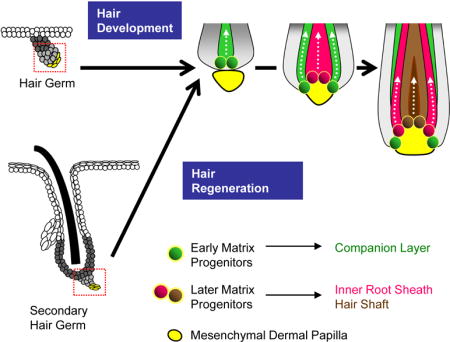
SUMMARY
During development and regeneration, matrix progenitors undergo terminal differentiation to form the concentric layers of the hair follicle. These differentiation events are thought to require signals from the mesenchymal dermal papilla (DP); however, it remains unclear how DP-progenitor cell interactions govern specific cell fate decisions. Here, we show that the hair follicle differentiated layers are specified asynchronously, with early matrix progenitors initiating differentiation prior to surrounding the DP. Furthermore, these early matrix cells can undergo terminal differentiation in the absence of Shh, BMP signaling, and DP maturation. Whereas early matrix progenitors form the hair follicle companion layer, later matrix populations progressively form the inner root sheath and hair shaft. Together, our findings characterize some of the earliest terminal differentiation events in the hair follicle, and reveal that the matrix progenitor pool can be divided into early and late phases based on distinct temporal, molecular and functional characteristics.
Keywords: keratin 79, hair follicle, progenitor cell, matrix cell, hair cycle, companion layer, inner root sheath
Graphical abstract
INTRODUCTION
The hair on our skin is a mammalian innovation that serves numerous functions, including thermoregulation, sensation and protection against the environment (Blanpain and Fuchs, 2009; Schneider and Paus, 2014). Hair follicles, which house the differentiated cells of the hair shaft, are initially formed from placodes that condense throughout the epidermis during embryonic development (Biggs and Mikkola, 2014). In mice, these placodes appear in several waves commencing at embryonic day 14.5 and ending at around birth. These epithelial buds subsequently extend into the underlying dermis (hair germ and hair peg stages) and surround the mesenchymal dermal papilla (DP), a key signaling and organizing center at the base of the follicle (Enshell-Seijffers et al., 2010; Rompolas et al., 2012; Woo et al., 2012).
Although hair follicle specification ceases around the time of birth, existing follicles are periodically renewed throughout life. This process, known as the hair cycle, consists of cyclic phases of rest (telogen), growth (anagen) and regression (catagen), and recapitulates many of the events seen during initial hair follicle development (Schmidt-Ullrich and Paus, 2005). During regeneration, hair follicle stem cells within the lower bulge and secondary hair germ contribute to the outer root sheath (ORS) and matrix progenitors, respectively (Greco et al., 2009; Panteleyev et al., 2001; Rompolas et al., 2013; Zhang et al., 2009). Eventually, these matrix progenitors will terminally differentiate into cells that form the concentric layers of the hair shaft, the inner root sheath (IRS) and the companion layer (CL).
How is the intricate and stereotyped radial configuration of these different layers achieved? Detailed lineage tracing studies have suggested that matrix cells, which juxtapose and receive anagen-inducing signals from the DP, display a spatial organization that determines their fate (Langbein et al., 2002; Legue and Nicolas, 2005; Sequeira and Nicolas, 2012). Matrix cells located more centrally in the anagen hair bulb form the innermost layers that comprise the hair shaft, whereas more peripherally-located matrix cells generate the outer differentiated layers, the IRS and CL (Sequeira and Nicolas, 2012).
These observations suggest that the location of matrix progenitors along the hair follicle medial-lateral axis largely governs their fate. However, it is important to note that these progenitors are not a static population, but rather one that expands and changes shape as the lower hair bulb envelopes the DP during development and regeneration (Muller-Rover et al., 2001; Paus et al., 1999) (Figure 1A–B). Thus, these progenitor populations may make varying cell fate decisions that hinge upon evolving spatial and temporal cues.
Figure 1. Asynchronous specification of terminally differentiated cell layers.
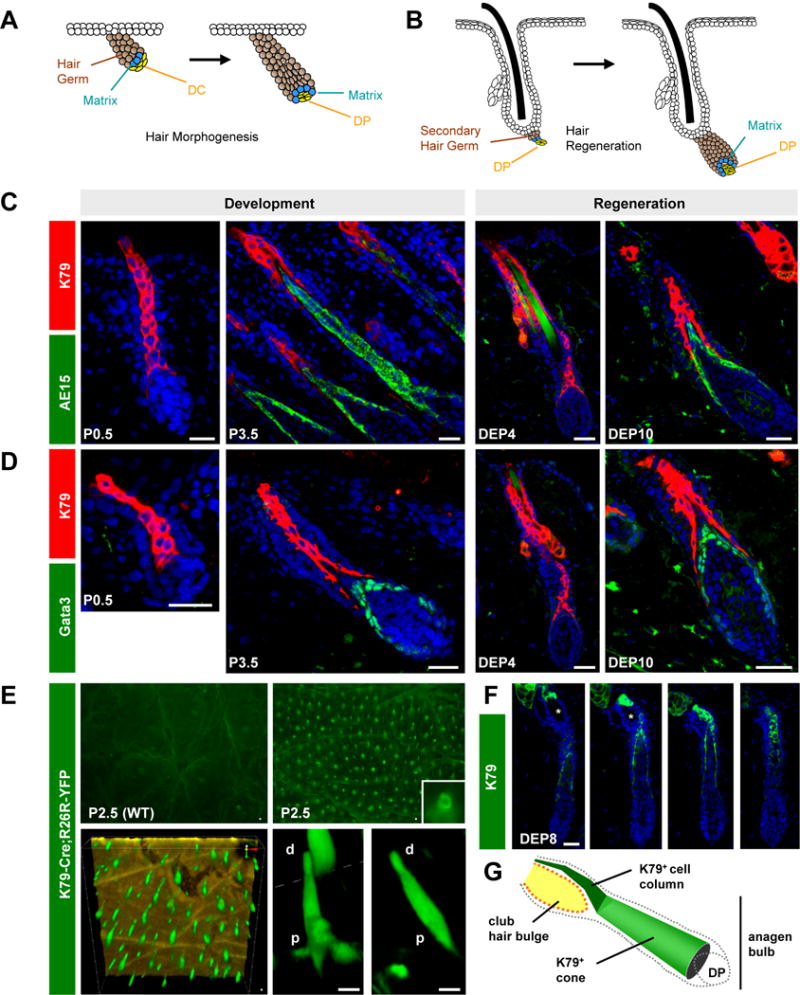
A. Schematic of early hair development. The mesenchymal dermal condensate (DC) matures into the dermal papilla (DP), which is surrounded by the growing hair germ (brown). Matrix progenitors (blue) are thought to differentiate only after surrounding the DP. B. Similarly, the secondary hair germ (brown) engulfs the DP during hair regeneration. Images not drawn to scale. C. K79 precedes AE15 during hair development and regeneration. D. K79 precedes Gata3. E. K79-Cre;R26R-YFP mice possess epifluorescent hair canals in P2.5 whole-mount skin viewed from the surface (top right). Bottom panels, confocal imaging from the skin underside, with K79+ cells (green) forming a cone that is wider at the base and narrower at the tip. The epidermis is colored gold (bottom left). Bottom right, magnified views of individual follicles. p, proximal; d, distal. F. Serial sections through an adult early anagen follicle, with K79+ cells (green) forming a cone that narrows into a column near the bulge (asterisk). G. Schematic of K79+ cone and column in the anagen follicle. P, postnatal day. DEP, days post-depilation. Scale bars, 50 μm.
Among the terminally differentiated cells in the growing hair follicle, the IRS and CL are thought to arise from adjacently-located matrix progenitors, and have been reported to share similar growth kinetics, morphology and expression of markers such as Cutl1/CDP (Ellis et al., 2001; Gu and Coulombe, 2007; Morioka, 2005; Rothnagel and Roop, 1995; Sequeira and Nicolas, 2012; Winter et al., 1998). Elaborate desmosomal and gap junction contacts between the CL and IRS have also been noted (Langbein et al., 2002), which may enable upward-moving IRS cells to “pull” CL cells up alongside the anagen follicle (Chapman, 1971; Orwin, 1971). Given the extensive similarities and physical connections between the IRS and CL, this has led to speculation that these layers may act as an interdependent complex, with the CL essentially serving as the outermost layer of the IRS (Ellis et al., 2001; Sequeira and Nicolas, 2012).
Our previous studies identified Keratin 79 (K79) as a marker of early differentiating cells that form the CL (Veniaminova et al., 2013). We now show that CL cells are specified prior to other terminally differentiated cells in the hair follicle. Given the early appearance of these cells, we traced their origins back to a primitive matrix population that differentiates both prior to DP engulfment and independently of BMP signaling and Shh. Finally, we provide evidence that K79 is not required for hair growth, that the CL is distinct from the IRS, and that CL cells are lost during hair regression.
RESULTS
Asynchronous formation of terminally differentiated cell layers in the hair follicle
We previously reported that K79 identifies an early population of terminally differentiated cells within hair germs during development and secondary hair germs during physiological hair cycling (Veniaminova et al., 2013). In both instances, K79+ cells form columns that extend outwards. To place the appearance of these cells in the context of other events that occur during hair growth, we began by assessing the specification of K79+ cells relative to other differentiated cells in the hair follicle.
IRS cells first appear in Stage 4 hair pegs and in Anagen IIIa regenerating follicles, which have fully engulfed the DP at this point (Muller-Rover et al., 2001; Paus et al., 1994). Interestingly, in earlier stage hair germs and in Anagen II regenerating follicles, K79+ cells already formed a solid column (Figure 1C–D). In contrast, IRS cells were not detected at these stages, as assessed by the markers trichohyalin (AE15) and Gata3 (Kaufman et al., 2003) (Figure 1C–D). When IRS cells eventually did appear in later stage follicles, these IRS cells pushed upwards through the middle of the existing K79+ column, causing those cells to separate into a cone-like configuration at the proximal end (Figure 1C–D).
We next generated transgenic mice expressing a Cre-GFP fusion protein under the control of the K79 promoter (K79-Cre) to better visualize these differentiated cells. When coupled with a Cre-inducible YFP reporter allele (K79-Cre;R26R-YFP), P2.5 animals displayed epifluorescent follicles (Figure 1E). Upon imaging the under-surface of freshly excised skin by confocal microscopy, we observed K79+ columns that appeared wider at the base, where the IRS cone was forming, and narrower at the tip (Figure 1E). Although the thickness of adult skin precluded us from performing similar imaging studies during regeneration, serial sections confirmed that K79+ cells also formed a wide, cone-like shape at the base that was continuous with a narrow column of cells extending up around the club hair bulge (Figure 1F–G). Altogether, these observations indicate that terminal differentiation occurs asynchronously in the growing hair follicle, and that K79+ cells are specified earlier than other terminally differentiated cell types.
The CL is specified prior to canonical K75 and K6 expression
Our previous studies suggested that K79+ cells become the CL, which is comprised of a flat layer of cells that initially expresses Keratin 75 (K75) and then Keratin 6 (K6) (Gu and Coulombe, 2007; Rothnagel et al., 1999; Smyth et al., 2004; Winter et al., 1998; Wojcik et al., 2001). Although the cellular origins of the CL have been somewhat controversial, several studies have argued that the CL is related to the IRS and that the two layers form concomitantly (Chapman, 1971; Gu and Coulombe, 2007; Orwin, 1971). However, since K79+ cells appear prior to the IRS, we decided to reassess whether canonical CL markers such as K75 and K6 might also be expressed in the early regenerating follicle.
Surprisingly, early K79+ cell columns did not express K75 (Figure 2A). Rather, we detected K79/K75 co-localization only in more advanced anagen follicles, with K75 expression originating proximally in the hair bulb and extending outward in a “bulb to bulge” direction, consistent with prior reports (Gu and Coulombe, 2007) (Figure 2B–C). We made similar observations with K6, which co-localized with K79+ cells in the CL only in later stage follicles, but whose expression extended in an opposing inward “bulge to bulb” direction, as has also previously been observed (Gu and Coulombe, 2007) (Figure 2D–E).
Figure 2. The CL undergoes dynamic maturation.
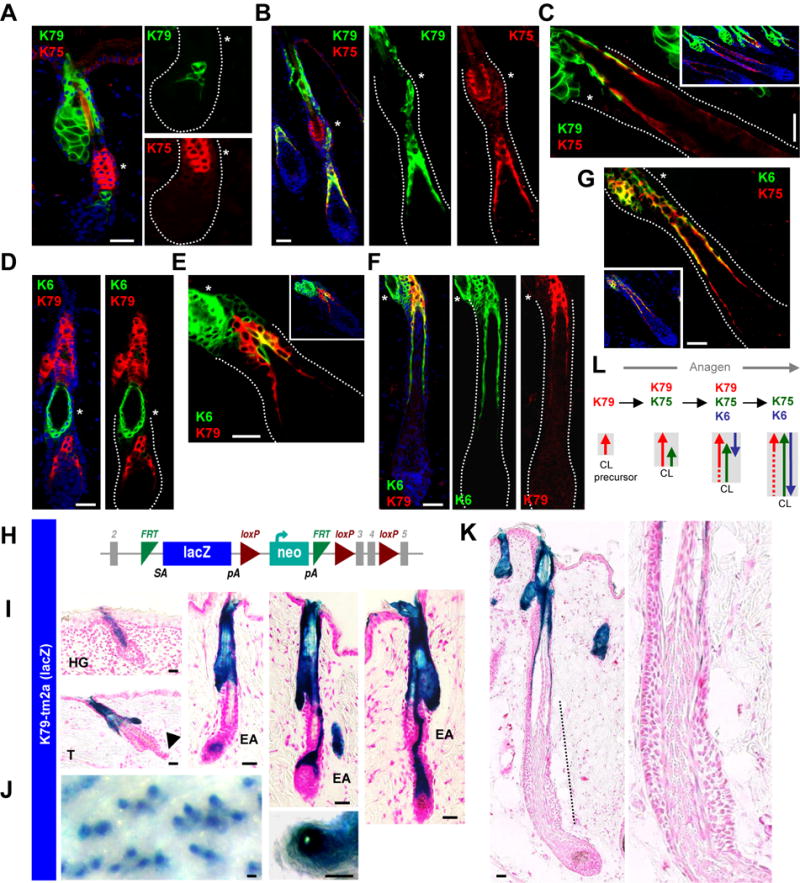
A. K79 appears prior to K75 in anagen II regenerating follicles. B. K79 and K75 co-localize in the CL during Anagen III. C. K79 is lost from the K75+ CL in Anagen IV-V follicles. D. K79 precedes K6 in Anagen II follicles. E. K6 later overlays with K79 in Anagen III follicles, beginning at the distal CL. F. K79 is lost from the K6+ CL in Anagen IV-V follicles. G. The mature CL at this stage is K75+K6+K79−. H. Schematic of the K79tm2a allele, where LacZ is inserted into the endogenous K79 locus. I. β-gal activity in K79tm2a/+ skin recapitulates K79 expression in developing hair germs (HG), during telogen (T) and early anagen (EA). Note the absence of β-gal/K79 in the telogen secondary hair germ (arrowhead). J. Whole-mount K79tm2a/+ telogen skin from 8 week old mice, showing labeled hair canals. K. β-gal activity is absent from the lower bulb of an Anagen V-VI follicle (dotted line), consistent with loss of K79. Right, magnified view of lower follicle. L. Schematic summarizing keratin shifts in the growing CL (gray box), with arrows indicating direction by which keratin expression appears. Dotted lines indicate weak or no expression. In panels with multiple boxes, these are separated channel views with the bulge indicated by an asterisk. Scale bars, 50 μm.
By mid-anagen, we further noted that K79 is largely lost from the CL, which now expresses only K75 and K6 (Figure 2C, F–G). To confirm these shifts in K79 expression, we generated mice in which a lacZ cassette, encoding β-galactosidase (β-gal), was inserted into the endogenous K79 locus, thereby inactivating the allele but also serving as a reporter for K79 promoter activity (K79tm2a mice) (Figure 2H). K79tm2a/+ mice did not display any overt abnormalities, while β-gal activity recapitulated the expression pattern for K79 in the skin (Quigley et al., 2016; Veniaminova et al., 2013). This included strong activity in columns extending out from hair germs; in the sebaceous glands and suprabasal cells of the infundibulum in adult follicles; and in the early CL (Figure 2I–J). β-gal activity, however, was absent from the lower late anagen follicle, consistent with the loss of K79 expression from the mature CL (Figure 2K). These observations indicate that the CL undergoes a dynamic maturation process throughout anagen, and that the CL is already specified before gaining canonical K75 and K6 expression. These findings may possibly explain why the exact origins of the CL have been difficult to elucidate, and also why asynchronous formation of this cell layer has not been previously documented (Figure 2L).
Early matrix progenitors give rise to K79+ cells
Terminal differentiation in the hair follicle is thought to occur only after matrix cells have surrounded the DP (Muller-Rover et al., 2001; Paus et al., 1994). However, since K79+ cells appear earlier than other differentiated cells, this suggested to us that a primitive matrix population might be functioning even prior to DP engulfment. We therefore delved deeper into the origins of K79+ cells, reasoning that if we can identify the earliest differentiation events that occur within the follicle, this will enable us to pinpoint the moment when matrix cells first become functional.
Previous studies have shown that Sonic hedgehog (Shh) is expressed in hair placodes and later restricted to the base of developing follicles (Chiang et al., 1999; Oro and Higgins, 2003; St-Jacques et al., 1998). Using mice that express EGFP-Cre from the endogenous Shh locus (Shh-EGFP-Cre), coupled with a Cre-inducible reporter allele, Levy et al., demonstrated that Shh+ progenitors give rise to the hair follicle, but not to the interfollicular epidermis (Levy et al., 2005). Even in the absence of a conditional reporter allele, Shh-EGFP-Cre mice display highly fluorescent Shh+ matrix progenitors (Harfe et al., 2004; Levy et al., 2005). Importantly, we took advantage of the fact that the immediate, Shh-negative progeny of these cells are also weakly fluorescent, likely due to the transient persistance of EGFP-Cre fusion protein. This is illustrated by the slightly expanded territory of EGFP+ cells relative to the Shh+ domain at the base of the hair germ (Figure 3A). Therefore, in contrast to conventional lineage tracing strategies where a cell progenitor and all its direct and indirect descendents become permanently marked, these Shh-EGFP-Cre mice provided us a convenient snapshot of Shh+ progenitors and only their most direct, weakly fluorescent progeny at all times.
Figure 3. Early matrix progenitors initiate terminal differentation in hair germs.
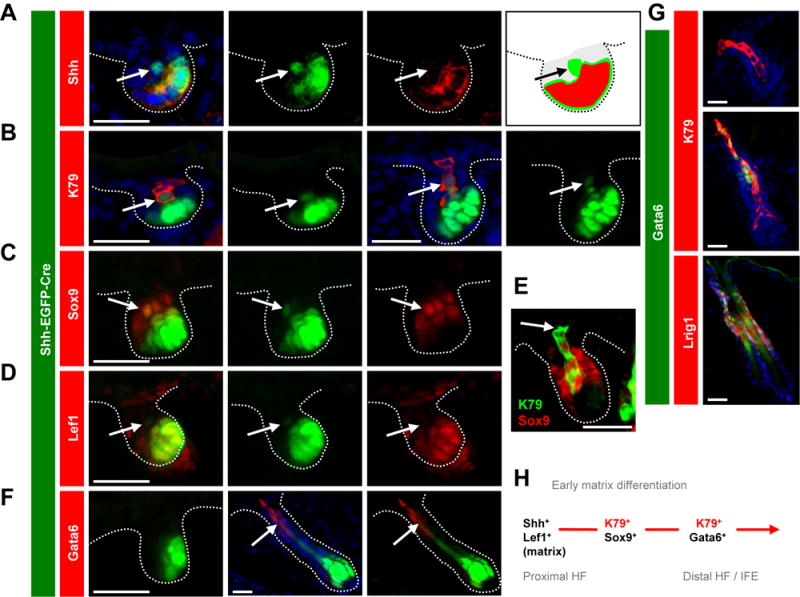
A-D. P2.5 hair germs from Shh-EGFP-Cre skin display EGFP expression in early matrix progenitors (bright green) and in their immediate progeny (light green, arrows). These progeny are Shh−, K79+, Sox9+ and Lef1−, as indicated (red). E. K79+ columns lose Sox9 distally in later stage hair germs in P2.5 skin (arrow). F. Gata6 is not in hair germs (left), but is present in distal cells in hair pegs (middle and right, arrows) at P2.5. G. In P2.5 skin, Gata6 is not in hair germs (top) but overlays with distal K79+ cells in hair pegs (middle). In adult telogen follicles, Gata6 overlays with Lrig1 (bottom). H. Schematic of early matrix differentiation. In panels with multiple boxes, these are separated channel views. Scale bars, 50 μm. See also Figures S1 and S6.
Using P2.5 Shh-EGFP-Cre newborn skin, we focused on hair germs that had initiated at around the time of birth and had not surrounded the DP. We observed that single K79+, EGFP-weak suprabasal cells were often centrally located immediately above EGFP-high, Shh+ matrix cell clusters (Figure 3B). We confirmed that these K79+ cells were Shh-negative, thereby suggesting that Shh+ matrix cells directly give rise to overlying K79+ suprabasal cells (Figure S1). Similarly, in slightly later stage hair germs, columns of K79+ cells were observed, with only the most proximal 1–2 cells retaining weak EGFP (Figure 3B). We further noted that the base of K79+ columns was comprised of Sox9+ cells that lacked Wnt pathway activity, as assessed by Lef1 expression (Figure 3C–E and Figure S1). As Sox9+/Wnt-negative cells in the early hair bud have been reported to specify future bulge stem cells (Nowak et al., 2008; Ouspenskaia et al., 2016), our findings here suggest that at least a subset of these cells, directly derived from early Shh+ progenitors and expressing K79, are already terminally differentiated.
Finally, we observed using Oncomine that expression of K79 is highly correlated with Gata6 in vulvar intraepithelial neoplasia (Santegoets et al., 2007). Gata6 mRNA is also enriched in sorted hair follicle stem cells (Rezza et al., 2016), in particular those expressing Lrig1 (Page et al., 2013), which give rise to suprabasal K79+ cells in the infundibulum (Veniaminova et al., 2013). In late stage hair pegs, we determined that Gata6 is expressed in distal K79+ cells, but is not present in earlier stage hair germs (Figure 3F). Furthermore, Gata6+ cells did not directly originate from Shh+ progenitors, but did overlay with Lrig1+ stem cells in the adult hair follicle isthmus (Jensen et al., 2009) (Figure 3F–G). Taken together, these data suggest that early matrix cells can undergo terminal differentiation even prior to DP engulfment. Subsequently, K79+ differentiated cells gain some markers (Gata6) while losing others (Shh, Sox9, Lef1) during maturation (Figure 3H).
Early and late matrix progenitors exhibit molecular differences
Thus far, we have defined early matrix progenitors based on their ability to form K79+ cells prior to surrounding the DP. To determine whether these progenitors are molecularly distinct from later matrix populations in the anagen bulb, we assessed the expression of canonical matrix markers, including nuclear localization of Lef1, Msx2 and phosphorylated Smad1/5 (pSmad).
To examine cycling hair follicles in synchrony, we focused our analysis on adult anagen follicles from Shh-EGFP-Cre mice that were depilated for either 6 or 8 days, timepoints before and after DP engulfment, respectively. Similar to during development, we confirmed that early Shh+ progenitors in the secondary hair germ directly give rise to K79+ cells by 6 days post-depilation (Figure 4A). We next co-localized canonical matrix markers with Shh expression, and observed that this early population exhibited strong Lef1 nuclear localization, confirming that these are true matrix cells (Figure 4B). For Msx2, we observed occasional nuclear staining, typically in cells located at the periphery of the Shh+ cell cluster (Figure 4C). Importantly, although we observed strong nuclear pSmad in the bulge, as previously reported (Andl et al., 2004; Genander et al., 2014), early Shh+ matrix progenitors typically displayed only diffuse pSmad staining (Figure 4D). In contrast, later matrix cells surrounding the DP exhibited nuclear localization of all 3 markers (Figure 4B–D). These findings suggest that early and late matrix progenitors activate overlapping but also distinct pathways, possibly due to evolving interactions with the DP, and that this may influence their subsequent cell fate decisions.
Figure 4. Early matrix progenitors are molecularly distinct from later matrix populations.
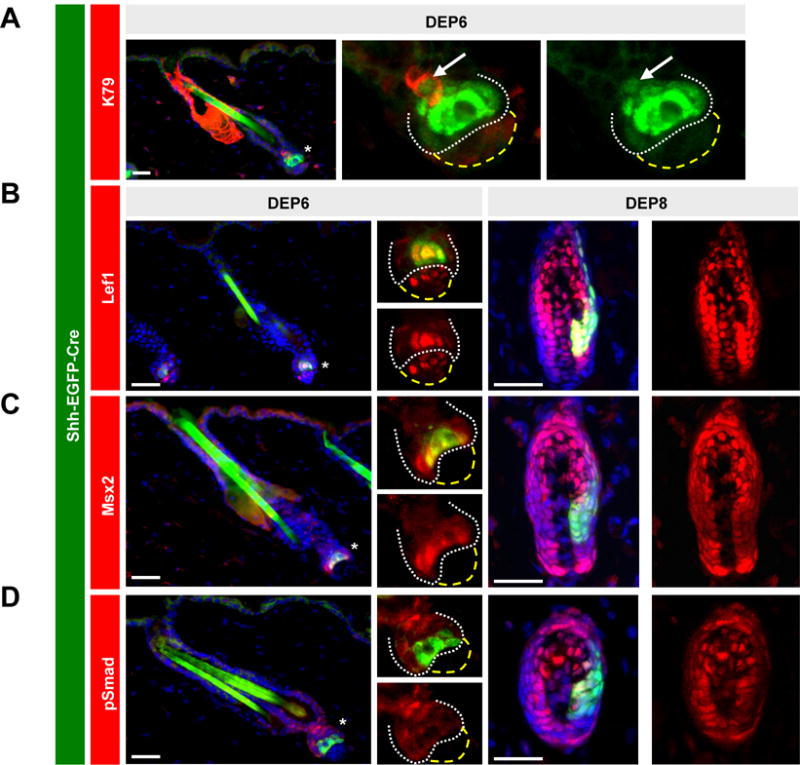
A. 6 days post-depilation, regenerating follicles from adult Shh-EGFP-Cre mice display EGFP expression in early matrix progenitors (bright green) and in their K79+ immediate progeny (light green, arrows). B-D. Left boxes, early matrix cells (green) from Shh-EGFP-Cre skin exhibit Lef1, occasional Msx2, and no pSmad nuclear localization, as indicated. Right boxes, later matrix cells display nuclear localization of all 3 canonical matrix markers. In panels with multiple boxes, these are separated channel views, magnified from the region of the lower follicle indicated by an asterisk. White dotted lines, lower regenerating follicle. Yellow dashed lines, DP. DEP, days post-depilation. Scale bars, 50 μm.
Neither BMP signaling, Shh nor DP maturation is required for early matrix cell differentiation
The absence of nuclear pSmad in early matrix progenitors suggests that these cells do not require BMP signaling to initiate differentiation. To test this functionally, we generated mice expressing Keratin 5 promoter-driven Cre recombinase coupled with conditional floxed and deleted alleles of BMP receptor 1A (K5;Bmpr1aflox/−). These mutants, which exhibited hind limb defects (Andl et al., 2004) (Figure 5A), developed hair pegs which specifically lacked epithelial BMP signaling (Figure 5B). Consistent with our hypothesis that early matrix progenitors initiate differentiation without nuclear pSmad, K5;Bmpr1Aflox/− follicles possessed normal K79+ cells, but no AE15+ IRS cells, even in later stage hair pegs (Figure 5B). Since early-arising K79+ cells eventually form the CL, these observations may explain why ablating BMP signaling in the hair follicle specifically disrupts IRS differentiation, while sparing the CL (Andl et al., 2004; Kobielak et al., 2003; Yuhki et al., 2004).
Figure 5. Early matrix progenitors differentiate in the absence of Bmpr1a, Shh or DP maturation.
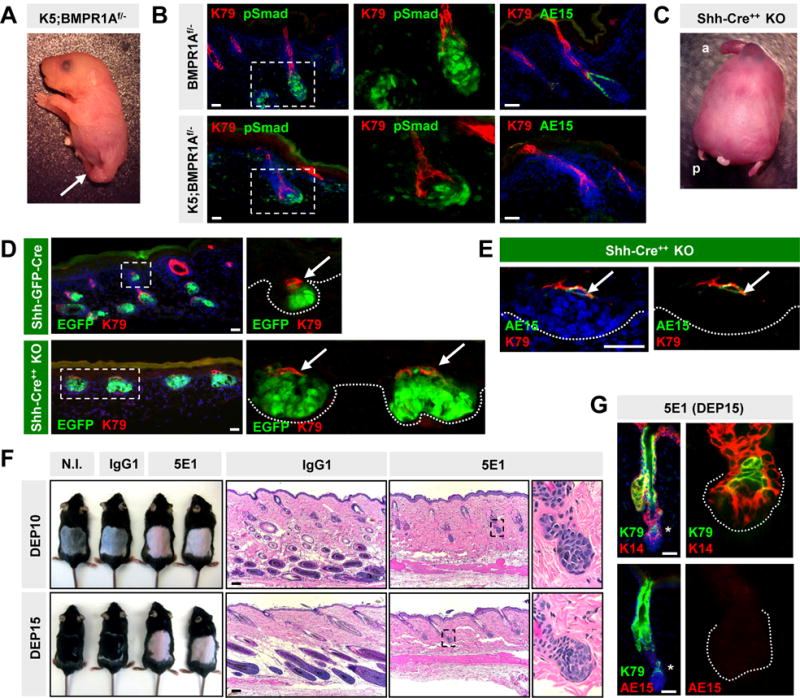
A. Newborn K5;Bmpr1a flox/− mutant with defective hindlimbs (arrow). B. K5;Bmpr1aflox/− mutant follicles at P0 lack pSmad specifically in the epithelial compartment, with normal pSmad in the DP. Mutant follicles form K79+ cells, but not AE15+ IRS. C. Newborn Shh null mutant, generated by homozygosing the Shh-EGFP-Cre allele (Shh-Cre++) (a, anterior; p, posterior). D. K79+ cells (arrows) are specified in aberrant Shh-null hair germs at P0 (bottom). Control littermates harboring a single Shh-EGFP-Cre allele develop normal follicles (top). E. A subset of K79+ cells aberrantly co-expresses AE15 (arrows) in Shh-null follicles at P0. F. Mice injected with anti-Shh antibody (5E1) do not re-enter anagen, unlike mice injected with isotype control IgG1, or not injected (N.I.). G. Matrix progenitors in 5E1-injected depilated skin can differentiate into K79+ cells, but do not form IRS. In panels with multiple images, these are separated channel views, magnified from either the boxed regions (B, D, F) or from the lower follicle (G) (asterisk). DEP, days post-depilation. Scale bars, 50 μm.
Given that early matrix progenitors initiate differentiation prior to surrounding the DP, this then led us to wonder whether these early progenitors depend upon DP maturation for function. Since Shh is required for DP maturation and maintenance (Chiang et al., 1999; St-Jacques et al., 1998; Woo et al., 2012), we examined whether specification of K79+ cells is perturbed in Shh−/− animals. As previously reported, P0 Shh−/− mutants display gross abnormalities in the head, tail and appendages, but possess largely normal epidermis (Figure 5C) (Chiang et al., 1999; St-Jacques et al., 1998). Although hair germs were initiated, these buds were enlarged and deformed, did not extend into the deeper dermis, and were juxtaposed by deteriorated or non-existant DPs. In spite of these defects, K79+ cells were still specified (Figure 5D). Interestingly, a subset of K79+ cells co-expressed AE15 (Figure 5E), markers that do not typically overlap (Figure 1C). These findings suggest that early matrix progenitors can form K79+ cells even in the absence of DP maturation, and that some of these cells subsequently gain aberrant AE15 expression.
To reassess these findings in the context of adult hair regeneration, we used the monoclonal antibody 5E1 to neutralize Shh prior to and after depilation. As previously shown, animals injected daily with 5E1 do not regenerate hair (Wang et al., 2000), in contrast to mice that were either uninjected or injected with an isotype-matched IgG1 antiserum (Figure 5F). In 5E1-injected depilated mice, cells in the secondary hair germ underwent initial expansion, similar to Shh−/− hair germs, but did not progress past Anagen II.
As was seen during development, Shh suppression did not prevent K79 specification at 10 or 15 days after depilation (Figure 5G); however, specification of IRS cells was fully inhibited by the 5E1 antibody (Figure 5G). Since similarly staged hair follicles were observed at both timepoints in 5E1-injected mice, this confirmed that these follicles were fully halted in early anagen, and not merely progressing through the hair cycle more slowly. Altogether, these results indicate that early matrix progenitors do not require BMP signaling, Shh or DP maturation to undergo terminal differentiation into K79+ cells. In contrast, later matrix populations require these signals to form the IRS.
Asynchronous maturation of terminally differentiated cell layers
Having now shown that early and late matrix populations initiate CL and IRS specification at different times, we next tested whether these progenitors also finish forming these layers asynchronously. Prior evidence supporting this possibility comes from electron microscopy studies by Morioka et al., who showed that the ORS directly abuts IRS cells without an intervening CL at the most proximal end of advanced follicles (Morioka, 2005) (diagrammed in Figure 6A). Thus, at the base of late anagen follicles, CL cells are sealed off from direct contact with matrix progenitors, implying that no new CL cells are being created at this point.
Figure 6. Matrix cells asynchronously complete the inner layers of the hair follicle.
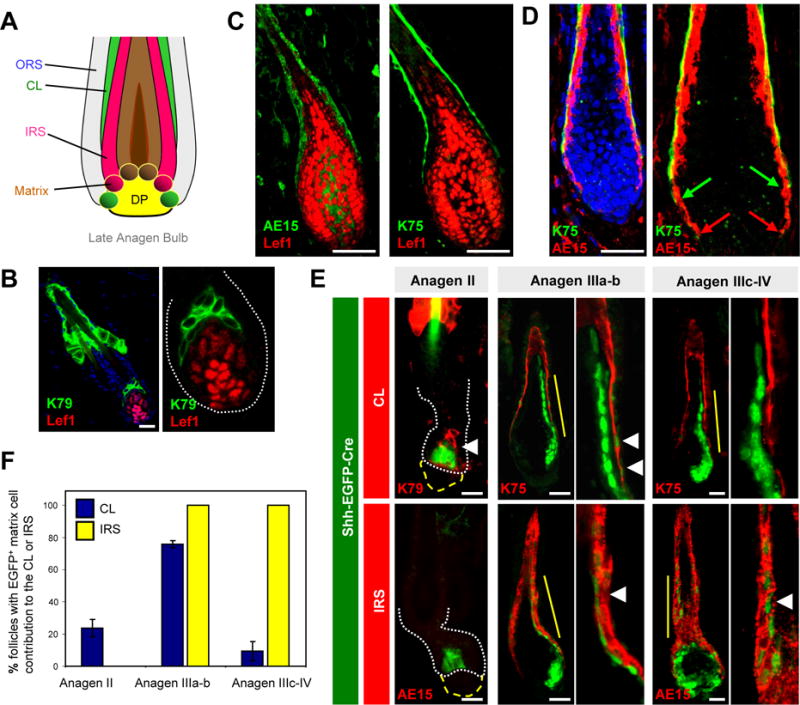
A. Schematic of the mature anagen bulb, adapted from Morioka et al., 2005. Note the lack of direct contact between the mature CL (green) and matrix. B. K79+ cells are juxtaposed with Lef1+ matrix progenitors in Anagen II follicles. C. IRS (left), but not mature CL (right), directly contacts matrix progenitors in Anagen IV-V follicles. D. IRS cells (red arrows) extend more proximally down the anagen bulb than does the CL (green arrows) in Anagen IV-V follicles. E. Left, in Anagen II follicles from Shh-EGFP-Cre mice, early matrix cells contribute only to the CL (arrowhead). Middle, in Anagen IIIa-b follicles, matrix cells contribute to both CL and IRS (arrowheads). Right, in Anagen IIIc-IV follicles, matrix cells contribute to IRS (arrowhead), but not to CL. For (B) and (D), right boxes are magnified views of the lower follicle without DAPI. For (E), magnified views are of areas indicated by the solid yellow line. White dotted lines, lower follicle. Yellow broken lines, DP. F. Quantitation of results from (E), scoring EGFP+ matrix contribution to the indicated layers at different hair stages. For scoring methodology, see Supplemental Experimental Procedures. Data are represented as mean ± SEM. Scale bars, 50 μm. See also Figure S2.
Consistent with these observations, we noted that in early anagen follicles, K79+ cell columns are directly juxtaposed with their Lef1+ matrix progenitors (Figure 6B). At later stages, however, only IRS cells, which are themselves partially Lef1+, directly contacted matrix cells (Figure 6C). Importantly, we also noticed that the IRS extends lower down the anagen bulb than does the CL (Figure 6D). This again suggests that, as the configuration of matrix progenitors and their differentiated progeny changes during anagen, CL cells are eventually blocked off from direct contact with progenitor cells.
This lack of direct contact suggested to us that not only do matrix progenitors initiate CL formation earlier than that of the other layers, but that these progenitors may also finish forming the CL earlier as well. To test this hypothesis, we again used the Shh-EGFP-Cre allele as a “timing mechanism” to trace only the most immediate contributions made by Shh+ matrix cells to the growing hair follicle.
In depilated skin from adult mice, Shh+ cells within Anagen II follicles formed only CL cells, since IRS cells are not present at this point (Figure 6E–F). In Anagen IIIa-b follicles, we observed efficient labeling of both the CL and IRS, indicating that Shh+ progenitors are directly forming both cell types. In more advanced Anagen IIIc-IV follicles, however, labeling was observed in the IRS and hair shaft, but only rarely in the CL. These labeling shifts were not due to selective expression of Shh in specific hair follicle layers, since Shh was restricted to the lower anagen bulb (Figure S2). In addition, we performed conventional lineage tracing experiments by inducibly and permanently labeling Shh+ progenitors during mid/late-anagen using Shh-CreERT2 mice, and observed that these cells formed IRS and hair shaft, but not CL (Figure S2). Altogether, these results, obtained using both permanent and transient fate mapping strategies, suggest that Shh+ matrix progenitors progressively form and complete the inner layers of the growing hair follicle asynchronously. Due to the limitations of our animal models, however, we cannot formally rule out the possibility that Shh− progenitors can continue forming the CL during late anagen. Similarly, it is also possible that a K75− CL precursor population might exist between the Lef1+ matrix progenitor pool and the mature CL, as has previously been suggested (Sequeira and Nicolas, 2012).
Fate of terminally differentiated CL cells
At the conclusion of anagen, the hair follicle regresses during catagen to re-enter telogen. This is accompanied by the upward movement of terminally differentiated cells, which are eventually expelled into the hair canal (Mesa et al., 2015; Stenn and Paus, 2001).
To trace the fate of CL cells during catagen, we turned back to K79-Cre;R26R-YFP mice described above (Figure 1E). These animals enable permanent labeling of cells that, at any given time, expressed K79. As expected, we observed fluorescent cells within the K79+ suprabasal domain of the infundibulum, but also efficient labeling of the entire mature CL, whose elongated cells and nuclei manifest a “beads on a string” morphology (Figure 7A–B). The permanent labeling of the entire mature CL occurred in spite of the apparent absence of K79 protein and K79 promoter/β-gal activity in this domain (Figure 7C, 2K). This suggests that mature CL cells, identified by K75 expression, may either express very low levels of K79 (enough to drive functional Cre expression), or at one time expressed K79 and subsequently downregulated its expression.
Figure 7. Fate of CL cells during hair regression.
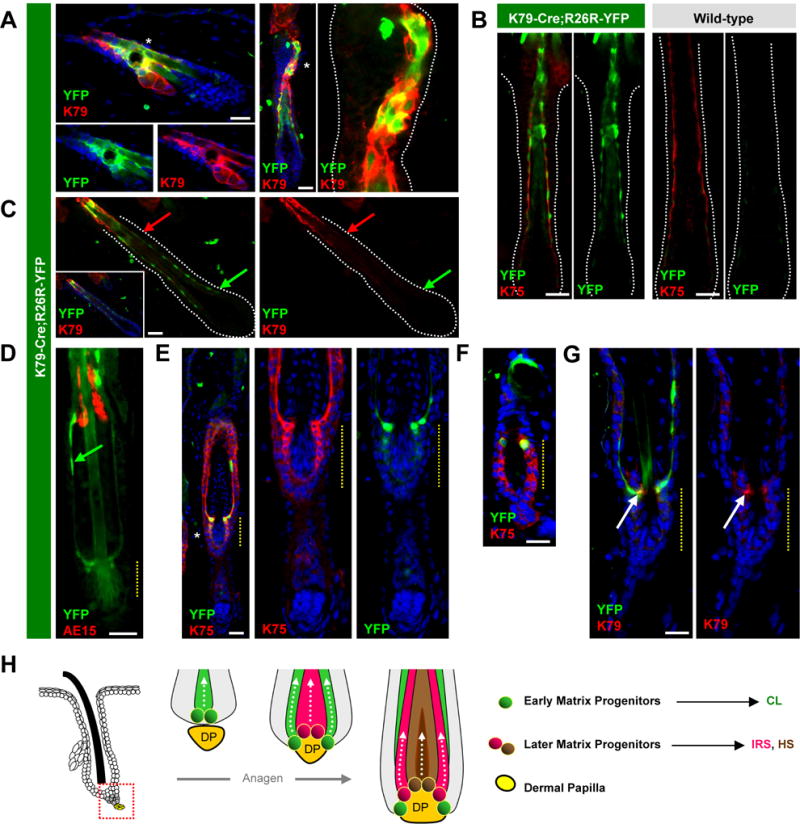
A. K79-Cre;R26R-YFP mice exhibit labeling (green) in a subset of K79+ suprabasal cells in the infundibulum during telogen (left) and in the CL of Anagen III follicles (right). B. The mature CL, identified by K75 (red), is labeled in these mice (left boxes, green), but not in control animals (right boxes), from Anagen IV-V follicles. C. CL labeling persists (arrows, green), even as K79 recedes (arrows, red) in Anagen IV-V follicles. D. In early catagen, the CL persists (green arrow), with partially evacuated IRS remnants (red). E. The regressing CL (green) appears continuous with upper cells of the future inner bulge (yellow dotted lines), both identified by K75 (red), during early catagen. F. In telogen, labeling persists in upper cells of the inner bulge (green). G. This is possibly due to weak re-expression of K79 (red) in cells below the CL during catagen (arrows). H. Schematic of hair regeneration, where early matrix cells (green) form the CL prior to surrounding the DP (yellow). After DP engulfment, later matrix cells (red and brown) occupy more central regions of the bulb and form the IRS and HS, with no further contribution to the CL. This model incorporates findings from Sequeira and Nicolas, 2012, where CL labeling is associated with labeling of proximal matrix cells abutting the DP. Alternative models, where matrix cells of varying innate differentiation potentials may already co-exist in early anagen, or where early matrix cells directly become later matrix cells without re-locating along the proximal-distal axis, are also plausible but not depicted here for clarity. In panels with multiple images, these are separated channel views, in some cases magnified from areas indicated by asterisks. Scale bars, 50 μm. See also Figures S3–S5.
In K79-Cre;R26R-YFP mice during early catagen, we observed labeled CL cells being drawn up the regressing follicle (Figure 7D). These cells maintained their elongated morphology, even after the deteriorating IRS had partially evacuated the hair bulb (Figure 7D). Intriguingly, the base of the regressing CL appeared continuous with the future inner bulge, which also expresses K75 (Figure 7E). As follicles re-entered telogen, we observed that the inner bulge was mostly unlabeled, consistent with previous reports that the CL does not give rise to these cells (Hsu et al., 2011) (Figure 7F). However, we occasionally noticed persistent fluorescent cells within the upper domain of the inner bulge, possibly due to weak re-expression of K79 in these cells specifically during catagen (Figure 7F–G). As for the ultimate fate of the CL, we observed thin YFP+K79− cells shed into the hair canal, suggesting that these are likely the remnants of this layer (Figure S3). In summary, we have lineage traced a discrete layer in the hair follicle and confirmed that K79+ cells form the CL. During catagen, the CL is eliminated, while cells immediately proximal to the CL persist in the upper reaches of the inner bulge, a region that has recently been reported to harbor at least 3 molecularly distinct subpopulations (Joost et al., 2016).
Functional testing of K79
Given that K79 is a defining marker of the earliest differentiating cells in the hair follicle, we lastly tested the requirement for this keratin in development and regeneration. To do this, we generated K79tm2a/tm2a homozygous mice, where both alleles of K79 are disrupted by lacZ, and confirmed that K79 was fully ablated throughout the skin (Figure S4).
K79tm2a/tm2a newborn mice developed hair normally and did not exhibit any overt skin phenotypes. Upon challenging adult mice to 2 consecutive cycles of depilation or hair plucking, mutant animals entered anagen and regrew hair similarly to littermate controls (Figures S4–S5). We also did not notice any obvious histological differences between mutant and control skin at different hair cycle stages, except that K79tm2a/tm2a mice possess disordered sebaceous glands (data not shown; manuscript in preparation). During anagen, expression of K75 and K6 in the CL was unaffected by loss of K79. Finally, we confirmed that the cells which normally express K79 are still present and located within the expected domains of mutant follicles in K79tm2a/tm2a mice, as determined by β-gal expression from the endogenous K79 locus. Altogether, these findings indicate that K79 is not required for hair growth, possibly due to functional redundancy with related keratin family members.
DISCUSSION
During morphogenesis and regeneration, the DP promotes hair growth by secreting factors such as TGF-β, Fgf7/10, Noggin and possibly Eda to responding cells at the base of the follicle (Botchkarev et al., 1999; Oshimori and Fuchs, 2012; Rezza et al., 2016; Rosenquist and Martin, 1996; Woo et al., 2012). These signals are thought to initially drive early cell proliferation in the hair germ or secondary hair germ (Greco et al., 2009; Panteleyev et al., 2001; Zhang et al., 2009), with terminal differentiation occuring later as the expanding follicle envelopes the DP (Muller-Rover et al., 2001; Schmidt-Ullrich and Paus, 2005). Once this occurs, matrix progenitors are thought to assume different cell fates along the hair follicle medial-lateral axis (Legue and Nicolas, 2005). How these choices are made remains unclear, although signaling gradients may lead to differential expression of matrix transcription factors such as Msx2, Dlx3 and Foxn1 (Cai et al., 2009; Hwang et al., 2008; Kobielak et al., 2003).
Our findings here offer several insights into this complicated process (Figure 7H). First, we show that K79 is the earliest terminal differentiation marker in the growing hair follicle, and that matrix progenitors initiate differentiation prior to surrounding the DP. Second, we find that specification of the differentiated layers of the follicle both commences and terminates asynchronously, as determined using Shh-EGFP-Cre mice. Third, we propose that the matrix progenitor pool can be divided into early and late phases that are distinguishable temporally (anagen stage), morphologically (physical relationship to the DP) and molecularly (BMP status). Although terminal differentiation occurs throughout both phases, specific cell fate choices evolve over time and rely on distinct signaling inputs, as proven functionally by analyzing Shh and Bmpr1a mutant mice, as well as by antibody-mediated Shh neutralization. Fourth, we observe using K79tm2a and K79-Cre;R26R-YFP mice that the CL undergoes a dynamic maturation process, and that CL-derived cells likely do not persist into telogen. Finally, fifth, we report that K79 is not required for normal hair growth, as assessed using K79 null animals.
In developing hair placodes, basal Shh+ cells divide perpendicularly to the basement membrane to generate Sox9+ cells that will eventually become the bulge (Nowak et al., 2008; Ouspenskaia et al., 2016). We also observed early specification of basal layer future bulge cells in Shh-EGFP-Cre placodes (Figure S6). In later stage hair germs and secondary hair germs, we noted that basal Shh+ cells form overlying, terminally differentiated K79+ suprabasal cells, firmly establishing that matrix progenitors are functional at this point. These findings are consistent with the observation that early cell divisions in the secondary hair germ are often oriented perpendicularly to both the basement membrane and the axis of hair growth, which may be a mechanism for generating K79+ cells (Zhang et al., 2010).
Here, we have used Shh-EGFP-Cre mice, in the absence of a traditional reporter allele, to visualize direct cellular relationships between Shh+ progenitors and their progeny. One limitation of this technique is that during anagen, Shh+ progenitors represent only a subset of the entire mature matrix pool (Figure 4). Indeed, we have occasionally noticed in late anagen follicles that Shh+ matrix cells appear to display reduced pSmad, relative to adjacent non-Shh matrix (Figure S2). In spite of this, gene expression studies have indicated that Shh+ progenitors are very similar to the overall matrix population (Rezza et al., 2016), suggesting that the behavior of this sub-population may be representative of the whole.
Earlier studies have questioned the origins of the CL, a structure hypothesized to function either as a slippage plane for hair growth, or as an anchor for the hair shaft (Hanakawa et al., 2004; Rothnagel and Roop, 1995). More recent reports have argued that the CL arises from matrix progenitors (Hsu et al., 2011; Sequeira and Nicolas, 2012), a conclusion supported here. Given the intimate cellular connections between the CL and IRS, including specialized structures termed “Flügelzellen” (Langbein et al., 2002), it has also been proposed that growth of the CL depends upon the upward movement of the IRS (Chapman, 1971; Orwin, 1971). Our findings, however, argue against this since the CL clearly forms before the other differentiated layers. Compellingly, hair follicles lacking Gata3, Bmpr1a or functional NuMA possess abnormal IRS without obvious defects in the CL (Andl et al., 2004; Kaufman et al., 2003; Kobielak et al., 2003; Seldin et al., 2016). Altogether, these findings unequivocally indicate that the CL can form independently of the IRS.
An important lingering question relates to how the matrix progenitor pool expands throughout anagen. In particular, it remains unclear how early and late matrix populations are related, how these cells assume their final medial-lateral positions along the DP axis, and how Shh expression becomes restricted (Hsu et al., 2011; Legue and Nicolas, 2005; Sequeira and Nicolas, 2012). Although we have divided the matrix progenitor pool into early and late phases here, it is also possible that matrix cells with varying innate potentials might co-exist early on, or exist along a continuum. In addition, further studies will be needed to determine whether early matrix progenitors can directly become later matrix cells, give rise to later matrix cell progeny, or remain as separate and independent populations throughout anagen.
EXPERIMENTAL PROCEDURES
Mice
The following strains were used: Shhtm1(EGFP/cre)Cjt/J (Shh-EGFP-Cre) and B6.129S6-Shhtm2(cre/ERT2)Cjt/J (Shh-CreERT2) (Harfe et al., 2004); Bmpr1aflox (Yuhki et al., 2004); and Gt(ROSA)26Sortm1(EYFP)Cos (R26R-YFP) (Srinivas et al., 2001). To generate Shh-null animals, Shh-EGFP-Cre mice were inter-crossed. To generate K79tm2a mice, embryonic stem cells from clone EPD0179-4-A12 were purchased from KOMP and microinjected into blastocysts. Founder animals were crossed with E2a-Cre mice to remove the neomycin cassette. K79tm2a mice were inter-crossed to generate K79-null mice. To generate K79-Cre mice, the 5,789 bp sequence upstream of the K79 transcriptional start site was PCR-amplified using BAC # RP24-152H23 and cloned into pCAG-Cre:GFP (Addgene #13776). Finally, the insert was purified and microinjected into (C57BL/6 × SJL) F2 mouse eggs and transferred into recipients. All studies were performed on mice of both genders in accordance with regulations established by the University of Michigan Unit for Laboratory Animal Medicine.
5E1 Experiments
One day prior to depilation, 8 week old mice were either uninjected, or injected with purified 5E1 anti-Shh antibody (Developmental Studies Hybridoma Bank), or purified IgG1 isotype-matched control antiserum (BioLegend). Mice received 200 μg of purified antibody daily for up to 15 days after depilation, similar to previously described (Peterson et al., 2015).
Immunohistochemistry
Antibodies and procedures used for immunohistochemistry are listed in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We are grateful to Anj Dlugosz, Yuji Mishina, Charlotte Mistretta and Marina Pasca di Magliano for sharing reagents. We also acknowledge Wanda Fillipak and Galina Gavrilina for generating transgenic mice, as well as the Transgenic Animal Model Core at the University of Michigan, supported by the NCI (P30CA046592). S.Y.W. acknowledges the support of the NIH (R01AR065409, R21CA209166); the University of Michigan Department of Dermatology; the Biological Sciences Scholars Program; and the Center for Organogenesis. A.L.M. was supported by the NIH Cellular and Molecular Biology Training Grant (T32GM007315).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
A.L.M, N.A.V and M.V.L. conceived and performed experiments. S.Y.W. conceived and performed experiments, wrote the manuscript and secured funding.
References
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- Biggs LC, Mikkola ML. Early inductive events in ectodermal appendage morphogenesis. Semin Cell Dev Biol. 2014;25–26:11–21. doi: 10.1016/j.semcdb.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, Lindner G, McMahon JA, Peters C, Lauster R, et al. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol. 1999;1:158–164. doi: 10.1038/11078. [DOI] [PubMed] [Google Scholar]
- Cai J, Lee J, Kopan R, Ma L. Genetic interplays between Msx2 and Foxn1 are required for Notch1 expression and hair shaft differentiation. Dev Biol. 2009;326:420–430. doi: 10.1016/j.ydbio.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RE. Cell migration in wool follicles of sheep. J Cell Sci. 1971;9:791–803. doi: 10.1242/jcs.9.3.791. [DOI] [PubMed] [Google Scholar]
- Chiang C, Swan RZ, Grachtchouk M, Bolinger M, Litingtung Y, Robertson EK, Cooper MK, Gaffield W, Westphal H, Beachy PA, et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev Biol. 1999;205:1–9. doi: 10.1006/dbio.1998.9103. [DOI] [PubMed] [Google Scholar]
- Ellis T, Gambardella L, Horcher M, Tschanz S, Capol J, Bertram P, Jochum W, Barrandon Y, Busslinger M. The transcriptional repressor CDP (Cutl1) is essential for epithelial cell differentiation of the lung and the hair follicle. Genes Dev. 2001;15:2307–2319. doi: 10.1101/gad.200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA. beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell. 2010;18:633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genander M, Cook PJ, Ramskold D, Keyes BE, Mertz AF, Sandberg R, Fuchs E. BMP signaling and its pSMAD1/5 target genes differentially regulate hair follicle stem cell lineages. Cell Stem Cell. 2014;15:619–633. doi: 10.1016/j.stem.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu LH, Coulombe PA. Keratin expression provides novel insight into the morphogenesis and function of the companion layer in hair follicles. J Invest Dermatol. 2007;127:1061–1073. doi: 10.1038/sj.jid.5700673. [DOI] [PubMed] [Google Scholar]
- Hanakawa Y, Li H, Lin C, Stanley JR, Cotsarelis G. Desmogleins 1 and 3 in the companion layer anchor mouse anagen hair to the follicle. J Invest Dermatol. 2004;123:817–822. doi: 10.1111/j.0022-202X.2004.23479.x. [DOI] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Mehrani T, Millar SE, Morasso MI. Dlx3 is a crucial regulator of hair follicle differentiation and cycling. Development. 2008;135:3149–3159. doi: 10.1242/dev.022202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, Watt FM. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009;4:427–439. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joost S, Zeisel A, Jacob T, Sun X, Manno GL, Lonnerberg P, Linnarsson S, Kasper M. Single-cell transcriptomics reveals that differentiation and spatial signatures shape epidermal and hair follicle heterogeneity. Cell Syst. 2016;3:1–17. doi: 10.1016/j.cels.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman CK, Zhou P, Pasolli HA, Rendl M, Bolotin D, Lim KC, Dai X, Alegre ML, Fuchs E. GATA3: an unexpected regulator of cell lineage determination in skin. Genes Dev. 2003;17:2108–2122. doi: 10.1101/gad.1115203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor 1A. J Cell Biol. 2003;163:609–623. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbein L, Rogers MA, Praetzel S, Aoki N, Winter H, Schweizer J. A novel epithelial keratin, hK6irs1, is expressed differentially in all layers of the inner root sheath, including specialized Huxley cells (Flugelzellen) of the human hair follicle. J Invest Dermatol. 2002;118:789–799. doi: 10.1046/j.1523-1747.2002.01711.x. [DOI] [PubMed] [Google Scholar]
- Legue E, Nicolas JF. Hair follicle renewal: organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development. 2005;132:4143–4154. doi: 10.1242/dev.01975. [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell. 2005;9:855–861. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Mesa KR, Rompolas P, Zito G, Myung P, Sun TY, Brown S, Gonzalez DG, Blagoev KB, Haberman AM, Greco V. Niche-induced cell death and epithelial phagocytosis regulate hair follicle stem cell pool. Nature. 2015;522:94–97. doi: 10.1038/nature14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka K. Outer rooth sheath and companion layer. In: Morioka K, editor. Hair Follicle. Springer; Tokyo: 2005. pp. 89–106. [Google Scholar]
- Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oro AE, Higgins K. Hair cycle regulation of Hedgehog signal reception. Dev Biol. 2003;255:238–248. doi: 10.1016/s0012-1606(02)00042-8. [DOI] [PubMed] [Google Scholar]
- Orwin DFG. Cell differentiation in the lower outer sheath of the romney wool follicle: a companion cell layer. Aust J Biol Sci. 1971;24:989–999. doi: 10.1071/bi9710989. [DOI] [PubMed] [Google Scholar]
- Oshimori N, Fuchs E. Paracrine TGF-beta signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell. 2012;10:63–75. doi: 10.1016/j.stem.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouspenskaia T, Matos I, Mertz AF, Fiore VF, Fuchs E. WNT-SHH antagonism specifies and expands stem cells prior to niche formation. Cell. 2016;164:156–169. doi: 10.1016/j.cell.2015.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Lombard P, Ng F, Gottgens B, Jensen KB. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell. 2013;13:1–12. doi: 10.1016/j.stem.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panteleyev AA, Jahoda CA, Christiano AM. Hair follicle predetermination. J Cell Sci. 2001;114:3419–3431. doi: 10.1242/jcs.114.19.3419. [DOI] [PubMed] [Google Scholar]
- Paus R, Handjiski B, Czarnetzki BM, Eichmuller S. A murine model for inducing and manipulating hair follicle regression (catagen): effects of dexamethasone and cyclosporin A. J Invest Dermatol. 1994;103:143–147. doi: 10.1111/1523-1747.ep12392542. [DOI] [PubMed] [Google Scholar]
- Paus R, Müller-Röver S, van der Veen C, Maurer M, Eichmüller S, Ling G, Hofmann U, Foitzik K, Mecklenburg L, Handjiski B. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- Peterson SC, Eberl M, Vagnozzi AN, Belkadi A, Veniaminova NA, Verhaegen ME, Bichakjian CK, Ward NL, Dlugosz AA, Wong SY. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell. 2015;16:400–412. doi: 10.1016/j.stem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley DA, Kandyba E, Huang P, Halliwill KD, Sjölund J, Pelorosso F, Wong CE, Hirst GL, Wu D, Delrosario R, et al. Gene Expression Architecture of Mouse Dorsal and Tail Skin Reveals Functional Differences in Inflammation and Cancer. Cell Rep. 2016;16:1153–1165. doi: 10.1016/j.celrep.2016.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezza A, Wang Z, Sennett R, Qiao W, Wang D, Heitman N, Mok KW, Clavel C, Yi R, Zandstra P, et al. Signaling networks among stem cell precursors, transit-amplifying progenitors, and their niche in developing hair follicles. Cell Rep. 2016;14:3001–3018. doi: 10.1016/j.celrep.2016.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Deschene ER, Zito G, Gonzalez DG, Saotome I, Haberman AM, Greco V. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature. 2012;487:496–499. doi: 10.1038/nature11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist TA, Martin GR. Fibroblast growth factor signalling in the hair growth cycle: expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev Dyn. 1996;205:379–386. doi: 10.1002/(SICI)1097-0177(199604)205:4<379::AID-AJA2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Rothnagel JA, Roop DR. The hair follicle companion layer: reacquainting an old friend. J Invest Dermatol. 1995;104:42S–43S. doi: 10.1038/jid.1995.59. [DOI] [PubMed] [Google Scholar]
- Rothnagel JA, Seki T, Ogo M, Longley MA, Wojcik SM, Bundman DS, Bickenbach JR, Roop DR. The mouse keratin 6 isoforms are differentially expressed in the hair follicle, footpad, tongue and activated epidermis. Differentiation. 1999;65:119–130. doi: 10.1046/j.1432-0436.1999.6520119.x. [DOI] [PubMed] [Google Scholar]
- Santegoets LA, Seters Mv, Helmerhorst TJ, Heijmans-Antonissen C, Hanifi-Moghaddam P, Ewing PC, van Ijcken WF, van der Spek PJ, van der Meijden WI, Blok LJ. HPV related VIN: highly proliferative and diminished responsiveness to extracellular signals. Int J Cancer. 2007;121:759–766. doi: 10.1002/ijc.22769. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–261. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- Schneider MR, Paus R. Deciphering the functions of the hair follicle infundibulum in skin physiology and disease. Cell Tissue Res. 2014;358:697–704. doi: 10.1007/s00441-014-1999-1. [DOI] [PubMed] [Google Scholar]
- Seldin L, Muroyama A, Lechler T. NuMA-microtubule interactions are critical for spindle orientation and the morphogenesis of diverse epidermal structures. eLife. 2016;5:e12504. doi: 10.7554/eLife.12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira I, Nicolas JF. Redefining the structure of the hair follicle by 3D clonal analysis. Development. 2012;139:3741–3751. doi: 10.1242/dev.081091. [DOI] [PubMed] [Google Scholar]
- Smyth I, Ellis T, Hetherington R, Riley E, Narang M, Mahony D, Wicking C, Rothnagel JA, Wainwright BJ. Krt6a-Cre transgenic mice direct LoxP-mediated recombination to the companion cell layer of the hair follicle and following induction by retinoic acid to the interfollicular epidermis. J Invest Dermatol. 2004;122:232–234. doi: 10.1046/j.0022-202X.2003.22122.x. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, McMahon JA, Lewis PM, Paus R, McMahon AP. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Veniaminova NA, Vagnozzi AN, Kopinke D, Do TT, Murtaugh LC, Maillard I, Dlugosz AA, Reiter JF, Wong SY. Keratin 79 identifies a novel population of migratory epithelial cells that initiates hair canal morphogenesis and regeneration. Development. 2013;140:4870–4880. doi: 10.1242/dev.101725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LC, Liu ZY, Gambardella L, Delacour A, Shapiro R, Yang J, Sizing I, Rayhorn P, Garber EA, Benjamin CD, et al. Conditional disruption of hedgehog signaling pathway defines its critical role in hair development and regeneration. J Invest Dermatol. 2000;114:901–908. doi: 10.1046/j.1523-1747.2000.00951.x. [DOI] [PubMed] [Google Scholar]
- Winter H, Langbein L, Praetzel S, Jacobs M, Rogers MA, Leigh IM, Tidman N, Schweizer J. A novel human type II cytokeratin, K6hf, specifically expressed in the companion layer of the hair follicle. J Invest Dermatol. 1998;111:955–962. doi: 10.1046/j.1523-1747.1998.00456.x. [DOI] [PubMed] [Google Scholar]
- Wojcik SM, Longley MA, Roop DR. Discovery of a novel murine keratin 6 (K6) isoform explains the absence of hair and nail defects in mice deficient for K6a and K6b. J Cell Biol. 2001;154:619–630. doi: 10.1083/jcb.200102079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo WM, Zhen HH, Oro AE. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes Dev. 2012;26:1235–1246. doi: 10.1101/gad.187401.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuhki M, Yamada M, Kawano M, Iwasato T, Itohara S, Yoshida H, Ogawa M, Mishina Y. BMPR1A signaling is necessary for hair follicle cycling and hair shaft differentiation in mice. Development. 2004;131:1825–1833. doi: 10.1242/dev.01079. [DOI] [PubMed] [Google Scholar]
- Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell. 2009;5:1–12. doi: 10.1016/j.stem.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, White BS, Shalloway DI, Tumbar T. Stem cell dynamics in mouse hair follicles: a story from cell division counting and single cell lineage tracing. Cell Cycle. 2010;9:1504–1510. doi: 10.4161/cc.9.8.11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


