turnover of other miRNAs compares to l7/l7*, by performing correlative live- and fixed-cell counting 2 h after microinjection when stable assembly of l7 was observed (Figure 3A). We found that a guide-strand labeled let-7 seed mutant (ml7-Cy5/ml7*), which cannot repress targets with l7 binding sites (Figure S3C) and consequently has few intracellular targets, was rapidly turned over and unable to assemble into active miRISC-mRNP complexes, as indicated by a shift into quadrant III (Figure 3B). Similarly, the artificial cxcr4 miRNA (cx) and a negative control dsRNA (ctrl), both of which have limited intracellular targets, were unstable and inefficiently assembled into diffusing complexes, also confining them to quadrant III (Figure 3B). By contrast, guide strand labeled miR-21 (m21-Cy5/m21*), which has ~300 distinct seed matched targets in human cells (predicted by targetscan 7.1), stably assembled into miRISC-mRNP complexes and was found in quadrant I, next to l7 (Figure 3B). These data suggest that miRNA stability is correlated with the abundance of intracellular targets. To directly test whether seed-matched targets enhance miRNA stabilization and assembly into miRISC-mRNP complexes, we co-microinjected random, seed-mismatched, or seed-matched mRNA targets with the ml7-Cy5/ml7* or cx-Cy5/cx* miRNAs. As predicted, seed-matched targets mediated miRNA protection and miRISC-mRNP assembly, whereas all mismatches do not (Figure 3C).
Based on our previous observation that l7 is stabilized by anti-let7 in the form of small-molecular weight complexes (Figure 2I), we reasoned that anti-miRs might act as “minimal” seed-matched targets. If so, seed-matched anti-miRs should generally mediate miRNA protection, just as the mRNA targets they mimic. We therefore co-microinjected various combinations of anti-miRs with either matched of mismatched miRNAs, confirming that only little anti-miR hybridized with the double-stranded miRNA in the injection needle (Figure S4A). As predicted, correlative live- and fixed-cell analysis showed that anti-cxcr4 miRNA robustly stabilized matched cx miRNA while not forming the slowly diffusing particles resembling miRISC-mRNP complexes, as evident from this combination appearing in quadrant II (Figure 3D). Indeed, an anti-miR generally shifts a matched or nearly matched miRNA into quadrant II (Figure 3D). Notably, anti-let-7 shifted the nearly matched mutant ml7-Cy5 into quadrant II, but stabilized it less than the matched l7-Cy5, as evident from its lower fixed-cell count (Figure 3D). This observation is consistent with the only partial relief of ml7-mediated reporter gene repression by anti-let-7 (Figure S4B). We conclude that target-mediated miRNA protection (Chatterjee et al., 2011) can be observed in human cells, involves both mRNA and anti-miR targets, and competes with miRNA degradation to sustain intracellular gene silencing.
Mature miRNAs with mRNA targets localize to the nucleus in rapidly diffusing complexes
To understand the sub-cellular distribution and fate of mature miRNAs, we performed iSHiRLoC and CCA upon microinjecting l7-Cy5/l7* miRNA either into the cytoplasm or the nucleus of U2OS cells, as confirmed by imaging of the co-injected Alexa 405-labeled high molecular weight dextran. Regardless of the compartment of injection, 2 h after microinjection a large majority of miRNAs had assembled into miRISC-mRNP complexes in the cytosol (quadrant I), with only few remaining in the nucleus as low molecular weight complexes (quadrants II and III; Figures 4A and S5A). Notably, the retention of miRNAs in the nucleus was ~2-fold higher when first introduced there (Figures 4A and S5A). Consistent with previous reports for small interfering RNAs (Ohrt et al., 2008), our data suggest that nuclear miRNAs assemble into low-molecular weight complexes compositionally different from cytoplasmic high-molecular weight miRISC-mRNP complexes.
Figure 4. Mature miRNAs localize in the nucleus as rapidly diffusing complexes, with the extent of localization dependent on miRNA sequence.
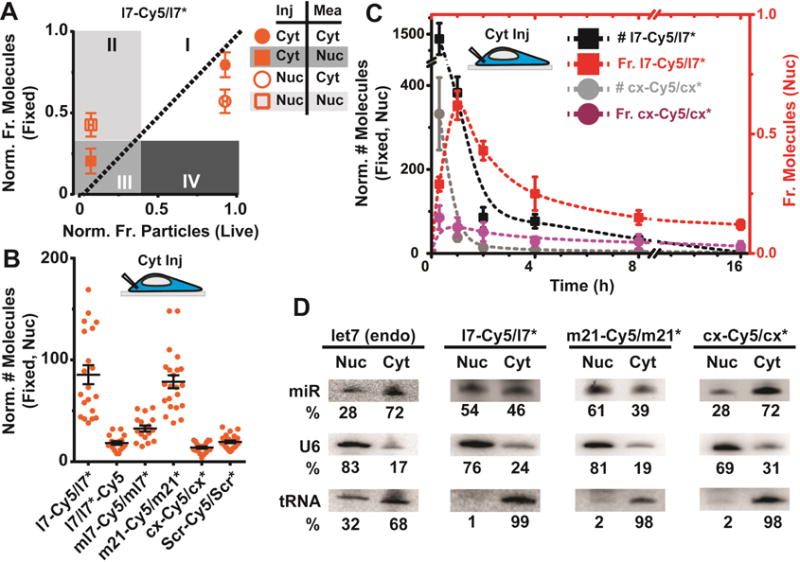
(A) CCA plot of l7-Cy5/l7* when injected (Inj) in the cytosol (Cyt) or nucleus (Nuc) and measured (Mea) in the appropriate compartments (n ≥ 2, ≥ 12 cells). (B) Scatter plot representing the number of appropriate miRNA molecules in the nucleus (n ≥ 2, ≥ 15 cells) when injected in the cytosol of U2OS cells. (C) Time course analysis of nuclear l7-Cy5/l7* or cx-Cy5/cx*, upon cytoplasmic injections. The molecular abundance in the nucleus (left y-axis, # l7-Cy5/l7* and # cx-Cy5/cx*) and the nuclear fraction (right Y-axis, Fr. l7-Cy5/l7* and Fr. cx-Cy5/cx*) of each miRNA is represented (n ≥ 2, ≥ 15 cells). t = 0, is an assumed data-point, considering that all miRNAs are delivered directly into the cytosol during injection. (D) Northern blot analysis of sub-cellular fractionated U2OS cells to detect endogenous (endo) or transfected miRNAs. Nuclear and cytoplasmic sub-cellular fractions are annotated as Nuc and Cyt. U6 small nuclear RNA (U6) and cytoplasmic tRNA-Lys serve as sub-cellular loading controls. Percent of signal in each sub-cellular compartment has been corrected for leakage of U6 and tRNA-Lys and is mentioned below each blot (n = 2).
To test the broader validity of our observations, we performed iSHiRLoC fixed-cell counting with a panel of miRNAs 2 h after microinjection. While l7-Cy5/l7* and m21-Cy5/m21* localized in the nucleus of U2OS cells to similar extents, miRNAs without mRNA targets (ml7-Cy5/ml7*, cx-cy5/cx*, and Scr-Cy5/Scr*, as well as the passenger strand of let-7, i.e. l7/l7*-Cy5) generally displayed lower nuclear localization, independent of the compartment of microinjection (Figures 4B and S5B). Similar observations were made when l7-Cy5/l7* or cx-Cy5/cx* were instead microinjected into HeLa cells (Figure S5C). Analysis of l7 and cx miRNAs over a time course showed that significantly higher fractions of l7 were retained in the nucleus at any given time than cx, irrespective of the compartment of injection (Figures 4C, S5D and Table S2). We further found that an initial rapid influx of l7 miRNAs into the nucleus was followed by a slow efflux, whereas cx showed very little influx to begin with (Figure 4C, S5D and Table S1). These data suggest that nuclear localization appears to be specific to miRNAs with potential mRNA targets and that a guide strand is preferentially retained in the nucleus over a passenger strand.
A previous report used oligofectamine-based transfection followed 24 h later by sub-cellular fractionation and Northern blotting to suggest that miR-29b accumulates in the nucleus of HeLa cells based on a hexanucleotide nuclear localization signal (Hwang et al., 2007). Using this experimental approach, U2OS cells transfected with fluorophore labeled miRNAs indicated a more pronounced retention of l7 and m21 over cx in the nucleus (Figure 4D), consistent with our iSHiRLoC results. Notably, we found that transfection with lipofectamine generally retained miRNAs in the nucleus at 24 h (Figure S5E), suggesting that this reagent is not ideal for localization studies. As a control, we also probed for endogenous l7 in U2OS cells, whose subcellular distribution closely matches that of our microinjected l7-Cy5/l7*, but was different from transfected miRNAs, further supporting the notion that transfection – in contrast to microinjection – is not an ideal method to track miRNA localization. Next, we performed sub-cellular fractionation and Northern blotting of HeLa cells transfected using oligofectamine and one of three different miR-29b variants to reproduce the previous work (Hwang et al., 2007). Surprisingly, we found that si-miR29a, a miRNA that does not to contain the hexanucleotide signal and was previously suggested to predominantly localize to the cytosol (Hwang et al., 2007), displayed similar nuclear abundance as si-miR29b and fluorophore-labeled miR-29b that both contain the signal (Figure S6A). Similarly, the extent of miR-29b nuclear localization after microinjection as measured by iSHiRLoC was similar to those of l7 and m21 in both U2OS and HeLa cells (Figure S6B and C). This suggests that miR-29b localizes significantly to the nucleus of human cells, but not more than other miRNAs with cellular mRNA targets that lack the previously described nuclear localization signal of miR-29b (Hwang et al., 2007). That is, nuclear miRNA localization appears to be primarily target-driven.
Intracellular miRNA unwinding and nuclear localization are dependent on Ago identity
Small ds-oligonucleotides, such as miRNAs, siRNAs and dsDNAs used in our assays, are <40 kDa and therefore have the potential to passively shuttle across the nuclear membrane. To test whether target-dependent retention of miRNAs in the cell nucleus requires a saturable protein factor, we used our previous strategy (Figure 2) of blocking RISC loading with competing dsRNAs. We performed iSHiRLoC fixed-cell counting 2 h after microinjecting mixtures of l7-Cy5/l7* with a 5-fold excess of unlabeled l7/l7*, m21/m21*, cx/cx* or dl7/dl7* either into the cytoplasm or nucleus of U2OS cells. Nuclear localization of l7-Cy5 was diminished ~10-fold upon co-injection of competing dsRNAs into the cytosol compared to the control and ~3-fold when the RNA mixture was delivered directly into the nucleus (Figure 5A). These data suggest that a saturable protein factor and perhaps Ago loading are critical for nuclear localization of miRNAs. Next, we used two-color iSHiRLoC to test whether miRNAs are unwound when microinjected directly into the nucleus. To capture the rapidly degrading P strand (Figure 1), we performed these assays at the earliest possible time point just 20 min after microinjection. Strikingly, only 8–15% of all l7 strands were still co-localized with l7*, although both strands were still found in the nucleus (Figure S7A–C). Additionally, l7 abundance was at least 2-fold higher than l7* levels (Figure S7C), very similar to our observations on miRNA unwinding upon cytoplasmic injection (Figure 1G–J). Our data are consistent with the notion that miRNAs can be potentially unwound in the nucleus, in addition to the cytoplasm, and selectively retained in the nucleus.
Figure 5. Ago proteins are critical for nuclear localization and unwinding of miRNAs.
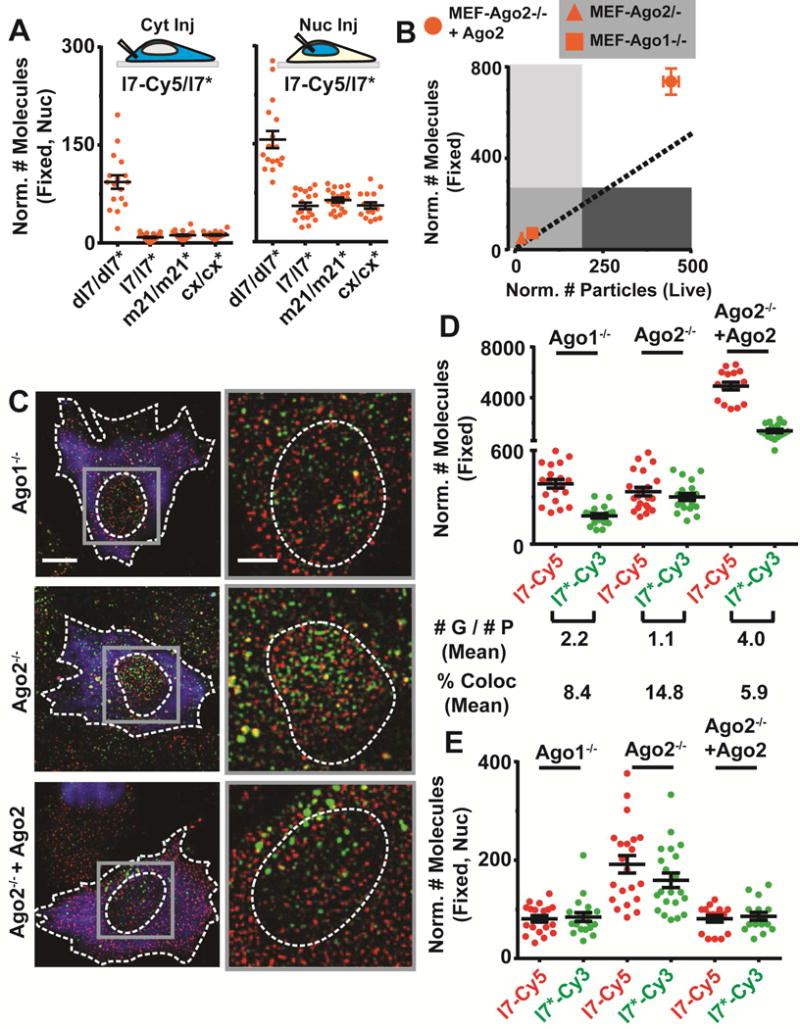
(A) Scatter plot representing the number of l7-Cy5/l7* molecules in the nucleus (n ≥ 2, ≥ 15 cells) when co-injected with a let-7 dsDNA (dl7-Cy5/dl7*), or unlabeled l7/l7*, m21/m21*, cx/cx* dsRNA. Samples were injected into the cytosol (left) or nucleus (right) of U2OS cells. (B) CCA plot of l7-Cy5/l7* when injected in the cytosol of various MEF cells (n ≥ 2, ≥ 10 cells). (C) Representative, pseudocolored images of appropriate MEF cells injected in the cytoplasm with Alexa-405 labeled 500 kDa dextran (blue) and l7-Cy5/l7*-Cy3 miRNA (Cy5 – red, Cy3 - green). Scale bar, 10 μm. Zoomed-in view of particles within grey is depicted to the right of each image. Scale bar 5 μm. (D, E) Scatter plot depicting the number of guide (G) and passenger (P) strand molecules throughout the appropriate MEF cell (D) or within the nucleus (E) is shown (n ≥ 2, ≥ 15 cells), when injected in the cytosol of U2OS cells. The mean G:P ratio is mentioned below plot (D), representing the relative enrichment of guide strands.
Mammalian genomes encode four Ago isoforms, the relative expression of which depends on the cell type (Meister, 2013). The isoforms are thought to be functionally redundant in mediating canonical RNA silencing, but only Ago2 possesses endonucleolytic RNA cleavage (“slicer”) activity. We therefore tested whether miRNA function and nuclear localization are dependent on Ago identity, by employing a panel of three isogenic mouse embryonic fibroblast (MEF) cells that largely lack Ago1 (MEF-Ago1−/−), lack Ago2 (MEF-Ago2−/−), or overexpress Ago2 40-fold over the other two MEFs (MEF-Ago2−/−+Ago2) (Broderick et al., 2011). Consequently, unlabeled ml7/ml7* repressed a cognate reporter with at least 10-fold less potency in MEF-Ago1−/− or MEF-Ago2−/− cells than in MEF-Ago2−/−+Ago2 cells (Figure S7D and Table S3). 3′ Fluorophore labeling of ml7 made these distinctions even more pronounced, as the miRNA was not functional in MEF-Ago1−/− or MEF-Ago2−/− cells even at high doses, whereas it robustly repressed its target in MEF-Ago2−/−+Ago2 cells (Figure S7E). Consistent with these observations, CCA conducted 2 h after microinjecting l7/l7* showed that the miRNA was highly destabilized in MEF-Ago1−/− and MEF-Ago2−/− cells (quadrant III, Figure 5B), whereas stable miRNA assembly into miRISC-mRNA complexes was observed in MEF-Ago2−/− +Ago2 cells (quadrant I, Figure 5B). These data support the notions that Argonaute proteins can be a limiting silencing factor in cells (Broderick et al., 2011; Janas et al., 2012) and that, consequently, miRNA stability and repression activity are enhanced by increasing intracellular Ago levels, at the very least Ago2. To further test whether the lack of specific Ago proteins affects miRNA maturation and nuclear localization, we performed two-color iSHiRLoC fixed-cell counting on l7-Cy5/l7*-Cy3 at the earliest possible time point after microinjection (20 min). We observed, first, that the relative abundances of l7 and l7* were almost identical in MEF-Ago2−/− cells, as expected if both strands are degraded to similar extents; in contrast, there was asymmetric retention of l7 over l7* in both MEF-Ago1−/− and MEF-Ago2−/−+Ago2 cells (Figures 5C and D). Consistent with our observation of limited stability upon depletion of either Ago1 or Ago2, however, the overall levels of l7 in MEF-Ago1−/− and MEF-Ago2−/− cells were similar, and significantly (~10-fold) lower than those in MEF-Ago2−/−+Ago2 cells (Figure 5D). Second, nuclear localization of both l7 and l7* was significantly (~2-fold) higher in MEF-Ago2−/− cells than in either MEF-Ago1−/− or MEF-Ago2−/−+Ago2 cells (Figure 5E). Taken together, our data indicate that miRNA strand selection and unwinding are active only in cells that contain sufficient Ago2 and that a miRNA tends to be stabilized and retained particularly in the cytosol of such cells, whereas Ago1 (and perhaps Ago3 and Ago4) stabilizes miRNAs particularly in the nucleus without unwinding them. Ago2 thus has expanded functions in cytosolic gene silencing, beyond its endonucleolytic capabilities and not served by Ago1/3/4, which appears to play a more dominant role in nuclear retention.
Seed-matched RNA targets enhance nuclear retention of mature miRNAs
To probe the role of RNA targets in nuclear retention of miRNAs directly, we performed iSHiRLoC fixed-cell counting of l7-Cy5/l7* and cx-Cy5/cx* miRNAs in U2OS cells treated with 5 μg/mL actinomycin D (ActD) for 4 h, a transcription inhibitor that drastically reduces levels of nascent transcripts (Bensaude, 2011, Figure S7F). As a result, the relative nuclear localization of l7-Cy5/l7*, 2 h post-injection and incubation in ActD containing media, was significantly reduced, ~3–4-fold for cytoplasmic injections and 5–6-fold for nuclear injections, whereas that of cx-Cy5/cx* remained almost unaltered (Figure 6A). To verify that targets are directly involved in nuclear retention of miRNAs, we co-microinjected either ml7-Cy5/ml7* or cx-Cy5/cx* miRNAs with random or seed-matched mRNA targets into the cytoplasm or nucleus of U2OS cells. We found that miRNAs are retained more strongly in their compartment of injection upon co-microinjection with cognate target (Figure 6B and C). Conversely, co-microinjection of mRNA with seed-matched miRNA allows for a particularly robust cytoplasmic mRNA expression (Figure 6D–F), suggesting that a significant fraction of the mature mRNA is exported from the nucleus and its expression then not effected by the nucleus-retained miRNA. Cytoplasmic co-microinjection resulted in robust RNA silencing, confirming that microinjection does not affect canonical RNA silencing (Figure 6D–F). Furthermore, we confirmed that a majority of the miRNA and mRNA molecules did not prematurely hybridize under our co-microinjection conditions in the needle (Figure S7G), suggesting that miRNA retention by nuclear RNA must involve active unwinding.
Figure 6. Seed-matched targets mediate nuclear localization of miRNAs.
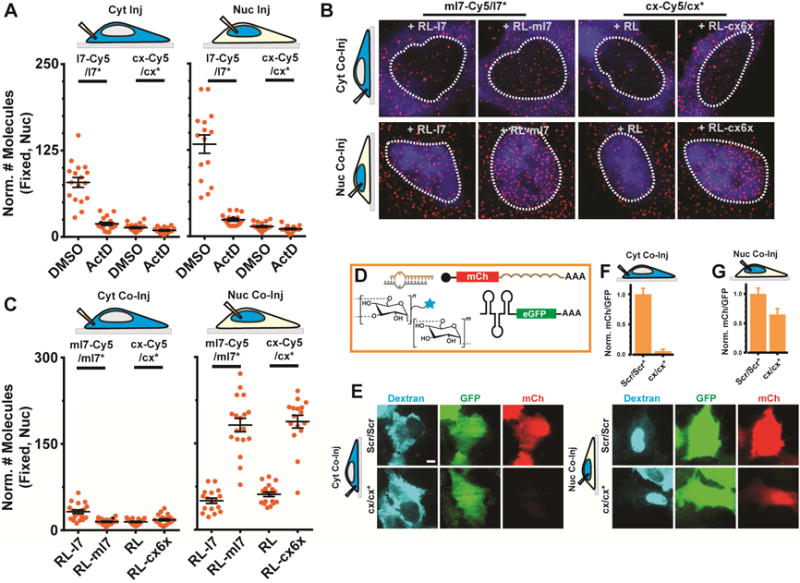
(A) Scatter plot representing the number of l7-Cy5/l7* or cx/cx* miRNA molecules in the nucleus of DMSO or ActD treated cells (n ≥ 2, ≥ 10 cells). Samples were injected into the cytosol (left) or nucleus (right) of U2OS cells and imaged 2 h after microinjection. (B) Representative, pseudocolored images of U2OS cells injected in the cytoplasm (top) or nucleus (bottom) with FITC labeled 500 kDa dextran (blue), guide strand labeled miRNA (ml7-Cy5/ml7* or cx-Cy5/cx*, Cy5 – red) and unlabeled mRNA (RL-l7, RL-ml7, RL-cx6x or RL). Scale bar, 5 μm. (C) Scatter plot depicting the quantifications of samples represented in (B) (n ≥ 2, ≥ 15 cells). (D) Top (left), schematic of assay. (E) Representative images of Dextran (blue), GFP (green) and mCherry (mCh, red) fluorescence in cells coinjected with a scrambled control RNA (Scr/Scr*) or cx/cx* in the cytosol (left panels) or nucleus (right panels) are shown. Scale bar, 10 μm. (F) Quantifications of mCh fluorescence relative to GFP fluorescence for each sample is shown in (E). Error bars, SEM.
DISCUSSION
Small ncRNAs have been found to perform widespread structural, catalytic and regulatory roles in the eukaryotic cell (Cech and Steitz, 2014). Yet, little is known about their spatiotemporal distribution and functionality within their native cellular environment. To bridge this gap, we here have refined our single-cell, single-molecule iSHiRLoC approach (Pitchiaya et al., 2012; Pitchiaya et al., 2013; Shankar et al., 2016) and applied it to mechanistically probe the miRNA-induced cytoplasmic RNA silencing pathway and its non-canonical nuclear counterpart. First, we validated our previous findings on the intracellular assembly of miRNAs (Pitchiaya et al., 2012), developed and validated a correlative live- and fixed-cell counting analysis to probe miRNA stability and function, and extended the applicability of iSHiRLoC to a wider range of cell types. Leveraging these advances, we further found that seed-matched RNA targets enhance miRNA stability and nuclear retention, with Ago1 playing a dominant role in recruiting miRNAs, without unwinding, into low molecular weight nuclear complexes. By contrast, Ago2 unwinds mature miRNAs and forms high-molecular weight miRISC-mRNP complexes primarily in the cytoplasm.
Our human cell data unveil a fundamental competition between incorporation of mature miRNAs into the intracellular RNA silencing and degradation pathways (Figure 7), which is shifted toward silencing by the availability of Ago proteins and RNA targets. These observations are consistent with reports that Ago is typically a limiting factor in enabling miRNA activity (Broderick et al., 2011; Janas et al., 2012) and that the introduction of high levels of siRNAs in knockdown experiments can have side effects by competing with intracellular miRNAs (Khan et al., 2009) (Figures 2G and 5A). We additionally find that threshold target abundance is potentially crucial for miRNA protection (Figure 3). Of note, it is possible that the 3′ fluorophore on our miRNA probes suppresses 3′ tailing and trimming, which in turn decouples 3′ end remodeling from target-mediated effects, allowing us to corroborate target-mediated miRNA protection as a viable pathway for controlling miRNA levels, as previously described to occur during developmental processes (Chatterjee et al., 2011).
Figure 7. Summary of our findings and model consistent with our data.
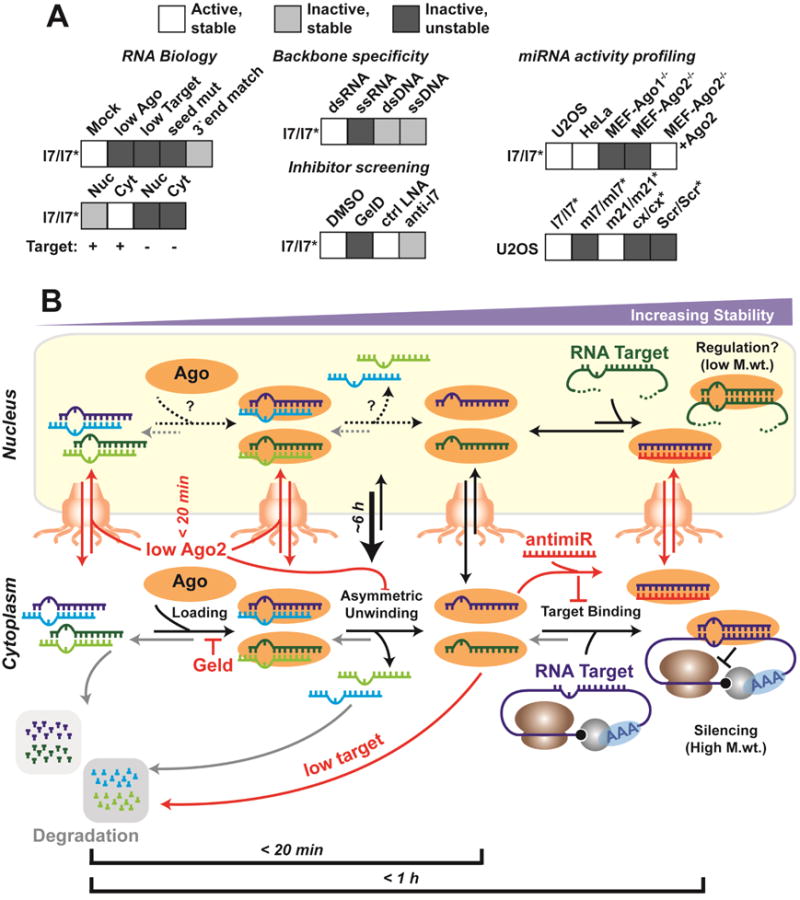
(A) Inferences from CCA of miRNAs. (B) A model depicting the time constants of assembly, turnover and nucleo-cytoplasmic transport of miRNAs.
Strikingly, we find that MEF cells lacking Ago2 exhibit miRNA unwinding and localization profiles (Figure 5) distinct from those lacking Ago1. More specifically, miRNA unwinding was reduced in cells containing Ago1 but lacking Ago2, leading to both strands of the miRNA localizing to the nucleus. This suggests that Ago2 mediates miRNA unwinding and promotes retention of miRNAs in the cytosol for canonical RNA silencing (Figure 7), expanding on the notion that mammalian Ago isoforms exhibit distinct miRNA binding/processing profiles and thus non-redundant functions (Meister, 2013).
Ago-bound small ncRNAs have been reported to perform important functions in the cellular nuclei of several lower organisms. For instance, small ncRNAs mediate RNA induced transcriptional silencing (RITS) and epigenetic regulation in yeast, C. elegans and plants, as well as transposon silencing in fruit flies (Castel and Martienssen, 2013). Moreover, the mammalian let-7 miRNA has been shown to regulate its own biogenesis, hypothetically via nuclear processing of its primary miRNA transcript (Zisoulis et al., 2012). The rapid uptake of our miRNA probes into the nucleus and their RNA target-dependent retention suggest that similar pathways may exist in mammals. Our data indicate that mammalian miRNAs play a role in nuclear RNA-dependent processes such as transcription or splicing. In support of this hypothesis, miRNAs that were co-microinjected with cognate targets into the nucleus of mammalian cells were retained in the nucleus most strongly, even though the co-microinjected mature mRNAs were, as expected, efficiently exported into the cytoplasm, where they faced little RNA silencing from the largely nucleus-retained miRNAs (Figure 6).
Very few methods currently enable detection of single functional miRNAs in live or fixed cells. Tsourkas and coworkers have developed methods to count endogenous miRNAs in situ (Lu and Tsourkas, 2009), but only in formaldehyde fixed cells that lose spatiotemporal features critical to living cells. Schwille and coworkers developed techniques based on microinjection and fluorescence correlation spectroscopy (FCS) to characterize diffusion properties of nuclear and cytoplasmic RISC (Ohrt et al., 2006; Ohrt et al., 2008) in live cells. However, the confocal illumination required by FCS only yields data from selected sub-sections of a mammalian cell at any given time, and deconvolving more than two molecular species of distinct mobility is challenging. Moreover, Schwille and coworkers typically investigated Ago2-bound miRNAs, with limited access to aspects of miRNA binding and turnover not requiring Ago2. We demonstrate here that our iSHiRLoC technique can account for all miRNA molecules introduced into a single cell at a defined zero time point, and provides an unbiased, quantitative survey of intracellular pathways involving miRNAs at single molecule resolution. In live cell experiments, our typical 50–100 ms time resolution elegantly filters out “free” miRNAs (with a diffusion constant of 10–20 μm2/s) and minimal RISC complexes (5–6 μm2/s), both of which can be detected complementarily by FCS (Ohrt et al., 2008). Complementarily, fixed-cell iSHiRLoC experiments account for these complexes, as exemplified by the ability to detect stable, yet non-functional miRNA-anti-miR complexes (Figure 3).
RNA therapeutics have exhibited remarkable promise in treating various pathologies (Kole et al., 2012) and a significant subset of these drugs target the miRNA pathway. For instance, anti-miR drugs that target miR-21 and miR-122 are currently in clinical trials for the treatment of Alport syndrome (Gomez et al., 2015) and pancreatic cancer (Sicard et al., 2013), respectively, as well as for hepatic pathologies (Haussecker and Kay, 2010). Moreover, miRNAs that mimic miR-34 activity (MRX34) have shown promise in targeting previously untreatable forms of cancer (Bouchie, 2013). iSHiRLoC uses individual cells as reaction vessels in ways that promise to facilitate screening for effective oligonucleotide based drugs such as anti-miRs, especially in pre-clinical cell culture models, which has the potential to propel single-cell, single-molecule analysis into the realm of therapeutics.
EXPERIMENTAL PROCEDURES
Plasmids
Description of plasmids pmG-mH3U, pmG-mH3UM, pmG-cx6x, pEF6-mCh-cx6x, pEF6-mH and pEF6-mHM can be found in Supplemental Experimental Procedures.
DNA and RNA oligonucleotides
All DNA and RNA oligonucleotides used for iSHiRLoC experiments were obtained from IDT with a 5′ Phosphate (P) and, in the case of fluorophore labeled oligonucleotides, a Cy3 or Cy5 dye at the 3′ end. G and P strands were heat-annealed in a 1:1.1 or 1:1 ratio, resulting in duplex miRNAs, and were frozen for further use. Negative control siRNA and siluc2 siRNA were purchased as ready-to-use duplex samples from Ambion and Dharmacon respectively. Oligonucleotide sequences are listed in Tables S4 and S5.
mRNA synthesis
In vitro transcriptions and mRNA purifications were performed as previously described (Pitchiaya et al., 2012).
Cell culture
HeLa (CCL-2, ATCC) and U2OS (HTB-96, ATCC) cells were propagated as per supplier’s protocol. MEF cells, namely MEF-Ago1−/−, MEF-Ago2−/− and MEF-Ago2−/− + Ago2 were obtained from Phil Zamore’s lab and were propagated as described (Broderick et al., 2011).
Luciferase reporter assays
100 μL of 10, 000 −20, 000 cells were seeded per well of a 96 well plate. Transfection conditions and luminescence readouts are as described (Pitchiaya et al., 2012; Pitchiaya et al., 2013).
EMSA
Appropriate RNA samples were mixed with non-denaturing gel-loading buffer (10 mM Tris-HCl, 100 mM NaCl, 10 mM EDTA, 0.1% SDS, 0.02% NP40, 10% glycerol) on ice and 15 μL of each sample was loaded onto each well of a pre-cast 20% TBE gel (Life Technologies). Gels were run at 200 V for ~2.5 h at 4 °C.
Quantitative Real-Time RT-PCR (qRT-PCR)
U2OS cells were treated with DMSO or ActD (5 μg/μL) in regular medium for 0 h or 4 h in 6-well plates, harvested, and total RNA was extracted and quantified by qRT-PCR. Primer sequences are listed in Table S6.
Biochemical fractionation and Northern blotting
Nucleo-cytoplasmic fractionation and Northern blotting were performed as described (Gagnon et al., 2014; Hwang et al., 2007). Northern blotting probe sequences are listed in Table S5.
Microinjection
Cells grown on DeltaT dishes (Bioptechs) were microinjected as described (Pitchiaya et al., 2012; Pitchiaya et al., 2013).
Microscopy and Image Analysis
Imaging and analysis was performed as described (Pitchiaya et al., 2012; Pitchiaya et al., 2013) with some minor modifications. Briefly, particle tracking analysis was performed by using tracks that spanned at least four video frames. Stepwise photobleaching analysis in fixed cells was done using custom written Lab-view codes and ImageJ.
Statistical analysis
Graphpad-Prizm and Origin were used for statistical analysis and plotting. For pairwise comparisons, p-values were calculated based on non-parametric unpaired t-tests with Kolmogorov-Smirnov test. For comparisons involving more than two samples, one-way-ANOVA tests were used with Geisser-Greenhouse correction.
Supplementary Material
HIGHLIGHTS.
iSHiRLoC and CCA quantify miRNA unwinding, turnover and activity inside cells
miRNA stability and nuclear retention is dependent on Argonaute and targets
miRNA unwinding, strand selection and cytoplasmic retention are Ago2-dependent
Nuclear miRNAs do not repress cognate targets
Acknowledgments
We thank D. Bartel, C. Mayr, C. Novina, R. Tsien, N. Kedersha and R. Singer for generous gifts of plasmids containing the 3′UTR of HMGA2, 3′UTR of cxcr4, mCherry ORF, Dcp1a ORF and the MS2 system of plasmids respectively, P. Zamore for isogenic MEF cells and A. Mapp and A. Chinnaiyan for access to their plate reader. This work was supported by National Institutes of Health (NIH) R01 grant GM081025 to N.G.W. and an NIH Cellular Biotechnology training grant fellowship to E.J.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Detailed description of experimental methods can be found in Supplemental Experimental Procedures. Supplemental Information includes Supplemental Experimental Procedures, seven figures and six tables.
AUTHOR CONTRIBUTION
S.P. designed and performed all assays. L.A.H. performed the northern blotting analysis. E.J.C. assisted with microinjection experiments. J.I.P. assisted with reporter assays and EMSA. S.P. and N.G.W. conceived the study and all authors wrote the manuscript together.
References
- Allo M, Buggiano V, Fededa JP, Petrillo E, Schor I, de la Mata M, Agirre E, Plass M, Eyras E, Elela SA, et al. Control of alternative splicing through siRNA-mediated transcriptional gene silencing. Nature structural & molecular biology. 2009;16:717–724. doi: 10.1038/nsmb.1620. [DOI] [PubMed] [Google Scholar]
- Ameres SL, Horwich MD, Hung JH, Xu J, Ghildiyal M, Weng Z, Zamore PD. Target RNA-directed trimming and tailing of small silencing RNAs. Science. 2010;328:1534–1539. doi: 10.1126/science.1187058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarini A, Chauhan H, Gardner TJ, Jayaprakash AD, Sachidanandam R, Brown BD. Kinetic analysis reveals the fate of a microRNA following target regulation in mammalian cells. Current biology: CB. 2011;21:369–376. doi: 10.1016/j.cub.2011.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensaude O. Inhibiting eukaryotic transcription: Which compound to choose? How to evaluate its activity? Transcription. 2011;2:103–108. doi: 10.4161/trns.2.3.16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchie A. First microRNA mimic enters clinic. Nature biotechnology. 2013;31:577. doi: 10.1038/nbt0713-577. [DOI] [PubMed] [Google Scholar]
- Broderick JA, Salomon WE, Ryder SP, Aronin N, Zamore PD. Argonaute protein identity and pairing geometry determine cooperativity in mammalian RNA silencing. Rna. 2011;17:1858–1869. doi: 10.1261/rna.2778911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanotto D, Lingeman R, Riggs AD, Rossi JJ. CRM1 mediates nuclear-cytoplasmic shuttling of mature microRNAs. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21655–21659. doi: 10.1073/pnas.0912384106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanotto D, Tommasi S, Li M, Li H, Yanow S, Pfeifer GP, Rossi JJ. Short hairpin RNA-directed cytosine (CpG) methylation of the RASSF1A gene promoter in HeLa cells. Molecular therapy : the journal of the American Society of Gene Therapy. 2005;12:179–183. doi: 10.1016/j.ymthe.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Castel SE, Martienssen RA. RNA interference in the nucleus: roles for small RNAs in transcription, epigenetics and beyond. Nature reviews Genetics. 2013;14:100–112. doi: 10.1038/nrg3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157:77–94. doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Chandradoss SD, Schirle NT, Szczepaniak M, MacRae IJ, Joo C. A Dynamic Search Process Underlies MicroRNA Targeting. Cell. 2015;162:96–107. doi: 10.1016/j.cell.2015.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Fasler M, Bussing I, Grosshans H. Target-mediated protection of endogenous microRNAs in C. elegans. Developmental cell. 2011;20:388–396. doi: 10.1016/j.devcel.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Grosshans H. Active turnover modulates mature microRNA activity in Caenorhabditis elegans. Nature. 2009;461:546–549. doi: 10.1038/nature08349. [DOI] [PubMed] [Google Scholar]
- Gagnon KT, Li L, Chu Y, Janowski BA, Corey DR. RNAi factors are present and active in human cell nuclei. Cell reports. 2014;6:211–221. doi: 10.1016/j.celrep.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez IG, MacKenna DA, Johnson BG, Kaimal V, Roach AM, Ren S, Nakagawa N, Xin C, Newitt R, Pandya S, et al. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. The Journal of clinical investigation. 2015;125:141–156. doi: 10.1172/JCI75852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha M, Kim VN. Regulation of microRNA biogenesis. Nature reviews Molecular cell biology. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- Haussecker D, Kay MA. miR-122 continues to blaze the trail for microRNA therapeutics. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18:240–242. doi: 10.1038/mt.2009.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homolya L, Hollo Z, Germann UA, Pastan I, Gottesman MM, Sarkadi B. Fluorescent cellular indicators are extruded by the multidrug resistance protein. The Journal of biological chemistry. 1993;268:21493–21496. [PubMed] [Google Scholar]
- Hwang HW, Wentzel EA, Mendell JT. A hexanucleotide element directs microRNA nuclear import. Science. 2007;315:97–100. doi: 10.1126/science.1136235. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Kobayashi M, Yoda M, Sakaguchi Y, Katsuma S, Suzuki T, Tomari Y. Hsc70/Hsp90 chaperone machinery mediates ATP-dependent RISC loading of small RNA duplexes. Molecular cell. 2010;39:292–299. doi: 10.1016/j.molcel.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Janas MM, Wang B, Harris AS, Aguiar M, Shaffer JM, Subrahmanyam YV, Behlke MA, Wucherpfennig KW, Gygi SP, Gagnon E, et al. Alternative RISC assembly: binding and repression of microRNA-mRNA duplexes by human Ago proteins. Rna. 2012;18:2041–2055. doi: 10.1261/rna.035675.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowski BA, Huffman KE, Schwartz JC, Ram R, Hardy D, Shames DS, Minna JD, Corey DR. Inhibiting gene expression at transcription start sites in chromosomal DNA with antigene RNAs. Nature chemical biology. 2005;1:216–222. doi: 10.1038/nchembio725. [DOI] [PubMed] [Google Scholar]
- Janowski BA, Huffman KE, Schwartz JC, Ram R, Nordsell R, Shames DS, Minna JD, Corey DR. Involvement of AGO1 and AGO2 in mammalian transcriptional silencing. Nature structural & molecular biology. 2006;13:787–792. doi: 10.1038/nsmb1140. [DOI] [PubMed] [Google Scholar]
- Janowski BA, Younger ST, Hardy DB, Ram R, Huffman KE, Corey DR. Activating gene expression in mammalian cells with promoter-targeted duplex RNAs. Nature chemical biology. 2007;3:166–173. doi: 10.1038/nchembio860. [DOI] [PubMed] [Google Scholar]
- Kai ZS, Pasquinelli AE. MicroRNA assassins: factors that regulate the disappearance of miRNAs. Nature structural & molecular biology. 2010;17:5–10. doi: 10.1038/nsmb.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee HL, Dishinger JF, Blasius TL, Liu CJ, Margolis B, Verhey KJ. A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nature cell biology. 2012;14:431–437. doi: 10.1038/ncb2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Betel D, Miller ML, Sander C, Leslie CS, Marks DS. Transfection of small RNAs globally perturbs gene regulation by endogenous microRNAs. Nature biotechnology. 2009;27:549–555. doi: 10.1038/nbt.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khudayberdiev SA, Zampa F, Rajman M, Schratt G. A comprehensive characterization of the nuclear microRNA repertoire of post-mitotic neurons. Frontiers in molecular neuroscience. 2013;6:43. doi: 10.3389/fnmol.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Villeneuve LM, Morris KV, Rossi JJ. Argonaute-1 directs siRNA-mediated transcriptional gene silencing in human cells. Nature structural & molecular biology. 2006;13:793–797. doi: 10.1038/nsmb1142. [DOI] [PubMed] [Google Scholar]
- Kole R, Krainer AR, Altman S. RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nature reviews Drug discovery. 2012;11:125–140. doi: 10.1038/nrd3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AK. The Whereabouts of microRNA Actions: Cytoplasm and Beyond. Trends in cell biology. 2015;25:601–610. doi: 10.1016/j.tcb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LC, Okino ST, Zhao H, Pookot D, Place RF, Urakami S, Enokida H, Dahiya R. Small dsRNAs induce transcriptional activation in human cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17337–17342. doi: 10.1073/pnas.0607015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JY, Ma LM, Guo YH, Zhang YC, Zhou H, Shao P, Chen YQ, Qu LH. Deep sequencing of human nuclear and cytoplasmic small RNAs reveals an unexpectedly complex subcellular distribution of miRNAs and tRNA 3′ trailers. PloS one. 2010;5:e10563. doi: 10.1371/journal.pone.0010563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nature reviews Cancer. 2015;15:321–333. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Hu J, Corey DR. Expanding the action of duplex RNAs into the nucleus: redirecting alternative splicing. Nucleic acids research. 2012;40:1240–1250. doi: 10.1093/nar/gkr780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Lavis LD, Betzig E. Imaging live-cell dynamics and structure at the single-molecule level. Molecular cell. 2015;58:644–659. doi: 10.1016/j.molcel.2015.02.033. [DOI] [PubMed] [Google Scholar]
- Lu J, Tsourkas A. Imaging individual microRNAs in single mammalian cells in situ. Nucleic acids research. 2009;37:e100. doi: 10.1093/nar/gkp482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtke JJ, Sebestyen MG, Wolff JA. The effect of cell division on the cellular dynamics of microinjected DNA and dextran. Molecular therapy : the journal of the American Society of Gene Therapy. 2002;5:579–588. doi: 10.1006/mthe.2002.0581. [DOI] [PubMed] [Google Scholar]
- Macdonald P, Johnson J, Smith E, Chen Y, Mueller JD. Brightness analysis. Methods in enzymology. 2013;518:71–98. doi: 10.1016/B978-0-12-388422-0.00004-2. [DOI] [PubMed] [Google Scholar]
- Matsui M, Chu Y, Zhang H, Gagnon KT, Shaikh S, Kuchimanchi S, Manoharan M, Corey DR, Janowski BA. Promoter RNA links transcriptional regulation of inflammatory pathway genes. Nucleic acids research. 2013;41:10086–10109. doi: 10.1093/nar/gkt777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G. Argonaute proteins: functional insights and emerging roles. Nature reviews Genetics. 2013;14:447–459. doi: 10.1038/nrg3462. [DOI] [PubMed] [Google Scholar]
- Morris KV, Chan SW, Jacobsen SE, Looney DJ. Small interfering RNA-induced transcriptional gene silencing in human cells. Science. 2004;305:1289–1292. doi: 10.1126/science.1101372. [DOI] [PubMed] [Google Scholar]
- Napoli S, Pastori C, Magistri M, Carbone GM, Catapano CV. Promoter-specific transcriptional interference and c-myc gene silencing by siRNAs in human cells. The EMBO journal. 2009;28:1708–1719. doi: 10.1038/emboj.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohrt T, Merkle D, Birkenfeld K, Echeverri CJ, Schwille P. In situ fluorescence analysis demonstrates active siRNA exclusion from the nucleus by Exportin 5. Nucleic acids research. 2006;34:1369–1380. doi: 10.1093/nar/gkl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohrt T, Mutze J, Staroske W, Weinmann L, Hock J, Crell K, Meister G, Schwille P. Fluorescence correlation spectroscopy and fluorescence cross-correlation spectroscopy reveal the cytoplasmic origination of loaded nuclear RISC in vivo in human cells. Nucleic acids research. 2008;36:6439–6449. doi: 10.1093/nar/gkn693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nature reviews Genetics. 2012;13:271–282. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- Pitchiaya S, Androsavich JR, Walter NG. Intracellular single molecule microscopy reveals two kinetically distinct pathways for microRNA assembly. EMBO reports. 2012;13:709–715. doi: 10.1038/embor.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchiaya S, Krishnan V, Custer TC, Walter NG. Dissecting non-coding RNA mechanisms in cellulo by Single-molecule High-Resolution Localization and Counting. Methods. 2013;63:188–199. doi: 10.1016/j.ymeth.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran V, Chen X. Degradation of microRNAs by a family of exoribonucleases in Arabidopsis. Science. 2008;321:1490–1492. doi: 10.1126/science.1163728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roush S, Slack FJ. The let-7 family of microRNAs. Trends in cell biology. 2008;18:505–516. doi: 10.1016/j.tcb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Ruegger S, Grosshans H. MicroRNA turnover: when, how, and why. Trends in biochemical sciences. 2012;37:436–446. doi: 10.1016/j.tibs.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Shankar S, Pitchiaya S, Malik R, Kothari V, Hosono Y, Yocum AK, Gundlapalli H, White Y, Firestone A, Cao X, et al. KRAS Engages AGO2 to Enhance Cellular Transformation. Cell reports. 2016;14:1448–1461. doi: 10.1016/j.celrep.2016.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard F, Gayral M, Lulka H, Buscail L, Cordelier P. Targeting miR-21 for the therapy of pancreatic cancer. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21:986–994. doi: 10.1038/mt.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting AH, Schuebel KE, Herman JG, Baylin SB. Short double-stranded RNA induces transcriptional gene silencing in human cancer cells in the absence of DNA methylation. Nature genetics. 2005;37:906–910. doi: 10.1038/ng1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann L, Hock J, Ivacevic T, Ohrt T, Mutze J, Schwille P, Kremmer E, Benes V, Urlaub H, Meister G. Importin 8 is a gene silencing factor that targets argonaute proteins to distinct mRNAs. Cell. 2009;136:496–507. doi: 10.1016/j.cell.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes & development. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisoulis DG, Kai ZS, Chang RK, Pasquinelli AE. Autoregulation of microRNA biogenesis by let-7 and Argonaute. Nature. 2012;486:541–544. doi: 10.1038/nature11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


