Abstract
Addiction has been conceptualized as a three-stage cycle—binge/intoxication, withdrawal/negative affect, and preoccupation/anticipation—that worsens over time and involves allostatic changes in hedonic function via changes in the brain reward and stress systems. Using the withdrawal/negative affect stage and negative reinforcement as an important source of motivation for compulsive drug seeking, we outline the neurobiology of the stress component of the withdrawal/negative affect stage and relate it to a derivative of the Research Domain Criteria research construct for the study of psychiatric disease, known as the Addictions Neuroclinical Assessment. Using the Addictions Neuroclinical Assessment, we outline five subdomains of negative emotional states that can be operationally measured in human laboratory settings and paralleled by animal models. We hypothesize that a focus on negative emotionality and stress is closely related to the acute neurobiological alterations that are experienced in addiction and may serve as a bridge to a reformulation of the addiction nosology to better capture individual differences in patients for whom the withdrawal/negative affect stage drives compulsive drug taking.
Keywords: Addiction, stress, neuroscience, negative affect, alcohol
Conceptual Framework
What Is Stress?
Selye1 defined stress as responses to demands (usually noxious) upon the body that historically have been defined by various physiological changes that include activation of the hypothalamic–pituitary–adrenal (HPA) axis. However, a definition of stress that is more compatible with its many manifestations in the organism is “anything which causes an alteration of psychological homeostatic processes.”2 In fact, in a seminal paper, Mason3 argued the importance of psychological stress for eliciting a stress response, even among physical stressors and that many physical challenges absent psychological stress are not stressful.
The physiological response that is most associated with a state of stress is an elevation of glucocorticoids that derive from the adrenal cortex. This response is controlled by the HPA axis. Vale et al.4 first demonstrated that corticotropin-releasing factor (CRF) initiates the HPA axis neuroendocrine stress response (adrenocorticotropic hormone and ultimately glucocorticoids) by binding CRF1 receptors in the anterior pituitary after release into portal blood. CRF from the paraventricular nucleus of the hypothalamus was then identified as the primary controller in the HPA axis. Glucocorticoids function to increase and maintain blood sugar by elevating gluconeogenesis, and they decrease immune function by blocking proinflammatory proteins. These responses facilitate mobilization of the body in response to acute stressors. However, we now know that neurocircuits in the brain mediate behavioral responses to stressors and play a major role in “psychological homeostasis.”
Of relevance for this review, comorbidity between addictive and stress-related disorders is high. In the third wave of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), the 12-month odds ratio for posttraumatic stress disorder (PTSD; i.e., the psychiatric disease most directly linked to stress exposure) and any substance use disorder was 1.3; the lifetime odds ratio was 1.5.5 Furthermore, in the National Comorbidity Survey-Replication, a diagnosis of PTSD at Time 1 was associated with odds ratios of 3.2 and 5.4 for alcohol and illicit drug dependence, respectively, at Time 2, 10 years later, among those individuals not substance dependent at Time 1.6
What Is Addiction?
Addiction can be defined in many different ways, but one definition that has been generally adopted in the field is that addiction is a chronic, relapsing disorder that is characterized by a compulsion to seek and take drugs and the loss of control over drug intake. Others have emphasized a further characteristic, notably “the emergence of a negative emotional state (e.g., dysphoria, anxiety, and irritability) that defines a motivational withdrawal syndrome when access to the drug is prevented.”7 Indeed, some theorists have argued that such a negative emotional state is the defining feature of dependence on a drug:
The notion of dependence on a drug, object, role, activity or any other stimulus-source requires the crucial feature of negative affect experienced in its absence. The degree of dependence can be equated with the amount of this negative affect, which may range from mild discomfort to extreme distress, or it may be equated with the amount of difficulty or effort required to do without the drug, object, etc.8
Using such a framework, addiction has been conceptualized as a three-stage cycle—binge/intoxication, withdrawal/negative affect, and preoccupation/anticipation—that worsens over time and involves allostatic changes in hedonic function via changes in the brain reward and stress systems. Allostasis is defined as stability through change via a feed-forward mechanism that readjusts parameters to a new hedonic set point but outside the homeostatic range. Two primary sources of reinforcement—positive and negative reinforcement—have been hypothesized to play a role in this allostatic process. Positive reinforcement is defined as the process by which the presentation of a stimulus increases the probability of a response. Negative reinforcement is defined as the process by which the removal of an aversive stimulus (or aversive state, in the case of addiction) increases the probability of a response.
Another framework with which to conceptualize drug addiction is the impulsivity–compulsivity continuum, in which impulsivity can be behaviorally defined as “actions which are poorly conceived, prematurely expressed, unduly risky, or inappropriate to the situation and that often result in undesirable consequences.”9 Impulsivity is a core deficit in substance abuse disorders.10 It can be measured in multiple ways, but two domains dominate: the choice of a smaller, immediate reward over a larger, delayed reward11 or the inability to inhibit behavior by changing the course of action or to stop a response once it is initiated.12 Operationally, delay-to-gratification tasks (e.g., delayed discounting tasks, impulsive choice) and the Stop-Signal or Go/No-Go task (behavioral impulsivity) have both been used as measures of the various domains of impulsivity.13,14
In contrast, “compulsivity can be characterized by perseverative, repetitive actions that are excessive and inappropriate to a situation.”15 Individuals who suffer from compulsions often recognize that the behaviors are harmful, but they nonetheless feel emotionally compelled to perform them. Performance of these behaviors reduces tension, stress, or anxiety.15,16 Operationally, in animal models, responding for a drug or alcohol in the face of adverse consequences17 or responding for a drug or alcohol on a progressive-ratio schedule of reinforcement18 has been argued to reflect compulsivity. Thus, in addition to the positive reinforcement associated with high impulsivity linked to the early stages of the addiction process, an additional source of motivation is recruited, namely negative reinforcement.
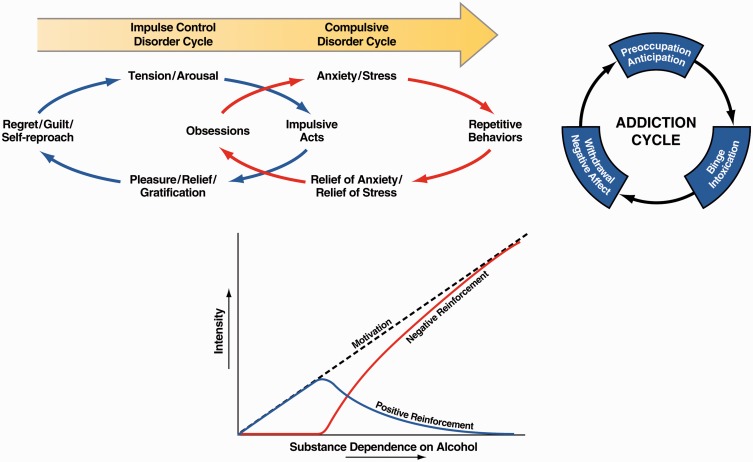
This impulsivity–compulsivity continuum has a nosological history. Subjects with classic atypical impulse control disorders, such as kleptomania, experience an increasing sense of tension or arousal before committing an impulsive act; pleasure, gratification, or relief at the time of committing the act; and regret, self-reproach, or guilt following the act.19 In contrast, subjects with classic compulsive-like disorders, such as obsessive–compulsive disorder, experience anxiety and stress before committing a compulsive repetitive behavior and relief from the stress by performing the compulsive behavior.19 We have argued that drug addiction progresses from a source of positive reinforcement that may indeed involve more elements of impulsivity to a source of negative reinforcement that may involve more elements of compulsivity (Figure 1).20 The three-stage cycle of addiction, with the embedded conceptual sources of motivation of positive and negative reinforcement that parallel impulsivity and compulsivity (Figure 1), are not unique to drug addiction and generalize to non-drug or “process” addictions. In a recent review,21 the authors identified three major domains of neurofunctional impairment related to gambling disorder, namely the loss of control, craving/withdrawal, and the neglect of other areas of life. These domains closely parallel the domains outlined in the three stages of the addiction cycle and the Addictions Neuroclinical Assessment (ANA) framework (see below).
Figure 1.
(Top left) Diagram showing the stages of impulse control disorder and compulsive disorder cycles related to the sources of reinforcement. In impulse control disorders, an increasing tension and arousal occurs before the impulsive act, with pleasure, gratification, or relief during the act. Following the act, there may or may not be regret or guilt. In compulsive disorders, there are recurrent and persistent thoughts (obsessions) that cause marked anxiety and stress followed by repetitive behaviors (compulsions) that are aimed at preventing or reducing distress.19 Positive reinforcement (pleasure/gratification) is more closely associated with impulse control disorders. Negative reinforcement (relief of anxiety or relief of stress) is more closely associated with compulsive disorders (taken with permission from Koob20). (Top right) Collapsing the cycles of impulsivity and compulsivity results in the addiction cycle, conceptualized as three major components: preoccupation/anticipation, binge/intoxication, and withdrawal/negative affect [taken with permission from Koob22). (Bottom) Change in the relative contribution of positive and negative reinforcement constructs during the development of substance dependence on alcohol (taken with permission from Koob20).
Neurobiology of Stress
Key highly conserved responses to stressors in the environment comprise fight or flight. A superstructure in the basal forebrain, the extended amygdala, processes fear, threats, and anxiety in humans (i.e., fight or flight responses)7,23 and engages the neurocircuitry of negative emotional states. The extended amygdala shares similarities in morphology, neurochemistry, and connectivity and is composed of the central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis (BNST), and a transition zone in the posterior medial part (shell) of the nucleus accumbens (NAcSh).24 The extended amygdala receives inputs from various regions of the brain that are involved in emotion, but most importantly the prefrontal cortex. The extended amygdala projects heavily to the hypothalamus and other midbrain structures that are involved in the expression of emotional responses.24,25 When animals are exposed to a stressor, they exhibit an enhanced freezing response to a conditioned fear stimulus, an enhanced startle response to a startle stimulus, the avoidance of open areas, open arms, and heights, and enhanced species-typical responses to an aversive stimulus. All of these responses are at least partially mediated by the extended amygdala. In psychopathology, dysregulation of the extended amygdala has been hypothesized to play a key role in disorders that are related to stress and negative emotional states, such as PTSD, general anxiety disorder, phobias, affective disorders, and addiction.26,27
Two neurochemical systems, CRF and dynorphin, play a key role in the extended amygdala to effect such behavioral changes. Both are also implicated in the psychopathology associated with the extended amygdala, and both are the focus of individual differences in stress pathology. The glucocorticoid response mobilizes the body for physiological responses to stressors; CRF plays another role by mobilizing the body’s behavioral response to stressors via brain circuits outside the hypothalamus. In an early study, CRF was intracerebroventricularly injected into the brain in naive rats, which produced hyperactivity and hyperarousal in a familiar environment but a very pronounced freezing-like response in a novel stressful environment.28 Subsequent work showed that a prominent system that mediates such responses to CRF and fear and anxiety in general is the extended amygdala. The administration of competitive CRF receptor antagonists was shown to have opposite anti-stress effects. This observation was critical because it confirmed a role for endogenous CRF in behavioral responses to stressors (for review, see Koob and Zorrilla29).
The dynorphin-κ opioid system also plays a key role in affecting behavioral responses to stressors. Dynorphins contain the leucine (leu)-enkephalin sequence at the N-terminal portion of the molecule and are endogenous ligands for the κ opioid receptor.30 Dynorphins are widely distributed in the central nervous system31 and play a role in neuroendocrine regulation, pain regulation, motor activity, cardiovascular function, respiration, temperature regulation, feeding behavior, and stress responsivity. Dynorphins produce aversive dysphoric-like effects in animals and humans and have been hypothesized to mediate behavioral responses to stressors and negative emotional states (for review, see Van’t Veer and Carlezon32).
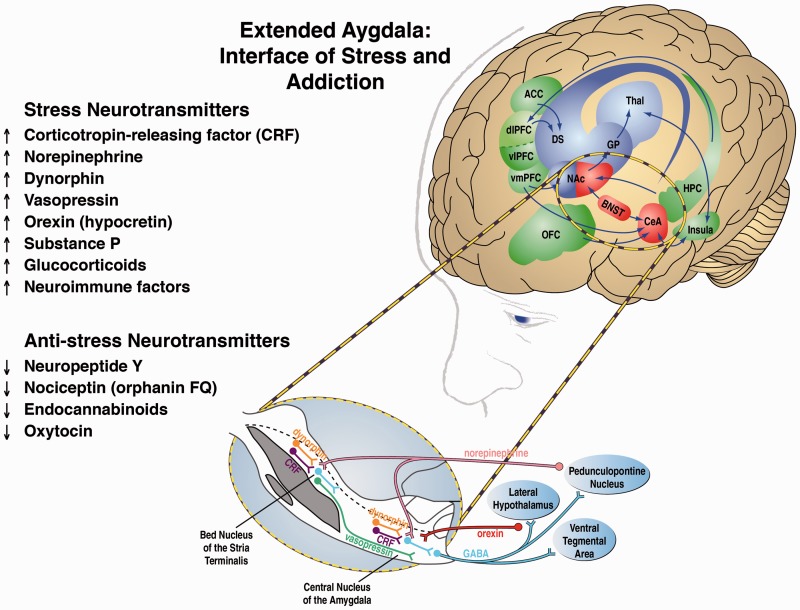
Other key neurotransmitter systems, all of which interact with the extended amygdala, that mediate behavioral responses to stressors include norepinephrine, vasopressin, hypocretin (orexin), substance P, proinflammatory cytokines, and key neurotransmitter systems that act in opposition to the brain stress systems, such as neuropeptide Y (NPY), nociceptin, and endocannabinoids. Altogether, these neurochemical systems set the tone and modulate emotional expression, particularly negative emotional states, via the extended amygdala (Figure 2).33 These stress systems and their relevance for addiction are comprehensively reviewed in Koob.22
Figure 2.
Neural circuitry associated with the three stages of the addiction cycle, with a focus on the withdrawal/negative affect stage and extended amygdala. The targets identified in this review that are relevant to the withdrawal/negative affect stage are listed on the left. On the right is the neurocircuitry of the pathophysiology of addiction. Binge/intoxication stage (blue): Drugs may engage associative mechanisms and reward neurotransmitters (such as dopamine and opioid peptides) in the nucleus accumbens shell and core (incentive salience, defined as a motivational response of the brain to reward-predicting stimuli) and then engage stimulus-response habits that depend on the dorsal striatum. Withdrawal/negative affect stage (red): The negative emotional state of withdrawal engages activation of the extended amygdala. The extended amygdala is composed of several basal forebrain structures, including the bed nucleus of the stria terminalis, central nucleus of the amygdala, and a transition zone in the medial portion (or shell) of the nucleus accumbens. Neurotransmitter systems engaged in the neurocircuitry of the extended amygdala that convey negative emotional states are indicated by upward-pointing arrows, and neurotransmitter systems that may buffer negative emotional states are indicated by downward-pointing arrows. Preoccupation/anticipation (craving) stage (green): This stage involves the prefrontal cortex and includes representations of contingencies, representations of outcomes, and executive function. An important neurotransmitter that is engaged in craving responses is glutamate. The magnified section (blue oval) illustrates the extended amygdala in detail. A major neurotransmitter in the extended amygdala is CRF, which projects to the brainstem where noradrenergic neurons provide a major projection reciprocally to the extended amygdala. Green/blue arrows indicate glutamatergic projections. Acb, nucleus accumbens; ACC, anterior cingulate cortex; BLA, basolateral amygdala; BNST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; CRF, corticotropin-releasing factor; DGP, dorsal globus pallidus; dlPFC, dorsolateral prefrontal cortex; NE, norepinephrine; OFC, orbitofrontal cortex; SNc, substantia nigra pars compacta; VGP, ventral globus pallidus; vlPFC and vmPFC, ventral prefrontal cortex; VTA, ventral tegmental area (modified with permission from Koob and Volkow34; see also Koob33 and Koob and Mason35).
Neurobiology of Addiction
The neurobiological basis of the binge/intoxication stage of the addiction cycle involves the activation of reward circuits and facilitation of incentive salience circuits. Drugs of abuse are rewarding but also confer motivational properties to previously neutral stimuli, a process known as incentive salience. Drug reward and drug-induced incentive salience are mediated largely by neurocircuitry in the basal ganglia. For most of the major drugs of abuse, animal studies have shown that their reinforcing actions are mediated by the release of dopamine and opioid peptides in the ventral striatum (NAc).34 Human imaging studies have shown that intoxicating doses of most drugs of abuse and alcohol release dopamine and opioid peptides into the ventral striatum.36,37 Activation of the ventral striatum leads to the recruitment of basal ganglia–globus pallidus–thalamic–cortical loops that engage the dorsal striatum in habit formation and habit strengthening that is hypothesized to be the beginning of compulsive-like responding for drugs.38
In the withdrawal/negative affect stage, two processes, possibly acting in parallel, are hypothesized to form the neurobiological basis for the loss of function in the reward systems (within-system neuroadaptation) in the ventral striatum and the recruitment of the brain stress systems (between-system neuroadaptation) in the extended amygdala.39 A within-system neuroadaptation was defined as the process by which the primary cellular response element to the drug (circuit A, reward circuit) adapts to neutralize the drug’s effects and have drug-opposite effects. Examples of within-system changes have been hypothesized to be molecular cellular changes within the reward circuits that are overactivated in the binge/intoxication stage and include the perturbations of intracellular signal transduction pathways, including changes in G-protein functioning and protein kinase A (PKA) activity and such transcription factors as cyclic adenosine monophosphate response element binding protein (CREB), and downstream ΔFosB, nuclear factor κB, and CDK5 that can modify gene expression.40 As dependence (defined as the manifestation of motivational withdrawal symptoms; i.e., elements of negative emotional states) develops, brain stress systems, such as CRF, norepinephrine, dynorphin, hypocretin, and substance P, are recruited, producing aversive or stress-like states.41,42 A between-system neuroadaptation was defined as a circuitry change in a circuit that is not circuit A, in which circuit B (stress circuit) may be triggered by activity in circuit A (i.e., the reward circuit). Within-system neuroadaptations can dynamically interact with between-system neuroadaptations, in which circuit B (i.e., the stress circuit) is activated either in parallel to affect a negative emotional state or in series to suppress the activity of circuit A to affect a negative emotional state.33 The CRF systems described above are recruited during repeated binge and withdrawal, with activation of the HPA axis, which, in turn, releases glucocorticoids, which, in turn, feed back to sensitize CRF systems in the extended amygdala that activate circuitry to drive negative emotional states (Table 1).33,43,44 The dynorphin-κ system has long been hypothesized to mediate negative emotional states of drug withdrawal by suppressing activity of the mesocorticolimbic dopamine system.42 Data to date suggest that these actions may be mediated by dynorphin activity in the NAcSh.45,46 The dynorphin-κ system may also interact with the CeA and be involved in promoting anxiety-like responses.47 In parallel, as noted above, there are anti-stress buffer systems in the extended amygdala that have the opposite effects to the stress-promoting modulatory systems. These include NPY, nociceptin, and endocannabinoids (Table 1). For example, NPY activation in the CeA has opposite effects to CRF.48 NPY blocks high compulsive-like alcohol administration, blocks the transition to excessive drinking with the development of dependence, and blocks the increase in γ-aminobutyric acid (GABA) release in the CeA that is produced by alcohol.49,50 The combination of decreases in reward neurotransmitter function and the recruitment of brain stress systems provides powerful motivation for reengaging in drug taking and drug seeking.
Table 1.
Molecular neurocircuits of the withdrawal/negative affect stage as focal points for neuroplasticity in addiction.
| Circuit | Neurotrasmitter | Modulatory response | Referencesa |
|---|---|---|---|
| Decreased reward neurotransmitters | |||
| VTA–NAc | CRF | ↑ | Grieder et al.51 |
| Habenula–VTA | Acetylcholine | ↓ | Fowler et al.52 |
| Increased stress neurotransmitters | |||
| CeA | CRF | ↑ | Funk et al.53 |
| Brainstem–BNST | Norepinephrine | ↑ | Delfs et al.54 |
| Hypothalamus–CeA | Hypocretin | ↑ | Schmeichel et al.55 |
| CeA | Substance P | ↑ | Barbier et al.56 |
| NAc shell | Dynorphin | ↑ | Carlezon et al.42 |
| CeA | Dynorphin | ↑ | Kallupi et al.47 |
| PVN/SON–CeA | Vasopressin | ↑ | Hernandez et al.57 |
| Decreased anti-stress neurotransmitters | |||
| ARC–CeA | Neuropeptide Y | ↓ | Heilig and Thorsell48 |
| CeA | Nociceptin | ↓ | Economidou et al.58 |
| CeA | Endocannabinoids | ↓ | Sidhpura and Parsons59 |
ARC: arcuate nucleus; CeA: central nucleus of the amygdala; NAc: nucleus accumbens; PVN: paraventricular nucleus; SON: supraoptic nucleus; VTA: ventral tegmental area.
References are key papers that show either direct evidence of the circuit outlined or hypothesize the existence of such modulation. The second column (Circuit) indicates either a neurotransmitter circuit or, where only one neuroanatomical site is listed, a local circuit. Arrows represent the direction of modulation.
Thus, multiple circuits that involve multiple modulatory neurotransmitter systems converge on the extended amygdala to mediate negative emotional states associated with the withdrawal/negative affect stage. Each theoretically conveys differential qualitative dimensions to the construct of a negative emotional state that forms a basis for the dimensions of a neuroclinical assessment for the withdrawal/negative affect stage of the addiction cycle (Table 1).
The preoccupation/anticipation (“craving”) stage mediates the impairment of executive control in addiction via prefrontal cortex circuits. Executive function can be defined as an overall control circuit that limits impulsive and compulsive responses, delays reinforcement, and makes appropriate choices and responses, among others. Two systems have been conceptualized: a Go system and a Stop system, which do not necessarily act in opposition.44 The Go system consists of parts of the anterior cingulate cortex, dorsal prefrontal cortex, and orbitofrontal cortex and engages habits via the basal ganglia. The Stop system consists of the ventral prefrontal cortex, orbitofrontal cortex, and other prefrontal regions that overlap with the Go system. Critically, Stop system projections inhibit the basal ganglia incentive salience system and extended amygdala stress system. In individuals with substance use disorders, there are disruptions of decision making, impairments in the maintenance of spatial information, and impairments in behavioral inhibition, all of which can drive craving and drug seeking. Craving, defined as the desire for a drug or alcohol in the absence of the drug, has been hypothesized to be divided into two domains: reward craving (drug seeking induced by drugs or stimuli linked to drugs) and relief craving (drug seeking induced by an acute stressor or a state of stress).60 The brain circuitry that mediates both of these constructs can parallel the hypothesized subcortical dysregulations associated with the binge/intoxication and withdrawal negative/affect stages and can contribute to relapse during protracted abstinence in the preoccupation/anticipation (“craving”) stage.
Neuroclinical Assessment: From Reward to Stress and Back
The nosological research framework termed Research Domain Criteria (RDoC) originated as part of the National Institute of Mental Health (NIMH) 2008 strategic plan, with the goal of creating a research framework for studying psychiatric disorders. The NIMH framework was conceptually grounded in neuroscience research and spanned five domains: Negative Valence Systems, Positive Valence Systems, Cognitive Systems, Systems for Social Processes, and Arousal and Regulatory Systems. RDoC domains are organized by units of analysis, ranging from genes to paradigms (for an overview of the RDoC matrix, see http://www.nimh.nih.gov/research-priorities/rdoc/research-domain-criteria-matrix.shtml; accessed 20 January 2017), and this approach has generated much conceptual and methodological discussion.61–65 We have proposed a more parochial, within-disorder, research approach, the Addictions Neuroclinical Assessment (ANA) framework,66 which captures information in three of the five original RDoC domains.
The ANA domains were derived from the conceptual framework outlined above, in which drug addiction derives from a three-stage cycle with conceptual roots in impulsivity and compulsivity, the recruitment of positive and negative reinforcement, and interactions between the neurobiological substrates of reward and stress. Three functional domains—executive function, incentive salience, and negative emotionality—were proposed as described above. The withdrawal/negative affect stage of this cycle, including stress and negative emotional states but not limited to withdrawal and representing the negative emotionality domain, is the focus of the discussion that follows.67
Negative Emotionality
Although often not emphasized, the reports of individuals who suffer from drug addiction are replete with descriptions of overall self-reported dysphoria and various manifestations of negative emotional states.68,69 Such descriptions include depression, anxiety, anhedonia, dysphoria, malaise, alexithymia, hyperkatifeia, emotional pain, physical pain, irritability, and sleep disturbances. A self-medication hypothesis has long infiltrated theories of addiction but has been dismissed, usually based on the grounds that both humans and animals will self-administer drugs without undergoing physical withdrawal. However, a rather common misunderstanding of tolerance and withdrawal in addiction is that they represent purely “physical” phenomena,70–73 rather than motivational constructs. Indeed, both tolerance (defined as increased reward seeking and taking more drug to produce the same effect)74 and withdrawal (defined as a motivational withdrawal syndrome characterized by dysphoria, anxiety, and irritability when the reward that is sought is unavailable)67,75 are present in all drug and behavioral addictions.76,77 For example, a complete assessment of reward constructs must include measurements of hypohedonia.78 Hypohedonia is widely documented as a clinical feature of addiction79–83 and is highly associated with increased craving for drugs of abuse84 and relapse.85
Opponent Process as a Guiding Principle
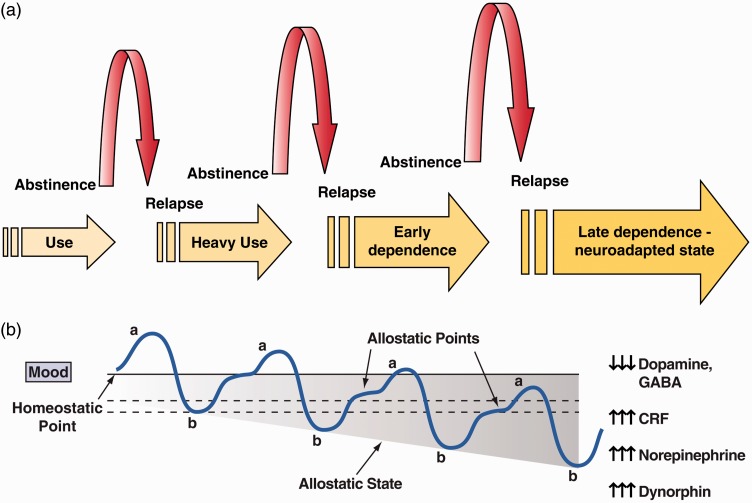
The interaction between reward and stress is dynamic both phenotypically and neurobiologically. Low levels of acute stress have long been considered rewarding. Glucocorticoids have rewarding properties and can even be self-administered by animals.86 However, chronic stress generally leads to malaise, irritability, and dysphoria, which drive mechanisms of negative reinforcement. Neurobiologically, accumulating evidence links excessive activation of the reward system as a causal mechanism for activation of the brain stress systems (see below). In the domain of motivation in addiction, the interaction between reward and stress was inextricably linked with hedonic, affective, or emotional states in the context of temporal dynamics by the opponent-process theory of motivation.87 Here, hedonic, affective, or emotional states, once initiated, are automatically modulated by mechanisms that reduce the intensity of hedonic feelings, presumably mediated by the central nervous system. Solomon and Corbit argued that there are affective or hedonic habituation (or tolerance) systems and affective or hedonic withdrawal (abstinence) systems. They defined two processes: the a-process and b-process. The a-process consists of either positive or negative hedonic responses. In the case of addiction, one would hypothesize that the a-process is a positive hedonic response to administration of a highly rewarding drug. The a-process occurs shortly after the presentation of a stimulus, correlates closely with the stimulus intensity, quality, and duration of the reinforcer, and shows tolerance. In contrast, the b-process appears after the a-process has terminated, is sluggish in onset, is slow to build up to an asymptote, is slow to decay, and gets larger with repeated exposure (Figure 3). The b-process would be the beginning of the development of the negative emotional state associated with the withdrawal/negative affect stage.
Figure 3.
(a) Schematic of the progression of drug and alcohol dependence over time, illustrating the shift in underlying motivational mechanisms. From initial, positive-reinforcing, pleasurable effects of drugs and alcohol, the addiction process progresses over time to being maintained by negative-reinforcing relief from a negative emotional state. Neuroadaptations that encompass the recruitment of extrahypothalamic CRF systems are key to this shift (taken with permission from Heilig and Koob88). (b) The a-process represents a positive hedonic or positive mood state, and the b-process represents the negative hedonic or negative mood state. The affective stimulus (state) has been argued to be the sum of both the a-process and the b-process. An individual who experiences a positive hedonic mood state from a drug of abuse with sufficient time between re-administering the drug is hypothesized to retain the a-process. An appropriate counteradaptive opponent process (b-process) that balances the activational process (a-process) does not lead to an allostatic state. The changes in the affective stimulus (state) in an individual with repeated frequent drug use may represent a transition to an allostatic state in the brain reward systems and, by extrapolation, a transition to addiction (see text). Notice that the apparent b-process never returns to the original homeostatic level before drug taking begins again, thus creating a greater and greater allostatic state in the brain reward system. The counteradaptive opponent-process (b-process) does not balance the activational process (a-process) but in fact shows a residual hysteresis. Although these changes that are illustrated in the figure are exaggerated and condensed over time, the hypothesis is that even during post-detoxification (a period of “protracted abstinence”), the reward system still bears allostatic changes. The following definitions apply: allostasis, the process of achieving stability through change; allostatic state, a state of chronic deviation of the regulatory system from its normal (homeostatic) operating level; allostatic load, the cost to the brain and body of the deviation, accumulating over time, and reflecting in many cases pathological states and accumulation of damage (Modified with permission from Koob and Le Moal41).
Such an opponent process has been demonstrated in animals.89 In an early study, chronic binge-like cocaine self-administration resulted in an opposite effect on brain stimulation reward thresholds (i.e., a measure of hedonic activity in the brain), namely an elevation of brain-stimulation reward thresholds.89 Subsequent studies showed that the elevation of brain reward thresholds that was associated with withdrawal from chronic administration of drugs of abuse is a common element of all drugs of abuse, including cocaine,89 amphetamine,90 opioids,91 cannabinoids,92 nicotine,93 and alcohol.94 A series of studies revealed elevations of brain reward thresholds during withdrawal in animal models. Key neuropharmacological evidence has been generated that shows that both reversing reward deficit neurotransmission and reversing stress surfeit neurotransmission can block the elevation of reward thresholds produced by drug withdrawal.95
A key component that drives negative emotional states in general and hypohedonia in particular and is associated with the withdrawal/negative affect stage of the addiction cycle is engagement of the brain stress systems, including both the HPA and extrahypothalamic systems.96 As noted above, the brain stress systems include such neurotransmitter systems as CRF, dynorphin, norepinephrine, hypocretin (orexin), substance P, and vasopressin. Equally compelling is evidence of the dysregulation of brain anti-stress systems, such as NPY, nociceptin, endocannabinoids, and oxytocin. Increased activity in brain stress systems and decreased activity in brain anti-stress systems are hypothesized to significantly contribute to negative emotionality.96
Neuroclinical Assessment: Anhedonia, Hypohedonia, and Dysphoria
The neurocircuitry of anhedonia, hypohedonia, and dysphoria to a large extent has been hypothesized to reflect “within-system” changes in the mesocorticolimbic dopamine system or opioid peptide systems the converge on the NAc. “Between-system” changes that mediate anhedonia, hypohedonia, and dysphoria include the activation of neurocircuits that are involved in stress (CRF in the CeA and BNST) or neurocircuits that feed back to suppress dopaminergic activity (CRF and/or dynorphin or acetylcholine in the VTA, NAc, and habenula; Table 1). Animal models with construct validity for anhedonia, hypohedonia, and dysphoric-like responding that have helped elucidate the respective neurocircuits include measures of brain stimulation reward thresholds (intracranial self-stimulation), sucrose preference, progressive-ratio responding, and the probabilistic reward task in animals (Table 2). Human laboratory assessments of anhedonia, hypohedonia, and dysphoria range from standard self-report measures, such as the Beck Depression Inventory and Hamilton Anxiety Rating Scale, to measures that focus selectively on negative reward constructs, such as the Fawcett-Clark Pleasure Scale (Table 3). More operational measures of anhedonia, hypohedonia, and dysphoria include the probabilistic reward task97–100 and Effort for Expenditure for Rewards Task,101 among others.
Table 2.
Animal models for negative emotional states.
| Negative emotionality | ||||
|---|---|---|---|---|
| Assessment | Measure | References | Repeated testing within subject | Animal model |
| Animal Model: anhedonia, hypohedonia, and dysphoria | Intracranial self-stimulation | Markou and Koob89 | Yes | Rat |
| Conditioned place aversion | Hand et al.102 | No | Rat | |
| Disrupted operant responding | Gellert and Sparber103 | Yes | Rat | |
| Drug discrimination | Gauvin and Holloway104 | Yes | Rat | |
| Sucrose preference | Hammami-Abrand Abadi et al.105 | Yes | Rat, mouse | |
| Probabilistic reward task | Pizzagalli et al.99 | Yes | Rat | |
| Neuroclinical: anxiety, stress reactivity, and irritability | Progressive-ratio responding | Wee et al.106 | Yes | Rat, mouse |
| Elevated plus maze | Fawcett et al.107 | No | Rat, mouse | |
| Defensive burying | Bagby et al.108 | No | Rat, mouse | |
| Light/dark box | Bourin and Hascoet109 | No | Rat, mouse | |
| Marble burying task | Njung’e and Handley110 | No | Rat, mouse | |
| Neuroclinical: pain and hyperkatifeia | Mechanosensitivity pain test | Edwards et al.111 | Yes | Rat, mouse |
| Thermal pain hypersensitivity (tail-flick test) | Raghavendra et al.112 | Yes | Rat, mouse | |
| Neuroclinical: malaise, sleep disturbances, and arousal | Electroencephalogram sleep measures (EEG activity) | Walker and Zornetzer113 | Yes | Rat, mouse |
| Locomotor activity | Pulvirenti and Koob114 | Yes | Rat, mouse | |
EEG: electroencephalogram.
Table 3.
Human laboratory tests for negative emotional states.
| Negative emotionality | ||||
|---|---|---|---|---|
| Negative emotional states | Measure | Reference | Time to complete | Type of task |
| Neuroclinical: anhedonia, hypohedonia, and dysphoria | Approach Avoidance Task | Heuer et al.115 | 10 min | Behavioral |
| Two-step Task (model-free model-based) | Sebold et al.116 | 15 min | Behavioral | |
| Beck Depression Inventory | Beck et al.117 | 5 min | Self-report | |
| Fawcett-Clark Pleasure Scale | Fawcett et al.107 | 5 min | Self-report | |
| Neuroclinical: anxiety, stress reactivity, and irritability | Cyberball | Williams and Jarvis118 | 10 min | Behavioral |
| Trier Social Stress Test | Kirschbaum et al.119 | 20 min | Behavioral | |
| Buss-Durkee Hostility Inventory | Buss and Durkee120 | 15 min | Self-report | |
| Neuroclinical: pain and hyperkatifeia | Cold Pressor Task | Lovallo121 | 10 min | Behavioral |
| Toronto Alexithymia Scale | Bagby et al.108 | 5 min | Self-report | |
| Facial Emotion Matching Task | Hariri et al.122 | 10 min | Neuroimaging | |
| Neuroclinical: malaise, sleep disturbances, and arousal | Malaise Inventory | Rodgers et al.123 | 5 min | Self-report |
| Pittsburgh Sleep Quality Index | Buysse et al.124 | 5 min | Self-report | |
| Behavioral Activation System | Carver and White125 | 5 min | Self-report | |
Neuroclinical Assessment: Anxiety, Stress Reactivity, and Irritability
The neurocircuitry of anxiety, stress, and irritability are hypothesized to involve “between-system” changes that include activation of neurocircuits involved in stress (CRF, norepinephrine, vasopressin, and hypocretin in the CeA and BNST; Table 1). Animal models with construct validity for anxiety-like behavior, stress reactivity, and irritability-like behavior that have helped elucidate the neurocircuitry associated with anxiety, stress, and irritability include the elevated plus maze, defensive withdrawal test, defensive burying test, marble burying test, and social interaction test (Table 2). Human laboratory assessments of anxiety, stress reactivity, and irritability range from standard self-report measures, such as the Beck Anxiety Inventory and Hamilton Depression Rating Scale, to those that focus selectively on trauma constructs, such as the Childhood Trauma Questionnaire (Table 3). More operational measures of anxiety, stress reactivity, and irritability include the Cyberball Test, Trier Social Stress Test, and Buss-Durkee Hostility Inventory (Table 3).
Neuroclinical Assessment: Pain and Hyperkatifeia
The neurocircuitry of pain and analgesia are hypothesized to involve “between-system” changes that include the activation of pain circuits and also neurocircuits that are involved in stress (CRF, norepinephrine, vasopressin, and substance P in the CeA and BNST; Table 1). Animal models with construct validity for pain and hyperalgesia that have helped elucidate the neurocircuitry associated with pain and the interaction between pain and stress include the hot plate test, tail flick test, and von Frey test (Table 2).
Both hyperalgesia and hyperkatifeia have been observed in humans during withdrawal from opioids and alcohol.126,127 Hyperalgesia can be defined as an increased sensitivity to pain. Hyperkatifeia (derived from the Greek word katifeia for dejection, sadness, or negative emotional state) is defined as the increased intensity of negative emotional/motivational symptoms and signs.128 Human laboratory assessments of hyperalgesia range from standard test batteries of pain thresholds to thermal, electrical stimulation, or pressure pain. More general tests of emotional liability include the Toronto Alexithymia Scale (Table 3). More operational measures of pain include tests that focus selectively on hyperalgesia (e.g., cold pressor test) and hyperkatifeia (e.g., Facial Emotion Matching Task; Table 3).
Neuroclinical Assessment: Malaise, Sleep Disturbances, and Arousal
The neurocircuitry of malaise, sleep disturbances, and arousal are hypothesized to involve both “within-system” changes in the mesocorticolimbic dopamine system for arousal and malaise, but also “between-system” changes in neurocircuits that are involved in malaise (CRF, norepinephrine, vasopressin, and hypocretin in the CeA and BNST) and sleep/arousal (hypocretin in the hypothalamus; Table 1). Indeed, hypocretin (orexin) has been shown to play a critical role not only in addiction, as described above, but also in regulating arousal and coordinating the alertness that is necessary to pursue goal-directed behaviors.129 Animal models with construct validity for malaise, sleep disturbances, and arousal that have helped elucidate the neurocircuitry associated with these constructs in humans include activity measures, electroencephalography, and observations of peripheral physiological arousal. Patients who are addicted to various agents have present self-reported malaise,130 sleep disturbances,131,132 and disruptions in arousal.133 Malaise may be defined as an undefined sense of illness or unease without a specific cause. Within addictive disorders, sleep disturbances often take the form of insomnia and changes in sleep architecture.131,132 Dysregulated arousal may appear as hyperarousal in response to stressful stimuli or drug cues compared with individuals who are not addicted.133 Relatedly, hyperarousal is a key diagnostic criterion for PTSD, which is highly comorbid with addiction to various substances.134 Human laboratory assessments of these constructs include polysomnography for the evaluation of sleep and electroencephalography and peripheral signals (e.g., galvanic skin response, respiration, and heart rate) for the evaluation of arousal, in addition to self-report measures, such as the Malaise Inventory, Pittsburgh Sleep Quality Index, and Behavioral Activation System Scale (Table 3).
Implications for Nosology of Addiction
Over time, the nosology of addictions has remained relatively static. The most recent iteration of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)135 combines the previous substance abuse and dependence categories into one, labeled Substance Use Disorder. This change also affords an assessment of disease severity based on symptom counts. Regardless, several problems exist with the current nosology, which may be addressed through the ANA and a focus on negative emotionality and stress. First, most of the specific diagnostic criteria load onto the same factor, despite the fact that in practice, considerable within-diagnosis heterogeneity exists and is a limiting factor in treatment outcome. Second, these criteria are largely not based on the neurobiology of addiction but rather on patient-reported symptoms. While patients’ who present complaints are a critical piece of diagnosis and treatment plan formulation, they are also insufficient for these tasks. For example, a patient who suffers from a particular form of cancer may complain of pain and fatigue; these presenting concerns, while important, do not form the basis of diagnosis. Instead, a diagnosis of cancer is made by considering alterations in patients’ biological systems, such as the presence of a tumor or an increase in cancer cells in the blood stream, which are diagnosed by imaging and/or blood tests. Currently, the presentation in subjects of the current diagnostic criteria of hedonic tolerance and motivational withdrawal (defined above) are most closely related to the actual neurobiological alterations that occur in addictions and may serve as a bridge to a reformulation of the nosology of addiction.
Even without a specific and definitive neurobiological marker, an emphasis on stress and negative affective states in addictive disorders, as discussed herein, could lead to the inclusion of these in future iterations of addiction diagnoses. For example, specifying whether an individual experiences significant dysphoria or relief craving during withdrawal, while still being symptom-based, would be one step closer toward a neurobiologically informed addiction diagnosis. It would also critically allow clinicians to identify treatments that would more closely align with a specific subtype of addiction. Overall, a strong emphasis on negative affective states that are associated with addiction could further the integration of neurobiology into the addiction nosology and improve treatment outcome. Given the significant public health costs associated with addictions, these improvements would be well worth the time and effort to further explore the role of stress and negative affect in addictions.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Selye H. A syndrome produced by diverse nocuous agents. Nature 1936; 138: 32. [DOI] [PubMed] [Google Scholar]
- 2.Burchfield S. The stress response: a new perspective. Psychosom Med 1979; 41: 661–672. [DOI] [PubMed] [Google Scholar]
- 3.Mason JW. A re-evaluation of the concept of “non-specificity” in stress theory. J Psychiatr Res 1971; 8: 323–333. [DOI] [PubMed] [Google Scholar]
- 4.Vale W, Spiess J, Rivier C, et al. Characterization of a 41-residue ovine hypothalamic peptide that stimulates the secretion of corticotropin and -endorphin. Science 1981; 213: 1394–1397. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein R, Smith SM, Chou SP, et al. The epidemiology of DSM-5 post traumatic stress disorder in the United States. Soc Psychiatry Psychiatr Epidemiol 2016; 51: 1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swendsen J, Conway KP, Degenhardt L, et al. Mental disorders as risk factors for substance use, abuse, and dependence. Addiction 2010; 105: 1117–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol 2008; 59: 29–53. [DOI] [PubMed] [Google Scholar]
- 8.Russell MAH. What is dependence? In: Edwards G. (ed). Drugs and drug dependence, Lexington: Lexington Books, 1976, pp. 182–187. [Google Scholar]
- 9.Durana JH, Barnes PA. A neurodevelopmental view of impulsivity and its relationship to the superfactors of personality. In: McCown WG, Johnson J, Shure MB. (eds). The impulsive client: Theory, research, and treatment, Washington, DC: American Psychological Association, 1993, pp. 23–37. [Google Scholar]
- 10.Allen TJ, Moeller FG, Rhoades HM, et al. Impulsivity and history of drug dependence. Drug Alcohol Depend 1998; 50: 137–145. [DOI] [PubMed] [Google Scholar]
- 11.Rachlin H, Green L. Commitment, choice and self-control. J Exp Anal Behav 1972; 17: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychol Sci 1997; 8: 60–64. [Google Scholar]
- 13.Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend 2002; 66: 265–273. [DOI] [PubMed] [Google Scholar]
- 14.Green L, Fristoe N, Myerson J. Temporal discounting and preference reversals in choice between delayed outcomes. Psychonom Bull Rev 1994; 1: 383–389. [DOI] [PubMed] [Google Scholar]
- 15.Berlin GS, Hollander E. Compulsivity, impulsivity, and the DSM-5 process. CNS Spectr 2014; 19: 62–68. [DOI] [PubMed] [Google Scholar]
- 16.Robbins TW, Gillan CM, Smith DG, et al. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn Sci 2012; 16: 81–91. [DOI] [PubMed] [Google Scholar]
- 17.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science 2004; 305: 1014–1017. [DOI] [PubMed] [Google Scholar]
- 18.Hopf FW, Lesscher HM. Rodent models for compulsive alcohol intake. Alcohol 2014; 48: 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed Washington, DC: American Psychiatric Press, 1994. [Google Scholar]
- 20.Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction: alcohol addiction as a reward deficit disorder. In: Sommer WH, Spanagel R. (eds). Behavioral neurobiology of alcohol addiction (series title: Current topics in nehavioral neuroscience, vol 13), Berlin: Springer-Verlag, 2013, pp. 3–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romanczuk-Seiferth N, van den Brink W, Goudriaan AE. From symptoms to neurobiology: pathological gambling in the light of the new classification in DSM-5. Neuropsychobiology 2014; 70: 95–102. [DOI] [PubMed] [Google Scholar]
- 22.Koob GF. A role for brain stress systems in addiction. Neuron 2008; 59: 11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci 2000; 23: 155–184. [DOI] [PubMed] [Google Scholar]
- 24.Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience 1988; 27: 1–39. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds SM, Zahm DS. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci 2005; 25: 11757–11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010; 35: 169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res 2003; 27: 232–243. [DOI] [PubMed] [Google Scholar]
- 28.Sutton RE, Koob GF, Le Moal M, et al. Corticotropin-releasing factor produces behavioural activation in rats. Nature 1982; 297: 331–333. [DOI] [PubMed] [Google Scholar]
- 29.Koob GF, Zorrilla EP. Neurobiological mechanisms of addiction: focus on corticotropin-releasing factor. Curr Opin Invest Drugs 2010; 11: 63–71. [PMC free article] [PubMed] [Google Scholar]
- 30.Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the κ opioid receptor. Science 1982; 215: 413–415. [DOI] [PubMed] [Google Scholar]
- 31.Watson SJ, Khachaturian H, Akil H, et al. Comparison of the distribution of dynorphin systems and enkephalin systems in brain. Science 1982; 218: 1134–1136. [DOI] [PubMed] [Google Scholar]
- 32.Van’t Veer A, Carlezon WA., Jr Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology 2013; 229: 435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koob GF. The dark side of emotion: the addiction perspective. Eur J Pharmacol 2015; 753: 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmac Rev 2010; 35: 217–238 [erratum: 35: 1051]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koob GF, Mason BJ. Existing and future drugs for the treatment of the dark side of addiction. Annu Rev Pharmacol Toxicol 2016; 56: 299–322. [DOI] [PubMed] [Google Scholar]
- 36.Volkow ND, Wang GJ, Telang F, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci 2007; 27: 12700–12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell JM, O’Neil JP, Janabi M, et al. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Trans Med 2012; 4: 116ra6. [DOI] [PubMed] [Google Scholar]
- 38.Belin D, Belin-Rauscent A, Murray JE, et al. Addiction: failure of control over maladaptive incentive habits. Curr Opin Neurobiol 2013; 23: 564–572. [DOI] [PubMed] [Google Scholar]
- 39.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science 1988; 242: 715–723. [DOI] [PubMed] [Google Scholar]
- 40.Edwards S, Koob GF. Neurobiology of dysregulated motivational systems in drug addiction. Future Neurol 2010; 5: 393–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 2001; 24: 97–129. [DOI] [PubMed] [Google Scholar]
- 42.Carlezon WA, Jr, Nestler EJ, Neve RL. Herpes simplex virus-mediated gene transfer as a tool for neuropsychiatric research. Crit Rev Neurobiol 2000; 14: 47–67. [DOI] [PubMed] [Google Scholar]
- 43.Vendruscolo LF, Barbier E, Schlosburg JE, et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci 2012; 32: 7563–7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 2016; 3: 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nealey KA, Smith AW, Davis SM, et al. κ-Opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol-dependent rats. Neuropharmacology 2011; 61: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlosburg JE, Whitfield TW, Jr, Park PE, et al. Long-term antagonism of κ opioid receptors prevents escalation of and increased motivation for heroin intake. J Neurosci 2013; 33: 19384–19392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kallupi M, Wee S, Edwards S, et al. Kappa opioid receptor-mediated dysregulation of gamma-aminobutyric acidergic transmission in the central amygdala in cocaine addiction. Biol Psychiatry 2013; 74: 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heilig M, Thorsell A. Brain neuropeptide Y (NPY) in stress and alcohol dependence. Rev Neurosci 2002; 13: 85–94. [DOI] [PubMed] [Google Scholar]
- 49.Gilpin NW, Misra K, Herman MA, et al. Neuropeptide Y opposes alcohol effects on gamma-aminobutyric acid release in amygdala and blocks the transition to alcohol dependence. Biol Psychiatry 2011; 69: 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gilpin NW, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced increases in alcohol drinking. Pharmacol Biochem Behav 2008; 90: 475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grieder TE, Herman MA, Contet C, et al. VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nat Neurosci 2014; 17: 1751–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fowler CD, Lu Q, Johnson PM, et al. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature 2011; 471: 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Funk CK, O’Dell LE, Crawford EF, et al. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci 2006; 26: 11324–11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delfs JM, Zhu Y, Druhan JP, et al. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature 2000; 403: 430–434. [DOI] [PubMed] [Google Scholar]
- 55.Schmeichel BE, Barbier E, Misra KK, et al. Hypocretin receptor 2 antagonism dose-dependently reduces escalated heroin self-administration in rats. Neuropsychopharmacology 2015; 40: 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barbier E, Vendruscolo LF, Schlosburg JE, et al. The NK1 receptor antagonist L822429 reduces heroin reinforcement. Neuropsychopharmacology 2013; 38: 976–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hernandez VS, Hernandez OR, Perez de la Mora M, et al. Hypothalamic vasopressinergic projections innervate central amygdala GABAergic neurons: implications for anxiety and stress coping. Front Neural Circuits 2016; 10: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Economidou D, Cippitelli A, Stopponi S, et al. Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal symptoms in the rat. Alcohol Clin Exp Res 2011; 35: 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sidhpura N, Parsons LH. Endocannabinoid-mediated synaptic plasticity and addiction-related behavior. Neuropharmacology 2011; 61: 1070–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verheul R, van den Brink W, Geerlings P. A three-pathway psychobiological model of craving for alcohol. Alcohol Alcohol 1999; 34: 197–222. [DOI] [PubMed] [Google Scholar]
- 61.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 2010; 167: 748–751. [DOI] [PubMed] [Google Scholar]
- 62.Carpenter WT. RDoC and DSM-5: what’s the fuss? Schizophr Bull 2013; 39: 945–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Casey BJ, Oliveri ME, Insel T. A neurodevelopmental perspective on the research domain criteria (RDoC) framework. Biol Psychiatry 2014; 76: 350–353. [DOI] [PubMed] [Google Scholar]
- 64.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med 2013; 11: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry 2014; 171: 395–397. [DOI] [PubMed] [Google Scholar]
- 66.Kwako LE, Momenan R, Litten RZ, et al. Addictions neuroclinical assessment: a neuroscience-based framework for addictive disorders. Biol Psychiatry 2016; 80: 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science 1997; 278: 52–58. [DOI] [PubMed] [Google Scholar]
- 68.Heilig M, Thorsell A, Sommer WH, et al. Translating the neuroscience of alcoholism into clinical treatments: from blocking the buzz to curing the blues. Neurosci Biobehav Rev 2010; 35: 334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sinha R, Fox HC, Hong KA, et al. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology 2009; 34: 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Brien CP. Drug addiction. In: Brunton LL, Chabner BA and Knollmann BC (eds) Goodman anbd Gilman s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, http://accessmedicine.mhmedical.com/content.aspx?bookid=1613§ionid=102159667 (2011, accessed 9 December 2016).
- 71.Benton D. The plausibility of sugar addiction and its role in obesity and eating disorders. Clin Nutr 2010; 29: 288–303. [DOI] [PubMed] [Google Scholar]
- 72.George O, Koob GF, Vendruscolo LF. Negative reinforcement via motivational withdrawal is the driving force behind the transition to addiction. Psychopharmacology 2014; 231: 3911–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Piazza PV, Deroche-Gamonet V. A multistep general theory of transition to addiction. Psychopharmacology 2013; 229: 387–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koob GF. Drug addiction: the yin and yang of hedonic homeostasis. Neuron 1996; 16: 893–896. [DOI] [PubMed] [Google Scholar]
- 75.Koob GF, Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Phil Trans R Soc B Biol Sci 2008; 363: 3113–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tao R, Huang X, Wang J, et al. Proposed diagnostic criteria for internet addiction. Addiction 2010; 105: 556–564. [DOI] [PubMed] [Google Scholar]
- 77.Wray I, Dickerson MG. Cessation of high frequency gambling and “withdrawal” symptoms. Br J Addict 1981; 76: 401–405. [DOI] [PubMed] [Google Scholar]
- 78.Snaith RP, Hamilton M, Morley S, et al. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry 1995; 167: 99–103. [DOI] [PubMed] [Google Scholar]
- 79.Hatzigiakoumis DS, Martinotti G, Giannantonio MD, et al. Anhedonia and substance dependence: clinical correlates and treatment options. Front Psychiatry 2011; 2: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heinz A, Schmidt LG, Reischies FM. Anhedonia in schizophrenic, depressed, or alcohol-dependent patients: neurobiological correlates. Pharmacopsychiatry 1994; 27(Suppl. 1): 7–10. [DOI] [PubMed] [Google Scholar]
- 81.Salo R, Nordahl TE, Galloway GP, et al. Drug abstinence and cognitive control in methamphetamine-dependent individuals. J Subst Abuse Treat 2009; 37: 292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salo R, Ursu S, Buonocore MH, et al. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: a functional magnetic resonance imaging study. Biol Psychiatry 2009; 65: 706–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martinotti G, Nicola MD, Reina D, et al. Alcohol protracted withdrawal syndrome: the role of anhedonia. Subst Use Misuse 2008; 43: 271–284. [DOI] [PubMed] [Google Scholar]
- 84.Janiri L, Martinotti G, Dario T, et al. Anhedonia and substance-related symptoms in detoxified substance-dependent subjects: a correlation study. Neuropsychobiology 2005; 52: 37–44. [DOI] [PubMed] [Google Scholar]
- 85.Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev 2007; 17: 337–345. [DOI] [PubMed] [Google Scholar]
- 86.Piazza PV, Deroche V, Deminiere JM, et al. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc Natl Acad Sci U S A 1993; 90: 11738–11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Solomon RL, Corbit JD. An opponent-process theory of motivation: 1. Temporal dynamics of affect. Psychol Rev 1974; 81: 119–145. [DOI] [PubMed] [Google Scholar]
- 88.Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci 2007; 30: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Markou A, Koob GF. Post-cocaine anhedonia: an animal model of cocaine withdrawal. Neuropsychopharmacology 1991; 4: 17–26. [PubMed] [Google Scholar]
- 90.Paterson NE, Myers C, Markou A. Effects of repeated withdrawal from continuous amphetamine administration on brain reward function in rats. Psychopharmacology 2000; 152: 440–446. [DOI] [PubMed] [Google Scholar]
- 91.Schulteis G, Markou A, Gold LH, et al. Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. J Pharmacol Exp Ther 1994; 271: 1391–1398. [PubMed] [Google Scholar]
- 92.Gardner EL, Vorel SR. Cannabinoid transmission and reward-related events. Neurobiol Dis 1998; 5: 502–533. [DOI] [PubMed] [Google Scholar]
- 93.Epping-Jordan MP, Watkins SS, Koob GF, et al. Dramatic decreases in brain reward function during nicotine withdrawal. Nature 1998; 393: 76–79. [DOI] [PubMed] [Google Scholar]
- 94.Schulteis G, Markou A, Cole M, et al. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A 1995; 92: 5880–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koob GF. Anti-reward, compulsivity, and addiction: seminal contributions of Dr. Athina Markou to motivational dysregulation in addiction. Psychopharmacology. Epub ahead of print 3 January 2017. DOI: 10.1007/s00213-016-4484-6. [DOI] [PubMed]
- 96.Koob GF, Buck CL, Cohen A, et al. Addiction as a stress surfeit disorder. Neuropharmacology 2014; 76: 370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pechtel P, Dutra SJ, Goetz EL, et al. Blunted reward responsiveness in remitted depression. J Psychiatr Res 2013; 47: 1864–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry 2005; 57: 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pizzagalli DA, Iosifescu D, Hallett LA, et al. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res 2008; 43: 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vrieze E, Ceccarini J, Pizzagalli DA, et al. Measuring extrastriatal dopamine release during a reward learning task. Hum Brain Map 2013; 34: 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Treadway MT, Buckholtz JW, Schwartzman AN, et al. Worth the “EEfRT”? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One 2009; 4: e6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hand TH, Koob GF, Stinus L, et al. Aversive properties of opiate receptor blockade: evidence for exclusively central mediation in naive and morphine-dependent rats. Brain Res 1988; 474: 364–368. [DOI] [PubMed] [Google Scholar]
- 103.Gellert VF, Sparber SB. A comparison of the effects of naloxone upon body weight loss and suppression of fixed-ratio operant behavior in morphine-dependent rats. J Pharmacol Exp Ther 1977; 201: 44–54. [PubMed] [Google Scholar]
- 104.Gauvin DV, Holloway FA. Cue dimensionality in the three-choice pentylenetetrazole-saline-chlordiazepoxide discrimination task. Behav Pharmacol 1991; 2: 417–428. [PubMed] [Google Scholar]
- 105.Hammami-Abrand Abadi A, Miladi-Gorji H, Bigdeli I. Effect of environmental enrichment on physical and psychological dependence signs and voluntary morphine consumption in morphine-dependent and morphine-withdrawn rats. Behav Pharmacol 2016; 27: 270–278. [DOI] [PubMed] [Google Scholar]
- 106.Wee S, Mandyam CD, Lekic DM, et al. α1-Noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol 2008; 18: 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fawcett J, Clark DC, Scheftner WA, et al. Assessing anhedonia in psychiatric patients: the Pleasure Scale. Arch Gen Psychiatry 1983; 40: 79–84. [DOI] [PubMed] [Google Scholar]
- 108.Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto Alexithymia Scale: I. Item selection and cross-validation of the factor structure. J Psychosom Res 1994; 38: 23–32. [DOI] [PubMed] [Google Scholar]
- 109.Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol 2003; 463: 55–65. [DOI] [PubMed] [Google Scholar]
- 110.Njung’e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav 1991; 38: 63–67. [DOI] [PubMed] [Google Scholar]
- 111.Edwards S, Vendruscolo LF, Schlosburg JE, et al. Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF1 receptor antagonism. Neuropharmacology 2012; 62: 1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Raghavendra V, Tanga FY, DeLeo JA. Attenuation of morphine tolerance, withdrawal-induced hyperalgesia, and associated spinal inflammatory immune responses by propentofylline in rats. Neuropsychopharmacology 2004; 29: 327–334. [DOI] [PubMed] [Google Scholar]
- 113.Walker DW, Zornetzer SF. Alcohol withdrawal in mice: electroencephalographic and behavioral correlates. Electroencephalogr Clin Neurophysiol 1974; 36: 233–243. [DOI] [PubMed] [Google Scholar]
- 114.Pulvirenti L, Koob GF. Lisuride reduces psychomotor retardation during withdrawal from chronic intravenous amphetamine self-administration in rats. Neuropsychopharmacology 1993; 8: 213–218. [DOI] [PubMed] [Google Scholar]
- 115.Heuer K, Rinck M, Becker ES. Avoidance of emotional facial expressions in social anxiety: the Approach-Avoidance Task. Behav Res Ther 2007; 45: 2990–3001. [DOI] [PubMed] [Google Scholar]
- 116.Sebold M, Deserno L, Nebe S, et al. Model-based and model-free decisions in alcohol dependence. Neuropsychobiology 2014; 70: 122–131. [DOI] [PubMed] [Google Scholar]
- 117.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev 1988; 8: 77–100. [Google Scholar]
- 118.Williams KD, Jarvis B. Cyberball: a program for use in research on interpersonal ostracism and acceptance. Behav Res Methods 2006; 38: 174–180. [DOI] [PubMed] [Google Scholar]
- 119.Kirschbaum C, Klauer T, Filipp SH, et al. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom Med 1995; 57: 23–31. [DOI] [PubMed] [Google Scholar]
- 120.Buss AH, Durkee A. An inventory for assessing different kinds of hostility. J Consult Psychol 1957; 21: 343. [DOI] [PubMed] [Google Scholar]
- 121.Lovallo W. The cold pressor test and autonomic function: a review and integration. Psychophysiology 1975; 12: 268–282. [DOI] [PubMed] [Google Scholar]
- 122.Hariri AR, Tessitore A, Mattay VS, et al. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage 2002; 17: 317–323. [DOI] [PubMed] [Google Scholar]
- 123.Rodgers B, Pickles A, Power C, et al. Validity of the malaise inventory in general population samples. Social Psychiatry Psychiatr Epidemiol 1999; 34: 333–341. [DOI] [PubMed] [Google Scholar]
- 124.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193–213. [DOI] [PubMed] [Google Scholar]
- 125.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Person Social Psychol 1994; 67: 319. [Google Scholar]
- 126.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006; 104: 570–587. [DOI] [PubMed] [Google Scholar]
- 127.Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev 2012; 36: 2179–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shurman J, Koob GF, Gutstein HB. Opioids, pain, the brain, and hyperkatifeia: a framework for the rational use of opioids for pain. Pain Med 2010; 11: 1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Boutrel B, Cannella N, de Lecea L. The role of hypocretin in driving arousal and goal-oriented behaviors. Brain Res 2010; 1314: 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res 2010; 1314: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schierenbeck T, Riemann D, Berger M, et al. Effect of illicit recreational drugs upon sleep: cocaine, ecstasy and marijuana. Sleep Med Rev 2008; 12: 381–389. [DOI] [PubMed] [Google Scholar]
- 132.Stein MD, Friedmann PD. Disturbed sleep and its relationship to alcohol use. Subst Abuse 2005; 26: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chaplin TM, Hong K, Fox HC, et al. Behavioral arousal in response to stress and drug cue in alcohol and cocaine addicted individuals versus healthy controls. Hum Psychopharmacol 2010; 25: 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pietrzak RH, Goldstein RB, Southwick SM, et al. Prevalence and axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord 2011; 25: 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th ed Washington, DC: American Psychiatric Publishing, 2013. [Google Scholar]