Abstract
Stress-associated disorders, including depression and anxiety, impact nearly 20% of individuals in the United States. The social, health, and economic burden imposed by stress-associated disorders requires in depth research efforts to identify suitable treatment strategies. Traditional medications (e.g., selective serotonin reuptake inhibitors, monoamine oxidase inhibitors) have significant limitations, notably a time lag for therapeutic response that is compounded by low rates of efficacy. Excitement over ketamine, a rapid acting antidepressant effective in treatment resistant patients, is tempered by transient dissociative and psychotomimetic effects, as well as abuse potential. Rodent stress models are commonly used to produce behavioral abnormalities that resemble those observed in stress-associated disorders. Stress models also produce molecular and cellular morphological changes in stress sensitive brain regions, including the prefrontal cortex and hippocampus that resemble alterations observed in depression. Rapid acting antidepressants such as ketamine can rescue stress-associated morphological and behavioral changes in rodent models. Here, we review the literature supporting a role for rapid acting antidepressants in opposing the effects of stress, and summarize research efforts seeking to elucidate the molecular, cellular, and circuit level targets of these agents.
Keywords: frontal cortex, ketamine, scopolamine, synapse, mTOR
Introduction
Depression is a devastating psychiatric disorder afflicting approximately 17% of individuals in the United States.1 Anxiety is commonly comorbid with depression and together these two illnesses represent approximately 50% of the disease burden associated with combined mental health and substance abuse disorders with burden peaking in early adulthood.2 Current medication options for depressed patients are limited by low response rates and delayed therapeutic effects.3,4 This is particularly concerning given that suicide risk is elevated in depressed individuals.3 The Centers for Disease Control and Prevention details a 25% increase in the suicide rate over the past 15 years and provides data suggesting that suicide is the second leading cause of death in early adulthood. Together, these points underscore why development of novel, more efficacious, and faster acting antidepressant treatments remains a significant unmet need. It is therefore not surprising that reports indicating that ketamine produces an antidepressant effect with rapid onset5 even in treatment resistant individuals6 has had a strong influence on the field of depression and spurred an abundance of preclinical mechanistic research.
Depression is a complex disorder associated with dysfunction in peripheral systems, as well as alterations to central nervous system structure and function. Numerous brain regions are affected by depression including the hippocampus and prefrontal cortex (PFC). Physiological changes in the PFC may be particularly important as this region is integral to top down regulation of behavior and control of stress reactivity.7 Clinical research has regularly noted volume loss in the PFC, as well as hippocampus of depressed individuals.8 Consistent with this, reduced neuron number and size,9,10 loss of synaptic contacts,11 changes in expression of synaptic proteins,11,12 and reductions in trophic factor expression13 are observed in postmortem analysis of tissue from depressed individuals.
Stress exposure is a risk factor for depression,14,15 and chronic stress is a common experimental model utilized to produce depression-like physiological and behavioral changes in animal models. These changes include altered neuronal structure and loss of trophic factor support.16 Conversely, there is evidence that a critical component of the therapeutic action of rapid acting antidepressants includes the induction of trophic factor signaling and subsequent synaptogenesis in the PFC.17,18 With ketamine, an NMDA receptor antagonist, these effects occur following a transient increase in glutamate neurotransmission within the PFC.19 The necessity for increased glutamate transmission and synaptic response has also been shown for another rapid acting antidepressant, scopolamine, an acetylcholine muscarinic (AChM) receptor antagonist.20
The present review will detail and discuss the efficacy of rapid acting antidepressants in stress paradigms typically used to model depression in the laboratory. Following this, the focus will shift to a review of the molecular and cellular underpinnings of the rapid and sustained antidepressant effects observed in rodents after ketamine administration. A greater understanding of the biological response to rapid acting antidepressants will aid in developing new, safer, therapies for individuals suffering from depression and other stress-associated disorders.
Rodent Stress Models and Rapid Acting Antidepressants
A large body of evidence demonstrates that repeated stress exposure causes dysfunction of various cellular processes in stress-related brain circuits, contributing to abnormal behavioral outcomes.21 Various types of stress in rodents have relevance to psychiatric disorders. Though variability exists based on the stress paradigm implemented, broadly speaking, stress exposure produces features of depression including, anhedonia, behavioral despair, increased anxiety, loss of weight, disrupted cognition, and aberrant social behavior. The ability of rapid acting antidepressants to reverse the effects of stress exposure, and even produce prophylactic effects in certain stress models, provides reverse-translational support for initial clinical results with ketamine. Importantly, these approaches also provide multiple models for extending our understanding of the biological response to rapid acting antidepressants and could potentially shed light on the pathophysiology of depression in humans.
Rapid Acting Antidepressant Efficacy in Chronic Unpredictable Stress (CUS) Models
Variants of the CUS exposure model are commonly used to produce a depression-like phenotype in rodents. The CUS paradigm consists of three weeks or more of exposure to a mix of stressors presented in a random fashion to prevent habituation that may occur upon repeated exposure to the same stressor. CUS exposure produces core behavioral symptoms of depression, notably anhedonia (i.e., reduced preference for a sweetened solution), and anxiety (i.e., in a novelty suppressed feeding test). Application of typical antidepressants in these behavioral models is associated with improvement but only after long-term (∼3 weeks) treatment.22–24 In contrast, rapid acting antidepressants demonstrate behavioral improvement after a single dose. Ketamine or RO25-6981, an NR2B selective antagonist, reverse the anhedonia and anxiety caused by CUS exposure; this includes reversal of the deficit in sucrose preference and increased latency to feed in the novelty suppressed feeding test.25 GLYX-13, currently described as a partial NMDA receptor glycine site agonist, also rapidly blocks stress-associated changes in the sucrose preference and novelty suppressed feeding test, and also facilitates extinction of contextual fear which is typically prolonged by stress.26 Finally, a single dose of scopolamine partially restores sucrose preference after CUS, and completely rescued the CUS-associated deficit after administration of three doses, a regimen typically utilized when treating depressed patients.27 The effects of rapid-acting antidepressants may also be long lasting, remaining up to 14 days after treatment in the case of ketamine.28 Beyond demonstrating rapid efficacy, these results suggest that the broad class of rapid acting antidepressants described may produce sustained effects through similar mechanisms as the behavioral profile after treatment shows broad overlap. A single dose of ketamine or RO25-6981 reverses the synaptic deficit in the PFC caused by chronic stress (Li et al., 2011). Therefore, synaptic remodeling may present a convergent mechanism that underlies the persistent behavioral changes produced by ketamine and other rapid acting antidepressants.
Synaptic Remodeling by Stress and Antidepressant Treatment
Alteration of synapse number and function is a hallmark of chronic stress exposure in rodents that may underlie the volume loss observed in depression. Atrophy of the dendritic field of the CA3 region of the hippocampus was initially described by McEwen and coworkers,29–31 where three weeks of restraint stress exposure produced a reduction in branching of the apical dendritic arbor of CA3 pyramidal neurons. Further work extended our understanding of neuroanatomical modeling by chronic restraint stress demonstrating dendrite atrophy and spine loss of pyramidal neurons in the PFC.32–35 In contrast, increased dendrite complexity of pyramidal neurons is observed in the amygdala and bed nucleus of the stria terminalis regions critical for fear and anxiety-like behaviors and regulation of the stress response.36–38 Other stress paradigms, such as CUS are also capable of producing similar changes in neuronal morphology.25,29,37 Moreover, rapid acting antidepressant administration is associated with reversal of stress-associated changes in neuronal morphology, and increased levels of synaptic proteins including PSD-95, synapsin-1, and GluA1, an α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptor subunit important in maintaining synaptic integrity. Importantly, these changes occur after a single treatment. In contrast, typical antidepressants that are capable of reversing or protecting against stress-associated neuronal remodeling require long-term (2–3 weeks) treatment.23,24,39 Thus restoration, or maintenance, of synaptic integrity appears to be critical for the behavioral response to both rapid and typical antidepressant treatments. The proposed mechanisms underlying these effects are discussed further below.
Other Models: Social Defeat Stress (SDS), Learned Helplessness, and Chronic Corticosterone
In the SDS model, subjects are repeatedly exposed to aggressive intruder males, resulting in a phenotype of reduced social interaction time in a susceptible subset of the stressed animals.40 Susceptible animals also demonstrate other depression-like behaviors, including decreased sucrose preference and increased anxiety, and these behaviors are reversed following a single dose of ketamine or GLYX-13.41,42 In a compelling study, Bagot et al.40 identified a subset of SDS susceptible animals that show a response to ketamine administration with an increase in social interaction time. RNA sequencing after treatment revealed a diverse array of effects in multiple limbic regions. Notably, a large proportion of the differentially expressed genes regulated by ketamine in the PFC were associated with genes that were differentially regulated between resilient and susceptible animals, suggesting that ketamine treatment produces a transcriptional profile that more closely resembles that of a resilient animal.
These findings are especially interesting given that recent reports demonstrate a prophylactic effect of ketamine exposure in other stress models. For instance, ketamine has been shown to reduce escape failures in the learned helplessness model of depression when given after uncontrollable stress,17,42,43 or when given up to two weeks before uncontrollable stress exposure.44,45 The prophylactic effects of ketamine were also shown in the SDS and chronic corticosterone administration models of depression.45 Taken together, the results demonstrate that rapid acting antidepressants effectively reverse the synaptic and behavioral deficits in rodent stress models, including social defeat. Studies are being conducted to further define the cellular mechanisms that promote stress recovery and resilience, as well as susceptibility.
Research on rapid acting antidepressants have been extended to metabolites of ketamine, demonstrating that (2R,6R)-hydroxynorketamine (HNK) is sufficient to produce the behavioral and synaptic antidepressant effects of ketamine.46 These studies demonstrate that ketamine is rapidly metabolized to (2R,6R)-HNK, that (2R,6R)-HNK itself is sufficient to produce antidepressant effects in a chronic social defeat test, as well as in the forced swim and learned helplessness tests, and that blockade of this metabolism can block the behavioral effects of ketamine. These studies also demonstrate that (2R,6R)-HNK increases glutamate AMPA receptor insertion and function in the hippocampus, providing a potential point of convergence with other rapid acting antidepressants that also increase synaptic integrity through insertion of AMPA GluA1 receptors.
Protein Synthesis is Critical for the Antidepressant Response to Rapid Acting Antidepressants
Chronic stress has consistently been shown to reduce dendritic arborization and spine number in the rodent PFC and hippocampus.25,33–35,47 Reversal of these changes within the PFC appears to be a convergent mechanism underlying the rapid and sustained response rapid acting antidepressants including ketamine,17,25,48 GLYX-13,49 and scopolamine.50 There are numerous lines of evidence demonstrating that spine formation following treatment with these rapid acting agents results in functional synapses that support restoration of synaptic inputs that are lost due to stress exposure. First, excitatory postsynaptic currents (EPSC) produced by both application of hypocretin/orexin or serotonin (5-HT) are reduced after stress25,47 and restored after ketamine administration.25 These changes are evident in stress naive animals as well.17,49,50 Second, stressed animals typically show suppression of long-term potentiation, a cellular model of synaptic learning and memory, and GLYX-13 is effective in ameliorating this deficit.26 Finally, in vivo imaging has demonstrated elevated spine generation following ketamine that leads to persistent spines in the dorsal frontal cortex that are indicative of functional synapses.48
Together, these findings indicate that rapid acting antidepressants effectively alter the ability of pyramidal cells in the PFC to receive afferent signals, thereby augmenting function in unstressed animals and even restoring function in stressed animals. The potential mechanisms behind these changes are discussed below. Included is a discussion of work conducted in the hippocampus that is likely relevant to PFC-associated changes as similar pathophysiological effects occur in each region with stress exposure.
Role of Mammalian Target of Rapamycin Complex 1 Signaling
The mammalian target of rapamycin complex 1 (mTORC1) pathway is a cellular integrator that regulates protein synthesis and thereby provides important control of cellular metabolism, growth, and survival.51 Activation of the mTORC1 pathway has been demonstrated to be a critical component of the response to rapid acting antidepressants.17,25,49 Rapamycin, a specific inhibitor of mTORC1 when administered acutely, has been shown to block the antidepressant effect of ketamine17 and GLYX-13.49 mTORC1 activation has multiple downstream effects, and its role in initiation of protein synthesis appears to be a necessary component for antidepressant activity. mTORC1 phosphorylates 4E-binding proteins (4E-BP) resulting in derepression of translation, and activates p70 ribosomal S6 protein kinases (p70S6K) resulting in direct stimulation of translation.51 Synaptoneurosome preparations collected 30 to 60 minutes after ketamine administration demonstrate increased phosphorylation of 4E-BP1 and p70S6K, both changes that would be expected to lead to increased protein translation.17 A similar effect is observed following administration of GLYX-1349 and RO25-6981.17 The induction of mTORC1 signaling by ketamine has been replicated by multiple laboratories, although there have also been negative reports (see Liu et al.49).
The synaptic proteins described above that are down-regulated by stress exposure (e.g., GluA1, PSD-95) are increased following rapid acting antidepressant administration, consistent with activation of the mTORC1 pathway resulting in increased protein translation, and reversal of the synaptic deficits caused by stress. Indeed, inescapable shock exposure,17 or three weeks of CUS exposure,25 caused reductions of multiple synaptic proteins that were rescued by NMDA antagonists in an mTORC1 dependent fashion.
Manipulations of effectors of the mTORC1 signaling pathway provide further support for its role in stress-associated outcomes. p70S6K has been reported to play an important role in depression-like behavior, demonstrated by viral manipulations capable of recapitulating stress-like outcomes (i.e., decreased p70S6K activity) or producing resiliency (i.e., increased p70S6K activity) in stress models.52 In addition, REDD1 (regulated in development and DNA damage response 1), an inhibitor of mTORC1 signaling,53 is increased by stress- and viral-mediated expression of REDD1 in the mPFC is sufficient to produce synapse loss and depressive-like behavior.12 Notably, in the same study, REDD1 was found to be increased in postmortem tissue from individuals with depression, consistent with the possibility that REDD1 could contribute to neuronal atrophy and depressive behaviors in patients.12 In contrast, REDD1 null mutant mice are resistant to the synaptic and behavioral deficits caused by chronic stress. Finally, inhibition of glycogen synthase kinase 3β, a negative regulator of mTORC1, has been shown to augment the effect of sub-threshold ketamine.54 However, glycogen synthase kinase 3β inhibition alone at the doses of the selective antagonists used does not produce an antidepressant response in the absence of ketamine-induced mTORC1 signaling.55
Together, these findings indicate that a transient increase in protein translation occurring shortly after administration of ketamine or other rapid acting antidepressants is sufficient to produce the sustained synaptic effects described above. Synaptic protein translation through mTORC1 signaling is a compelling therapeutic target for stress-associated disease as it provides a convergent mechanism for rapid antidepressant action that is not observed with typical antidepressants.17
Role of BDNF Translation and Signaling
Numerous studies have reported that brain derived neurotrophic factor (BDNF), a major neurotrophic factor in brain, is significantly decreased in the PFC and hippocampus by stress exposure.16 In addition, loss of growth/neurotrophic support is further demonstrated by studies reporting that the expression of vascular endothelial growth factor and insulin like growth factor 1 are also decreased by stress exposure.16 BDNF plays diverse roles in the CNS, such as regulating neuronal number and guidance during development, but is also involved in neuronal plasticity, function, and survival in adult brain.56 Transcription, trafficking, translation, and release of BDNF is driven by neuronal activity mainly through activation of glutamate receptors and voltage-dependent calcium channels, and is critical for maintaining synaptic integrity.57 BDNF binds preferentially to the tropomyosin receptor kinase B (TrkB) receptor to engage intracellular signaling pathways, including cascades that result in activation of mTORC1.51,58 Clinical studies report that levels of BDNF in serum are decreased in depressed patients and then increased in response to treatment with typical antidepressants.59 Reduced BDNF is also observed in postmortem tissue of untreated depressed individuals.60 Additionally, a BDNF polymorphism exists at codon 66 (Val66-Met) that disrupts BDNF trafficking and activity dependent release. The Val66-Met polymorphism has been associated with depression, and reduced hippocampal volume61 supporting a critical role for BDNF in neuronal integrity.
Typical antidepressants, such as selective serotonin reuptake inhibitors, selective norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, agents that modulate the levels of brain monoamines, have limited efficacy and a delayed therapeutic response of weeks to months.3 These typical antidepressants increase BDNF mRNA expression with a similar delayed time course of weeks. Other effective treatments such as electroconvulsive shock treatment rapidly increase BDNF and TrkB mRNA levels in the PFC and hippocampus, and greater increases are observed with chronic treatment.62 Exercise, an activity with stress-opposing effects, also increases BDNF expression, again along a time course that is consistent with the delay in antidepressant behavioral response in rodent models.63,64 Moreover, the antidepressant behavioral responses to monoamine-based antidepressant treatment and exercise require BDNF indicating that increased BDNF expression is a convergent mechanism of antidepressant action.16
Given the requirement for BNDF signaling in the pathophysiology and treatment of depression, BDNF also received early attention in mechanistic studies of rapid acting antidepressants. Interestingly, these studies demonstrate a key role for BDNF, including evidence for rapid release and translation of this neurotrophic factor in the actions of ketamine rather than a delayed increase in mRNA expression. Ketamine produces a rapid paradoxical burst of glutamate in the mPFC,19,65 and numerous reports demonstrate that the antidepressant behavioral response to ketamine requires AMPA receptor activity at the time of application.17,28,66–68 Glutamate-induced AMPA receptor activation and depolarization can stimulate BDNF release and lead to activation of TrkB-mTORC1 signaling.69 Evidence for ketamine stimulation of AMPA-dependent, BDNF release has been demonstrated in primary cortical cultures.70,71 BDNF release in these primary culture experiments is also sensitive to calcium signaling through voltage-gated calcium channels and NMDA receptors, and TrkB activation following BDNF release is necessary for mTORC1 pathway modulation.69–71 In addition, BDNF deletion or conditional TrkB knock down block the antidepressant response to ketamine.18 A requirement for BDNF release is demonstrated by studies reporting that the antidepressant response to ketamine is blocked by infusion of a BDNF neutralizing antibody into the mPFC prior to systemic administration of ketamine.71 In addition, BNDF Val66-Met mice that have impaired dendritic trafficking and release of BDNF do not show synaptogenic or antidepressant behavioral responses to ketamine.72
There are also reports that ketamine rapidly stimulates the translation of BDNF in the hippocampus, independent of elevations in mRNA. This is thought to occur via inhibition of spontaneous NMDA receptor activity, which is linked to inactivation of eukaryotic elongation factor 2 (eEF2) kinase and increased BDNF protein translation.18,68,73 Notably, eEF2 kinase knock-out animals are insensitive to the acute effects of ketamine administration.68 It is currently unclear whether this mechanism is sufficient to produce the long-term effects of ketamine administration. Also, it is unclear if eEF2 kinase inhibition alone is capable of restoring behavior in chronic stress-depression models to nonstress levels, nor has this mechanism for BDNF translation been demonstrated in the PFC after ketamine (see Zanos et al.46).
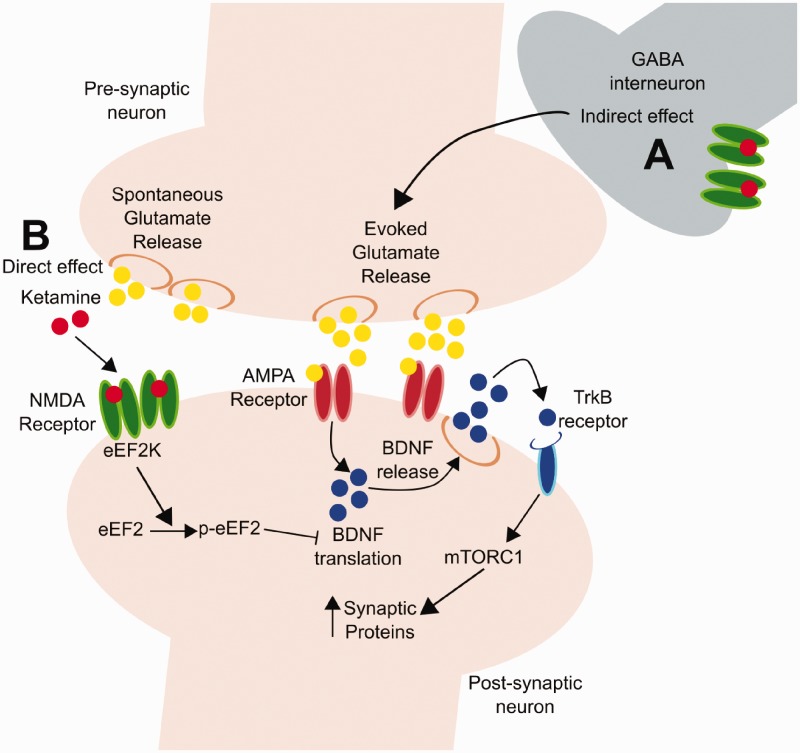
Activation of eEF2 kinase and mTORC1 signaling could function in concert as both stimulate the translation of BDNF as well as synaptic proteins. BDNF translation via eEF2 kinase inhibition may support BDNF release produced by the initial rapid burst of glutamate. Increased BDNF release would not only increase mTORC1 signaling but also act via additional signaling pathways to stimulate synapse formation,74 and reverse the synaptic deficits caused by chronic stress and depression. Increased translation of BDNF and synapse formation could also contribute to the long-term, sustained effects of ketamine by increasing the response to glutamate signaling (Figure 1).
Figure 1.
Proposed mechanisms for synaptic strengthening following ketamine administration. (a) The indirect hypothesis posits that ketamine produces a glutamate burst via blockade of NMDA receptors on GABAergic interneurons; this glutamate burst then causes activity dependent release of BDNF and activation of TrkB-mTORC1 signaling that increases levels of synaptic proteins AMPA receptor insertion and function. (b) The direct hypothesis states that the effects of ketamine occur via blockade of NMDA receptors located on principal neurons that are activated by spontaneous glutamate release; this results in inhibition of eEF2K and increased translation of BDNF.
Circuit Level Response to Rapid Acting Antidepressants
The previous discussion implicates BDNF-TrkB and mTORC1 signaling as downstream, mechanistic targets of rapid acting antidepressants. These findings suggest that drilling down on synaptic changes associated with stress pathology will provide insight into novel therapeutic targets for stress-associated diseases. However, there are some brain regions, including the amygdala and nucleus accumbens, that unlike the mPFC exhibit increased complexity after stress exposure.36,37 Given the regionally complex nature of neuronal remodeling following stress, studies to identify cell and circuit specific alterations involved in the behavioral deficits caused by chronic stress should also aid in identifying novel targets.
Critical Microcircuits in Rapid Antidepressant Responses
A key question in the field of rapid acting antidepressants is what the initial cellular trigger is and whether this involves local indirect actions on microcircuits or direct effects on principle neurons (Turner and Hall, 2015; Duman et al., 2016).75,76 The neuronal population of the PFC is comprised of approximately 70% pyramidal cells with the remainder consisting of a mixed population of GABAergic interneurons.77,78 The indirect hypothesis suggests that disinhibition of pyramidal cells, through antagonism of NMDA receptors on GABAergic interneurons, produces the rapid glutamate burst that is observed after ketamine administration. Increased glutamate causes activity-dependent BDNF release, which then produces downstream effects on mTORC1 signaling, synapse formation, and antidepressant behavioral responses. Alternatively, the direct mechanism posits that ketamine blocks NMDA receptors directly on principle glutamatergic neurons in the hippocampus and/or mPFC.18 This cellular trigger hypothesis is directly linked with blockade of spontaneous NMDA receptor activity and eEF2 kinase and increased BDNF translation. However, one difficulty with this hypothesis is that the rapid glutamate burst and AMPA receptor-mediated depolarization would eliminate the impact of spontaneous glutamate activity that occurs primarily in the absence of neuronal activity.
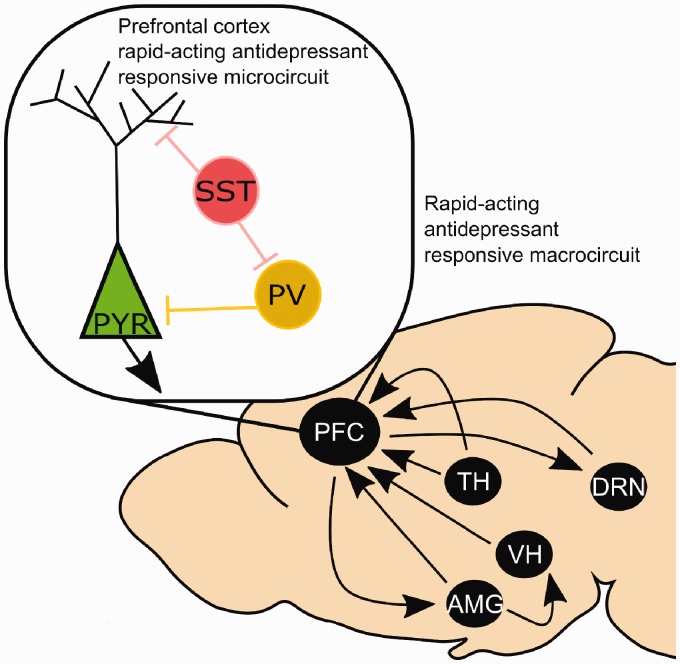
Beyond the rapid antidepressant response, GABAergic signaling is itself a compelling target for therapy in stress-associated diseases. Reduced GABA levels have been observed in depression, and a return to normal states upon remission during treatment.79–82 Low GABA levels have also been linked to susceptibility to post traumatic stress disorder.83,84 Thus, a rich literature is emerging, which demonstrates the importance of maintaining an appropriate balance of excitation and inhibition in the PFC for normal neuronal functioning. At the microcircuit level, there are interesting interactions between GABAergic interneurons and mPFC pyramidal cells that are only beginning to be investigated (Figure 2). There are numerous GABAergic interneuron subtypes that differ in functional properties and expression of signaling proteins (for review of interneuron subtypes and properties see earlier works).77,78
Figure 2.
Macro and microcircuits involved in the response to rapid acting antidepressants. Blockade of NMDA receptors on pyramidal neurons (PYR) and/or interneurons (somatostatin, SST; parvalbumin, PV) in the prefrontal cortex (PFC) increases glutamatergic transmission and facilitates BDNF and mTORC1 mediated synaptic strengthening. Modification of amygdala (AMG), thalamic (TH), ventral hippocampal (VH), and dorsal raphe nucleus (DRN) inputs to the PFC are observed after ketamine treatment. It is also likely that reciprocal projections to these regions contribute to rapid-acting antidepressant responses, although further work is required to characterize these effects.
Investigation of postmortem tissue has demonstrated a reduction in somatostatin (SST) content suggesting that the SST GABAergic subtype may be particularly impacted in depression.85–87 SST interneurons exert inhibitory effects on pyramidal cells, with GABAergic axons targeting the dendritic field allowing control of pyramidal cell responses to incoming signals. SST interneurons have also been demonstrated to potently suppress activity of microcircuits at rest88 suggesting that SST inhibition is critical for disinhibition of pyramidal cells. Manipulating the excitation/inhibition balance via regulation of SST GABA interneurons has been shown to play a role in depression-like behavior,89,90 and reductions of SST function and the resulting hyperactivity of glutamatergic neurons could lead to excitotoxicity,91 potentially contributing to the synaptic deficits in mPFC and hippocampus caused by stress. Consistent with the finding that SST neurons may potently limit microcircuit activity, removal of the AChM1 receptor subtype that is targeted by scopolamine to decrease SST interneuron activity blocks the antidepressant actions of this rapid acting agent.20 Studies are currently underway to determine if the actions of ketamine are also mediated by regulation of SST interneurons via NMDA receptors on SST inhibitory interneurons.
Pavalbumin (PV) interneurons gate pyramidal cell activity via axonal contacts with pyramidal cell somata. Inhibition of PV neurons does not appear to contribute as a target of ketamine as constitutive NMDA receptor knockdown on PV interneurons does not alter the antidepressant effects of ketamine92; knockdown of AChM1 receptors on PV neurons also does not block the antidepressant actions of scopolamine.20 However, reduced excitatory drive onto PV cells has been reported in helpless animals after inescapable footshock, and chemogenetic inhibition of PV cells can render resilient animals helpless.93 Therefore, PV interneurons likely play an important role in behavioral output as determined by the balance of excitation and inhibition present in the PFC. Further, PV and SST interneurons directly interact, with SST interneurons providing inhibitory tone to PV cells91 so that manipulation of SST neurons could impact PV function. Further studies of these as well as other classes of GABA interneurons in stress and antidepressant actions will prove insightful and could help elucidate critical components of pathophysiology of stress and treatment responses.
MDD Circuits and Interactions With Other Brain Regions
The changes observed in the mPFC dendritic field suggest that rapid acting antidepressant administration may alter the balance between inhibitory and excitatory transmission making the mPFC more receptive to distal inputs. Moreover, these findings indicate that mPFC projections are also enhanced and lead to improved top down, cortical control of other brain regions and their functional output (Figure 2).
Dorsal raphe, serotonin, and stressor control circuits
Increased 5-HT sensitivity in the PFC has been observed 24 h after treatment with NMDA antagonists and scopolamine.17,50 5-HT signaling is mediated by neurons located in the dorsal raphe nucleus (DRN) that synthesize 5-HT and project to multiple forebrain regions. Numerous reports indicate that bidirectional activity between the PFC and DRN is necessary for the response to ketamine.94–96 PFC interactions with the dorsal DRN have long been implicated in the learned helplessness model of depression, particularly with regard to the controllability of the stress response.97,98 Stressor controllability limits subsequent behavioral deficits and limits later 5-HT release in the amygdala during stressful experience. In contrast, exposing a rodent to uncontrollable stressors leads to helpless, depressive behaviors and increases 5-HT release in the amygdale.99 Notably, exposure to uncontrollable stress does not engage the mPFC-DRN circuit in the same manner as controllable stress. Ketamine has recently been demonstrated to be protective in the learned helplessness model.44 Especially interesting is the finding that ketamine functionally alters PFC to DRN activity, allowing for engagement even when animals are exposed to uncontrollable stress. Understanding the cellular mechanisms underlying the sustained prophylactic effects of ketamine provides an interesting opportunity for identifying the cellular mechanisms that contribute to stress effects, including stress resistance and resilience.
Arousal, attention, and cognition circuits
Hypocretin terminals in the mPFC are evident on thalamocortical projections and have been implicated in arousal and attention processes.100,99 Ketamine as well as GLYX-13 administration increase hypocretin-induced EPSCs in the mPFC and are reported to enhance attention.49 In a mouse model of the serial reaction time task, ketamine or GLYX-13 administration (24 h earlier) reduces failures to respond on trials presented with a very brief stimulus.49 Notably, ketamine, but not GLYX-13 treated animals display more premature responses, a measure of impulsivity, when the time between stimuli is increased, and this is associated with increased 5-HT2A receptor sensitivity (i.e., induced EPSCs) observed after ketamine, but not GLYX-13.49
Numerous reports also suggest that administration of a single low dose of ketamine can affect cognition after the initial drug exposure period. Studies utilizing the Wisconsin card sorting task report an impairment in strategy switching in depressed individuals.102 In an analogous rodent task, attentional set shifting, chronic stress is reported to impair cognitive flexibility in both an orbitofrontal cortex (OFC) and PFC dependent manner depending on the stressor utilized.35,103–105 The deficit in cognitive flexibility observed in the attentional set shifting task is rescued by ketamine treatment, in both OFC104 and PFC dependent paradigms.105,106 Notably, in the OFC dependent paradigm, ketamine’s beneficial effect appears to function in part through JAK/STAT3 signaling.102 Further investigation of reciprocal connections between the PFC and thalamus, and cortical-cortical connectivity between the OFC and PFC are necessary to better understand the impact of ketamine on attention and cognition.
Fear and emotion circuits
Ketamine has been shown to modulate corticotropin releasing factor mediated input to the mPFC from amygdala projections in a fashion that would be expected to reduce amygdala control of mPFC stress reactivity.107 mPFC connections to the amygdala may also be regulated by ketamine. Connections between the mPFC and amygdala are implicated in fear and anxiety states.108,109 Failure to extinguish fear memories is a hallmark of stress models. Extinction is enhanced by ketamine,110 GLYX-13,26 and scopolamine.111,112 The well-defined fear circuitry, particularly that between the mPFC and amygdala, could be an optimal circuit target for the increased synaptic changes that result from ketamine administration, which should facilitate extinction. Projections from the ventral hippocampus to the mPFC have also been implicated in the response to ketamine113 and are known to regulate anxiety-like behaviors often impacted by stress.114,115 Ventral hippocampal activity is critical for termination of the stress response and therefore plasticity in ventral hippocampus projections to mPFC could directly impact the stress response, though this remains to be tested after ketamine administration.
Conclusions
Mechanistic studies of the molecular, cellular, and circuit-level actions of rapid acting antidepressants have seen great progress since the early reports of rapid antidepressant behavioral responses to ketamine. The effectiveness of ketamine with treatment resistant patients and its single dose effectiveness in clinical studies and rodent stress models point to the need to further elucidate the molecular and cellular mechanisms underlying the rapid actions of ketamine and to identify novel targets that offer safer alternatives. Studies demonstrating the synaptic pathways that mediate changes in the balance of excitation and inhibition after antidepressant treatment will be especially interesting. Recent work demonstrating that optogenetic excitation in the mPFC can recapitulate rapid antidepressant responses116 opens the door to cell type and circuit specific manipulations that will aid in mapping interacting brain areas that underlie these effects. Circuit-level manipulations together with cell type specific, inducible manipulations of signaling pathways provide tools that will continue to produce a better understanding of the rapid antidepressant response, particularly as it relates to stress pathology.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Duman has received Honoraria from Lilly, Lundbeck, Johnson & Johnson, Naurex,and Forest Laboratories; consulting fees from Taisho, Naurex, and Johnson & Johnson; and research support from Allergan, Lundbeck, Sunovion, Johnson & Johnson, Navitor, Naurex, Taisho, and Forest Research Institute. This research was supported by NIMH Grants MH045481 and MH093897 to R.S.D.
References
- 1.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003; 289: 3095–3105. [DOI] [PubMed] [Google Scholar]
- 2.Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet (London, England) 2013; 382: 1575–1586. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatr 2006; 163: 28–40. [DOI] [PubMed] [Google Scholar]
- 4.Gaynes BN, Warden D, Trivedi MH, et al. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv 2009; 60: 1439–1445. [DOI] [PubMed] [Google Scholar]
- 5.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatr 2000; 47: 351–354. [DOI] [PubMed] [Google Scholar]
- 6.Zarate CA, Jr., Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatr 2006; 63: 856–864. [DOI] [PubMed] [Google Scholar]
- 7.Arnsten AF. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat Neurosci 2015; 18: 1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev 2009; 33: 699–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajkowska G, Miguel-Hidalgo JJ, Wei J, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatr 1999; 45: 1085–1098. [DOI] [PubMed] [Google Scholar]
- 10.Rajkowska G, O’Dwyer G, Teleki Z, et al. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology 2007; 32: 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang HJ, Voleti B, Hajszan T, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med 2012; 18: 1413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ota KT, Liu RJ, Voleti B, et al. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med 2014; 20: 531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karege F, Vaudan G, Schwald M, et al. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res 2005; 136: 29–37. [DOI] [PubMed] [Google Scholar]
- 14.Hettema JM, Kuhn JW, Prescott CA, et al. The impact of generalized anxiety disorder and stressful life events on risk for major depressive episodes. Psychol Med 2006; 36: 789–795. [DOI] [PubMed] [Google Scholar]
- 15.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatr 1999; 156: 837–841. [DOI] [PubMed] [Google Scholar]
- 16.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatr 2006; 59: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 17.Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Autry AE, Adachi M, Nosyreva E, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011; 475: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moghaddam B, Adams B, Verma A, et al. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 1997; 17: 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wohleb ES, Wu M, Gerhard DM, et al. GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Investig 2016; 126: 2482–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duman RS. Neurobiology of stress, depression, and rapid acting antidepressants: remodeling synaptic connections. Depress Anxiety 2014; 31: 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo L, Tan RX. Fluoxetine inhibits dendrite atrophy of hippocampal neurons by decreasing nitric oxide synthase expression in rat depression model. Acta Pharmacol Sin 2001; 22: 865–870. [PubMed] [Google Scholar]
- 23.Ampuero E, Rubio FJ, Falcon R, et al. Chronic fluoxetine treatment induces structural plasticity and selective changes in glutamate receptor subunits in the rat cerebral cortex. Neuroscience 2010; 169: 98–108. [DOI] [PubMed] [Google Scholar]
- 24.Morais M, Santos PA, Mateus-Pinheiro A, et al. The effects of chronic stress on hippocampal adult neurogenesis and dendritic plasticity are reversed by selective MAO-A inhibition. J Psychopharmacol 2014; 28: 1178–1183. [DOI] [PubMed] [Google Scholar]
- 25.Li N, Liu RJ, Dwyer JM, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatr 2011; 69: 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgdorf J, Kroes RA, Zhang XL, et al. Rapastinel (GLYX-13) has therapeutic potential for the treatment of post-traumatic stress disorder: characterization of a NMDA receptor-mediated metaplasticity process in the medial prefrontal cortex of rats. Behav Brain Res 2015; 294: 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarria A, Wohleb ES, Voleti B, et al. Rapid antidepressant actions of scopolamine: role of medial prefrontal cortex and M1-subtype muscarinic acetylcholine receptors. Neurobiol Dis 2015; 82: 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeng S, Zarate CA, Jr., Du J, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatr 2008; 63: 349–352. [DOI] [PubMed] [Google Scholar]
- 29.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience 1995; 69: 83–88. [DOI] [PubMed] [Google Scholar]
- 30.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci 2001; 933: 265–277. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res 1992; 588: 341–345. [DOI] [PubMed] [Google Scholar]
- 32.Radley JJ, Anderson RM, Hamilton BA, et al. Chronic stress-induced alterations of dendritic spine subtypes predict functional decrements in an hypothalamo-pituitary-adrenal-inhibitory prefrontal circuit. J Neurosci 2013; 33: 14379–14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radley JJ, Rocher AB, Miller M, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebr Cortex 2006; 16: 313–320. [DOI] [PubMed] [Google Scholar]
- 34.Radley JJ, Rocher AB, Rodriguez A, et al. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol 2008; 507: 1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liston C, Miller MM, Goldwater DS, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 2006; 26: 7870––7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res 2003; 965: 290–294. [DOI] [PubMed] [Google Scholar]
- 37.Vyas A, Mitra R, Shankaranarayana Rao BS, et al. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 2002; 22: 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience 2004; 128: 667–673. [DOI] [PubMed] [Google Scholar]
- 39.Magarinos AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol 1999; 371: 113–122. [DOI] [PubMed] [Google Scholar]
- 40.Bagot RC, Cates HM, Purushothaman I, et al. Ketamine and imipramine reverse transcriptional signatures of susceptibility and induce resilience-specific gene expression profiles. Biol Psychiatr 2016; 81: 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang B, Zhang JC, Han M, et al. Comparison of R-ketamine and rapastinel antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 2016; 233: 3647–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang C, Shirayama Y, Zhang JC, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatr 2015; 5: e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirayama Y, Hashimoto K. Effects of a single bilateral infusion of R-ketamine in the rat brain regions of a learned helplessness model of depression. Eur Arch Psychiatr Clin Neurosci. Epub ahead of print 1 August 2016. [DOI] [PubMed] [Google Scholar]
- 44.Amat J, Dolzani SD, Tilden S, et al. Previous ketamine produces an enduring blockade of neurochemical and behavioral effects of uncontrollable stress. J Neurosci 2016; 36: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brachman RA, McGowan JC, Perusini JN, et al. Ketamine as a prophylactic against stress-induced depressive-like behavior. Biol Psychiatry 2016; 79: 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zanos P, Moaddel R, Morris PJ, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016; 533: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A 2008; 105: 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phoumthipphavong V, Barthas F, Hassett S, et al. Longitudinal effects of ketamine on dendritic architecture in vivo in the mouse medial frontal cortex. eNeuro 2016; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu RJ, Duman C, Kato T, et al. GLYX-13 produces rapid antidepressant responses with key synaptic and behavioral effects distinct from ketamine. Neuropsychopharmacology. Epub ahead of print 19 October 2016. DOI:10.1038/npp.2016.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voleti B, Navarria A, Liu RJ, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry 2013; 74: 742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lipton JO, Sahin M. The neurology of mTOR. Neuron 2014; 84: 275–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dwyer JM, Maldonado-Aviles JG, Lepack AE, et al. Ribosomal protein S6 kinase 1 signaling in prefrontal cortex controls depressive behavior. Proc Natl Acad Sci U S A 2015; 112: 6188–6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katiyar S, Liu E, Knutzen CA, et al. REDD1, an inhibitor of mTOR signalling, is regulated by the CUL4A-DDB1 ubiquitin ligase. EMBO Rep 2009; 10: 866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu RJ, Fuchikami M, Dwyer JM, et al. GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology 2013; 38: 2268–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma XC, Dang YH, Jia M, et al. Long-lasting antidepressant action of ketamine, but not glycogen synthase kinase-3 inhibitor SB216763, in the chronic mild stress model of mice. PLoS One 2013; 8: e56053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lipsky RH, Marini AM. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann N Y Acad Sci 2007; 1122: 130–143. [DOI] [PubMed] [Google Scholar]
- 57.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem 2003; 10: 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci 2006; 361: 1545–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aydemir O, Deveci A, Taneli F. The effect of chronic antidepressant treatment on serum brain-derived neurotrophic factor levels in depressed patients: a preliminary study. Prog Neuropsychopharmacol Biol Psychiatry 2005; 29: 261–265. [DOI] [PubMed] [Google Scholar]
- 60.Chen B, Dowlatshahi D, MacQueen GM, et al. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry 2001; 50: 260–265. [DOI] [PubMed] [Google Scholar]
- 61.Frodl T, Schule C, Schmitt G, et al. Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch Gen Psychiatry 2007; 64: 410–416. [DOI] [PubMed] [Google Scholar]
- 62.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 1995; 15: 7539–7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duman CH, Schlesinger L, Russell DS, et al. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res 2008; 1199: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neeper SA, Gómez-Pinilla F, Choi J, et al. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res 1996; 726: 49–56. [PubMed] [Google Scholar]
- 65.Chowdhury GM, Zhang J, Thomas M, et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry 2017; 22: 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koike H, Chaki S. Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behav Brain Res 2014; 271: 111–115. [DOI] [PubMed] [Google Scholar]
- 67.Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res 2011; 224: 107–111. [DOI] [PubMed] [Google Scholar]
- 68.Nosyreva E, Szabla K, Autry AE, et al. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci 2013; 33: 6990–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jourdi H, Hsu YT, Zhou M, et al. Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci 2009; 29: 8688–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lepack AE, Bang E, Lee B, et al. Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology 2016; 111: 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lepack AE, Fuchikami M, Dwyer JM, et al. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol 2015; 18: pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu RJ, Lee FS, Li X-Y, et al. BDNF Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry 2012; 71: 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gideons ES, Kavalali ET, Monteggia LM. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc Natl Acad Sci U S A 2014; 111: 8649–8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 2013; 14: 7–23. [DOI] [PubMed] [Google Scholar]
- 75.Miller OH, Moran JT and Hall BJ. Two cellular hypotheses explaining the initiation of ketamine's antidepressant actions: Direct inhibition and disinhibition. Neuropharmacology 2016; 100: 17–26. [DOI] [PubMed]
- 76.Ghosal S, Hare B and Duman RS. Prefrontal cortex GABAergic deficits and circuit dysfunction in the pathophysiology and treatment of chronic stress and depression. Curr Opin Behav Sci 2017; 14: 1–8. [DOI] [PMC free article] [PubMed]
- 77.Markram H, Toledo-Rodriguez M, Wang Y, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 2004; 5: 793–807. [DOI] [PubMed] [Google Scholar]
- 78.Tremblay R, Lee S, Rudy B. GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 2016; 91: 260–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanacora G, Mason GF, Rothman DL, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry 1999; 56: 1043–1047. [DOI] [PubMed] [Google Scholar]
- 80.Hasler G, Neumeister A, van der Veen JW, et al. Normal prefrontal gamma-aminobutyric acid levels in remitted depressed subjects determined by proton magnetic resonance spectroscopy. Biol Psychiatry 2005; 58: 969–973. [DOI] [PubMed] [Google Scholar]
- 81.Hasler G, van der Veen JW, Tumonis T, et al. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 2007; 64: 193–200. [DOI] [PubMed] [Google Scholar]
- 82.Dubin MJ, Mao X, Banerjee S, et al. Elevated prefrontal cortex GABA in patients with major depressive disorder after TMS treatment measured with proton magnetic resonance spectroscopy. J Psychiatry Neurosci 2016; 41: E37–E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vaiva G, Boss V, Ducrocq F, et al. Relationship between posttrauma GABA plasma levels and PTSD at 1-year follow-up. Am J Psychiatry 2006; 163: 1446–1448. [DOI] [PubMed] [Google Scholar]
- 84.Vaiva G, Thomas P, Ducrocq F, et al. Low posttrauma GABA plasma levels as a predictive factor in the development of acute posttraumatic stress disorder. Biol Psychiatry 2004; 55: 250–254. [DOI] [PubMed] [Google Scholar]
- 85.Seney ML, Tripp A, McCune S, et al. Laminar and cellular analyses of reduced somatostatin gene expression in the subgenual anterior cingulate cortex in major depression. Neurobiol Dis 2015; 73: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sibille E, Morris HM, Kota RS, et al. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol 2011; 14: 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tripp A, Kota RS, Lewis DA, et al. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol Dis 2011; 42: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gentet LJ, Kremer Y, Taniguchi H, et al. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat Neurosci 2012; 15: 607–612. [DOI] [PubMed] [Google Scholar]
- 89.Lin LC, Sibille E. Somatostatin, neuronal vulnerability and behavioral emotionality. Mol Psychiatry 2015; 20: 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soumier A, Sibille E. Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice. Neuropsychopharmacology 2014; 39: 2252–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang W, Zhang L, Liang B, et al. Hyperactive somatostatin interneurons contribute to excitotoxicity in neurodegenerative disorders. Nat Neurosci 2016; 19: 557–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pozzi L, Pollak Dorocic I, Wang X, et al. Mice lacking NMDA receptors in parvalbumin neurons display normal depression-related behavior and response to antidepressant action of NMDAR antagonists. PLoS One 2014; 9: e83879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perova Z, Delevich K, Li B. Depression of excitatory synapses onto parvalbumin interneurons in the medial prefrontal cortex in susceptibility to stress. J Neurosci 2015; 35: 3201–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fukumoto K, Iijima M, Chaki S. The antidepressant effects of an mGlu2/3 receptor antagonist and ketamine require AMPA receptor stimulation in the mPFC and subsequent activation of the 5-HT neurons in the DRN. Neuropsychopharmacology 2016; 41: 1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fukumoto K, Iijima M, Chaki S. Serotonin-1A receptor stimulation mediates effects of a metabotropic glutamate 2/3 receptor antagonist, 2S-2-amino-2-(1S,2S-2-carboxycycloprop-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), and an N-methyl-D-aspartate receptor antagonist, ketamine, in the novelty-suppressed feeding test. Psychopharmacology (Berl) 2014; 231: 2291–2298. [DOI] [PubMed] [Google Scholar]
- 96.Nishitani N, Nagayasu K, Asaoka N, et al. Raphe AMPA receptors and nicotinic acetylcholine receptors mediate ketamine-induced serotonin release in the rat prefrontal cortex. Int J Neuropsychopharmacol 2014; 17: 1321–1326. [DOI] [PubMed] [Google Scholar]
- 97.Amat J, Baratta MV, Paul E, et al. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature Neurosci 2005; 8: 365–371. [DOI] [PubMed] [Google Scholar]
- 98.Amat J, Paul E, Zarza C, et al. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci 2006; 26: 13264–13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baratta MV, Zarza CM, Gomez DM, et al. Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. Eur J Neurosci 2009; 30: 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lambe EK, Aghajanian GK. Hypocretin (orexin) induces calcium transients in single spines postsynaptic to identified thalamocortical boutons in prefrontal slice. Neuron 2003; 40: 139–150. [DOI] [PubMed] [Google Scholar]
- 101.Lambe EK, Olausson P, Horst NK, et al. Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: correlation with improved attention in rat. J Neurosci 2005; 25: 5225–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Merriam EP, Thase ME, Haas GL, et al. Prefrontal cortical dysfunction in depression determined by Wisconsin Card Sorting Test performance. Am J Psychiatry 1999; 156: 780–782. [DOI] [PubMed] [Google Scholar]
- 103.Bondi CO, Rodriguez G, Gould GG, et al. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology 2007; 33: 320–331. [DOI] [PubMed] [Google Scholar]
- 104.Patton MS, Lodge DJ, Morilak DA, et al. Ketamine corrects stress-induced cognitive dysfunction through JAK2/STAT3 signaling in the orbitofrontal cortex. Neuropsychopharmacology. 2016. DOI:10.1038/npp.2016.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nikiforuk A, Popik P. Ketamine prevents stress-induced cognitive inflexibility in rats. Psychoneuroendocrinology 2014; 40: 119–122. [DOI] [PubMed] [Google Scholar]
- 106.Jett JD, Boley AM, Girotti M, et al. Antidepressant-like cognitive and behavioral effects of acute ketamine administration associated with plasticity in the ventral hippocampus to medial prefrontal cortex pathway. Psychopharmacology (Berl) 2015; 232: 3123–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu RJ, Ota KT, Dutheil S, et al. Ketamine strengthens CRF-activated amygdala inputs to basal dendrites in mPFC layer V pyramidal cells in the prelimbic but not infralimbic subregion. A key suppressor of stress responses. Neuropsychopharmacology 2015; 40: 2066–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Adhikari A, Lerner TN, Finkelstein J, et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature 2015; 527: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cho JH, Deisseroth K, Bolshakov VY. Synaptic encoding of fear extinction in mPFC-amygdala circuits. Neuron 2013; 80: 1491–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Girgenti MJ, Ghosal S, LoPresto D, et al. Ketamine accelerates fear extinction via mTORC1 signaling. Neurobiol Dis 2017; 100: 1–8. [DOI] [PMC free article] [PubMed]
- 111.Zelikowsky M, Hast TA, Bennett RZ, et al. Cholinergic blockade frees fear extinction from its contextual dependency. Biol Psychiatry 2013; 73: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Santini E, Sepulveda-Orengo M, Porter JT. Muscarinic receptors modulate the intrinsic excitability of infralimbic neurons and consolidation of fear extinction. Neuropsychopharmacology 2012; 37: 2047–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carreno FR, Donegan JJ, Boley AM, et al. Activation of a ventral hippocampus-medial prefrontal cortex pathway is both necessary and sufficient for an antidepressant response to ketamine. Mol Psychiatry 2016; 21: 1298–1308. [DOI] [PubMed] [Google Scholar]
- 114.Adhikari A, Topiwala MA, Gordon JA. Single units in the medial prefrontal cortex with anxiety-related firing patterns are preferentially influenced by ventral hippocampal activity. Neuron 2011; 71: 898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Padilla-Coreano N, Bolkan SS, Pierce GM, et al. Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron 2016; 89: 857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fuchikami M, Thomas A, Liu R, et al. Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proc Natl Acad Sci U S A 2015; 112: 8106–8111. [DOI] [PMC free article] [PubMed] [Google Scholar]