Abstract
Importance
Cardiac rehabilitation (CR) improves survival after acute myocardial infarction (AMI) and CR referral has been introduced as a performance measure of high quality care. The association of CR participation with patients’ health status (e.g. quality of life, symptoms, and functional status) is poorly defined.
Objective
To examine the association of CR with health status outcomes after AMI
Design, Setting, and Partcipants
A retrospective cohort study was conducted of patients enrolled in 2 AMI registries: PREMIER, from January 1, 2003, to June 28, 2004, and TRIUMPH, from April 11, 2005, to December 31, 2008. The analytic cohort was restricted to 4929 patients with data available on baseline health status, 6- or 12-month follow-up health status, and participation in CR. Data analysis was performed from 2014 to 2015.
Exposures
Participation in at least 1 CR session within 6 months of hospital discharge.
Main outcomes and measures
Patient health status was quantified using the Seattle Angina Questionnaire (SAQ) and the 12-Item Short-Form Health Survey (SF-12). The primary outcomes of interest were the mean differences in SAQ domain scores during the 12 months after AMI between patients who did and did not participate in CR. Secondary outcomes were the mean differences in the SF-12 summary scores and all-cause mortality.
Results
After successfully matching the cohorts of the 4929 patients (3328 men and 1601 women; mean [SD] age, 60.0 [12.2] years) for the propensity to participate in CR and comparing the groups using linear, mixed-effects models, mean differences in the SAQ and SF-12 domain scores were similar at 6 and 12 months between the 2012 patients participating in CR (3 were unable to be matched) and the 2894 who did not participate (20 were unable to be matched). At 6 months, the mean difference was –0.76 (95%CI, –2.05 to0.52) for the SAQ quality of life score, –1.53 (95% CI, –2.57 to –0.49) for the SAQ angina frequency score,0.38 (95% CI, –0.51 to 1.27) for the SAQ treatment satisfaction score, –0.42 (95% CI, –1.65 to0.79) for the SAQ physical limitation score,0.50 (95%CI, –0.22 to 1.22) for the SF-12 physical component score, and 0.13 (95% CI, –0.53 to 0.79) for the SF-12 mental component score. At 12 months, the mean difference was –0.89 (95% CI, –2.20to0.43) for the SAQ quality of life score, –1.05 (95% CI, –2.12 to 0.02) for the SAQ angina frequency score, 0.38 (95% CI, –0.54 to 1.29) for the SAQ treatment satisfaction score, –0.14 (95% CI, –1.41 to 1.14) for the SAQ physical limitation score, 0.17 (95%CI, –0.57 to 0.92) for the SF-12 physical component score, and 0.12 (95% CI, –0.56 to 0.80) for the SF-12 mental component score. In contrast, the hazard rate of all-cause mortality (up to 7 years) associated with participating in CR was 0.59 (95% CI,0.46–0.75).
Conclusions and Relevance
In a cohort of 4929 patients with AMI, we found that those who did and did not participate in CR had similar reported health status during the year following AMI; however, participation in CR did confer a significant survival benefit. These findings underscore the need for increased use of validated patient-reported outcome measures to further examine if and how health status can be maximized for patients who participate in CR.
Introduction
Cardiac rehabilitation (CR) is an important component of secondary prevention after acute myocardial infarction (AMI).1,2 Supported by systematic reviews and meta-analyses documenting reduced mortality,3–6 the American Heart Association (AHA) and American College of Cardiology (ACC) have designated CR referral as a class IA recommendation and endorsed it as a performance measure for the quality of care of patients with AMI.2,7,8 While health status improvement is among the commonly cited goals and benefits of CR,8,9 there is sparse evidence on the effect of CR on patient-reported health status (e.g. patients’ symptoms, function, and quality of life).
Examining the health status benefits of CR can enable clinicians and CR providers to better inform patients about the potential benefits of participating in CR. To better examine the association of CR with health status outcomes after AMI, we used detailed, patient-centered data from two large US, multi-center AMI registries, the Prospective Registry Evaluating outcomes after Myocardial Infarction: Events and Recovery (PREMIER)10 and the Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients’ Health status study (TRIUMPH) 11, to compare patient-reported health status between those who did and did not participate in CR.
Methods
Participants and Data Collection
Details regarding the study designs, patient selection criteria, site characteristics, and follow-up assessments of the PREMIER and TRIUMPH studies have been previously described.10,11 Briefly, both studies were US, multi-center, prospective, observational AMI registries with similar study protocols. A total of 2498 patients from 19 medical centers were enrolled into PREMIER (January 21, 2003 to June 28, 2004) while TRIUMPH (April 11, 2005 to December 31, 2008) enrolled 4340 patients across 21 sites (9 sites participated in both registries). PREMIER and TRIUMPH study sites included large academic medical centers, single-payer systems, inner-city hospitals, and non-university hospitals. Eligible patients were required to present to or be transferred to an enrolling site within 24 hours of presentation. Elevated biomarkers and either prolonged ischemic signs/symptoms or ischemic electrocardiographic changes were used to confirm AMI. Data regarding baseline patient demographics, co-morbidities, health status in addition to hospital course and management were obtained through chart abstraction and detailed, structured interviews conducted by trained research staff during hospital admission. Follow-up data regarding CR participation and health status were obtained through centralized, standardized telephone interviews at 1, 6, and 12 months after hospital discharge. Each of the 30 participating sites obtained Institutional Research Board approval and all patients provided written informed consent for study enrollment, data collection, and follow-up.
Study population and analytic cohort
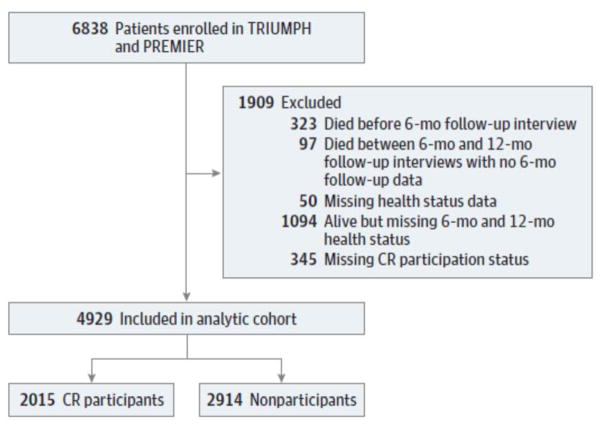
A total of 6838 patients with AMI were enrolled in the TRIUMPH and PREMIER studies. For our primary analysis, we restricted our cohort to those patients who had baseline health status data, follow-up health status data at 6 or 12 months, and information regarding CR participation available. Accordingly, excluded patients were those who died prior to 6 month follow-up (n=323), died between 6- and 12-month follow-up without any follow-up data (n=97), missing baseline health status data (n=50), missing 6- and 12-month follow up health status data (n=1094), and missing CR participation status (n=345) leaving a final analytic cohort of 4929 patients (Figure 1).
Figure 1. Flow Diagram.
Study population and analytic cohort. CR indicates cardiac rehabilitation; PREMIER, Prospective Registry Evaluating Myocardial Infarction: Events and Recovery; and TRIUMPH, Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status study.
Cardiac Rehabilitation
Information about participation in CR was obtained through centralized, standardized telephone interviews at 1 and 6 months after hospital discharge. Cardiac rehabilitation programs at participating sites from both registries were institutional based. During the 1- and 6-month follow-up telephone interviews, patients were asked if they had attended and participated in a CR program since hospitalization for their “heart attack or heart problem.” CR participants were defined as those who confirmed participating in a CR program (minimum of 1 session) within 6 months of hospital discharge following AMI.
Health Status Assessment
Patients’ health status was assessed at baseline and each follow-up interview using the Seattle Angina Questionnaire (SAQ) and the 12-item Short-Form Health Survey (SF-12). The SAQ is a validated, disease-specific instrument consisting of 19 items that measure 5 clinically relevant domains of health status in coronary artery disease (CAD) patients. For this analysis, four domains were included - quality of life (QoL), angina frequency (AF), treatment satisfaction (TS) and physical limitation (PL). Domain scores range from 0–100, with lower scores indicating worse health status (e.g. more angina, more limitation in physical activities due to angina and worse QoL).12 Prior work has suggested that a change of approximately 5 points in the SAQ QoL, AF, TS, and PL domain scores is clinically significant.12,13 The SF-12 is a generic health status measure consisting of 12-items derived to provide overall summary scales of the SF-36 physical and mental components (PCS and MCS). Scoring is norm-based and standardized to a mean of 50 with a standard deviation of 10, with lower scores indicating worse health status.14
Outcomes
The primary outcomes of interest were the mean differences in SAQ QoL, AF, TS, PL domain scores at 6- and 12-months after hospital discharge between patients who did and did not participate in CR. Secondary outcomes of interest were the mean differences in the SF-12 PCS and MCS scores and all-cause mortality (up to 7 years after AMI). Mortality was determined through follow-up interviews and querying the Social Security Death Index.
Data Analysis
Data analysis was performed between 2014 and 2015. We first measured the distribution of baseline patient characteristics between patients who did and did not participate in CR using standardized differences, defined as the mean difference between groups divided by the pooled standard deviation of the two groups. This measure of distribution is not as sensitive to sample size as traditional tests and provides a sense of the relative magnitude of differences, with a standardized difference of >10% typically considered to represent a meaningful imbalance.15 Next, to ensure an appropriate balance of patient characteristics between groups prior to comparing outcomes, we derived propensity scores for the likelihood of participating in CR within 6 months of hospital discharge for AMI. We constructed non-parsimonious logistic regression models using 45 variables to predict the likelihood of CR participation.9,16,17 Explanatory variables included in the propensity model (Table 1) were among larger conceptual domains that occurred before hospital discharge (and before the opportunity to participate in CR) and that were hypothesized to potentially be associated with participation in CR. These included demographic, psychosocial, and socioeconomic characteristics; clinical status at presentation; co-morbidities; revascularization during hospitalization for AMI; new events during hospitalization for AMI; health status at time of AMI; and medications prescribed at hospital discharge. Multiple imputation data sets were created to handle missing patient variables when constructing the propensity scores. We then conservatively matched (caliper width of 0.2 times the pooled standard deviation of the logit propensity score) CR participants to non-participants using an optimal strategy allowing many-to-many matching.18 After matching, standardized differences of all covariates were calculated to ensure that each was < 10%, indicating an adequate balance of covariate distribution between groups. For the analysis of health status outcomes, we used a linear, mixed effects model to estimate the mean difference of SAQ and SF-12 scores at 6 and 12 months between propensity-matched patients participating and not participating in CR. This model allowed for the incorporation of all eligible patients with baseline and follow-up health status scores at 6 or 12 months while assessing for interactions between groups and time. Mortality differences (up to 7 years post-AMI) between propensity-matched pairs of patients participating and not participating in CR were examined using a proportional hazards model to estimate hazard rates.
Table 1.
Baseline Characteristics of Study Population According to Participation in Cardiac Rehabilitation (CR) within 6 Months After Myocardial Infarction
| CR Participation | Standardized Difference (%)a | ||
|---|---|---|---|
|
| |||
| Yes n = 2015 | No n = 2914 | ||
|
| |||
| Demographics | |||
|
| |||
| Mean Age (SD), y | 60.2 (11.7) | 59.9 (12.5) | 2.9 |
|
| |||
| Sex | 12.0 | ||
| Male | 1427 (70.8%) | 1901 (65.2%) | |
| Female | 588 (29.2%) | 1013 (34.8%) | |
|
| |||
| Race: Caucasian | 1684 (83.6%) | 1837 (63.0%) | 47.7 |
|
| |||
| Payor: None/Self-Pay | 190 (9.4%) | 596 (20.5%) | 32.5 |
|
| |||
| Mean PHQ Depression Score (SD) | 4.7 (4.8) | 5.6 (5.4) | 18.4 |
|
| |||
| Mean ENRICHD Social Support Score (SD) | 22.5 (3.7) | 21.9 (4.5) | 14.3 |
| Marital Status | 29.3 | ||
| Married | 1377 (68.3%) | 1515 (52.0%) | |
| Divorced | 259 (12.9%) | 504 (17.3%) | |
| Separated | 38 (1.9%) | 134 (4.6%) | |
| Widowed | 167 (8.3%) | 358 (12.3%) | |
| Single (never married) | 19 (7.0%) | 343 (11.8%) | |
| Common law | 19 (0.9%) | 43 (1.5%) | |
| Other | 13 (0.6%) | 17 (0.6%) | |
|
| |||
| Socioeconomics | |||
|
| |||
| Insurance coverage for medications | 1715 (85.1%) | 2071 (71.1%) | 34.6 |
|
| |||
| Medical costs have been an economicburden | 26.2 | ||
| Severe burden | 131 (6.6%) | 348 (12.1%) | |
| Moderate burden | 131 (6.6%) | 291 (10.1%) | |
| Somewhat burden | 188 (9.4%) | 312(10.8%) | |
| A little | 181 (9.1%) | 270 (9.4%) | |
| No burden at all | 1362 (68.3%) | 1656(57.6%) | |
| Missing (n) | 22 | 37 | |
|
| |||
| Avoided getting health care due to cost | 297 (14.7%) | 730 (25.1%) | 26.2 |
|
| |||
| Not taken medication due to cost | 27.6 | ||
| Always | 19 (0.9%) | 88 (3.1%) | |
| Frequently | 53 (2.6%) | 171 (5.9%) | |
| Occasionally | 100 (5.0%) | 236 (8.2%) | |
| Rarely | 109 (5.4%) | 182(6.3%) | |
| Never | 1723 (86.0%) | 2207 (76.5%) | |
| Missing (n) | 11 | 30 | |
|
| |||
| Currently working for pay | 23.7 | ||
| Yes, I work full time | 914 (45.8%) | 1015 (35.1%) | |
| Yes, I work part time for pay | 198 (9.9%) | 258 (8.9%) | |
| No, I dont currently work for pay | 885 (44.3%) | 1617 (56.0%) | |
| Missing (n) | 18 | 24 | |
|
| |||
| Clinical status at presentation with AMI | |||
|
| |||
| Killip Class | 13.1 | ||
| I | 1752 (91.9%) | 2356 (86.6%) | |
| II | 117 (6.1%) | 293 (10.8%) | |
| III | 18 (0.9%) | 46 (1.7%) | |
| IV | 19 (1.0%) | 25 (0.9%) | |
| Unknown | 109 | 194 | |
|
| |||
| Mean Left Ventricular Ejection Fraction (SD), % | 48.4 (12.3) | 48.1 (13.6) | 7.3 |
|
| |||
| Type of AMI | |||
|
| |||
| NSTEMI | 1105 (54.9%) | 1126 (38.9%) | 13.5 |
| STEMI | 907 (45.1%) | 1769 (61.1%) | |
|
| |||
| Co-morbidities | |||
|
| |||
| Chronic Lung Disease | 130 (6.5%) | 291 ( 10.0%) | 12.9 |
|
| |||
| Chronic Renal Failure | 81 (4.0%) | 241 (8.3%) | 17.8 |
|
| |||
| Congestive Heart Failure | 81 (4.0%) | 291 (10.0%) | 23.5 |
|
| |||
| Diabetes | 463 (23.0%) | 911 (31.3%) | 18.7 |
|
| |||
| Hypercholesterolemia | 1077 (53.4%) | 1422 (48.8%) | 9.3 |
|
| |||
| Hypertension | 1220 (60.5%) | 1929 (66.2%) | 11.5 |
|
| |||
| Peripheral Arterial Disease | 80 (4.0%) | 202 (6.9%) | 13.1 |
|
| |||
| Prior Permanent Pacemaker | 29 (1.4%) | 49 (1.7%) | 2.0 |
|
| |||
| Prior Angina | 270 (13.4%) | 511 (17.5%) | 11.5 |
|
| |||
| Prior CABG | 186 (9.2%) | 393 (13.5%) | 13.4 |
|
| |||
| Prior MI | 298 (14.8%) | 678 (23.3%) | 21.7 |
|
| |||
| Prior PCI | 297 (14.7%) | 633 (21.7%) | 18.2 |
|
| |||
| Prior CVA or Prior TIA | 103 (5.1%) | 236 (8.1%) | 12.1 |
|
| |||
| History of Smoking | 1116 (55.4%) | 1813 (62.2%) | 13.9 |
|
| |||
| Revascularization during hospitalization for AMI | |||
|
| |||
| PCI or CABG (primary or other) | 1732 (86.0%) | 2042 (70.1%) | 39.1 |
|
| |||
| New Events during hospitalization for AMI | |||
|
| |||
| Atrial Fibrillation/Flutter | 146 (7.2%) | 197 (6.8%) | 1.9 |
|
| |||
| Bleeding | 220 (10.9%) | 259 (8.9%) | 6.8 |
|
| |||
| Cardiogenic Shock | 70 (3.5%) | 65 (2.2%) | 7.5 |
|
| |||
| Congestive Heart Failure | 104 (5.2%) | 184 (6.3%) | 5.0 |
|
| |||
| CVA | 8 (0.4%) | 23 (0.8%) | 5.1 |
|
| |||
| Reinfarction | 11 (0.5%) | 20 (0.7%) | 1.8 |
|
| |||
| Renal Failure | 49 (2.4%) | 122 (4.2%) | 9.8 |
|
| |||
| VT/VF Requiring Treatment | 145 (7.2%) | 141 (4.8%) | 9.9 |
|
| |||
| Baseline Health Status at time of AMI | |||
|
| |||
| Mean SAQ Quality of Life (SD) | 66.0 (21.8) | 62.2 (23.9) | 16.2 |
|
| |||
| Mean SAQ Treatment Satisfaction (SD) | 95.2 (9.1) | 93.5 (11.2) | 16.8 |
|
| |||
| Mean SAQ Angina Frequency (SD) | 87.9 (17.8) | 84.1 (22.0) | 19.1 |
|
| |||
| Mean SAQ Physical Limitation (SD) | 90.5 (17.4) | 83.4 (24.2) | 33.7 |
| Missing | 241 | 498 | |
|
| |||
| Mean SF-12 Physical Component Score (SD) | 45.4 (11.5) | 41.8 (12.5) | 29.7 |
| Missing | 71 | 129 | |
|
| |||
| Mean SF-12 Mental Component Score (SD) | 51.4 (10.7) | 49.2 (11.7) | 19.7 |
| Missing | 71 | 129 | |
|
| |||
| Medications prescribed at hospital discharge | |||
|
| |||
| Beta Blocker | 1857 (92.2%) | 2583 (88.6%) | 12.0 |
|
| |||
| ACE or ARB | 1503 (74.6%) | 2161 (74.2%) | 1.0 |
|
| |||
| Aspirin | 1920 (95.3%) | 2712 (93.1%) | 9.5 |
|
| |||
| Statin | 1791 (88.9%) | 2462 (84.5%) | 13.0 |
Abbreviations: ACE, angiotensin converting enzyme; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; CR, cardiac rehabilitation; CVA, cerebrovascular accident; ENRICHD, Enhancing Recovery in Coronary Heart Disease; NSTEMI, non–ST elevation myocardial infarction; PCI, percutaneous coronary intervention; PHQ, Patient Health Questionnaire; SAQ, Seattle Angina Questionnaire; SF-12, 12-Item Short-Form Health Survey; STEMI, ST-elevation myocardial infarction; TIA, transient ischemic attack; VF, ventricular fibrillation; VT, ventricular tachycardia.
A standardized difference of more than 10% for a patient characteristic is generally accepted to denote inadequate balance between groups.
To examine the robustness of our findings related to health status outcomes from our primary analysis, we conducted several additional sensitivity analyses. First, to assess for any survivor bias, patients who died within the first year and had neither 6- or 12-month follow-up health status data were included (n=420) and assigned a score of zero (worst possible score) for all SAQ and SF-12 domain scores at that particular time point. Second, for evaluation of any bias resulting from excluding surviving patients who were missing 6- and 12-month follow up health status data, we used inverse probability weighting. We first compared the characteristics of the analytic cohort versus alive patients excluded due to missing follow-up health status data (n= 1094). A logistic model was then constructed where patients were weighted on the inverse of the probability to be missing follow-up health status data, to enable greater weight in the outcomes to patients who were most like those that were alive but missing follow-up health status data. Lastly, to examine for any potential selection bias related to CR referral, we reran our primary analysis after restricting our analytic cohort to only those patients who were referred to CR (n=3957). The results of all sensitivity analyses were comparable with those from our original analysis and so only the primary analysis is reported. All analyses were conducted with SAS, version 9.3 (SAS Institute, Cary, North Carolina), and R, version 2.15.3 and 95% CIs were calculated for all point estimates.
Results
Among 4929 eligible patients (3328 men and 1601 women; mean [SD] age, 60.0 [12.2] years) with baseline health status data, 3743 (75.9%) had6-and12-month health status data available, while 711 (14.4%) and 475 (9.6%) only had 6- and 12-month follow-up health status data available, respectively. A total of 2015 patients (40.9%) reported participation in CR within 6 months of hospitalization for AMI. Before propensity score matching, several important baseline patient covariates were not well balanced between the groups (standardized differences >10%), although the mean (SD) age of patients who did and did not participate in CR was similar (60.2 [11.7] vs 59.9 [12.5] years) (Table). Compared with those who did not participate in CR, a higher proportion of participants were male (70.8% vs 65.2%), white (83.6% vs 63%), married (68.3% vs 52%), employed full-time (45.8%vs 35.1%), had health insurance coverage for medications (85.1%vs 71.1%), and received coronary revascularization during hospitalization for AMI(86% vs 70.1%). Moreover, patients who participated in CR avoided seeking health care owing to cost less often (14.7% vs 25.1%) and were generally healthier than those who did not participate in CR, with fewer participants having a prior MI (14.8% vs 23.3%) or history of smoking (55.4% vs 62.2%).
Although the standardized differences for all mean baseline SAQ and SF-12 scores were greater than 10%, only the standardized difference of the SAQPL score between patients who did and did not participate in CR was clinically significant (≥5 points),with participants having a mean(SD) higher score (90.5 [17.4] vs 83.4 [24.2]; standardized difference, 33.7%). During the year after AMI, the mean health status scores of patients who did and did not participate in CR improved in all domains except for SAQ TS, with small differences observed between groups (Figure 2 and eFigure 1 in the Supplement).
Figure 2. Mean Seattle Angina Questionnaire (SAQ) Domain Scores After Acute Myocardial Infarction.
A, Mean SAQ quality of life scores.
B, Mean SAQ angina frequency scores.
C, Mean SAQ treatment satisfaction scores.
D, Mean SAQ physical limitation scores.
CR indicates cardiac rehabilitation.
Propensity-matched health status scores and mortality analyses
After using propensity score methods to assemble a matched cohort of patients who participated in CR with characteristics similar to those who did not participate, 3 participants and 20 nonparticipants were unable to be matched and were excluded from further analyses (eFigure 2 in the Supplement). The distribution of observed patient characteristics were similar between the matched cohort of patients who participated in CR (n = 2012) and those who did not participate (n = 2894), as all standardized differences were less than 10% (eFigure 3 in the Supplement).
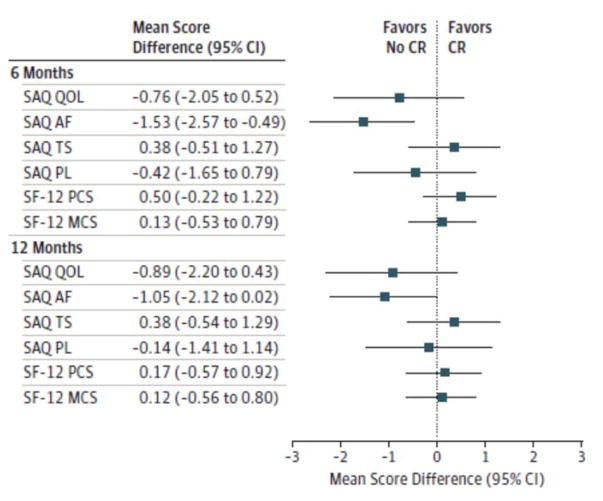
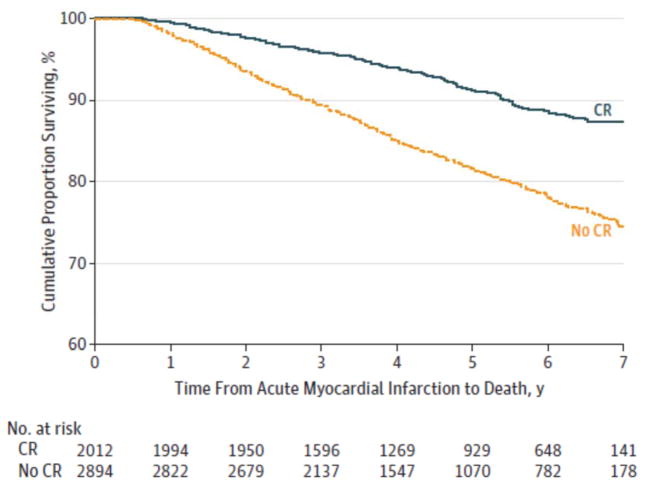
In the propensity score–matched analysis of health status outcomes, mean SAQ QoL, AF, TS, and PL scores at 6 and 12 months were clinically similar for both patients who participated in CR and those who did not participate. Although mean SAQ AF scores at 6 months were statistically significantly higher in those who did not participate than in CR patients who did participate (between-group difference, 1.53 points), this difference was well below the generally accepted threshold of clinical importance (≥5 points). For generic health status measurement, mean SF-12 physical component summary and mental component summary scores at 6 and 12 months were also similar (Figure 3). The results of the sensitivity analyses examining for any referral, survivor, or loss to follow-up bias were comparable with the above results. In the analysis of all-cause mortality during 7 years of follow-up in the propensity-matched patients who did and did not participate in CR, the participants had a 41%lower hazard rate of mortality compared with nonparticipants (hazard rate, 0.59; 95% CI, 0.46–0.75) (Figure 4).
Figure 3. Seattle Angina Questionnaire (SAQ) and 12-Item Short-Form Health Survey (SF-12) Domain Scores.
Mean differences at 6 and 12 months after acute myocardial infarction are shown between propensity-matched patients who participated in cardiac rehabilitation (CR) and those who did not participate in CR. A mean difference of ≥ 5 points is the minimal threshold for a clinically significant difference between participants and nonparticipants. AF indicates angina frequency; MCS, mental component summary; PCS, physical component summary; PL, physical limitation; QoL, quality of life; and TS, treatment satisfaction.
Figure 4. Survival During the 7 Years After Acute Myocardial Infarction.
Propensity-matched patients who participated in cardiac rehabilitation (CR) and those who did not participate in CR (hazard rate, 0.59; 95%CI, 0.46–0.75).
Discussion
In nearly 5000 post-AMI patients across 31 US centers, we found that, on average, both those who participated in at least 1 CR session and non-participants had improvements in their disease-specific and overall health status over the 12 months after hospital discharge. After propensity matching, the disease-specific and generic health status scores of patients who did and did not participate in CR were similar at both 6 and 12 months after AMI. Although health status did not clinically differ between groups, the hazard rate of long-term mortality was 41% lower in patients who participated in CR. To our knowledge, this is the largest effectiveness study to examine the association between CR and health status outcomes in post-AMI patients.
Although many studies have examined the effect of CR on morbidity and mortality in post-AMI patients,4,19,20 only a limited number of studies have examined its’ effect on health status using validated instruments, with most using generic measures. For instance, among 63 trials included in a 2016 Cochrane review of exercise based CR in patients with coronary artery disease, most of whom had experienced AMI, only 20 trials used a validated health status measure. While the majority of these studies found an improvement of health status scores in both the treatment and control arms, the significant heterogeneity in the measuring and reporting of quality of life precluded the Cochrane Collaborative from performing a meta-analysis of the health status benefits of CR.21
Among the 20 trials that used validated health status measures, only 3 studies in the post-AMI setting used disease-specific measures with heterogeneity in the type of intervention. The first, a study by Oldridge et al22, used the interviewer-administered Quality of Life after Myocardial Infarction questionnaire and found similar emotional and physical limitation domain scores at 4, 8, and 12 months in 201 AMI patients randomized to 8 weeks of CR or usual care. The second, a multi-center Australian study by Heller et al,23 used the MacNew Heart Disease Health-Related Quality of Life questionnaire in 450 patients with AMI who were randomized to usual care or an intervention, which consisted of education information on diet and exercise; they found that the intervention group had statistically higher emotional domain scores at 6-month follow-up as compared with usual care (5.4 vs. 5.2; p= 0.04), although the mean difference of 0.2 points between groups is not considered to be clinically significant. Lastly, a China based study by Wang et al, 24 used the Chinese Myocardial Infarction Dimensional Assessment Scale25,26 to assess disease specific QoL in 160 AMI patients from 2 centers who were randomized to home based CR, consisting of a self-help manual or usual care. Although the study did find statistically significant better scores in 3 out of 7 domains of CR participants at 6 month follow-up; the point difference between groups was only 4.2, 4.9, and 8.3 points in the dependency, physical activity, and concern over medications domains, respectively, and the clinical significance of these is not known. Our findings, although observational, substantially expand prior work by examining the association between CR and health status outcomes using well-accepted measures of disease-specific and generic health status in a much larger contemporary, ‘real-world’ AMI cohort.
Only two prior observational studies have used the SAQ to examine the effect of CR on health status in CAD patients, however neither study was conducted exclusively in the post-AMI setting. The first, by Goss et al 27, was a prospective, observational multi-center study examining the association between CR participation and health status in 691 patients during 12 months after coronary artery bypass grafting (CABG) surgery. This 13-center study conducted in Washington state found that the SAQ and 36-item Short-Form survey domain scores improved for all patients one year after CABG surgery, independent of CR participation. Tavella and Beltrame28 conducted a smaller, more contemporary prospective, single-center Australian study that examined the effect of participation in CR on health status in 150 patients with coronary artery disease over 6 months following coronary angiography. They found that all patients had improvements in SAQ and 36-item Short-Form survey domain scores independent of CR participation. Our findings of comparable changes in disease-specific and generic health status of both patients who did and did not participate in CR are similar to the results of these two studies, although our analyses focused exclusively upon patients with a recent AMI.
Although improvement in health status is a commonly cited benefit of CR participation after an AMI,8,9 our results, and prior work, underscore the paucity of data to support this statement.3,5 Much of the uncertainty surrounding this association is due to the lack of adequately powered, high-quality studies capturing the type, intensity, and frequency of CR while using validated, disease-specific health status measures. Despite no differences in health status, the association of participation in CR with survival after an AMI, and the reported underuse of CR,16 are compelling reasons to refer patients to, and ensure their participation in CR. Nevertheless, it is important that patients are provided with accurate, evidence-based information that CR is associated with better survival but not better health status, given that prior work has suggested that many patients primarily expect improvement in health status from participation in CR.29–32 Moreover, future work should explore whether or not the type, intensity, frequency, duration, or any other additional components of CR may improve health status, as improved angina control and better quality of life are primary goals of treatment after AMI. Our findings also underscore the need for increased use of validated cardiovascular health status measures in both clinical studies and the real-world setting to provide opportunities to further examine if and how these patient-centered outcomes can be maximized for patients who participate in CR.
Our study findings should be interpreted in the context of the following potential limitations. First, participation in CR was self-reported using structured follow-up interviews, and we did not verify patients’ reported participation at each site. However, prior work found a nearly perfect agreement between self-reported and site-verified CR participation.33 Second, we did not capture the type, intensity, frequency, and length of patients’ CR participation. However, our results are similar to prior randomized control trials and observational studies using disease-specific health status measures.22,27,28,34 Moreover, our demonstration of a survival benefit with participation In CR, similar to prior studies, further supports our characterization of participation in CR given our replication of the previously established benefits of this intervention.3 Nevertheless, future research efforts may be able to better quantify the quantity and quality of participation in CR and identify a dose-response association that was missed with our crude categorization of CR participation. Third, the two groups in our analytic cohort had significant differences at baseline in several characteristics and, while we were able to successfully match our cohorts using propensity-based methods in addition to conduction several sensitivity analyses, we cannot exclude unmeasured confounding.
Conclusion
In a large, contemporary, multi-center cohort of 4,929 patients with AMI, we found that patients who did and did not participate in CR had similar disease-specific and generic health status during the year following hospital discharge; however, participation in CR did confer a significant survival benefit. Our results underscore the need for further investigation of the impact of participation in CR on health status to identify if and how CR programs can better maximize health status outcomes for patients after AMI.
Supplementary Material
Acknowledgments
Funding/Support: The Prospective Registry Evaluating Outcomes After Myocardial Infarction: Events and Recovery (PREMIER) study was funded by CV Therapeutics, Palo Alto, California. The Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH) study was funded by grant P50 HL 077113 from the National Heart, Lung, and Blood Institute. This study was also funded in part by CV Outcomes, Inc, Kansas City, Missouri.
Footnotes
Author Contributions: Drs Kureshi and Spertus had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kureshi, Kennedy, Jones, Spertus.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Kureshi.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Kureshi, Kennedy, Jones.
Administrative, technical, or material support: Kureshi, Buchanan, Spertus.
Study supervision: Spertus.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Drs Kureshi, Qintar, and Fendler reported receiving support Award Number T32HL110837 from the National Heart, Lung, and Blood Institute of the National Institutes of Health. Dr Spertus reported holding a copyright of the Seattle Angina Questionnaire. No other conflicts were reported.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Thomas RJ. Cardiac Rehabilitation/Secondary Prevention Programs: A Raft for the Rapids: Why Have We Missed the Boat? Circulation. 2007;116(15):1644–1646. doi: 10.1161/CIRCULATIONAHA.107.728402. [DOI] [PubMed] [Google Scholar]
- 2.Thomas RJ, King M, Lui K, et al. AACVPR/ACC/AHA 2007 Performance Measures on Cardiac Rehabilitation for Referral to and Delivery of Cardiac Rehabilitation/Secondary Prevention ServicesEndorsed by the American College of Chest Physicians, American College of Sports Medicine, American Physical Therapy Association, Canadian Association of Cardiac Rehabilitation, European Association for Cardiovascular Prevention and Rehabilitation, Inter-American Heart Foundation, National Association of Clinical Nurse Specialists, Preventive Cardiovascular Nurses Association, and the Society of Thoracic Surgeons. Journal of the American College of Cardiology. 2007;50(14):1400–1433. doi: 10.1016/j.jacc.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Heran BS, Chen JM, Ebrahim S, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;(7):CD001800. doi: 10.1002/14651858.CD001800.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2011;162(4):571–584. e572. doi: 10.1016/j.ahj.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. The American journal of medicine. 2004;116(10):682–692. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Clark AM, Hartling L, Vandermeer B, McAlister FA. Meta-analysis: secondary prevention programs for patients with coronary artery disease. Annals of internal medicine. 2005;143(9):659–672. doi: 10.7326/0003-4819-143-9-200511010-00010. [DOI] [PubMed] [Google Scholar]
- 7.Krumholz HM, Anderson JL, Bachelder BL, et al. ACC/AHA 2008 performance measures for adults with ST-elevation and non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to develop performance measures for ST-elevation and non-ST-elevation myocardial infarction): developed in collaboration with the American Academy of Family Physicians and the American College of Emergency Physicians: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, Society for Cardiovascular Angiography and Interventions, and Society of Hospital Medicine. Circulation. 2008;118(24):2596–2648. doi: 10.1161/CIRCULATIONAHA.108.191099. [DOI] [PubMed] [Google Scholar]
- 8.Wenger NK. Current status of cardiac rehabilitation. J Am Coll Cardiol. 2008;51(17):1619–1631. doi: 10.1016/j.jacc.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 9.Arena R, Williams M, Forman DE, et al. Increasing Referral and Participation Rates to Outpatient Cardiac Rehabilitation: The Valuable Role of Healthcare Professionals in the Inpatient and Home Health Settings: A Science Advisory From the American Heart Association. Circulation. 2012;125(10):1321–1329. doi: 10.1161/CIR.0b013e318246b1e5. [DOI] [PubMed] [Google Scholar]
- 10.Spertus JA, Peterson E, Rumsfeld JS, Jones PG, Decker C, Krumholz H. The Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER)--evaluating the impact of myocardial infarction on patient outcomes. Am Heart J. 2006;151(3):589–597. doi: 10.1016/j.ahj.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Arnold SV, Chan PS, Jones PG, et al. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH): design and rationale of a prospective multicenter registry. Circulation. Cardiovascular quality and outcomes. 2011;4(4):467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 13.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. The American journal of cardiology. 1994;74(12):1240–1244. doi: 10.1016/0002-9149(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 14.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Mamdani M, Sykora K, Li P, et al. Reader’s guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ. 2005;330(7497):960–962. doi: 10.1136/bmj.330.7497.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balady GJ, Ades PA, Bittner VA, et al. Referral, Enrollment, and Delivery of Cardiac Rehabilitation/Secondary Prevention Programs at Clinical Centers and Beyond: A Presidential Advisory From the American Heart Association. Circulation. 2011;124(25):2951–2960. doi: 10.1161/CIR.0b013e31823b21e2. [DOI] [PubMed] [Google Scholar]
- 17.Oldridge N, Gottlieb M, Guyatt G, Jones N, Streiner D, Feeny D. Predictors of health-related quality of life with cardiac rehabilitation after acute myocardial infarction. J Cardiopulm Rehabil. 1998;18(2):95–103. doi: 10.1097/00008483-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC. A Tutorial and Case Study in Propensity Score Analysis: An Application to Estimating the Effect of In-Hospital Smoking Cessation Counseling on Mortality. Multivariate behavioral research. 2011;46(1):119–151. doi: 10.1080/00273171.2011.540480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA : the journal of the American Medical Association. 1988;260(7):945–950. [PubMed] [Google Scholar]
- 20.O’Connor GT, Buring JE, Yusuf S, et al. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation. 1989;80(2):234–244. doi: 10.1161/01.cir.80.2.234. [DOI] [PubMed] [Google Scholar]
- 21.Anderson L, Oldridge N, Thompson DR, et al. Exercise-Based Cardiac Rehabilitation for Coronary Heart DiseaseCochrane Systematic Review and Meta-Analysis. Journal of the American College of Cardiology. 2016;67(1):1–12. doi: 10.1016/j.jacc.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 22.Oldridge N, Guyatt G, Jones N, et al. Effects on quality of life with comprehensive rehabilitation after acute myocardial infarction. The American journal of cardiology. 1991;67(13):1084–1089. doi: 10.1016/0002-9149(91)90870-q. [DOI] [PubMed] [Google Scholar]
- 23.Heller RF, Knapp JC, Valenti LA, Dobson AJ. Secondary prevention after acute myocardial infarction. The American journal of cardiology. 1993;72(11):759–762. doi: 10.1016/0002-9149(93)91058-p. [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Chair SY, Thompson DR, Twinn SF. Effects of home-based rehabilitation on health-related quality of life and psychological status in Chinese patients recovering from acute myocardial infarction. Heart & Lung: The Journal of Acute and Critical Care. 2012;41(1):15–25. doi: 10.1016/j.hrtlng.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Wang W, Lopez V, Thompson DR. A Chinese Mandarin translation and validation of the Myocardial Infarction Dimensional Assessment Scale (MIDAS) Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2006;15(7):1243–1249. doi: 10.1007/s11136-006-0065-1. [DOI] [PubMed] [Google Scholar]
- 26.Thompson DR, Jenkinson C, Roebuck A, Lewin RJ, Boyle RM, Chandola T. Development and validation of a short measure of health status for individuals with acute myocardial infarction: the myocardial infarction dimensional assessment scale (MIDAS) Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2002;11(6):535–543. doi: 10.1023/a:1016354516168. [DOI] [PubMed] [Google Scholar]
- 27.Goss JR, Epstein A, Maynard C. Effects of cardiac rehabilitation on self-reported health status after coronary artery bypass surgery. J Cardiopulm Rehabil. 2002;22(6):410–417. doi: 10.1097/00008483-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Tavella R, Beltrame JF. Cardiac rehabilitation may not provided a quality of life benefit in coronary artery disease patients. BMC Health Serv Res. 2012;12:406. doi: 10.1186/1472-6963-12-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper AF, Jackson G, Weinman J, Horne R. A qualitative study investigating patients’ beliefs about cardiac rehabilitation. Clinical Rehabilitation. 2005;19(1):87–96. doi: 10.1191/0269215505cr818oa. [DOI] [PubMed] [Google Scholar]
- 30.Wittmer M, Volpatti M, Piazzalonga S, Hoffmann A. Expectation, satisfaction, and predictors of dropout in cardiac rehabilitation. European Journal of Preventive Cardiology. 2012;19(5):1082–1088. doi: 10.1177/1741826711418163. [DOI] [PubMed] [Google Scholar]
- 31.Cooper AF, Weinman J, Hankins M, Jackson G, Horne R. Assessing patients’ beliefs about cardiac rehabilitation as a basis for predicting attendance after acute myocardial infarction. Heart. 2007;93(1):53–58. doi: 10.1136/hrt.2005.081299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redfern J, Ellis ER, Briffa T, Freedman SB. High risk-factor level and low risk-factor knowledge in patients not accessing cardiac rehabilitation after acute coronary syndrome. The Medical journal of Australia. 2007;186(1):21–25. doi: 10.5694/j.1326-5377.2007.tb00783.x. [DOI] [PubMed] [Google Scholar]
- 33.Kayaniyil S, Leung YW, Suskin N, Stewart DE, Grace SL. Concordance of self- and program-reported rates of cardiac rehabilitation referral, enrollment and participation. The Canadian journal of cardiology. 2009;25(4):e96–99. doi: 10.1016/s0828-282x(09)70063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofman-Bang C, Lisspers J, Nordlander R, et al. Two-year results of a controlled study of residential rehabilitation for patients treated with percutaneous transluminal coronary angioplasty. A randomized study of a multifactorial programme. Eur Heart J. 1999;20(20):1465–1474. doi: 10.1053/euhj.1999.1544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.