Abstract
Purpose
To evaluate the utility of Endothelial/Descemet's membrane complex (En/DM) characteristics in diagnosing corneal graft rejection.
Design
Diagnostic reliability study.
Methods
139 eyes (96 corneal grafts post penetrating keratoplasty or Descemet's stripping automated endothelial keratoplasty: 40 clear, 23 actively rejecting, 24 rejected and 9 non-immunological failed grafts, along with 43 age-matched control eyes) were imaged using high-definition optical coherence tomography. Images were used to describe En/DM and measure central corneal thickness (CCT) and central En/DM thickness (DMT). En/DM rejection index (DRI) was computed to detect the relative En/DM thickening to the entire cornea.
Results
In actively rejecting grafts, DMT and DRI were significantly greater than controls and clear grafts (28, 17 and 17 μm and 1.5, 1 and 1, respectively; P<0.001). Rejected grafts had the highest DMT and DRI compared to all groups (59 μm and 2.1; P<0.001). DMT and DRI showed excellent accuracy, significantly better than that of CCT, in differentiating actively rejecting from clear grafts (100% and 96% sensitivity; 92.5% and 92.5% specificity), actively rejecting from rejected grafts (88% and 83% sensitivity; 91% and 83% specificity) and non-immunological failed from rejected grafts (100% and 100% sensitivity; 88% and 100% specificity). DMT correlated significantly with rejection severity (P<0.001).
Conclusions
In corneal grafts, in vivo relative thickening of the En/DM is diagnostic of graft rejection as measured by DMT and DRI. These indices have excellent accuracy, sensitivity and specificity in detecting graft immunological status, superior to CCT. DMT is a quantitative index that correlates accurately with the severity of rejection.
Introduction
Every year, 120,000 corneal transplants are performed worldwide.1 This makes it, by far, the most commonly performed solid organ transplantation.2 More than 50% of those grafts experience at least one episode of rejection of which 27% ultimately fail by the 3rd year.3-6 Those failures impose a heavy burden on the health care system and on patients' quality of life and productivity.7
Immunological rejection against corneal graft endothelial cells is the single most important cause of graft failure.7,8 The immunological injury to endothelial cells is irreversible as those cells are unable to regenerate. Thus, the sooner that one can initiate treatment, the longer the corneal graft will survive.9 This fact highlights the importance of the early and accurate diagnosis of graft rejection to preserve corneal grafts and prolong their survival.10-12 The currently available diagnostic techniques include slit-lamp examination (SLE), central corneal thickness (CCT) and corneal endothelial cell density (ECD). These measurements provide valuable clinical information. Nevertheless, they fall short in monitoring corneal graft health by not detecting mild rejection, nor predicting graft survival.5,8,12-15 Therefore, the search for a novel diagnostic technique to improve the standard of care for monitoring corneal grafts is highly needed.
There is strong evidence in solid organ transplantation literature that detecting thickening of allograft basement membranes on electron and light microscopic studies is a sign of solid organ graft rejection.16-20 In kidney transplantation, multi-layering and thickening of the basement membrane of peritubular capillaries of renal grafts is an established technique for the diagnosing chronic renal rejection and predicting graft survival.16-18 Likewise, thickening of the epithelial basement membrane of bile ducts in liver grafts and of bronchiolar basement membrane in lung grafts are diagnostic signs of graft rejection and predictors of graft survival.19,20 Detecting those signs in renal, hepatic and lung grafts requires invasive tissue biopsies. In contrast to those organs, the cornea is a transparent organ. This transparency, with the advent of the in vivo microscopic imaging techniques such as high definition spectral domain optical coherence tomography (HD-OCT) and specular microscopy, allows for non-invasive optical evaluation of corneal grafts. For instance, morphologic changes in corneal endothelial cells as demonstrated by specular microscopy were found to be indicative of pending allograft rejection following Descemet's membrane endothelial keratoplasty (DMEK).21 Despite that strong body of evidence in solid organ transplantation literature and the non-invasiveness of implementing this technique to corneal grafts, no studies exist in the literature evaluating the use of the corneal endothelial basement membrane, the Descemet's membrane (DM), for the diagnosis of corneal graft rejection.
In this study, we report for the first time, the use of HD-OCT to detect corneal endothelial/Descemet's membrane complex (En/DM) microstructural characteristics following corneal graft transplantation and validate our methodology and results by conducting a HD-OCT/histopathology correlation study. We then quantify those in vivo En/DM structural changes using novel diagnostic indices, central En/DM thickness (DMT) and En/DM rejection index (DRI), that we found to be highly sensitive and specific in characterizing the immunological status of corneal grafts, most importantly active corneal graft rejection. We also present data demonstrating that DMT and DRI are much better indicators than one of the standard-of-care diagnostic techniques, namely central corneal thickness (CCT), in assessing the health of the corneal graft.
Methods
Study Population
Our study included 139 eyes, 96 consecutive corneal grafts (penetrating keratoplasty, PK and Descemet's stripping automated endothelial keratoplasty, DSAEK) and 43 age-matched control eyes. Patients were recruited at two clinical sites, the Bascom Palmer Eye Institute (BPEI) of University of Miami and the Saint Louis University Eye Institute (SLUEI) of Saint Louis University. Written informed consent, approved by University of Miami and Saint Louis University Institutional Review Boards (IRB), was obtained from all patients. Eyes with corneal infections or endothelial pathologies such as Fuchs endothelial dystrophy and posterior polymorphous dystrophy (PPMD) as diagnosed clinically under high biomicroscopic magnification were excluded from the study. Slit lamp examination (SLE) by a cornea specialist was performed on all eyes to confirm the absence of any of the exclusion criteria and on corneal grafts to determine their immunological statuses. Active endothelial graft rejection was diagnosed by detecting new keratic precipitates or Khodadoust line in the presence of anterior chamber cells and new corneal graft edema. Corneal graft edema was confirmed at a subsequent clinical exam to ensure persistence of edema. Clinically stratifying corneal grafts rejection based on the severity of rejection episodes was, however, not attempted. Rejected grafts, on the other hand, were diagnosed by detecting corneal graft edema with prior history of a causative rejection episode and absence of signs of active rejection. Failed grafts secondary to non-immunological causes were those grafts that never cleared after surgery, endured significant surgical trauma and did not have signs of prior or active corneal graft rejection.
Image Acquisition and Processing
To describe the En/DM complex structural characteristics and measure its thickness, a horizontal 2-dimensional HD-OCT image centered on the corneal vertex was captured for all studied corneas. In the BPEI site, a custom-made HD-OCT was used. Details of the device were described in our previous reports.22,23 Briefly, it uses a super-luminescent diode light source (SLD) with a center wavelength of 840 nm and has an axial resolution of ∼ 3 μm in tissue (the refractive index is ∼1.39).24 In SLUEI site, a comparable HD-OCT (Cirrus HD-OCT, Carl Zeiss Meditec, Dublin, CA) was used. This device uses an SLD with the same wavelength (840 nm) and has a resolution of ∼5 μm in tissue. A corneal reflex artifact with a hyper-reflective area on the corneal surface was created with a reflex saturation beam to identify corneal vertex in order to get accurate pachymetry measurements in the custom-built HD-OCT,25 while with the Cirrus HD-OCT, the patient's own fixation on the fixation target was used to ensure the scan was centered on the corneal vertex.
As previously reported by our group, the two most posterior hyper-reflective bands of the cornea on HD-OCT images represented the 2 interfaces of the En/DM. Validation studies of our image acquisition and analysis techniques were published in our previous work.22 This method has been reported in previous studies.26,27 Note that, as we were unable to separate the Descemet's membrane from the corneal endothelium on the HD-OCT images, we referred to them as the En/DM complex. Graft CCT was measured using the obtained HD-OCT images. All HD-OCT measurements were determined by a single unmasked reader (MAS).
Diagnostic indices
We used two diagnostic indices to describe the structural characteristics of the En/DM complex, En/DM thickness (DMT) and En/DM rejection index (DRI). DMT was measured as the distance between the two most posterior hyper-reflective bands of the central cornea on the HD-OCT image.22,26 DRI was formulated to isolate the specific thickening of En/DM from the generalized thickening of a swollen cornea. We computed DRI as DMT divided by CCT multiplied by a constant. We have used “33” as the constant in the aforementioned equation since on using that constant, the mean DRI of the age-matched control group in our study was equal to 1. Note that in cases of DSAEK grafts, the stromal tissue transplanted with the En/DM graft, as measured by HD-OCT from the center of the donor stroma-Descemet's interface to the center of the donor stroma-recipient stroma interface, was excluded from the CCT measurements for the sake of standardization.
Validation of HD-OCT measurements
In order to validate our in vivo HD-OCT findings, we conducted an ex vivo study that included 18 corneal specimens. Preoperative HD-OCT measurements were taken for 9 eyes undergoing PK (4 eyes using the custom-built HD-OCT at the BPEI site and 5 using the Cirrus HD-OCT at the SLUEI site). HD-OCT/Histopathologic correlation was performed for CCT and DMT. Histologic specimens were fixed in 10% buffered formalin, dehydrated, and embedded in paraffin. Slides were sectioned at 5 μm then were stained with periodic acid Schiff. Specimens were analyzed using a microscope (Olympus Optical Co., Tokyo, Japan). The mean thickness of Endothelial/Descemet's membrane complex was determined by averaging thickness measurements in 3 representative randomly selected central high power field per slide (original magnification, X 400).
To further validate the use of DMT in the diagnosis of graft rejection, we obtained 9 globes enucleated secondary to posterior melanoma but with no corneal or angle involvement. DMT measurements were obtained using aforementioned methodology and compared to measurements obtained from the 9 eyes that underwent PK for graft rejection.
Statistical Analysis
Statistical analyses were performed by the Biostatistics Department of the Bascom Palmer Eye Institute using SPSS software version 22.0 (SPSS, Chicago, IL, USA). Means of CCT, DMT and DRI were compared between all groups using one-way analysis of variance. Tamhane post-hoc comparisons were performed to account for differences in variance between groups. To assess the correlation between the in vivo HD-OCT and ex vivo histopathological measurements, we used Pearson correlations. We also examined agreement with the Bland-Altman method. Mean of ex vivo DMT measurements of the rejected grafts and control corneas as measured by light microscopy were compared using t-test. The accuracy, sensitivity and specificity of DMT, DRI and CCT in differentiating between studied groups were determined using a forward stepwise logistic regression model and by generating receiver operating characteristic curves (ROC). In order to determine if DMT and DRI would be descriptive of graft rejection severity, linear regression analyses of those indices and rejection severity described by CCT were computed. Two-sided p-values less than 0.05 were considered statistically significant. Values are presented as means ± standard deviation.
Results
Our study included 139 eyes from 115 patients; the breakdown from these patients were 40 clear grafts (28 PK and 12 DSAEK), 23 actively rejecting grafts (18 PK and 5 DSAEK), 24 rejected grafts (20 PK and 4 DSAEK), 9 non-immunological failed grafts (6 PK and 3 DSAEK) and 43 age-matched control eyes. Table 1 summarizes the different characteristics of all groups. There were no statistically significant differences between mean ages of study groups. There were no statistically significant differences between the mean CCT of post-PK corneas vs. the mean CCT of post-DSAEK corneas (after excluding the donor stromal thickness), or between the DMT or DRI of PK vs. DSAEK grafts, or between eyes recruited at BPEI and SLUEI within the same study group (Fig. 1).
Table 1. Characteristics of our study groups.
| Normal control group | Clear grafts | Actively rejecting grafts | Rejected grafts | Non-immunological failed grafts | ||
|---|---|---|---|---|---|---|
| Number of eyes | 43 | 40 | 23 | 24 | 9 | |
| Number of Patients | 31 | 36 | 22 | 23 | 9 | |
| Age (in years) | 70 ±9 | 67 ±16 | 65 ±17 | 67 ±17 | 64 ±13 | |
| Gender | Female | 18 | 19 | 11 | 11 | 4 |
| Male | 13 | 17 | 11 | 12 | 5 | |
| CCT in μm | 543 ±37 | 527 ±69 | 614 ±111 | 928 ±371 | 748 ±125 | |
| DMT in μm | 17 ±2 | 17 ±3 | 28 ±6 | 59 ±29 | 24 ±5 | |
| DRI | 1 ±0.1 | 1 ±0.2 | 1.5 ±0.2 | 2.1 ±0.5 | 1 ±0.1 | |
Note that 7 patients contributed eyes to two different study groups (study included a total of 115 patients).
Values are presented as means ± standard deviation.
CCT: central corneal thickness.
DMT: Endothelial/Descemet's membrane complex (En/DM) thickness.
DRI: En/DM rejection index, defined as DMT divided by CCT multiplied by 33.
Figure 1.

Boxplot distributions of central corneal thickness (CCT, left), central Endothelium/Descemet's membrane complex thickness (DMT, middle), and Endothelium/Descemet's membrane complex rejection index (DRI, right) of eyes recruited at Bascom Palmer Eye Institute (BPEI) and Saint Louis University Eye Institute (SLUEI). There were no statistically significant differences between mean CCT, DMT, or DRI between eyes recruited at BPEI and SLUEI, within the same study group. (P=0.490, 0.162, and 0.204, respectively)
CCT: central corneal thickness
DMT: Endothelium/Descemet's membrane complex thickness
DRI: Endothelium/Descemet's membrane complex rejection index
BPEI: Bascom Palmer Eye Institute
SLUEI: Saint Louis University Eye Institute
Using HD-OCT, we characterized the En/DM of all studied eyes. In normal controls, En/DM was visualized as a band formed by two smooth hyper-reflective lines with a translucent space in between. En/DM of clear grafts had similar characteristics to normal controls (Fig. 2a and 2b). On the other hand, in actively rejecting grafts, En/DM was visualized as a thickened band formed by two hyper-reflective lines, with a posterior line that has irregular hyper-reflectivity and occasional nodular excrescences (Fig. 2c and 2d). En/DM of rejected grafts had a similar appearance to actively rejecting grafts but with a more irregular posterior hyper-reflective line and with nodular excrescences (Fig. 1e and 1f). Interestingly, in non-immunological failed grafts, En/DM was similar in appearance to normal controls and clear grafts but thicker and with no identifiable nodular excrescences (Fig. 2g and 2h).
Figure 2.
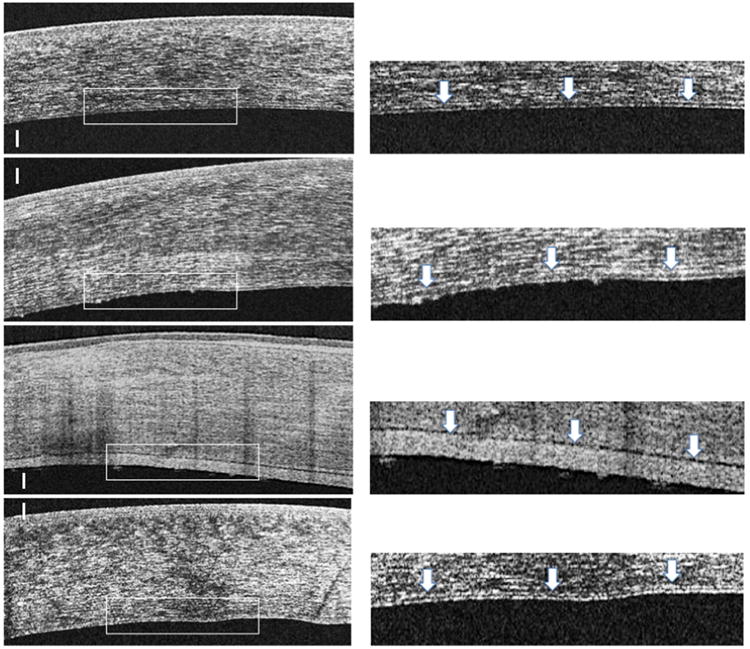
High definition spectral domain optical coherence tomography (HD-OCT) images of a clear corneal graft (first row, left), an actively rejecting graft (second row, left), a rejected graft (third row, left) and non-immunological failed grafts (fourth row, left). Presets (right column) show magnified images of the posterior part of the corresponding cornea on the left. In the presets, arrows are indicating the Endothelial/Descemet's membrane complex (En/DM). In clear grafts (first raw), En/DM was visualized as a band formed by two smooth hyper-reflective lines with a translucent space in between (first row, right, arrows). In actively rejecting grafts (second row), En/DM was visualized as a thickened band formed by two hyper-reflective lines. The anterior line and the translucent space in between the lines were similar to those of the clear graft, while the posterior line had a broader hyper-reflectivity and occasional nodular excrescences (second row, right, arrows). In rejected corneal grafts (third row), En/DM had a similar appearance to actively rejecting grafts but with much thicker and more irregular posterior hyper-reflective line and nodular excrescences (third row, right, arrows). On the other hand, En/DM of non-immunological failed grafts (fourth row) was similar in appearance to clear grafts but with a generalized thickening of the hyper-reflective lines and the translucent space in between and with no identifiable nodular excrescences (fourth row, right, arrows). Bars are 100 μm.
In our study, we have disclosed that corneal graft rejection causes the En/DM complex to develop significant thickening that can be quantified using our two thickness indices, DMT and DRI. (Table 1) While, mean DMT of clear grafts was not statistically significantly different from normal controls, mean DMT of actively rejecting grafts was thicker compared with normal controls, clear grafts, and non-immunologically failed grafts with high statistical significance (P<0.001). Furthermore, DMT of rejected corneal grafts demonstrated a thickness that was significantly greater than all groups (P<0.001). Interestingly, grafts with non-immunological failure had significantly thicker DMT than clear grafts and controls (P<0.001 for both comparisons) but thinner than that in actively rejecting and rejected grafts (P<0.001 for both comparisons).
Mean CCT was statistically significant for discriminating between all study groups (Table 2, P<0.001). However, while mean DMT of rejected grafts was 347% thicker than normal controls and clear grafts, CCT of rejected grafts was thickened by only 71% compared to normal controls and 76% compared to clear grafts (928, 543 and 527 μm, respectively; P<0.004). Thickening of rejected grafts En/DM was clearly out of proportion to the total graft thickening. To highlight the observed localized En/DM thickening and deduct the effect of the generalized corneal swelling on this layer, we formulated the DRI. DRI of actively rejecting and rejected grafts were highly statistically significantly different from controls, clear grafts and non-immunologically failed grafts (P<0.005).
Table 2. Pairwise comparisons of central corneal thickness, Endothelial/Descemet's membrane complex thickness, and Endothelial/Descemet's membrane complex rejection index means in different study groups.
| Dependent Variable | (I) group | (J) group | Mean Difference (I-J) | Standard Error | Significanced | 95% Confidence Interval for Differenced | |
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| CCT | 1 PK/DSAEK control | 3 rejected graft | -347.292*,b,c | 13.195 | 0 | -374.73 | -319.85 |
| 4 Actively rejecting graft | -94.516*,b,c | 13.374 | 0 | -122.33 | -66.703 | ||
| 5 graft failure | -224.413*,b,c | 18.628 | 0 | -263.15 | -185.67 | ||
| 6 Normal age matched | -25.685b,c | 11.525 | 0.037 | -49.653 | -1.716 | ||
| 3 Rejected graft | 4 Actively rejecting graft | 252.776*,b,c | 14.806 | 0 | 221.985 | 283.567 | |
| 5 graft failure | 122.879*,b,c | 19.681 | 0 | 81.95 | 163.808 | ||
| 6 Normal age matched | 321.607*,b,c | 13.16 | 0 | 294.24 | 348.974 | ||
| 4 Actively rejecting graft | 5 graft failure | -129.896*,b,c | 19.802 | 0 | -171.08 | -88.716 | |
| 6 Normal age matched | 68.832*,b,c | 13.339 | 0 | 41.091 | 96.572 | ||
| 5 Non-Immunological graft failure | 6 Normal age matched | 198.728*,b,c | 18.603 | 0 | 160.041 | 237.415 | |
| DMT | 1 PK/DSAEK control | 3 rejected graft | -36.116*,b,c | .560 | 0 | -37.28 | -34.951 |
| 4 Actively rejecting graft | -12.074*,b,c | .567 | 0 | -13.254 | -10.894 | ||
| 5 graft failure | -7.640*,b,c | .790 | 0 | -9.283 | -5.996 | ||
| 6 Normal age matched | -.328*,b,c | 0.489 | 0.51 | -1.345 | 0.689 | ||
| 3 rejected graft | 4 Actively rejecting graft | 24.042*,b,c | 0.628 | 0 | 22.735 | 25.348 | |
| 5 graft failure | 28.476*,b,c | 0.835 | 0 | 26.739 | 30.212 | ||
| 6 Normal age matched | 35.788*,b,c | 0.558 | 0 | 34.627 | 36.949 | ||
| 4 Actively rejecting graft | 5 graft failure | 4.434*,b,c | 0.84 | 0 | 2.687 | 6.181 | |
| 6 Normal age matched | 11.746*,b,c | 0.566 | 0 | 10.569 | 12.923 | ||
| 5 Non-Immunological graft failure | 6 Normal age matched | 7.312*,b,c | 0.789 | 0 | 5.671 | 8.953 | |
| DRI | 1 PK/DSAEK control | 3 rejected graft | -.950*,b,c | .027 | 0 | -1.007 | -0.893 |
| 4 Actively rejecting graft | -.488*,b,c | .028 | 0 | -0.546 | -0.431 | ||
| 5 graft failure | -.018b,c | 0.038 | 0.649 | -0.098 | 0.062 | ||
| 6 Normal age matched | .031b,c | 0.024 | 0.211 | -0.019 | 0.08 | ||
| 3 rejected graft | 4 Actively rejecting graft | .461*,b,c | 0.031 | 0 | 0.398 | 0.525 | |
| 5 graft failure | .932*,b,c | 0.041 | 0 | 0.848 | 1.017 | ||
| 6 Normal age matched | .981*,b,c | 0.027 | 0 | 0.924 | 1.037 | ||
| 4 Actively rejecting graft | 5 graft failure | .471*,b,c | 0.041 | 0 | 0.386 | 0.556 | |
| 6 Normal age matched | .519*,b,c | 0.028 | 0 | 0.462 | 0.576 | ||
| 5 Non-Immunological graft failure | 6 Normal age matched | .048b,c | 0.038 | 0.221 | -0.031 | 0.128 | |
CCT: central corneal thickness.
DMT: Endothelial/Descemet's membrane complex (En/DM) thickness.
DRI: En/DM rejection index, defined as DMT divided by CCT multiplied by 33.
PK: penetrating keratoplasty.
DSAEK: Descemet's stripping automated endothelial keratoplasty.
Based on estimated marginal means
The mean difference is significant at the .050 level.
An estimate of the modified population marginal mean (I).
An estimate of the modified population marginal mean (J).
Adjustment for multiple comparisons: Least Significant Difference (equivalent to no adjustments)
In contrast, non-immunological failed grafts had a mean DMT, which, while significantly thicker (41% increase) than normal controls and clear grafts (P=0.02), was similar to the increased thickness of the whole cornea (38% increase in CCT when compared to normal controls and a 42% increase in CCT when compared to clear grafts. Interestingly, the mean DRI of this group was 1±0.1, which is the same as controls and clear grafts. This highlights the lack of a localized thickening of the En/DM in non-immunological failed graft, but rather a generalized thickening of the whole corneal graft including the En/DM. On comparing non-immunological failed grafts to rejected grafts, DMT and DRI were highly significantly different between the 2 groups (24 vs. 59 μm and 1 vs. 2.1, respectively; P<0.001). On the other hand, on comparing the non-immunological failed grafts to the actively rejecting grafts, it was noted that DMT did not differentiate between the 2 groups (24 vs. 28, respectively; P=0.4). Nevertheless, DRI was able to differentiate between the two groups, in a highly statistically significant manner (1 vs. 1.5, respectively; P<0.001).
In order to evaluate the clinical usefulness of CCT, DMT, and DRI in terms of their accuracy, sensitivity, and specificity in detecting the immunological status of corneal grafts and diagnosing active corneal graft rejection, we used a forward stepwise logistic regression model. We also assessed their utility with receiver-operating characteristic (ROC) curves for each group (Fig. 3) to allow a comparison of these indices to standard-of-care clinical tests. For discriminating between clear grafts and those actively rejecting, DMT was the only variable to independently enter the model (p<0.001). The p-values for CCT and DRI were p=0.218 and p=0.201, respectively (Fig 3a). DMT was also the only statistically significant variable to enter the model (p<0.001) in differentiating rejected grafts from those actively rejecting (Fig 3b). DRI was, however, the only independent variable to statistically significantly enter the model (p<0.001) in discriminating non-immunological failed grafts and those actively rejecting (Fig. 3c). The p-values for CCT and DMT were p=0.066 and p=0.067, respectively. Likewise, DRI was the only independent variable to enter the model (p<0.001) in discriminating non-immunological failed and rejected grafts (Fig. 3d). The p-values for CCT and DMT were p=0.078 and p=0.094, respectively. Areas under ROC curve (AUC) for the three measurements in all groups are shown in Table 3.
Figure 3.
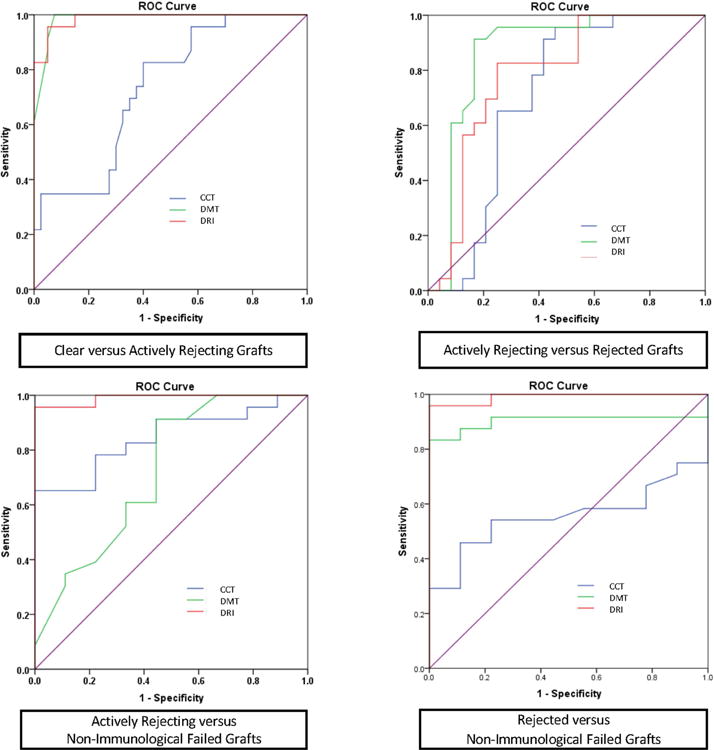
Combined receiver operating characteristics (ROC) graphs of central corneal thickness (CCT), endothelial/Descemet's membrane complex (En/DM) thickness (DMT), En/DM rejection index (DRI) in differentiating clear grafts from actively rejecting grafts (upper left), actively rejecting from rejected grafts (upper right), actively rejecting from non-immunological failed grafts (lower left) and rejected from non-immunological failed grafts (lower right).
ROC: Receiver operating characteristics graphs
CCT: Central corneal thickness
DMT: Endothelium/Descemet's membrane complex thickness
DRI: Endothelium/Descemet's membrane complex rejection index
Table 3. Forward stepwise logistic regression model to evaluate diagnostic performance of Endothelial/Descemet's membrane complex thickness, Endothelial/Descemet's membrane complex rejection index and central corneal thickness in detecting corneal grafts immunological statuses.
| Clear vs. Actively rejecting grafts | Actively rejecting vs. Rejected graft | Rejected vs. Non-immunological failed graft | Actively rejecting vs. Non-immunological failed graft | ||
|---|---|---|---|---|---|
| DMT | P value | P<0.001 | P<0.001 | P=0.094 | P=0.067 |
| AUC | 0.986 | 0.866 | 0.903 | 0.722 | |
| Sensitivity | 100% | 96% | 92% | 61% | |
| Specificity | 92.5% | 75% | 78% | 22% | |
| DRI | Cut-off | 21 μm | 37 μm | 29 μm | 29 |
| P value | P=0.201 | P=0.344 | P<0.001 | P<0.001 | |
| AUC | 0.987 | 0.786 | 0.991 | 0.990 | |
| Sensitivity | 96 | 83% | 96% | 96% | |
| Specificity | 95 | 75% | 100% | 100% | |
| CCT | Cut-off | 1.3 | 1.7 | 1.2 | 1.3 |
| P value | P=0.218 | P=0.244 | P=0.078 | P=0.066 | |
| AUC | 0.734 | 0.707 | 0.556 | 0.845 | |
| Sensitivity | 78% | 70% | 50% | 83% | |
| Specificity | 60% | 63% | 78% | 67% | |
| Cut-off | 549 μm | 638 μm | 808 μm | 716 μm | |
DMT: Endothelial/Descemet's membrane complex (En/DM) thickness.
DRI: En/DM rejection index, defined as DMT divided by central corneal thickness multiplied by 33.
AUC: area under the curve.
Vs.: versus.
CCT: central corneal thickness.
Specificity, sensitivity and cut-off values are chosen to maximize total diagnostic accuracy (minimize total number of errors).
DMT and DRI ROC curves showed excellent accuracy for the diagnosis of active corneal graft rejection (AUC=0.986 and 0.987, respectively). DMT achieved 100% sensitivity and 92.5% specificity (optimal cut-off value, OCV, of 21 μm), whereas DRI achieved 96% sensitivity and 95% specificity (OCV of 1.3) in differentiating actively rejecting grafts from clear grafts. On the other hand, CCT showed lower accuracy (AUC=0.734) and only achieved 78% sensitivity and 60% specificity (OCV of 549 μm) in differentiating actively rejecting grafts from clear grafts. Likewise, DMT and DRI showed excellent accuracy in differentiating actively rejecting from rejected grafts (AUC of 0.866 and 0.786, respectively). DMT achieved 96% sensitivity and 75% specificity (OCV of 37 μm), while DRI achieved 83% sensitivity and 75% specificity (OCV of 1.7). On the other hand, CCT had an AUC of only 0.707 and achieved only 70% sensitivity and 63% specificity (OCV of 654 μm) in differentiating between those two groups.
In our study, we also evaluated the diagnostic performance of DMT, DRI and CCT in differentiating non-immunological failed grafts from rejected grafts. CCT failed to differentiate between these 2 groups, with an AUC of 0.556, whereas DMT and DRI demonstrated an excellent ability to differentiate between them (AUC of 0.903 and 0.991, respectively). DMT achieved 92% sensitivity and 78% specificity (OCV of 29 μm), and DRI achieved 96% sensitivity and 100% specificity (OCV 1.3 μm) in differentiating non-immunological failed from rejected grafts. We then evaluated the performance of DMT, DRI and CCT in differentiating actively rejecting from non-immunological failed grafts. DMT and CCT had a low accuracy in differentiating between the 2 groups (AUC=0.722 and 0.845, respectively). Nevertheless, DRI showed excellent accuracy (AUC=0.99) with a 96% sensitivity and 100% specificity (OCV of 1.3) in differentiating actively rejecting from non-immunologically failed grafts.
To test whether DMT and DRI could be used as quantitative indices to describe the severity of graft rejection, we correlated each of them with CCT. In control eyes, DMT and DRI did not show significant correlations with CCT. On the other hand, DMT of clear, actively rejecting, and rejected graft groups showed a significant linear correlation with CCT (r=0.5, 0.8, and 0.9; P<0.001, respectively). DRI did not show a significant correlation with CCT in any of the groups.
Using our HD-OCT/histopathological correlation study, we were able to successfully validate our study. We demonstrated a large and highly statistically significant correlations between the in vivo CCT and DMT measurements obtained using the HD-OCT, at both of our clinical sites, to the ex vivo measurements of the same grafts measured by light microscopy (DMT, r=0.75, p=0.020; CCT, r=0.94, p=0.005). It is noteworthy that all in vivo measurements were significantly larger than those obtained ex vivo and that 4 out of the 9 rejected grafts had a fibrous retrocorneal membrane. Furthermore, our ex vivo study demonstrated that the mean DMT thickness of rejected grafts measured using the light microscope (16.2 ±13.8 μm) was statistically significantly thicker than the ex vivo DMT of clear corneas of eyes enucleated secondary to posterior melanoma without corneal or angle involvement (5.5±1.2 μm; p=0.049, un-pooled variance t-test). Note that there was no statistically significant difference in age between the rejected graft groups and the controls (69±13 and 62±6 years, respectively).
Discussion
The key to protect corneal grafts is the early and accurate diagnosis of graft rejection to promptly initiate treatment to prevent irreversible damage to the graft.28 The currently available diagnostic techniques fall short in this aspect. They lack the sufficient sensitivity and specificity to detect mild and early graft rejection that would enable the clinician to accurately determine the graft immunological status or predict its survival. 5,8,12-15 Thus, a search for novel diagnostic techniques to address those limitations is warranted.
Corneal endothelial cells secrete their basement membrane, the Descemet's membrane (DM). As normal subjects grow older their endothelial cells decrease in number but their DM grows in thickness.29,30 Our previous work has shown that En/DM complex grows 1.2 μm per decade in normal corneas. Nevertheless, in cases where the endothelial cells become stressed it grows at a much higher rate to reach double or triple its thickness.22 Based on those observations and on solid organ transplantation literature that established thickening of basement membranes of allografts as a sign of graft rejection, we conducted our study. We were able, herein, for the first time in the literature, to characterize in vivo the En/DM complex in corneal grafts and correlate those structural changes to the mmunological status of corneal grafts.
Using HD-OCT, the corneal endothelium and DM were visualized as one structure and thus we refer to them as the En/DM complex.22,26 In normal controls, the En/DM complex was visualized as a band formed of two smooth hyper-reflective lines with a translucent space in between. This was in agreement with previous reports.22,26 In clear corneal grafts, En/DM appeared to have the same structural characteristics as the normal age-matched controls. This observation was confirmed by the fact that there were no statistically significant differences between means of either DMT or DRI of clear grafts and the control age-matched group. In actively rejecting grafts, Mean DMT was highly statistically significantly thicker than in normal controls and clear grafts. Furthermore, the DMT of rejected grafts showed an even greater and thus a more significant thickening. The thickening of the En/DM, described by DMT, in actively rejecting and rejected grafts was more pronounced than the thickening of the total cornea. That indicated a preferential thickening of the En/DM during the immunological rejection. We have managed to isolate and highlight the observed localized thickening by devising the DRI index, which was formulated using the control subjects in our study as described above. Using that index, we were able to highlight the advantage of measuring DMT as a means of characterizing the immunological status of the transplanted cornea. In actively rejecting grafts, DRI increased significantly compared to normal controls and clear grafts. In rejected grafts, DRI showed even greater and thus more significant thickening than that of the actively rejecting grafts. This indicated that immunological rejection caused En/DM to thicken more significantly than the rest of the corneal graft.
Examining corneal grafts that failed due to non-immunological causes was critical to understanding the En/DM structural changes caused by the endothelial pump failure from those due to the immunological rejection. Interestingly, En/DM in non-immunological failed grafts was similar in appearance to clear grafts and control eyes but with a generalized thickening. The mean DMT of the non-immunological failed grafts was approximately 150% that of normal controls and clear grafts. Their mean CCT was also approximately 150% that of normal controls and clear grafts. This was further highlighted by a mean DRI of 1 for these grafts. This underlines the fact that preferential En/DM thickening is not due to generalized corneal edema, but is intimately associated with immunological rejection. Furthermore, the preferential thickening of the En/DM is likely due to immunologically driven responses against the allogeneic cornea that is not seen in non-immunological failed grafts. Our study has revealed excellent accuracy, sensitivity and specificity of DMT and DRI in detecting the immunological status of corneal grafts. It has also demonstrated that the diagnostic performances of those indices were superior to CCT, one of the standard of care tests for detecting the immunological status of corneal grafts.
Lastly, our study has demonstrated that DMT can be used to quantify the severity of the immunological rejection. To test that, we correlated En/DM indices to CCT, an indicator of the severity of rejection. In control eyes, there was no significant correlation. That was in agreement with our previous report that has shown that normal controls with thicker corneas do not have thicker En/DM.22 On the other hand, actively rejecting and rejected grafts' DMT showed significant correlations with CCT. Interestingly, there was also a significant correlation between DMT of clear grafts and their CCT. Further studies are needed to determine if that correlation could indicate that DMT was detecting a subclinical endothelial dysfunction in those clear grafts. DRI, on the other hand, did not show a significant correlation to CCT in any of the groups.
We validated our in vivo findings using an ex vivo HD-OCT/histopathological correlation study. Our results demonstrated that HD-OCT measurements obtained by the custom-build HD-OCT at BPEI and the Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, CA) at the SLUEI site correlated with ex vivo histopathologic measurements. The correlations were strong and highly statistically significant. HD-OCT measurements were thicker compared with histopathologic measurements, which can be attributed to relative dehydration following histopathologic processing. Moreover, we have confirmed our in vivo findings by showing that the En/DM of rejected grafts was significantly thicker than that of control corneas obtained from eyes enucleated secondary to posterior melanoma with no corneal or angle involvement.
We are not aware of any study in the literature that has reported the in vivo characteristics of En/DM in corneal graft rejection and evaluated using them for the diagnosis of graft rejection. Furthermore, to our knowledge, there are no in vitro studies that have described human corneal grafts' En/DM structural changes during active rejection. The former is probably due to the lack of imaging devices with sufficient resolution to depict the En/DM in vivo. The latter is owing to lack of histologic specimens to study the En/DM in active rejection, as re-grafting surgery is avoided during the active rejection episode and deferred until after graft failure has set in, to increase the chances of survival of the future graft. Thus, only animal model studies of active corneal graft rejection were used to study active rejection.
Inomata et al 31 demonstrated enlargement of the endothelial cells and thickening of their nuclei together with infiltration of En/DM with lymphocytes, monocytes, and fibrin during active corneal graft rejection of albino rabbits' corneal grafts using electron microscopy. Morphologic changes in corneal endothelial cells as demonstrated by specular microscopy were found to be indicative of pending allograft rejection following Descemet's membrane endothelial keratoplasty (DMEK).21 That together with deposition of immunological and metabolic end products on the endothelial interface of the En/DM is a possible mechanism of the observed thickening of En/DM in active graft rejection in our study. On the other hand, for rejected corneal grafts, Kurz and D'Amico 32 disclosed, using light microscopy, a thickened, laminated DM with numerous small rounded excrescences, a picture similar to the HD-OCT images of our rejected grafts. They also demonstrated the presence of retrocorneal membranes in some of their cases, another possible mechanism for the severely thickened En/DM in our rejected group. In fact, 4 out of the 9 rejected grafts that we have examined by light microscopy had fibrous retrocorneal membranes. The excrescences observed on HD-OCT in our grafts with rejection and in their reported cases were likely deposits of the inflammatory cells driving the rejection, clinically known as keratic precipitates (KPs). Future studies are needed to further reveal the pathogenesis behind the observed structural changes of En/DM complex in graft rejection.
Our study is not without its limitations. Using the HD-OCT, we were not able to delineate the corneal endothelium (and fibrous retrocorneal membrane, if present) from the DM. Hence we referred to the visualized structure as the En/DM complex. We only imaged the central part of the corneal graft and were not able to create a tomographic map of the En/DM complex to highlight peripheral localized changes that may be missed by only measuring the central thickness of the En/DM. Those study limitations are secondary to limitations of the current imaging technology. Single readings of HD-OCT measurements were obtained by a single examiner who was not masked to the group, and therefore inter- and intra-observer variability were not determined. Other limitations of the study are not correlating the rejection duration, clinically determined severity of graft rejection, ECD and data on corneal graft endothelium with En/DM indices. A future prospective longitudinal study that uses a masked reading center and test the aforementioned correlations is therefore warranted to address those limitations. Moreover, we acknowledge that the same subject data were used both to create and test the predicted model. Future prospective studies are warranted to assess the prediction mode with a dataset of new subjects and determine whether DMT and DRI can predict graft survival and guide treatment to prolong graft survival. Developing an HD-OCT technology to create an entire tomographic map of the graft's En/DM may enable future studies to evaluated the En/DM in its entirely and evaluate its use in the diagnosis of subclinical graft rejection.
In conclusion, our study has disclosed, for the first time in the literature, the in vivo microstructural characteristics of corneal grafts' En/DM and correlated those changes to their immunological status. Our study has shown that corneal grafts' En/DM structural characteristics can be accurately quantified using our indices, DMT and DRI. Those indices have shown excellent accuracy, sensitivity and specificity in detecting the immunological status of the graft. Furthermore, DMT has shown to be a quantitative index that correlates accurately with the severity of rejection. As a consequence of this study, we believe that DMT and DRI might have the predictive potential to aid the clinician in detecting immunologically-driven rejections, potentially leading to prolonged corneal allograft survival. A longitudinal study of DMT and DRI in patients who received a PK is, however, warranted in order to establish the utility of these novel measurements in detecting graft rejection in the pre-clinical stage.
Acknowledgments
Funding/support: This study was supported by a NEI K23 award (K23EY026118), NEI core center grant to the University of Miami (P30 EY014801), Research to Prevent Blindness (RPB) and the American Society of Cataract and Refractive Surgery (ASCRS) Foundation.
The funding organization had no role in the design or conduct of this research.
Footnotes
Financial Disclosures: United States Non-Provisional Patent Application. Application No.: 14/247903 (MA, VLP, JW and SHY). Patent is owned by University of Miami. Dr. Yoo is a consultant of Alcon, Allergan, Carl Zeiss Meditec, Bioptigen, Bausch and Lomb and Transcend Medical. Dr. Yoo has grants from AMO and Avedro. Dr. Victor L. Perez is a consultant of Alcon Ltd.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Human organ and tissue transplantation. Report by the Secretariat Executive Board, EB112/5, 112th session, Provisional agenda item 4.3. [Accessed February 15, 2017]; Available at: www.who.int/gb/ebwha/pdf_files/EB112/eeb1125.pdf.
- 2.Aldave AJ, DeMatteo J, Glasser DB, et al. Report of the Eye Bank Association of America medical advisory board subcommittee on fungal infection after corneal transplantation. Cornea. 2013;32(2):149–154. doi: 10.1097/ICO.0b013e31825e83bf. [DOI] [PubMed] [Google Scholar]
- 3.Alldredge OC, Krachmer JH. Clinical types of corneal transplant rejection. Their manifestations, frequency, preoperative correlates, and treatment. Arch Ophthalmol. 1981;99(4):599–604. doi: 10.1001/archopht.1981.03930010599002. [DOI] [PubMed] [Google Scholar]
- 4.Williams KA, Muehlberg SM, Lewis RF, Coster DJ. How successful is corneal transplantation? A report from the Australian Corneal Graft Register. Eye (Lond) 1995;9(Pt 2):219–227. doi: 10.1038/eye.1995.43. [DOI] [PubMed] [Google Scholar]
- 5.Ing JJ, Ing HH, Nelson LR, Hodge DO, Bourne WM. Ten-year postoperative results of penetrating keratoplasty. Ophthalmology. 1998;105(10):1855–1865. doi: 10.1016/S0161-6420(98)91030-2. [DOI] [PubMed] [Google Scholar]
- 6.Anne WK, Lowe MT, Bartlett CM, Kelly L, Coster DJ. The Australian Corneal Graft Registry 2007 Report Flinders. University Press; Adelaide: 2007. [Accessed February 15, 2017]. Available at: http://hdl.handle.net/2328/25859. [Google Scholar]
- 7.Wilson SE, Kaufman HE. Graft failure after penetrating keratoplasty. Surv Ophthalmol. 1990;34(5):325–356. doi: 10.1016/0039-6257(90)90110-h. [DOI] [PubMed] [Google Scholar]
- 8.Musch DC, Schwartz AE, Fitzgerald-Shelton K, Sugar A, Meyer RF. The effect of allograft rejection after penetrating keratoplasty on central endothelial cell density. Am J Ophthalmol. 1991;111(6):739–742. doi: 10.1016/s0002-9394(14)76782-0. [DOI] [PubMed] [Google Scholar]
- 9.Lee HS, Kim MS. Influential factors on the survival of endothelial cells after penetrating keratoplasty. Eur J Ophthalmol. 2009;19(6):930–935. doi: 10.1177/112067210901900606. [DOI] [PubMed] [Google Scholar]
- 10.Hill JC, Maske R, Watson P. Corticosteroids in corneal graft rejection. Oral versus single pulse therapy. Ophthalmology. 1991;98(3):329–333. doi: 10.1016/s0161-6420(91)32291-7. [DOI] [PubMed] [Google Scholar]
- 11.Yamazoe K, Yamazoe K, Shimazaki-Den S, Shimazaki J. Prognostic factors for corneal graft recovery after severe corneal graft rejection following penetrating keratoplasty. BMC ophthalmol. 2013;13:5. doi: 10.1186/1471-2415-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claerhout I, Beele H, De Bacquer D, Kestelyn P. Factors influencing the decline in endothelial cell density after corneal allograft rejection. Invest Ophthalmol Vis Sci. 2003;44(11):4747–4752. doi: 10.1167/iovs.03-0536. [DOI] [PubMed] [Google Scholar]
- 13.Benetz BA, Lass JH, Gal RL, et al. Endothelial Morphometric Measures to Predict Endothelial Graft Failure After Penetrating Keratoplasty. JAMA ophthalmol. 2013:1–8. doi: 10.1001/jamaophthalmol.2013.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lass JH, Sugar A, Benetz BA, et al. Endothelial cell density to predict endothelial graft failure after penetrating keratoplasty. Arch Ophthalmol. 2010;128(1):63–69. doi: 10.1001/archophthalmol.2010.128.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verdier DD, Sugar A, Baratz K, et al. Corneal thickness as a predictor of corneal transplant outcome. Cornea. 2013;32(6):729–736. doi: 10.1097/ICO.0b013e31827b14c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aita K, Yamaguchi Y, Horita S, et al. Thickening of the peritubular capillary basement membrane is a useful diagnostic marker of chronic rejection in renal allografts. Am J Transplant. 2007;7(4):923–929. doi: 10.1111/j.1600-6143.2006.01708.x. [DOI] [PubMed] [Google Scholar]
- 17.Roufosse CA, Shore I, Moss J, et al. Peritubular capillary basement membrane multilayering on electron microscopy: a useful marker of early chronic antibody-mediated damage. Transplantation. 2012;94(3):269–274. doi: 10.1097/TP.0b013e31825774ab. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu T, Ishida H, Shirakawa H, et al. Clinicopathological analysis of transplant glomerulopathy cases. Clin Transplant. 2009;23(20):39–43. doi: 10.1111/j.1399-0012.2009.01008.x. [DOI] [PubMed] [Google Scholar]
- 19.Taddesse-Heath L, Kovi J. Electron microscopic findings in hepatic allograft rejection. J Natl Med Assoc. 1994;86(10):779–782. [PMC free article] [PubMed] [Google Scholar]
- 20.Siddiqui MT, Garrity ER, Martinez R, Husain AN. Bronchiolar basement membrane changes associated with bronchiolitis obliterans in lung allografts: a retrospective study of serial transbronchial biopsies with immunohistochemistry. Mod Pathol. 1996;9(3):320–328. [PubMed] [Google Scholar]
- 21.Monnereau C, Bruinsma M, Ham L, et al. Endothelial cell changes as an indicator for upcoming allograft rejection following descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2014;158(3):485–495. doi: 10.1016/j.ajo.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 22.Shousha MA, Perez VL, Wang J, et al. Use of ultra-high-resolution optical coherence tomography to detect in vivo characteristics of Descemet's membrane in Fuchs' dystrophy. Ophthalmology. 2010;117(6):1220–1227. doi: 10.1016/j.ophtha.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shousha MA, Karp CL, Canto AP, et al. Diagnosis of ocular surface lesions using ultra-high-resolution optical coherence tomography. Ophthalmology. 2013;120(5):883–891. doi: 10.1016/j.ophtha.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin RC, Shure MA, Rollins AM, Izatt JA, Huang D. Group index of the human cornea at 1.3-microm wavelength obtained in vitro by optical coherence domain reflectometry. Opt Lett. 2004;29(1):83–85. doi: 10.1364/ol.29.000083. [DOI] [PubMed] [Google Scholar]
- 25.Huang D, Izatt JA, Yasuno Y, et al. Future direction of anterior segment optical coherence tomography. In: Steinert RF, Huang D, editors. Anterior segment optical coherence tomography. SLACK Inc.; Thorofare, NJ: 2008. pp. 165–173. [Google Scholar]
- 26.Tao A, Chen Z, Shao Y, et al. Phacoemulsification induced transient swelling of corneal Descemet's Endothelium Complex imaged with ultra-high resolution optical coherence tomography. PLoS One. 2013;8(11):e80986. doi: 10.1371/journal.pone.0080986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutchings N, Simpson TL, Hyun C, et al. Swelling of the human cornea revealed by highspeed, ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51(9):4579–4584. doi: 10.1167/iovs.09-4676. [DOI] [PubMed] [Google Scholar]
- 28.Guilbert E, Bullet J, Sandali O, et al. Long-term rejection incidence and reversibility after penetrating and lamellar keratoplasty. Am J Ophthalmol. 2013;155(3):560–569e562. doi: 10.1016/j.ajo.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 29.Jakus MA. Studies on the cornea. II. The fine structure of Descement's membrane. J Biophys Biochem Cytol. 1956;2(4 Suppl):243–252. doi: 10.1083/jcb.2.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy C, Alvarado J, Juster R. Prenatal and postnatal growth of the human Descemet's membrane. Invest Ophthalmol Vis Sci. 1984;25(12):1402–1415. [PubMed] [Google Scholar]
- 31.Inomata H, Smelser GK, Polack FM. The fine structural changes in the corneal endothelium during graft rejection. Invest ophthalmol. 1970;9(4):263–271. [PubMed] [Google Scholar]
- 32.Kurz GH, D'Amico RA. Histopathology of corneal graft failures. Am J Ophthalmol. 1968;66(2):184–199. doi: 10.1016/0002-9394(68)92063-1. [DOI] [PubMed] [Google Scholar]


