Abstract
Objective
Alendronate (ALN) inhibits osteoclastic bone resorption and triggers osteostimulative properties both in vivo and in vitro, as shown by increase in matrix formation. This study aimed to explore the efficacy of 1% ALN gel as local drug delivery (LDD) in adjunct to scaling and root planing (SRP) for the treatment of chronic periodontitis among smokers.
Material and Methods
75 intrabony defects were treated in 46 male smokers either with 1% ALN gel or placebo gel. ALN gel was prepared by adding ALN into carbopol-distilled water mixture. Clinical parameters [modified sulcus bleeding index, plaque index, probing depth (PD), and periodontal attachment level (PAL)] were recorded at baseline, at 2 months, and at 6 months, while radiographic parameters were recorded at baseline and at 6 months. Defect fill at baseline and at 6 months was calculated on standardized radiographs by using the image analysis software.
Results
Mean PD reduction and mean PAL gain were found to be greater in the ALN group than in the placebo group, both at 2 and 6 months. Furthermore, a significantly greater mean percentage of bone fill was found in the ALN group (41.05±11.40%) compared to the placebo group (2.5±0.93%).
Conclusions
The results of this study showed 1% ALN stimulated a significant increase in PD reduction, PAL gain, and an improved bone fill compared to placebo gel in chronic periodontitis among smokers. Thus, 1% ALN, along with SRP, is effective in the treatment of chronic periodontitis in smokers.
Keywords: Alendronate, Chronic periodontitis, Smoking, Regeneration
Introduction
Bacterial biofilm has been considered one of the main aetiological factors in periodontal diseases10. Additionally, extensive researches suggest that host-derived enzymes, cytokines, and other mediators play a direct role in extracellular matrix (ECM) destruction in periodontitis20. The bacteria, therefore, initiate disease by activating host mechanisms that then destroy the supporting structures of the periodontium. This justifies how initiation and progression of periodontal diseases can be inhibited by interfering with host factors.
Conventionally, periodontal disease is treated by mechanical periodontal therapy, but certain cases may require adjunctive chemical periodontal therapy. Chemical periodontal therapy may involve various host modulators for controlling periodontal tissue destruction.
Bisphosphonates (BPs) are carbon-substituted pyrophosphate (P-C-P) analogs that include potent inhibitors of bone resorption, which have been effectively used to control osteolysis or reduce bone loss in Paget’s disease, metastatic bone disease, hypercalcemia of malignancy1, and osteoporosis16.
Alendronate sodium (ALN) is an amino bisphosphonate and, once taken up by bone, it acts as an antiosteolytic agent. ALN binds to resorption surfaces and is locally released during the acidification associated with osteoclastic activity. This release leads to a rise in the local concentration of ALN, resulting in an alteration in the ruffled border membrane characteristic of osteoclasts without destroying the cells. Therefore, ALN seems to have a potential to be used as an inhibitor of alveolar bone resorption in the treatment of periodontitis.
Previous studies have shown the effects of systemic ALN in human and other animal models in decreasing bone loss and increasing alveolar bone density12,15,17. Additionally, studies have observed that topical application of ALN was highly effective in reducing alveolar bone resorption after mucoperiosteal flap surgery3,4,22,24,30.
Evidence from cross-sectional and case-control studies in various populations and an abundant number of reviews on the subject have shown that adult smokers are about two to four times more likely to have periodontitis than nonsmokers. Previous studies indicated that smokers did not respond to non-surgical periodontal therapy2,13. Additionally, smokers showed less probing depth reduction and attachment gain, compared to nonsmokers, for periodontal surgical treatment11,23.
Considering the abovementioned facts, the current study is designed to evaluate the efficacy of 1% ALN gel as local drug delivery along with scaling and root planing (SRP) for the treatment of intrabony defects among smokers with chronic periodontitis.
Material and methods
Source of data
In this 6 month follow-up longitudinal interventional study, a total of 52 male smokers (age range: 30-50 years old) with chronic periodontitis was selected from the outpatient section of the Department of Periodontics, Government Dental College & Research Institute, Bangalore. The research protocol was presented to the Ethical Committee and Review Board of the institution. After ethical approval, all subjects were verbally informed and written informed consent was taken for participation in the research. The study was conducted from March 2010 to April 2011.
Selection criteria
Systemically healthy subjects with probing depth (PD) ≥5 mm or periodontal attachment level (PAL) ≥4 to 6 mm and a radiographic vertical bone loss ≥3 mm with no history of periodontal therapy or use of antibiotics in the preceding 6 months were included in the study. Smoking history was collected by self-report after a standardized questionnaire. Subjects were classified as smokers and nonsmokers based on criteria established by the Centers for Disease Control and Prevention (CDC): “current smokers” were defined as those who had smoked 100 or more cigarettes over their lifetime and smoked at the time of interview; “former smokers” had smoked 100 or more cigarettes over their lifetime but were not currently smoking; and “nonsmokers” had not smoked 100 or more cigarettes over their lifetime. Former smokers were excluded in an attempt to make a clear discrimination between smokers and nonsmokers. Subjects with the following characteristics were excluded from the study: known systemic disease; known or suspected allergy to the ALN/bisphosphonate group; systemic ALN/bisphosphonate therapy; aggressive periodontitis; use of smokeless tobacco in any form; alcoholism; immunodeficiency.
46 subjects (out of 52 enrolled), who matched clinical and radiographic parameters, were recruited for double-blind clinical study (Figure 1). Multiple sites from the same patients were also considered in case of fulfilling selection criteria. A total of 75 sites were randomly (by computer generated system) assigned to either ALN or placebo group. 37 sites (2 failed) and 38 sites (4 failed) completed the study in the ALN group and placebo group, respectively. Another clinician (AS) treated subjects enrolled to either group. All pre- and posttreatment clinical parameters were recorded by an examiner (ARP) who was masked to the type of treatment received by the subjects, while another clinician (AS) provided treatment to both groups.
Figure 1. Study flow chart.
In the ALN group, sites were treated with SRP followed by 1% ALN gel (10 mg/ml) local drug delivery, while in the placebo group sites were treated with SRP followed by placebo gel placement. Subjects were blinded for allocation into ALN or placebo group. SRP was performed at baseline until the root surface was considered smooth and clean by the operator (AS). No antibiotics or anti-inflammatory agent were prescribed after treatment. Clinical parameters, including modified sulcus bleeding index (mSBI)18, full mouth and site-specific plaque score (PI)28, PD, and PAL, were recorded at baseline (before the SRP) and at 2 and 6 months. A custom-made acrylic stent and a no. 15 color-coded University of North Carolina periodontal probe were used to standardize the measurement of clinical parameters.
Intra-examiner calibration
30 sites were examined twice for intra-examiner calibration. Calibrations were considered for measurements similar to 1 mm at the 95% level.
Radiographic assessment of Intrabony Defects (IBD)
Bone fill was evaluated at baseline and at 6 months using an image analyzer (Scion image Corporation, Frederick, MA, USA). IBD was measured on the radiograph by measuring the vertical distance from the crest of the alveolar bone to the base of the defect. Individually customized bite blocks and a parallel-angle technique were used to obtain films as reproducible as possible. All radiographs were reviewed in a single reference center by a masked evaluator. For assessment, radiographs were scanned with a 6400 DPI scanner (Epson Perfection V700, Bangalore, India) by an evaluator who was blinded to the surgical procedure performed in the subjects. The radiographic IBD depth was measured by computer aided software program, as previously used25.
Primary and secondary outcome measures
The primary outcome of the study was PD and PAL. The secondary outcomes included complete bone defect fill.
Formulation of 1% ALN gel
ALN gel was prepared as described by Reddy, et al.24 (2005). Briefly, ALN (Apex Pharma, Ankleshwar, Gujarat, India) was dissolved in a required amount of distilled water to achieve 1% ALN concentration. A weighed quantity of carbopol 934P (2% w/w) was taken and added to the distilled water. The mixture was gradually stirred and carbopol was allowed to soak for 2 h. 1% triethanolamine was added to neutralize the carbopol solution and to form the gel. The pH was adjusted to 6.8. Finally, the required amount of methylparaben (0.1%) and propylparaben (0.05%) were dissolved in ethanol and added to the gel. The placebo gel was prepared by the abovementioned procedure without adding the active ingredient (ALN).
Local drug delivery
The prepared ALN gel (10 mg/ml) was dispensed into the periodontal pockets with intrabony defects using a syringe with a blunt cannula. Patients were instructed not to use forceful brushing or interdental aids at the treated sites until the appointment after 2 months and to avoid chewing sticky or hard food.
Statistical analysis
Power analysis calculations were performed before the study was initiated. To achieve 90% power and detect mean differences of the clinical parameters between groups, 30 sites in each group were required. The categorical variable (site-specific PI) was expressed as percentage and the continuous variable (Full mouth PI, mSBI, PD, PAL, and IBD depth), as mean ± standard deviation. Site-specific PI was compared by using Chi-squared test or Fisher’s exact test when the expected frequency was less than 5. Normality assumption was tested using Shapiro-Wilk’s W test. If the continuous variable followed a normal distribution, a comparison would be carried out in the treatment group using student’s t test. Statistical significance was defined as p<0.05. Statistical analysis was performed with SPSS version 15, SPSS Inc., Chicago, IL, USA.
Results
46 subjects (multiple sites/subject) out of 52 completed the study (Figure 1). All subjects tolerated the drug well without any complications or adverse reactions. Soft tissues healed within normal limits, and no significant visual differences were noted.
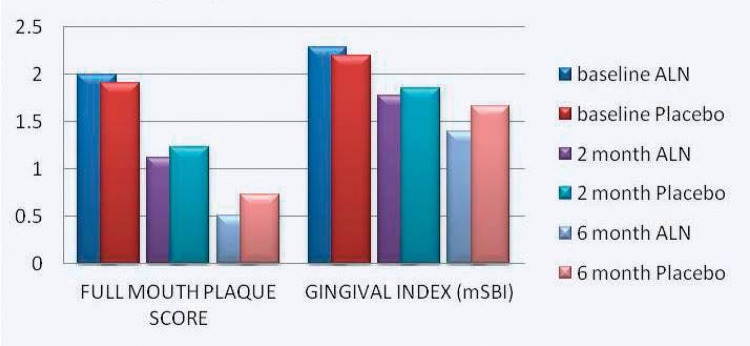
Both groups showed improvement in site specific and full mouth PI score, but improvement was not statistically significant among both groups at any time point for site-specific PI. Improvement in full mouth PI score was significantly higher in the ALN group (p<0.05) compared to the placebo group at 6 months (Figure 2).
Figure 2. Full mouth plaque scores and gingival index for alendronate and placebo groups at different time intervals.
In both groups, mSBI showed no difference at baseline, at 2 months, and at 6 months (Figure 2).
Clinical parameters PD and PAL showed no difference in intergroup comparison at baseline and showed significant PD reduction and PAL gain at 2 and 6 months at p<0.05 (Tables 1, 2).
Table 1. Probing depth, periodontal attachment level, and intrabony defect depth in alendronate and placebo groups at different time interval.
| Parameters (mm) | Visits | ALN | Placebo | t-value† | p-value |
|---|---|---|---|---|---|
| Probing depth | Baseline 2 Month 6 Month | 7.84 ±2.04 5.05 ±1.78 3.68 ±1.93 | 7.62 ±1.97 6.38 ±1.84 5.57±1.88 | 0.21 9.87 18.253 | 0.645 <0.002* <0.001* |
| Periodontal attachment level | Baseline 2 Month 6 Month | 6.43 ±1.77 4.05 ±1.94 2.49 ±1.53 | 6.19 ±1.54 5.22 ±1.73 4.41±1.83 | 0.4 7.36 23.79 | 0.531 <0.008* <0.001* |
| Intrabony defect depth | Baseline 6 Month | 5.18 ±1.00 3.07 ±0.94 | 5.10 ±0.95 4.97 ±0.95 | 0.123 74.03 | 0.727 <0.001* |
*Statistically significant at p<0.05
† t test
Table 2. Change in mean probing depth, periodontal attachment level, and intrabony defect and percentage change in bone fill in alendronate and placebo groups from baseline.
| Clinical parameters | % change from baseline | ALN group | Placebo group | t- value† | p- value |
|---|---|---|---|---|---|
| Mean PD | 2 Month 6 Month | 2.78 ± 1.20 4.16 ± 1.23 | 1.24± 0.83 2.05± 0.94 | 41.01 68.1 | <0.001* <0.001* |
| Mean PAL (mm) | 2 Month 6 Month | 2.38 ± 0.95 3.95 ± 0.88 | 0.97 ± 0.92 1.78 ±1.22 | 41.317 75.789 | <0.001* <0.001* |
| Mean IBD (mm) | 6 Month | 2.10 ± 0.69 | 0.12 ± 0.04 | 302.01 | <0.001* |
| Bone defect fill | 6 Month | 41.05 ±11.40 | 2.5 ± 0.93 | 420.07 | <0.001* |
*Statistically significant at p<0.05
† t test
Radiographic parameter IBD showed statistically significant mean reduction of 2.10±0.69 mm in the alendronate group, in comparison to the placebo group (0.12±0.04 mm) (Table 2). Alendronate sites presented a significantly higher vertical defect fill (41.05±11.40%) than placebo sites (2.5±0.93%) at 6 months (Table 2).
Discussion
This study has evaluated the clinical efficacy of 1% ALN gel along with SRP for the treatment of IBD in smokers with chronic periodontitis and showed significant radiographic bone fill and improvement in clinical parameters compared to placebo gel.
ALN and other ingredients of gel formulation are approved by the Food and Drug Administration (USFDA) for oral uses. Studies24,26 have explored different properties for various concentrations of ALN and found 1% ALN gel is the optimum dosage for LDD, thus 1% ALN was the choice for the current research.
ALN is a bisphosphonate that acts as a potent inhibitor of bone resorption. It is now generally accepted that the main cell by which bisphosphonates mediate their action is the osteoclast. Various mechanisms to be involved are inhibition of osteoclast recruitment, osteoclast adhesion, osteoclast activity, and shortening of osteoclast lifespan (apoptosis)5. Several reports have shown that bisphosphonates not only induce osteoblasts to secrete inhibitors of osteoclast-mediated resorption, but also stimulate the formation of osteoblast precursors and mineralized nodules, thereby promoting early osteoblastogenesis7.
Histometric analyses showed more percentage of bone in the furcation area that was treated with 1 mL sodium ALN irrigation (10-5M), when compared to control groups of experimental periodontitis in rats at 7 and 15 days3. Immunohistochemical analyses also expressed stronger osteoprotegerin immunolabeling, weaker receptor activator of nuclear factor-kB ligand immunolabeling, and fewer tartrate-resistant acid phosphatase-positive cells in rats3. Collectively, this study showed signs of shift from inflammation to periodontal health.
To our knowledge, there have been no studies reporting the use of 1% ALN gel as LDD in the treatment of chronic periodontitis in smokers. Therefore, a direct comparison with other studies is not possible. Comparing changes in clinical and radiographic parameters with 1% ALN gel as the local drug delivery used in the treatment of subjects with CP and aggressive periodontitis (AgP) in previous studies26,27, the improvement in clinical parameters in this study was higher in AgP subjects but lower in subjects with CP, while radiographic bone fill was higher in CP subjects but lower in AgP subjects. PD reduction was lower (4.16±1.23) in CP subjects (4.48±1.27 mm), as reported in previous study26, but greater compared to AgP subjects (3.88±1.39 mm) at 6 months, as observed in previous study27. Similarly, PAL gain was lower (3.95±0.88) in CP subjects (4.03±0.84 mm), but greater compared to AgP subjects (3.27±1.11 mm) at 6 months. Conversely, percentage change in bone fill was nearly equal but lower (41.05±11.40) in AgP subjects (46.1±9.48%) and nearly equal but greater in CP subjects (40.4±11.7%)26,27.
The results of our study are in line with previous studies that have reported that ALN was highly effective in reducing alveolar bone resorption following mucoperiosteal flap surgery24,30. This study found a percentage of IBD fill in the ALN group of 41.05±11.40%, compared to 2.5±0.93% in the placebo group at 6 months. This is in accordance with our previous studies26,27. ALN LDD also showed significant percentage of bone fill (40.87±2.73%) compared to atorvastatin LDD (34.61±5.11%) in a recent clinical trial22. Considering the abovementioned facts and results of previous studies, it can be proposed that direct subgingivally placed ALN will be a better approach for periodontal healing of bony defects in smokers with chronic periodontitis. The probable mechanism for better bone fill in the current study can be explained by the fact that tobacco components have shown to have direct effects on certain bone resorptive mediators. Tappia, et al.29 (1995) reported that smokers exposed to bacterial lipopolysaccharide had significantly higher plasma levels of TNF alpha and IL-6 than nonsmokers, while other study reported that abrogation of interleukin-6 production by alendronate in human osteoblastic cells can occur, which could also affect osteoclastic activity8.
This study has considered the technique of subgingivally delivering ALN directly into pockets of smokers with chronic periodontitis as the local drug delivery systems that offers the advantages of high concentrations at the target site with reduced dosage, fewer applications, and high patient acceptability9. Compared to a systemic regimen, local delivery may offer important benefits in terms of adverse reactions and patient compliance, as also reported in previous study19,26,27.
The current study reported presence of bone fill after 6 months from baseline, while ALN was found to be present in gingival crevicular fluid sample only at 1 month and absent at 2 months, as reported in a previous study26. This can be explained by the release of BPs from bounded bone mineral during bone resorption by osteoclasts. This could lead to a localized accumulation of BPs, which could directly perturb osteoclastic activity or indirectly target osteoblasts and macrophages, resulting in decreased osteoclastic chemotaxis and activity6. Carbopol was used as vehicle for preparation of ALN gel in this study, which is considered to provide intimate contact and prolong the residing time of a dosage form in the periodontal pocket, by specific interfacial forces in a process commonly referred as mucoadhesion, after LDD14,21.
Conclusion
This study showed 1% ALN gel caused significant improvement in periodontal clinical parameters in smokers with chronic periodontitis compared to control subjects. 1% ALN can be used as a local drug delivery system along with SRP for nonsurgical management of periodontitis.
Acknowledgments
The authors thank Apex Healthcare Limited, Ankleshwar, Gujarat, India, for providing a gift sample of ALN; Department of Pharmaceutics, Al-Ameen College of Pharmacy, Bangalore, India, for helping us prepare ALN and placebo gel; and Mr. Jagannatha PS, for carrying all required statistics. The authors declare no conflict of interest related to this study.
References
- 1.Adami S, Zamberlan M, Mian M, Dorizzi R, Rossini M, Braga B, et al. Duration of the effect of intravenous alendronate in postmenopausal women and in patients with primary hyperparathyroidism and Paget’s disease of bone. Bone Miner. 1994;25(2):75–82. doi: 10.1016/s0169-6009(08)80249-8. [DOI] [PubMed] [Google Scholar]
- 2.Ainamo J, Ainamo A. Risk assessment of recurrence of disease during supportive periodontal care. Epidemiological considerations. J Clin Periodontol. 1996;23(3 Pt 2):232–239. doi: 10.1111/j.1600-051x.1996.tb02082.x. [DOI] [PubMed] [Google Scholar]
- 3.Almeida J, Ervolino E, Bonfietti LH, Novaes VC, Theodoro LH, Fernandes LA, et al. Adjuvant therapy with sodium alendronate for the treatment of experimental periodontitis in rats. J Periodontol. 2015;86(10):1166–1175. doi: 10.1902/jop.2015.150166. [DOI] [PubMed] [Google Scholar]
- 4.Binderman I, Adut M, Yaffe A. Effectiveness of local delivery of alendronate in reducing alveolar bone loss following periodontal surgery in rats. J Periodontol. 2000;71(8):1236–1240. doi: 10.1902/jop.2000.71.8.1236. [DOI] [PubMed] [Google Scholar]
- 5.Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev. 1998;19(1):80–100. doi: 10.1210/edrv.19.1.0325. [DOI] [PubMed] [Google Scholar]
- 6.Fleisch H. Bisphosphonates–preclinical. In: Fleisch H, editor, editor. Bisphosphonates in bone disease: from the laboratory to the patient. San Diego: Academic Press; 2000. pp. 34–55. [Google Scholar]
- 7.Giuliani N, Pedrazzoni M, Negri G, Passeri G, Impicciatore M, Girasole G. Bisphosphonates stimulate formation of osteoblast precursors and mineralized nodules in murine and human bone marrow cultures in vitro and promote early osteoblastogenesis in young and aged mice in vivo. Bone. 1998;22(5):455–461. doi: 10.1016/s8756-3282(98)00033-7. [DOI] [PubMed] [Google Scholar]
- 8.Giuliani N, Pedrazzoni M, Passeri G, Girasole G. Bisphosphonates inhibit IL-6 production by human osteoblast-like cells. Scand J Rheumatol. 1998;27(1):38–41. doi: 10.1080/030097498441155. [DOI] [PubMed] [Google Scholar]
- 9.Greenstein G, Tonetti M. The role of controlled drug delivery for periodontitis. The Research, Science and Therapy Committee of the American Academy of Periodontology. J Periodontol. 2000;71(1):125–140. doi: 10.1902/jop.2000.71.1.125. [DOI] [PubMed] [Google Scholar]
- 10.Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontology 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 11.Haffajee AD, Socransky SS. Relationship of cigarette smoking to the subgingival microbiota. J Clin Periodontol. 2001;28(5):377–388. doi: 10.1034/j.1600-051x.2001.028005377.x. [DOI] [PubMed] [Google Scholar]
- 12.Jeffcoat MK, Cizza G, Shih WJ, Genco R, Lombardi A. Efficacy of bisphosphonates for the control of alveolar bone loss in periodontitis. J Int Acad Periodontol. 2007;9(3):70–76. [PubMed] [Google Scholar]
- 13.Johnson GK, Hill M. Cigarette smoking and the periodontal patient. J Periodontol. 2004;75(2):196–209. doi: 10.1902/jop.2004.75.2.196. [DOI] [PubMed] [Google Scholar]
- 14.Jones DS, Brown AF, Woolfson AD. Rheological characterization of bioadhesive, antimicrobial, semisolids designed for the treatment of periodontal diseases: transient and dynamic viscoelastic and continuous shear analysis. J Pharm Sci. 2001;90(12):1978–1990. doi: 10.1002/jps.1149. [DOI] [PubMed] [Google Scholar]
- 15.Lane N, Armitage GC, Loomer P, Hsieh S, Majumdar S, Wang HY, et al. Bisphosphonate therapy improves the outcome of conventional periodontal treatment: results of a 12-month, randomized, placebo-controlled study. J Periodontol. 2005;76(7):1113–1122. doi: 10.1902/jop.2005.76.7.1113. [DOI] [PubMed] [Google Scholar]
- 16.Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, et al. Effect of oral alendronate on bone mineral density and incidence of fractures in postmenopausal osteoporosis. New Engl J Med. 1995;333(22):1437–1443. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 17.Menezes AM, Rocha FA, Chaves HV, Carvalho CB, Ribeiro RA, Brito GA. Effect of sodium alendronate on alveolar bone resorption in experimental periodontitis in rats. J Periodontol. 2005;76(11):1901–1909. doi: 10.1902/jop.2005.76.11.1901. [DOI] [PubMed] [Google Scholar]
- 18.Mombelli A, van Oosten MA, Schurch E, Jr, Lang NP. The microbiota associated with successful or failing implants. Oral Microbiol Immunol. 1987;2(4):145–151. doi: 10.1111/j.1399-302x.1987.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 19.Needleman IG, Pandya NV, Smith SR, Foyle DM. The role of antibiotics in the treatment of periodontitis (Part 2 - Controlled drug delivery) Eur J Prosthodont Restor Dent. 1995;3(3):111–117. [PubMed] [Google Scholar]
- 20.Offenbacher S. Periodontal diseases: pathogenesis. Ann Periodontol. 1996;1(1):821–878. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- 21.Perioli L, Ambrogi V, Rubini D, Giovagnoli S, Ricci M, Blasi P, et al. Novel mucoadhesive buccal formulation containing metronidazole for the treatment of periodontal disease. J Control Rel. 2004;95(3):521–533. doi: 10.1016/j.jconrel.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Pradeep AR, Kanoriya D, Singhal S, Garg V, Manohar B, Chatterjee A. Comparative evaluation of subgingivally delivered 1% alendronate versus 1.2% atorvastatin gel in treatment of chronic periodontitis: a randomized placebo-controlled clinical trial. 10.1111/jicd.12215.J Investig Clin Dent. 2016 Apr 19; doi: 10.1111/jicd.12215. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Preber H, Bergström J. The effect of non-surgical treatment on periodontal pockets in smokers and non-smokers. J Clin Periodontol. 1986;13(4):319–323. doi: 10.1111/j.1600-051x.1986.tb02229.x. [DOI] [PubMed] [Google Scholar]
- 24.Reddy GT, Kumar PM, Veena KM. Formulation and evaluation of alendronate sodium gel for the treatment of bone resorptive lesions in periodontitis. Drug Deliv. 2005;12(4):217–222. doi: 10.1080/10717540590952663. [DOI] [PubMed] [Google Scholar]
- 25.Sharma A, Pradeep AR. Autologous platelet rich fibrin in the treatment of mandibular degree II furcation defects: a randomized clinical trial. J Periodontol. 2011;82(10):1396–1403. doi: 10.1902/jop.2011.100731. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A, Pradeep AR. Clinical efficacy of 1% alendronate gel as local drug delivery system in the treatment of chronic periodontitis: a randomized controlled clinical trial. J Periodontol. 2012;83(1):11–18. doi: 10.1902/jop.2011.110091. [DOI] [PubMed] [Google Scholar]
- 27.Sharma A, Pradeep AR. Clinical efficacy of 1% alendronate gel in adjunct to mechanotherapy in the treatment of aggressive periodontitis: a randomized controlled clinical trial. J Periodontol. 2012;83(1):19–26. doi: 10.1902/jop.2011.110206. [DOI] [PubMed] [Google Scholar]
- 28.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 29.Tappia PS, Troughton KL, Langley-Evans SC, Grimble RF. Cigarette smoking influences cytokine production and antioxidant defences. Clin Sci (Lond) 1995;88(4):485–489. doi: 10.1042/cs0880485. [DOI] [PubMed] [Google Scholar]
- 30.Veena HR, Prasad D. Evaluation of an aminobisphosphonate (alendronate) in the management of periodontal osseous defects. J Indian Soc Periodontol. 2010;14(1):40–45. doi: 10.4103/0972-124X.65438. [DOI] [PMC free article] [PubMed] [Google Scholar]