Abstract
This paper examines health status differences between England and the United States, with an emphasis on the implications of any health disparities for health care cost differences between the two countries. We first document health status differences in disease prevalence, disability, mortality and co‐morbidity. We find higher disease prevalence in the US than in England (confirming previous findings) but much smaller differences between the two countries in disability and mortality. We attribute the smaller differences in disability to the fact that disability measures rely primarily on subjective questions on experiencing disabilities, which are reported differently in the two countries. Smaller mortality differences are most likely due to a combination of earlier disease diagnosis and more effective disease treatment in the US. Co‐morbidity is a common and important dimension of disease in both countries that is often neglected in scientific papers, especially by economists.
We find, however, that disease prevalence has little implication for out‐of‐pocket health care costs in the US except for relatively few individuals with particular diseases. Instead, costs are more associated with incidence than prevalence and with those who are going to die in the next year or two. Co‐morbidity is associated with higher costs but even this association is limited to a relatively small fraction of people who are co‐morbid.
Keywords: health, disability, mortality, international, I10, I12, I13
Policy points
The observed ‘cost’ or ‘value for money’ of a country's health care system should only be viewed in the context of the underlying health of the relevant population. Thus, when comparing the US and UK systems of health care, one needs to also understand the nature of any health disparities between the two countries.
There are well‐documented differences in disease prevalence between the two countries, with substantially lower rates of disease in the population aged 50 and over in England than in the US.
Differences in disability between the two populations are smaller and more nuanced, depending on the age group and the measures of severity being considered. The same is true for mortality differences conditional on having a disease, most likely due to earlier disease diagnosis and more effective disease treatment in the US.
Co‐morbidity rates are higher in the US although by no more than would be expected given the higher prevalence of the individual diseases concerned. Co‐morbidity is often neglected in scientific papers but is an important characteristic of the patterning of disease in a population that is relevant to both health care costs and mortality outcomes.
I. Introduction
Spending on health care varies dramatically across countries, partly because of differences in institutions, but also presumably due to differences in underlying health. In this paper, we focus on comparisons between the United States and England. We select those two countries because the quality of data on the issues examined is excellent and there appear to be dramatic differences between the two countries in health status and in out‐of‐pocket spending on health care. Previous work by Banks, Smith and co‐authors has shown that, on many dimensions of health, Americans do worse than the English.1 This holds true for self‐reported lifetime prevalence of several major diseases, as well as biomarkers such as blood pressure, glycated haemoglobin (HbA1c) and grip strength.2
Intuitively, one might expect worse health to be associated with higher spending on health care. Higher disease prevalence, particularly for diseases expensive to treat such as cancer, will translate into higher demand for medication, surgical procedures and doctor time. However, there are offsetting effects. An incurable virus that kills 100 per cent of its victims within 24 hours may be ‘cheap’ for the health care system. Differences in institutions could imply different spending for the same underlying health if they have different approaches to a ‘reasonable’ treatment for the same conditions. Some countries may ‘over‐treat’ while others may ‘under‐treat’ the same disease.
The dimension of health being measured may also be important. Some diseases are inexpensive to treat, while others are expensive to treat especially at the time of incidence. In this paper, we look at health status as measured by self‐reported lifetime prevalence, using survey respondents’ answers to the question ‘Has a doctor ever diagnosed you with [condition]?’. Since personal health care costs depend on disability, we also explore disability as a dimension of health, once again comparing the US and England. We also examine the health care costs of the prevalence and disability dimensions of health.
Building on our previous health status research, we extend our earlier work on England–US comparisons along the following dimensions. First, we examine an indicator of disability in addition to disease prevalence. Disability is a more subjective indicator than doctor‐diagnosed disease, so reporting differences between the two countries could affect results. Second, we address co‐morbidity, defined as having at least two of the major conditions we study. In the age groups we study, many individuals are co‐morbid, so studying diseases in isolation may be misleading. Co‐morbidity could affect our overall assessment of health status in the two countries as well as the costs associated with disease.
Finally, we consider mortality as another dimension of health and also as an indicator that provides information about the morbidity burden within a country. A low mortality rate combined with high morbidity rates has obvious implications for health care costs. After showing descriptively the relative prevalence rates in England and the US, we explore the relationship between prevalence and four‐/eight‐year survival.
The paper is organised as follows. Section II. discusses how we measure various dimensions of health. Section III. makes comparisons between England and the US in terms of health indicators, co‐morbidity, mortality and survival rates. Section IV. presents results from survival models and Section V. looks at out‐of‐pocket medical expenses. Section VI. concludes.
II. Measuring dimensions of health
1. Lifetime prevalence of disease
We use household survey data to compare dimensions of self‐reported health in the US and England using the Health and Retirement Study (HRS, for the US) and the English Longitudinal Study of Ageing (ELSA, for England). These surveys were designed for comparability, so most questions are asked in both surveys and worded in similar ways.
The first health measure we use is each individual's response to the question ‘Has a doctor ever diagnosed you with [condition]?’.3 This question does not, however, capture whether the individual is currently suffering from the condition, merely whether they have at some point in their life been diagnosed with it. Past work using this measure of health to compare the US and England has shown that the English have lower disease prevalence than Americans do.4 ELSA and HRS also contain information from nurse visits, when biomarkers are collected. The biomarkers support the conclusions of the disease diagnosis responses, so this measure is capturing something real.
2. Disability
We examine disability as another potential indicator of health care costs. To measure disability, we use survey respondents’ answers to the question ‘Do you have any difficulty with [task]?’. Disability as measured in this way is a subjective indicator. The subjectivity of the measure could be a problem for comparisons between countries with different social norms regarding difficulty thresholds. We have examined this issue in prior work and have shown that Americans set lower thresholds for good and excellent health than the English.5
Next is the challenge of compiling a binary indicator of disability from a set of 22 questions, each of which asks about a different aspect of an individual's capabilities. We choose a (relatively arbitrary) cut‐off point of four, such that anyone who reports difficulty with four or more activities is measured as disabled. When we use this threshold, we will refer to ‘moderate’ disability. We also describe most of our results using an alternative cut‐off of eight, referring to this as ‘severe’ disability. Figure 1 shows the distribution within our sample of 55‐ to 84‐year‐olds of the total number of reported disabilities for ELSA and HRS.
Figure 1.
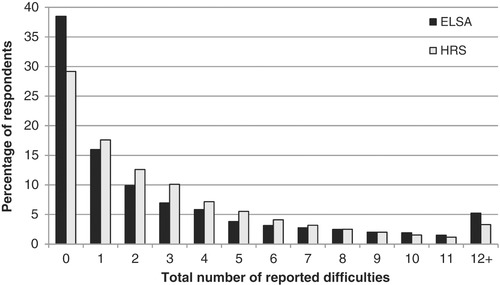
Distribution of disabilities in England and the US
Note: Sample is core respondents aged 55–84 interviewed in 2002–03. Unweighted. See Table A.1 in the online appendix for a list of activities included in the disability index.
Proportionately more people in the US report any limitation, and they are more likely to report between one and seven limitations than English respondents are. However, the tail of the ELSA distribution is fatter than the tail of the HRS distribution; in other words, there are proportionately more reports of severely disabled people in ELSA than in HRS. The results will therefore be sensitive to whether we use a cut‐off point of four or eight (the higher cut‐off point will tend to depress the disability rate in HRS).
3. Mortality
There are two ways of identifying that a respondent in HRS or ELSA has died. The first is when, as a consequence of attempts to interview respondents in future waves, the survey organisation finds that the respondent is deceased. This information is largely contained in the so‐called ‘exit’ interview in both surveys, in which a proxy interview takes place typically with a close relative of the now deceased respondent. The second way is by matching the respondent to a country's national death index, which typically includes information about date and cause of death of respondents regardless of their participation in subsequent waves of the survey.
HRS data on respondent mortality are remarkably close to those obtained from the American life table. At all ages 50 and above, the two mortality curves closely overlap with the only difference being the larger random component in the survey data, especially at older ages when numbers of living respondents in the HRS sample become relatively thin. Over this age range, there does not appear to be any systematic difference between the national death registry and HRS‐based estimates of the four‐year probability of mortality by age.
However, as demonstrated in Banks, Muriel and Smith (2010), this close correspondence is not the case in England. After age 65, mortality among ELSA respondents is somewhat lower than mortality in the English national death index.6 The explanation for this discrepancy is that compared with HRS, ELSA is an immature survey in the sense that it has not yet reached population representative steady state. ELSA's baseline sample in 2002 was drawn from the non‐institutionalised population, thereby leaving out people living in nursing homes – whose mortality prospects, especially at older ages, are higher than average. A similar bias existed in the original HRS sample of older respondents (AHEAD sample of those aged 70–80 fielded in 1993). Since respondents are subsequently followed into nursing homes in both HRS and ELSA samples, this bias no longer exists in the older HRS sample but is still relevant in ELSA.
The tables in this paper correct for this issue by excluding respondents who are resident in a nursing home in their baseline HRS interview. This renders any comparison between the US and England to a comparison between their private residential populations rather than the populations as a whole. The results in this paper should be interpreted with the caveat that differences in health care costs may arise due to the different propensity of England and the US to institutionalise their sick and because of differences in the cost of treating health conditions in and out of nursing homes.
III. Comparison between England and the US
1. Comparing health indicators in England and the US
Table 1 compares diagnosed disease prevalence rates in England and the US using the 2002 baseline wave of ELSA and the 2002 wave of HRS. Prevalence rates are presented for the full age sample of those aged 55 and over and for two age subsamples – 55‐ to 69‐year‐olds and 70‐ to 84‐year‐olds. We also present prevalence rates for those with low and high education in Table 2.7 Low education is defined as having 0–12 years of schooling in the US and as leaving education at or before the compulsory school‐leaving age in England. In both countries, high education is the complement of that.
Table 1.
Prevalence rates in 2002
| Condition | All aged 55 and over | Aged 55–69 | Aged 70–84 | |||
|---|---|---|---|---|---|---|
| HRS | ELSA | HRS | ELSA | HRS | ELSA | |
| Hypertension | 51.9 | 40.7*** | 46.1 | 36.3*** | 59.8 | 47.8*** |
| Heart attacka | 8.4 | 7.0*** | 5.9 | 4.9** | 12.2 | 9.7*** |
| Stroke | 6.9 | 5.4*** | 4.2 | 3.1*** | 9.5 | 7.8*** |
| Diabetes | 15.1 | 8.2*** | 13.9 | 6.8*** | 17.7 | 10.7*** |
| Lung disease | 10.7 | 6.6*** | 9.7 | 5.7*** | 12.3 | 7.9*** |
| Cancer | 14.5 | 7.2*** | 11.1 | 6.1*** | 19.0 | 8.4*** |
| Moderate disability | 31.4 | 30.8 | 24.1 | 22.4** | 38.2 | 38.7 |
| Severe disability | 11.6 | 14.5*** | 7.8 | 10.6*** | 14.1 | 17.1*** |
Heart attack prevalence in HRS comes from the 2010 wave, the first wave it was available.
Note: Sample is all core ELSA sample members aged 55 and over in wave 1, i.e. including the population aged 85 and over. ELSA results are weighted. To make HRS and ELSA comparable, nursing home residents are not included in the HRS sample. ‘Low education’ is defined in HRS as having 0–12 years of schooling and in ELSA as leaving school before the compulsory school‐leaving age. ‘High education’ covers everyone else, i.e. everyone with more than 12 years of schooling in HRS and everyone leaving school after the compulsory school‐leaving age in ELSA. ‘Moderate disability’ is defined as reporting four or more limitations (out of 22). ‘Severe disability’ is defined using an alternative threshold of eight limitations. ***, ** and * indicate significant difference between countries at 1 per cent, 5 per cent and 10 per cent level, according to ‘t’ tests.
Table 2.
Prevalence rates in 2002, by education level
| Aged 55–69 | ||||
|---|---|---|---|---|
| Condition | Low education | High education | ||
| HRS | ELSA | HRS | ELSA | |
| Hypertension | 49.8 | 39.2*** | 42.6 | 32.9*** |
| Heart attacka | 7.6 | 6.0** | 4.9 | 3.5*** |
| Stroke | 5.1 | 4.2* | 3.2 | 1.9*** |
| Diabetes | 15.8 | 7.8*** | 12.1 | 5.7*** |
| Lung disease | 12.7 | 7.4*** | 6.6 | 3.6*** |
| Cancer | 11.0 | 5.8*** | 11.2 | 6.5*** |
| Moderate disability | 31.7 | 28.6*** | 16.4 | 14.8 |
| Severe disability | 11.0 | 14.9*** | 4.5 | 5.4 |
| Aged 70–84 | ||||
|---|---|---|---|---|
| Condition | Low education | High education | ||
| HRS | ELSA | HRS | ELSA | |
| Hypertension | 61.8 | 47.8*** | 57.1 | 47.6*** |
| Heart attacka | 13.4 | 10.9*** | 10.6 | 7.8*** |
| Stroke | 10.3 | 9.0 | 8.1 | 5.9** |
| Diabetes | 19.3 | 10.8*** | 15.2 | 10.7*** |
| Lung disease | 14.1 | 9.1*** | 9.4 | 6.0*** |
| Cancer | 17.0 | 8.5*** | 22.2 | 8.3*** |
| Moderate disability | 42.9 | 42.4 | 30.9 | 32.5 |
| Severe disability | 16.4 | 20.0*** | 10.5 | 12.3 |
For notes, see Table 1.
As previously found,8 the American population has higher disease prevalence than the English population for all diseases shown. Overall, the variation by condition and age is greater than that between countries (see, for example, Tables B.1 and B.2 in the online appendix). Unsurprisingly, lifetime prevalence is higher among the older age group, and less severe conditions such as hypertension and diabetes are common relative to past heart attacks and strokes.
Including disability using a cut‐off of four limitations reveals that the prevalence of moderate disability is at similar high rates in England and the US and rises with age. This is in contrast to diagnosed diseases, where prevalence rates are higher in the US than in England for all conditions considered. For the higher disability cut‐off of eight limitations, severe disability is reported at a higher rate in England than in the US.
Given higher lifetime disease prevalence in the US, it is perhaps surprising that disability rates are not more different between the two countries for the threshold of four limitations and that rates of severe disability are actually higher in England than in the US. One interpretation is that disease prevalence is just one dimension of health, and taking a broader definition reduces the imbalance between England and the US.9 This interpretation is problematic since disability is a subjective measure and there is solid evidence that there are differences in subjective thresholds whereby English residents are more likely than Americans to say they are disabled for the same objective disability.10
Disability does match the other health conditions shown in terms of its sharp differences by education – it is unevenly distributed, particularly when measured using the cut‐off of eight rather than four limitations. In the younger age group (55–69), low‐educated people are around twice as likely to be disabled as high‐educated people, in both the US and England. In the older age group, this difference falls from twice as likely to a third more likely to be disabled in the low‐education groups in both countries.
2. Co‐morbidity
Co‐morbidity is an important dimension of disease since both the health and health care cost consequences of a particular condition may differ for individuals according to the number of other diseases or disabilities they have. Table 3 summarises a simple measure of co‐morbidity, defined as the fraction of individuals with more than one condition, conditional on having at least one. The upper panel excludes disability whereas the lower one includes it. Co‐morbidity increases with age, and Americans are more co‐morbid than the English, whether or not one includes disability in one's measure of co‐morbidity. This latter finding is perhaps unsurprising since prevalence is higher in the US than in England, and one would expect prevalence of the conditions to be positively correlated. The inclusion of disability leads to a large increase in rates of co‐morbidity in the US and particularly in England. Disability can be seen to be highly correlated with prevalence of the diseases that we consider, especially in England.
Table 3.
Co‐morbidity rates
| Co‐morbidity rates (including stroke, cancer, lung disease and diabetes) | ||||||
|---|---|---|---|---|---|---|
| Age | All | Low education | High education | |||
| HRS | ELSA | HRS | ELSA | HRS | ELSA | |
| 55–69 | 20.7 | 11.0*** | 24.2 | 13.3*** | 15.7 | 7.2*** |
| 70–84 | 24.6 | 15.3*** | 26.3 | 16.3*** | 21.9 | 13.6*** |
| 85 and over | 27.5 | 12.6*** | 29.9 | 12.6*** | 21.1 | 12.6 |
| All, 55 and over | 23.1 | 13.1*** | 25.7 | 14.6*** | 18.8 | 10.3*** |
| Co‐morbidity rates (adding moderate disability) | ||||||
|---|---|---|---|---|---|---|
| Age | All | Low education | High education | |||
| HRS | ELSA | HRS | ELSA | HRS | ELSA | |
| 55–69 | 36.0 | 25.6*** | 40.3 | 29.5*** | 29.2 | 18.6*** |
| 70–84 | 41.7 | 32.2*** | 44.3 | 33.9*** | 37.1 | 29.0*** |
| 85 and over | 46.9 | 32.0*** | 48.8 | 32.1*** | 38.6 | 31.9 |
| All, 55 and over | 39.5 | 29.2*** | 43.0 | 31.8*** | 33.4 | 24.4*** |
Note: Co‐morbidity is defined as the number of respondents reporting more than one condition as a percentage of the number reporting any condition. For comparability, the conditions include stroke, cancer, lung disease and diabetes in the upper panel. Moderate disability (reporting four or more limitations) is also included in the lower panel. ***, ** and * indicate significant difference between countries at 1 per cent, 5 per cent and 10 per cent level, according to ‘t’ tests.
In England, the large difference between co‐morbidity rates of the high‐/low‐educated in the younger age group disappears in the older ones, whereas this difference persists in the US. From these cross‐sectional results, one cannot identify separate age and cohort effects; survival bias or real cohort differences could be driving this result.
Table 4 portrays the extent of co‐morbidity between individual diseases and our two measures of disability by presenting the excess rate of prevalence for those with each disease over the prevalence rate for the entire population aged 55 and over. To illustrate, in the US, hypertensive prevalence is 51.9 per cent overall for this age group but it is 23.6 percentage points higher (at 75.5 per cent) among those who reported that they had previously had a stroke. The fact that all the numbers in Table 4 are positive indicates that co‐morbidity is very common for all diseases and disability in both countries. Co‐morbidity should not be ignored in describing health status, although this often happens. In both countries, co‐morbidity is particularly high between hypertension and stroke and diabetes and many people who have had strokes, diabetes and lung disease suffer from disability, which is often severe. Using our measures of excess prevalence in Table 4, the extent of co‐morbidity between diseases is very similar in the two countries.
Table 4.
Excess disease prevalence rates for those with a co‐morbid condition relative to sample totals
| HRS | |||||||
|---|---|---|---|---|---|---|---|
| Hypertension | Stroke | Diabetes | Lung disease | Cancer | Moderate disability | Severe disability | |
| Sample total prevalence rate | 51.9 | 6.9 | 15.1 | 10.7 | 14.5 | 31.4 | 11.6 |
| Excess for those with: | |||||||
| Hypertension | ‐ | 3.1 | 6.6 | 1.6 | 0.9 | 7.6 | 3.6 |
| Stroke | 23.6 | ‐ | 11.7 | 6.9 | 4.0 | 30.7 | 23.9 |
| Diabetes | 22.5 | 5.3 | ‐ | 2.9 | 1.8 | 15.9 | 9.4 |
| Lung disease | 7.7 | 4.5 | 4.1 | ‐ | 5.1 | 25.9 | 14.1 |
| Cancer | 3.3 | 1.9 | 1.8 | 3.7 | ‐ | 7.2 | 4.0 |
| Moderate disability | 12.6 | 6.7 | 7.7 | 8.8 | 3.3 | ‐ | 25.3 |
| Severe disability | 16.3 | 14.1 | 12.3 | 13.0 | 5.1 | ‐ | ‐ |
| ELSA | |||||||
|---|---|---|---|---|---|---|---|
| Hypertension | Stroke | Diabetes | Lung disease | Cancer | Moderate disability | Severe disability | |
| Sample total prevalence rate | 40.7 | 5.4 | 8.2 | 6.6 | 7.2 | 30.8 | 14.5 |
| Excess for those with: | |||||||
| Hypertension | ‐ | 3.0 | 5.1 | 0.4 | 0.4 | 6.6 | 4.0 |
| Stroke | 22.2 | ‐ | 6.7 | 2.3 | 1.9 | 31.2 | 27.3 |
| Diabetes | 24.8 | 4.4 | ‐ | 1.4 | 0.7 | 17.7 | 11.6 |
| Lung disease | 1.9 | 1.8 | 1.8 | ‐ | 0.3 | 29.0 | 23.4 |
| Cancer | 2.3 | 1.4 | 0.8 | 0.3 | ‐ | 7.9 | 4.8 |
| Moderate disability | 9.0 | 5.0 | 4.7 | 6.3 | 1.8 | ‐ | 32.7 |
| Severe disability | 11.3 | 9.5 | 6.5 | 10.6 | 2.3 | ‐ | ‐ |
Note: Cells contain the absolute percentage point difference between column‐heading prevalence rates of those with a row‐heading condition. ELSA and HRS samples are weighted. Sample is those aged 55 and over. ‘Moderate disability’ is defined as reporting four or more limitations (out of 22). ‘Severe disability’ is defined using an alternative threshold of eight limitations.
There is a question of whether the between‐country difference in levels of co‐morbidity is genuine, or whether it reflects in part a different approach to diagnosis between American and English institutions. Health professionals in the US are often thought to diagnose diseases more aggressively than in England, and some of the differences between countries may well reflect that.
3. Mortality by lifetime prevalence of disease
Table 5 displays four‐year survival rates organised by disease prevalence in 2002–03 in HRS and ELSA for two age groups: ages 55–69 and ages 70–84. Four‐year survival rates among those who do not have that specific disease are remarkably similar in England and the US for both age groups. Differences in survival between the two countries are larger among those with a specific disease in 2002–03, with a general tendency toward higher survival probabilities in England than in the US for the older age group and somewhat higher survival rates in the US for the younger age group.
Table 5.
Survival rates between 2002–03 and four years later, by disease prevalence in 2002–03
| Prevalence in 2002–03 | Aged 55–69 | Aged 70–84 | |||
|---|---|---|---|---|---|
| HRS | ELSA | HRS | ELSA | ||
| Heart attack | Yes | NA | 87.6 | NA | 80.3 |
| No | NA | 96.3 | NA | 84.9 | |
| Stroke | Yes | 87.8 | 86.2 | 68.3 | 73.5 |
| No | 96.0 | 96.2 | 84.4 | 85.4 | |
| Diabetes | Yes | 91.1 | 91.7 | 72.4 | 79.0** |
| No | 96.4 | 96.2 | 85.1 | 85.1 | |
| Lung disease | Yes | 90.4 | 87.4 | 70.1 | 72.5 |
| No | 96.2 | 96.4 | 84.6 | 85.5 | |
| Cancer | Yes | 90.6 | 86.9* | 76.3 | 74.9 |
| No | 96.3 | 96.5 | 84.4 | 85.3 | |
| Moderate disability | Yes | 91.5 | 91.1 | 72.2 | 78.4*** |
| No | 97.0 | 97.4 | 89.4 | 88.8 | |
| Severe disability | Yes | 86.7 | 86.9 | 60.1 | 74.3*** |
| No | 96.5 | 97.1 | 86.6 | 86.9 | |
| ALL | ‐ | 95.7 | 95.9 | 82.9 | 84.4* |
Note: ELSA sample is weighted. ‘Moderate disability’ is defined as reporting four or more limitations (out of 22). ‘Severe disability’ is defined using an alternative threshold of eight limitations. ***, ** and * indicate significant difference between countries at 1 per cent, 5 per cent and 10 per cent level, according to ‘t’ tests.
High survival rates for those with a particular disease are partly an indication of a well‐functioning health care system. It may also be that there is a lower threshold of severity of the disease for diagnosis of that particular disease, i.e. that only those with a more severe level of the disease are diagnosed in England compared with the United States.
4. Survival by education level
The positive relationship between health and socio‐economic status is well documented. Table 2 demonstrated differences in lifetime disease prevalence by education, and Table 6 confirms the relationship in mortality by presenting ratios of four‐year mortality rates of the less‐educated group compared with the more‐educated group. To illustrate, 55‐ to 69‐year‐olds with high education in England are 1.63 times as likely to survive for the next four years as those with low education. This difference falls to 1.36 times among the elderly English. Lower survival prospects for the less‐educated also exist in the US. The excess mortality of the less‐educated in both countries is fully consistent with the excess disease prevalence of the less‐educated that was documented in Table 2. While the relative survival by education level is roughly the same in our two countries for the older age group, the low‐education ‘survival penalty’ for 55‐ to 69‐year‐olds is much larger in England. Once again, this is consistent with our prevalence data in Table 2, since the relative excess disease prevalence of less‐educated 55‐ to 69‐year‐olds is higher for most diseases in England.
Table 6.
Relative four‐year mortality probability of low‐educated compared with high‐educated, conditional on prevalence
| Prevalence in 2002–03 | Aged 55–69 | Aged 70–84 | |||
|---|---|---|---|---|---|
| HRS | ELSA | HRS | ELSA | ||
| Stroke | Yes | 1.00 | 1.84 | 1.37 | 1.26 |
| No | 1.32 | 1.52 | 1.36 | 1.33 | |
| Diabetes | Yes | 1.10 | 1.52 | 1.23 | 1.39 |
| No | 1.25 | 1.60 | 1.39 | 1.35 | |
| Lung disease | Yes | 1.58 | 1.03 | 1.09 | 1.14 |
| No | 1.24 | 1.60 | 1.40 | 1.33 | |
| Cancer | Yes | 1.36 | 2.32 | 1.58 | 1.43 |
| No | 1.32 | 1.52 | 1.37 | 1.32 | |
| Moderate disability | Yes | 1.29 | 1.59 | 1.19 | 1.25 |
| No | 0.96 | 1.11 | 1.28 | 1.23 | |
| Severe disability | Yes | 1.12 | 1.22 | 1.13 | 1.56 |
| No | 1.16 | 1.28 | 1.33 | 1.16 | |
| ALL | ‐ | 1.32 | 1.63 | 1.38 | 1.36 |
Note: ELSA sample is weighted. ‘Moderate disability’ is defined as reporting four or more limitations (out of 22). ‘Severe disability’ is defined using an alternative threshold of eight limitations.
The next question is whether there are differences in mortality between the high‐ and low‐educated, conditional on having had a past diagnosis of a particular condition. In other words, are differences in prevalence rates enough to explain the survival differences between the high‐ and low‐educated? Table 6 shows that not only do the low‐educated have higher disease prevalence rates, but conditional on lifetime disease prevalence they also experience higher mortality. This relationship exists across all conditions we consider, although the difference is small in some cases, such as strokes in HRS and lung disease in ELSA. Possible reasons for the relationship include differential access to health care, more co‐morbidities among the low‐educated, differences in the severity of conditions between groups since the less‐educated may have the condition for longer before being diagnosed, and differences in the ability of people to manage their conditions.
The combination of high prevalence and high life expectancy may contribute to a higher financial cost to the health care system, and potentially to a higher cost to the individual. To the extent that this varies across different groups, it might explain differential precautionary saving behaviour or differences in out‐of‐pocket spending. It may also explain some of the health care spending differences between countries, as the US has relatively high prevalence and relatively low mortality conditional on prevalence.
5. Survival by prevalence and co‐morbidities
We showed above that there are differences between the English and the Americans in rates of disease co‐morbidity. Table 7 illustrates how this feeds through into survival by displaying disease mortality rates for those who are not co‐morbid and those who are. Survival is significantly lower if a co‐morbidity is present than if it is not, which is bad news for Americans, who are more co‐morbid (see Table 3). In addition, the difference between co‐morbid and single‐condition individuals in terms of survival seems to be greater in the US. At older ages, co‐morbidity is associated with a larger drop in survival probability than at younger ages in both England and the US. Co‐morbidity is, in general and especially at older ages, a very common phenomenon that has tended to be neglected in health status and mortality analysis.
Table 7.
Four‐year mortality, by health condition, with and without co‐morbidity
| Prevalence in 2002–03 | Aged 55–69 | Aged 70–84 | |||
|---|---|---|---|---|---|
| HRS | ELSA | HRS | ELSA | ||
| Stroke | Only | 8.0 | 10.3 | 8.0*** | 18.2** |
| + co‐morbidity | 13.7 | 14.7 | 38.0 | 29.9 | |
| Diabetes | Only | 5.2*** | 5.7* | 16.3*** | 10.4*** |
| + co‐morbidity | 11.8 | 11.3 | 33.6 | 26.9 | |
| Lung disease | Only | 5.5** | 4.0*** | 13.8*** | 17.9** |
| + co‐morbidity | 10.7 | 18.4 | 35.6 | 31.1 | |
| Cancer | Only | 7.2** | 9.8** | 15.3*** | 17.7*** |
| + co‐morbidity | 12.2 | 18.8 | 30.1 | 32.4 | |
| Moderate disability | Only | 4.4*** | 5.6*** | 18.1*** | 15.9*** |
| + co‐morbidity | 12.2 | 15.7 | 35.1 | 29.3 | |
| Severe disability | Only | 7.8*** | 8.7*** | 28.7*** | 18.6*** |
| + co‐morbidity | 16.5 | 18.5 | 45.8 | 33.8 | |
Note: ELSA sample is weighted. ‘Moderate disability’ is defined as reporting four or more limitations (out of 22). ‘Severe disability’ is defined using an alternative threshold of eight limitations. ***, ** and * indicate significant difference between ‘only’ and ‘+ co‐morbidity’ at 1 per cent, 5 per cent and 10 per cent level, according to ‘t’ tests.
IV. Survival models
So far, we have presented sample averages of our measures of health status and mortality. In this section, we summarise results from estimated models that allow us to identify associations between survival, prevalence and personal attributes. We estimate a set of ordinary least squares (OLS) models, where the dependent variable is exact four‐year survival, i.e. whether or not the individual survived to four years exactly after the (lifetime) prevalence of the condition was recorded. We first estimate a basic model, with independent variables including interacted sex/age indicators, education (a low education indicator variable) and income tercile (indicator variables for low and middle income, with high income tercile the omitted group). We then estimate expanded models sequentially adding more controls, including indicators of previous diagnoses (as shown in previous sections of this paper) and moderate disability indicator, marital status, work status and lifestyle indicators (obesity, smoking, exercise, drinking). The third model then adds an indicator of co‐morbidity with an interaction of co‐morbidity with being aged 75 and over. We show the full results in Table B.3 in the online appendix but summarise key results here in the text and in Table 8.
Table 8.
OLS regression coefficients
| Dependent variable: four‐year survival | |||
|---|---|---|---|
| Basic (1) | + priors, behaviours and work (2) | + co‐morbidity and interactions (3) | |
| HRS | |||
| Low education | –0.87 | 0.34 | 0.37 |
| (0.57) | (0.56) | (0.55) | |
| Bottom household income tercile | –6.57*** | –2.03*** | –2.22*** |
| (0.71) | (0.73) | (0.73) | |
| Middle household income tercile | –2.31*** | –0.32 | –0.48 |
| (0.67) | (0.66) | (0.66) | |
| ELSA | |||
| Low education | –2.05*** | –0.97 | –1.00* |
| (0.61) | (0.60) | (0.60) | |
| Bottom household income tercile | –3.50*** | 0.15 | 0.06 |
| (0.80) | (0.80) | (0.80) | |
| Middle household income tercile | –1.53** | 0.16 | 0.11 |
| (0.75) | (0.74) | (0.74) | |
Note: For the full results, see Table B.3 in the online appendix. Model (1) also controls for being male, in five‐year age groups (excluded group is aged 55–59) and five‐year‐age‐group–male interaction terms. Model (2) also controls for prior diagnoses of cancer, diabetes, lung disease, stroke, moderate disability and heart attack (the last for ELSA only), marital status (baseline is married or cohabiting), obesity indicators, smoking status, exercise and drinking habits, and current work status. Model (3) includes an indicator for co‐morbidity and the interaction between co‐morbidity and being aged 75 or over. Sample is core respondents aged between 55 and 84. Standard errors are shown in parentheses. ***, ** and * indicate significant at 1 per cent, 5 per cent and 10 per cent level.
In the most basic model (1) – i.e. controlling for sex and age (in five‐year age bands) – low education is significantly (negatively) associated with survival only in England. The probability of survival is highest in the highest income tercile, but this effect largely disappears in England and is much diminished in the US when the other controls are added to the model. Model (2) shows the estimation results if we include indicators of prior health conditions, and behaviours such as smoking, drinking, obesity and exercise. Including these characteristics attenuates the relationship between education and survival. The relationship between income and survival in the US is much weaker than in the basic model, which suggests that the covariates account for much of the pathway between income and survival. Accounting for these covariates in England, income tercile does not have a statistically significant relationship with survival. Model (3) shows the results from the full model, including an indicator for co‐morbidity, which is also interacted with an indicator for being aged 75 or over. Co‐morbidity especially after age 74 has a very negative association with survival in both countries. The income effect persists in the US and remains non‐existent in England.
Elsewhere in this special issue, a negative relationship between income and health costs has been found. The results here would imply that low‐income individuals in the US have worse survival chances than high‐income individuals, beyond what can be explained by their higher prevalence of disease or higher propensity to engage in risky health behaviours. This could be indicative of greater need (perhaps the low‐educated have more extreme versions of the characteristics controlled for) or worse access to health care conditional on need.
In England, differences in survival between education and income groups are operating through their associations with other observable factors. The association between education or income and survival is explained by the relationship between the former and other factors (which may be proxying for lifetime resources). But low education or income in and of itself does not seem to be associated with lower survival and therefore greater need for health care.
Table 9 summarises results from the model with the full set of controls. Columns (a) and (b) show the results for the full HRS/ELSA samples, whereas columns (c)–(f) show the model estimated using samples split by education group, i.e. fully interacted with education. Even when including the complete set of controls described above, there are strong and large negative associations between health conditions and survival. The major exception is diabetes in England, where there is no discernible relationship between diabetes and survival. However, when we estimate models for eight‐year survival (not shown), there is the expected negative and statistically significant relationship between diabetes and survival.
Table 9.
OLS regression coefficients with full set of controls
| Dependent variable: four‐year survival | ||||||
|---|---|---|---|---|---|---|
| HRS | ELSA | HRS | HRS | ELSA | ELSA | |
| (a) Full sample | (b) Full sample | (c) Low education | (d) High education | (e) Low education | (f) High education | |
| Prior diagnosis of cancer | –3.68*** | –7.41*** | –4.53*** | –3.07*** | –10.21*** | –3.80** |
| (0.81) | (1.18) | (1.16) | (1.12) | (1.70) | (1.57) | |
| Prior diagnosis of diabetes | –5.02*** | –0.46 | –5.29*** | –4.69*** | –0.82 | 0.04 |
| (0.84) | (1.16) | (1.14) | (1.23) | (1.59) | (1.65) | |
| Prior diagnosis of lung disease | –3.76*** | –5.37*** | –3.28** | –4.62*** | –5.60*** | –5.24** |
| (0.96) | (1.32) | (1.27) | (1.49) | (1.71) | (2.11) | |
| Prior diagnosis of heart attack | –0.36 | –0.03 | –0.70 | |||
| (1.27) | (1.69) | (1.94) | ||||
| Prior diagnosis of stroke | –6.07*** | –3.38** | –6.28*** | –5.52*** | –5.00*** | –0.26 |
| (1.15) | (1.44) | (1.51) | (1.81) | (1.85) | (2.38) | |
| Moderate disability prior | –6.43*** | –2.93*** | –7.01*** | –5.48*** | –3.73*** | –1.44 |
| (0.74) | (0.80) | (0.98) | (1.14) | (1.08) | (1.18) | |
| Co‐morbid | 1.23 | –2.64* | 0.74 | 1.91 | –0.98 | –6.41*** |
| (1.25) | (1.51) | (1.68) | (1.89) | (1.99) | (2.33) | |
| Co‐morbid and 75 or over | –15.17*** | –8.75*** | –14.24*** | –15.60*** | –11.67*** | –1.40 |
| (1.29) | (1.68) | (1.67) | (2.07) | (2.18) | (2.68) | |
Note: For the full results, see Tables B.3–B.5 in the online appendix. Models (a) and (b) include (uninteracted) controls for education. ‘Moderate disability’ is defined as reporting four or more limitations (out of 22). Sample is core respondents aged between 55 and 84. Standard errors are shown in parentheses. ***, ** and * indicate significant at 1 per cent, 5 per cent and 10 per cent level.
Comparing columns (a) and (b) in Table 9, there are differences between the countries in terms of the negative associations between health conditions and survival. Cancer has a larger negative coefficient in England than in the US, and the reverse is true for disability. Comparing columns (c) and (d), there do not appear to be large differences between the high‐ and low‐educated in the US in terms of the relationship between disease prevalence and survival. However, a comparison of columns (e) and (f) reveals larger differences between education groups in England. Cancer in England is associated with a reduction in four‐year survival chance that is almost three times larger for the low‐educated than for the high‐educated. For stroke and disability, the high‐educated in England experience no direct reduction in their survival chances, whereas the low‐educated do.
The final two covariates shown in Table 9 are indicators for co‐morbidity on its own and also interacted with an indicator for being aged 75–84. The statistically significant coefficients imply that there is some negative association between being co‐morbid and survival, beyond the direct effects of the individual conditions. In the US, co‐morbidity matters much more for the population aged 75–84 and there does not seem to be a difference between education groups. In ELSA, co‐morbidity matters much more for the older age group in the low‐educated group. For the high‐educated, co‐morbidity matters irrespective of age. In Section III..2, we showed that co‐morbidity is higher in the US than in England, though it is higher in England relative to each disease's lower underlying prevalence. These results show that not only is co‐morbidity higher in the US but also it has a worse effect on survival. These findings do not depend on education level. In England, co‐morbidity matters differently for different education groups.
The co‐morbid elderly are probably particularly expensive to treat, and there are proportionately more of them in the US than in England. But the implications for health care costs are ambiguous, as their lower chances of survival mean that they might not live for as long in that ‘expensive’ state.
One final interesting result, shown in Tables B.4 and B.5 in the online appendix, is the relationship between disability and survival estimated on subsamples split by marital status. In both countries, disability is associated with a larger reduction in survival chances for single people than for those in couples, which could be associated with the quality of care (or the amount of informal care) available to individuals in those circumstances. An interesting avenue for future research might be to explore the mechanisms underlying this result.
V. Out‐of‐pocket medical expenditures and health status
In this section, we discuss the implications of our various measures of health status for out‐of‐pocket (OOP) medical expenditures. Onsets of illnesses could be a major driver of the amount that individuals have to pay for their medical care. If so, these OOP costs should also depend on the nature of the disease onset. The severity of the illness could also contribute to the distribution of OOP costs in the population.
Because of the universal National Health Service, OOP medical expenses are trivial in England, and not surprisingly there are no data on out‐of‐pocket expenses in ELSA. To illustrate, for those aged 45 and above, OOP medical expenses as a fraction of after‐tax income are about 3 per cent in England compared with about 15 per cent in the US.11 Thus there is really no need to have OOP data in England since, in this aspect of health, the story is really a US story and the US data alone summarise the salient differences between the countries.
Table 10 provides a detailed description of distributional patterns of OOP over a two‐year period for those who were at least 60 years old, as derived from the 2002 wave of HRS. The HRS asks each respondent how much they spent on OOP medical costs over the last two years and what the nature of those medical expenditures was. The first three rows in Table 10 describe the distribution of those expenditures for three samples: (1) the combined full sample of those 2002 HRS respondents who had survived since 2000 and those who had died between the 2000 and 2002 samples; (2) the subsample who died between 2000 and 2002; and (3) the (complementary) subsample of survivors.
Table 10.
Two‐year out‐of‐pocket individual medical expenditures
| Dollars | ||||||
|---|---|---|---|---|---|---|
| Mean | P25 | Median | P75 | P90 | P95 | |
| Total survivors and non‐survivors | 6,175 | 664 | 2,940 | 7,239 | 12,980 | 18,904 |
| Total non‐survivors | 9,898 | 480 | 3,460 | 9,960 | 22,188 | 49,860 |
| Total survivors | 5,842 | 684 | 2,894 | 7,080 | 12,488 | 17,760 |
| Total no insurance | 3,761 | 315 | 1,220 | 3,262 | 7,272 | 12,070 |
| Survivors only | ||||||
| Hospital | 299 | 0 | 0 | 0 | 0 | 750 |
| Nursing home stays | 405 | 0 | 0 | 0 | 0 | 0 |
| Outpatient | 67 | 0 | 0 | 0 | 0 | 140 |
| Doctor visits | 398 | 0 | 25 | 200 | 700 | 2,000 |
| Dental | 422 | 0 | 13 | 400 | 1,200 | 2,000 |
| Prescription drugs | 2,131 | 0 | 480 | 1,680 | 4,800 | 7,200 |
| Home health care | 26 | 0 | 0 | 0 | 0 | 0 |
| Special health facility | 10 | 0 | 0 | 0 | 0 | 0 |
| Medicare HMO | 264 | 0 | 0 | 0 | 1,080 | 1,584 |
| Private insurance | 1,721 | 0 | 0 | 2,640 | 5,568 | 7,800 |
| Long‐term care insurance | 360 | 0 | 0 | 0 | 0 | 3,000 |
Note: Sample is respondents aged 60 and over in the 2002 wave of HRS. Expenditures are for the period 2000–02. P25 is the 25th percentile, P75 is the 75th percentile and so on. An HMO is a health maintenance organisation.
For the combined sample of survivors and those who died, mean OOP medical expenses over a two‐year period were $6,175 but the distribution of expenditures was very uneven across this population. Median OOP medical expenses were less than $3,000, but one in every 20 respondents spent almost $20,000 or more over these two years.
As the second and third rows of Table 10 illustrate, the level and distribution of medical expenses were very different for survivors and for those who died between 2000 and 2002. Mean medical expenses for non‐survivors were about $10,000, almost twice the level observed for survivors over the same period. This comparison is especially stark at the upper end of the OOP medical expenses distribution. At the 95th percentile of their respective distributions, survivors spent about $18,000 while non‐survivors spent almost $50,000. However, the impact of these very high expenses associated with death on the full sample of survivors and non‐survivors is relatively modest. For the combined sample, medical expenses at the 95th percentile are a bit below $19,000, much less than observed in the non‐survivor sample.
In addition to data on total OOP medical expenses for these groups, Table 10 shows for the survivor sample the component categories of medical expenses that make up the totals. By far the two biggest categories are prescription drug expenditures and payments for private insurance. These two categories alone comprise almost three‐quarters of all OOP medical expenses. This concentration into these two categories does differ within some subsamples. For example, for the non‐survivor sample, nursing home expenses accounted for 37 per cent of all OOP expenses. The distribution of medical expenses in all the categories in Table 10 is very heterogeneous, with median expenditures across respondents being zero in most categories.
Table 11 provides the same type of information on OOP medical expenditure as does Table 10 but now for a full six‐year period using the 2002, 2004 and 2006 waves of HRS. These data are for the sample of HRS who survived over the six‐year period between 2000 and 2006. This table shows that while six‐year OOP expenditures are relatively modest for the average person, some older Americans are at risk of quite high expenses indeed. Ten per cent of HRS respondents spent at least $37,000 on medical care and 5 per cent spent at least $51,000.
Table 11.
Six‐year out‐of‐pocket individual medical expenditures for survivors
| Dollars | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mean | P25 | Median | P75 | P90 | P95 | Persistence: | ||
| 2004/2002 | 2006/2002 | |||||||
| Total | 18,460 | 4,564 | 12,330 | 23,198 | 37,284 | 51,031 | 0.58 | 0.51 |
| Total no insurance | 11,915 | 2,280 | 5,724 | 12,480 | 23,919 | 36,840 | 0.53 | 0.45 |
| Hospital | 779 | 0 | 0 | 0 | 1,000 | 3,000 | 0.20 | 0.13 |
| Nursing home stays | 1,490 | 0 | 0 | 0 | 0 | 300 | 0.44 | 0.38 |
| Outpatient | 345 | 0 | 0 | 0 | 0 | 0 | 0.16 | 0.10 |
| Doctor visits | 1,084 | 0 | 250 | 850 | 2,600 | 5,000 | 0.44 | 0.38 |
| Dental | 1,457 | 0 | 500 | 1,705 | 4,000 | 6,000 | 0.59 | 0.53 |
| Prescription drugs | 5,899 | 648 | 2,472 | 6,000 | 12,224 | 18,960 | 0.58 | 0.27 |
| Home health care | 108 | 0 | 0 | 0 | 0 | 0 | 0.07 | 0.11 |
| Special health facility | 56 | 0 | 0 | 0 | 0 | 70 | 0.15 | 0.16 |
| Medicare HMO | 638 | 0 | 0 | 0 | 2,088 | 4,080 | 0.28 | 0.21 |
| Private insurance | 5,295 | 0 | 1,968 | 8,424 | 15,336 | 20,016 | 0.54 | 0.45 |
| Long‐term care insurance | 1,250 | 0 | 0 | 0 | 4,000 | 9,200 | 0.72 | 0.71 |
Note: Sample is respondents aged 60 and over in the 2002, 2004 and 2006 waves of HRS. Expenditures are for the period 2000–06. P25 is the 25th percentile, P75 is the 75th percentile and so on. An HMO is a health maintenance organisation.
The final two columns in Table 11 provide a metric of the association of medical costs over time for these HRS respondents. The coefficients in these two columns represent the estimated coefficient of 2004 wave OOP medical costs on 2002 wave OOP medical costs and the estimated coefficient of 2006 wave OOP medical costs on 2002 wave OOP medical costs. Equivalently, these are autoregressive coefficients from an AR(1) regression. OOP medical costs are significantly correlated over time, implying a significant risk of high levels of OOP medical costs for older Americans – a financial risk that residents of the UK largely do not face.12
We also stratified the results shown in Table 11 into three groups defined by whether the respondent was eligible for Medicare or not.13 While we do not show the detailed results here, they can be easily summarised. Fifty‐eight per cent of our sample were Medicare eligible in all six years, 10 per cent were never Medicare eligible and the remaining 32 per cent were Medicare eligible in some but not all years. The distributions of OOP medical expenses in the three groups are remarkably close. For example, median medical expenses are $12,476 for those Medicare eligible in all years compared with $13,012 for those never Medicare eligible over this six‐year period.
Not surprisingly, the distributions differ more in some of the categories that make up total OOP expenses. The most salient difference appears in the insurance categories. Total mean expenses on insurance (the last three categories in Table 11) are $8,616 for those who were never Medicare eligible compared with $6,919 for those always Medicare eligible. This difference is even starker in the tails. To illustrate, private insurance expenses at the 90th percentile are about $22,000 for the never‐Medicare‐eligible group compared with about $14,000 for the always‐Medicare‐eligible group. The principal offsets to this are higher nursing home stay costs and drug expenses in the always‐Medicare‐eligible group, which are primarily due to age.
Table 12 illustrates the relationship between the prevalence of our disease measures and OOP medical costs. For diabetes and lung disease, having the disease has little impact on OOP medical costs except at the very upper tail of the distribution. The other two diseases included in Table 12 – stroke and cancer – do show some effect of having those diseases on OOP medical costs, although the impact on costs for the relevant median respondent is quite modest. The cost impact once again is borne by relatively few people with these diseases, but for them the cost implications can be substantial.
Table 12.
Six‐year out‐of‐pocket individual medical expenditures
| Dollars | ||||||
|---|---|---|---|---|---|---|
| Mean | P25 | Median | P75 | P90 | P95 | |
| Diabetes | ||||||
| Has diabetes | 18,883 | 4,548 | 12,713 | 23,833 | 37,961 | 52,939 |
| No diabetes | 18,369 | 4,566 | 12,244 | 23,120 | 37,178 | 50,841 |
| Only diabetes | 17,269 | 4,417 | 12,700 | 21,500 | 34,469 | 44,438 |
| Has diabetes and another diseasea | 18,348 | 5,020 | 11,790 | 23,801 | 38,112 | 46,028 |
| Lung disease | ||||||
| Has lung disease | 19,145 | 4,345 | 12,356 | 23,882 | 37,961 | 52,556 |
| No lung disease | 18,386 | 4,588 | 12,330 | 23,132 | 37,178 | 50,841 |
| Only lung disease | 16,919 | 3,984 | 12,416 | 23,520 | 35,505 | 48,704 |
| Has lung disease and another diseasea | 20,563 | 4,548 | 11,965 | 23,522 | 38,512 | 57,569 |
| Stroke | ||||||
| Has stroke | 26,132 | 4,250 | 12,880 | 26,992 | 53,084 | 86,100 |
| No stroke | 17,902 | 4,590 | 12,310 | 23,024 | 36,680 | 49,165 |
| Only stroke | 27,005 | 3,380 | 12,072 | 24,890 | 41,420 | 84,784 |
| Has stroke and another diseasea | 27,670 | 4,794 | 15,060 | 31,466 | 82,542 | 106,841 |
| Cancer | ||||||
| Has cancer | 20,709 | 5,719 | 13,729 | 25,248 | 40,179 | 57,975 |
| No cancer | 18,083 | 4,400 | 13,081 | 22,842 | 36,800 | 50,841 |
| Only cancer | 17,348 | 5,652 | 12,416 | 23,302 | 36,675 | 47,584 |
| Has cancer and another diseasea | 23,408 | 5,800 | 14,940 | 26,500 | 45,421 | 84,942 |
| Estimates of additional six‐year out‐of‐pocket medical expenditures due to disease prevalence and co‐morbidity | ||||||
|---|---|---|---|---|---|---|
| Mean | Quantile 25 | Quantile 50 | Quantile 75 | Quantile 90 | Quantile 95 | |
| Diabetes only | –1,172 | –89 | 434 | –1,663 | –2,728 | –6,612 |
| (–0.71) | (–0.14) | (0.47) | (–1.11) | (–1.09) | (–1.05) | |
| Diabetes, co‐morbid | –4,630 | 50 | –1,228 | 832 | –5,203 | –20,992 |
| (–1.42) | (0.04) | (–0.66) | (0.28) | (–1.04) | (–1.68) | |
| Lung disease only | –1,522 | –479 | 210 | 285 | –1,842 | –2,346 |
| (–0.82) | (–0.68) | (0.20) | (0.17) | (–0.65) | (–0.33) | |
| Lung disease, co‐morbid | –1,501 | 375 | 652 | –2,612 | –1,907 | 4,092 |
| (–0.47) | (0.31) | (0.36) | (–0.90) | (–0.39) | (0.34) | |
| Stroke only | 8,564 | –1,126 | –194 | 1,727 | 4,073 | 33,734 |
| (2.82) | (–0.97) | (–0.11) | (0.63) | (0.88) | (2.91) | |
| Stroke, co‐morbid | 8,716 | –596 | 1,802 | 8,445 | 40,362 | 48,249 |
| (2.17) | (–0.39) | (0.79) | (2.31) | (6.58) | (3.15) | |
| Cancer only | –1,093 | 1,164 | 568 | 82 | –672 | –3,466 |
| (–0.78) | (2.17) | (0.72) | (0.06) | (–0.31) | (–0.65) | |
| Cancer, co‐morbid | 5,968 | 1,289 | 2,022 | 2,690 | 8,667 | 33,226 |
| (1.88) | (1.06) | (1.12) | (0.93) | (1.78) | (2.73) | |
| Constant | 18,441 | 4,506 | 12,266 | 23,163 | 37,347 | 51,050 |
| (59.63) | (38.02) | (70.05) | (82.40) | (79.04) | (43.19) | |
Not counting hypertension.
Note: Sample is respondents aged 60 and over in the 2002, 2004 and 2006 waves of HRS. Expenditures are for the period 2000–06. P25 is the 25th percentile, P75 is the 75th percentile and so on.(Continued)
Note: Sample is respondents aged 60 and over in the 2002, 2004 and 2006 waves of HRS. t‐statistics are shown in parentheses.
To illustrate, the OOP costs of having had a stroke compared with not having had a stroke previously over a six‐year period are essentially zero at the median and only about $4,000 at the 75th percentile. If instead we examine the 90th and 95th percentiles, the extra OOP medical costs of having had a stroke would be about $16,000 and $37,000 respectively. A similar if less pronounced pattern exists for cancer. Most past cancer victims do not experience any additional OOP medical costs, but a few of them, presumably with the more complicated long‐term effects of the disease, do experience some extra costs; but even these costs, since they are measured over a six‐year period, are relatively modest.
The upper panel of Table 12 also demonstrates that another factor affecting OOP costs is co‐morbidities, especially at the extremes of the distributions. For all diseases in the table except diabetes, there are significant additional costs at the 95th percentile. These additional costs are particularly high for those who have had a stroke, where being co‐morbid results in more than $40,000 of additional OOP costs at the 90th percentile.
The lower panel of Table 12 shows regression coefficient estimates and associated t‐statistics on six‐year OOP medical expenses associated with having four diseases – diabetes, lung disease, stroke and cancer. For each disease, we have two variables, one indicating that the respondent only has that disease and the other indicating that the respondent also has another disease not counting hypertension. We estimate a number of models, summarised in Table 12 – an OLS (the mean) and quantile regressions at the 25th, 50th (median), 75th, 90th and 95th quantiles.
OOP medical expenses in all models are dominated by the constant term, indicating once again that the disease prevalence is not driving OOP expenses. There are no statistically significant effects on OOP expenses for diabetes or lung disease, whether co‐morbid or not, at any quantile. In contrast, having had a stroke, especially if one is co‐morbid, has a statistically significant effect but largely at the higher quantiles. Cancer effects are also concentrated at the higher quantiles but they exist only for co‐morbid diseases.
VI. Conclusions
This paper examines the issue of health status differences between England and the United States, with an emphasis on the implications of those differences for health care cost differences between the two countries. We first document health status differences in disease prevalence, disability, mortality and co‐morbidity. Confirming previous findings,14 we find higher disease prevalence in the US than in England but much smaller differences between the two countries in disability and mortality. We attribute the smaller differences in disability to the subjectivity of the questions on experiencing disabilities. Compared with the English, Americans place a much higher subjective threshold on being disabled, so we attribute the much more similar levels of disabilities between the two countries to these disability threshold differences. The more common mortality differences are most likely due to a combination of earlier disease diagnosis and more effective disease treatment in the US. Co‐morbidity is a very common and important dimension of disease in both countries that is often neglected in scientific papers, especially by economists.
We find, however, that disease prevalence has little implication for out‐of‐pocket health care costs in the US except for relatively few individuals with a particular disease. Instead, costs are more associated with incidence than prevalence and with those who are going to die in the next year or two. Co‐morbidity is also associated with higher OOP costs, but even this association is limited to a relatively small fraction of people who are co‐morbid.
Supporting information
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Appendix A: Descriptive tables
Appendix B: Full results
Submitted July 2015.
The research was funded by grants from the National Institute on Aging (R37‐AG025529 and P01‐AG022481). Banks and Keynes are grateful to the Economic and Social Research Council for co‐funding through the ESRC Centre for the Microeconomic Analysis of Public Policy at IFS (grant reference ES/M010147/1). Since completing this work, Keynes has left IFS to join The Economist.
Footnotes
Banks and Smith, 2012.
There is one difference between the surveys, which is that in England respondents are shown a card with a list of conditions, whereas in the US respondents are asked individually about each of the conditions listed. Banks, Muriel and Smith (2010) showed evidence that this led to under‐reporting in England of more minor conditions such as hypertension, though we have tried to correct for this by incorporating information using the earlier Health Survey for England.
Banks et al., 2006.
Banks et al., 2008.
Government Actuary's Department.
Since much of this special issue looks at health care costs by income group rather than by education, a similar analysis is presented with groups split by quintile of household income, for the ELSA sample only, in Tables A.2–A.4 in the online appendix.
Banks et al., 2006.
As claimed in Cieza et al. (2015).
Banks et al., 2008.
Banks et al., 2015.
Banks et al., 2015.
Medicare is a public health insurance programme in the US covering people older than 65 as well as disabled young people.
Banks et al., 2006.
Contributor Information
James Banks, Email: james_b@ifs.org.uk.
Soumaya Keynes, Email: soumaya_k@ifs.org.uk.
James P. Smith, Email: smith@rand.org
References
- Banks, J. , Blundell, R. , Levell, P. and Smith, J. P. (2015), ‘Life‐cycle consumption patterns at older ages in the US and the UK: can medical expenditures explain the difference?’, Institute for Fiscal Studies (IFS), Working Paper no. WP15/12.
- Banks, J. , Kapteyn, A. , Smith, J. P. and Van Soest, A. (2008), ‘Work disability is a pain in the ****, especially in England, the Netherlands, and the United States’, in Cutler D. and Wise D. (eds), Health in Older Ages: The Causes and Consequences of Declining Disability among the Elderly, Chicago, IL: University of Chicago Press. [Google Scholar]
- Banks, J. , Marmot, M. , Oldfield, Z. and Smith, J. P. (2006), ‘Disease and disadvantage in the United States and in England’, Journal of the American Medical Association, vol. 295, pp. 2037–45. [DOI] [PubMed] [Google Scholar]
- Banks, J. , Muriel, A. and Smith, J. P. (2010), ‘Disease prevalence, disease incidence, and mortality in the United States and in England’, Demography, vol. 47, pp. S211–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks, J. and Smith, J. P. (2012), ‘International comparisons in health economics: evidence from aging studies’, Annual Reviews in Economics, vol. 4, pp. 57–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieza, A. , Oberhauser, C. , Bickenbach, J. , Jones, R. , Ustun, T. , Kostanjsek, N. , Morris, J. N. and Chatterji, S. (2015), ‘The English are healthier than the Americans: really?’, International Journal of Epidemiology, vol. 44, pp. 229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J. P. (1999), ‘Healthy bodies and thick wallets: the dual relation between health and economic status’, Journal of Economic Perspectives, vol. 13, no. 2, pp. 144–66. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Appendix A: Descriptive tables
Appendix B: Full results


