Abstract
Acanthamoeba keratitis (AK) is a rare but sight-threatening disease caused by pathogenic species of Acanthamoeba. Despite its ubiquitous nature, the incidence of AK is relatively low compared to other forms of infectious keratitis. Although contact lens wear is a major risk factor, exposure to contaminated water and ocular trauma are also associated with AK. Once a patient develops AK the prognosis is very poor unless an aggressive treatment regimen is initiated early. Some of the intriguing features of AK are the lack of immunological memory, resistance of the dormant cyst form to treatment, differences between the pathogenic strains and soil isolates of Acanthamoeba and the unique role of the innate immune system in controlling this disease. Understanding the series of steps involved in the pathogenesis of the disease and the host immune response against Acanthamoeba antigens is crucial for developing effective therapeutic strategies targeting the disease.
Keywords: Acanthamoeba keratitis, corneal pathology, ocular immunology, mucosal immunity
Introduction
Acanthamoebae are microscopic, free-living protozoa that are frequently isolated from soil, water, air, and nasopharyngeal mucosa of healthy individuals. The life cycle of Acanthamoeba consists of two stages; the trophozoite and the cyst. The trophozoites (10 to 25µm) are the vegetative forms which feed on organic matter, other microbes, and divide mitotically. When exposed to harsh conditions such as lack of nutrients or extreme heat or cold, the trophozoites differentiate in to a double walled cyst form (8 to 12µm). The outer layer of the cysts is composed of polysaccharides and the inner layer is made up of cellulose. The cysts are resistant to repeated cycles of freeze-thawing and also remarkably high doses of ultraviolet and gamma irradiation. The cysts are metabolically inactive and can remain viable for up to 20 years under dry conditions. Pathogenic Acanthamoeba species cause two distinctly different diseases: a) granulomatous amoebic encephalitis (GAE) and b) amoebic keratitis. GAE is most commonly seen in immunocompromised patients whereas the most common disease caused by Acanthamoeba species is amoebic keratitis, which occurs in immunocompetent individuals [1].
Acanthamoeba keratitis (AK) is a progressive sight-threatening disease caused by at least eight species of Acanthamoeba: A. castellanii, A. culbertsoni, A. polyphaga, A. hatchetti, A. rhysodes, A. lugdunesis, A. quina, and A. griffini [2]. Contact lens wear is the most common risk factor and accounts for > 80 percent of the cases of AK. The disease can be managed if diagnosed and treated during the early stages. As the disease progresses an aggressive treatment regimen involving hourly around the clock application of antimicrobials is needed. Corticosteroids are sometimes used to dampen inflammation in severe cases but they often need to be used in combination with antimicrobials such as polyhexamethylene biguanide (PHMB) or propamidine isethionate (Brolene®). However, corticosteroids can cause extensive ocular damage and in some cases, induce the excystment of the cysts to the active trophozoite forms, which necessities coverage with antimicrobials [3]. Despite aggressive therapy, some patients fail to respond to treatment and corneal transplantation is needed to restore vision. Although Acanthamoeba spp. are ubiquitous and the leading risk factor for AK, contact lens wear, is practiced by over 30 million individuals in the United States, AK is extraordinarily rare with less than 150 cases occurring each year in the U.S. [4,5]. Moreover, serological surveys indicate that 90 to 100 percent of the adult population with no history of AK has serum antibodies specific for Acanthamoeba antigens – a finding that suggests that exposure to Acanthamoebae is commonplace, yet the disease is rare. These observations have prompted investigators to search for other risk factors and conditions that contribute to the development of AK.
Pathogenesis of Acanthamoeba Keratitis
One of the fundamental tenets of parasitology is the concept of host specificity. It is widely believed that the first step in the development of AK is initiated by binding of the trophozoites to the corneal epithelium. Early in vitro studies revealed that Acanthamoeba trophozoites demonstrated a strong predilection to bind to the corneal epithelium of only three mammalian species – human, pig, and Chinese hamster – but failed to adhere to the corneal epithelium of common laboratory animals such as mouse, rat, cotton rat, and rabbit [6]. These in vitro results were confirmed in vivo with the finding that AK could be transmitted through the application of trophozoite-laden contact lenses in pigs and Chinese hamsters, but not in any of the mouse or rat strains tested [6].
Contact Lens Wear as a Risk Factor for Acanthamoeba Keratitis
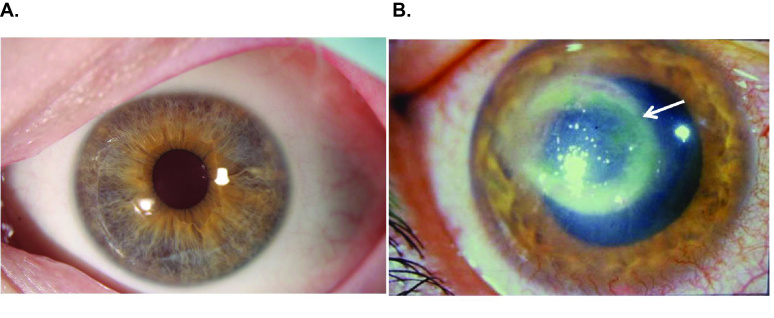
The characteristic features of AK include severe pain, radial keratoneuritis, and stromal ring-like infiltrate (Figure 1). It is widely believed that contact lenses serve as vectors for transmitting Acanthamoeba trophozoites to the ocular surface. However, studies in animal models have revealed that contact lenses not only serve to deliver infectious trophozoites to the cornea, but they also alter the ocular surface rendering it more receptive to binding of trophozoites. That is, cornea organ culture studies revealed that contact lens wear upregulated the expression of mannosylated glycoproteins on the surface of the corneal epithelium [7]. The mannose receptor on trophozoites facilitates the parasite’s binding to the corneal surface. This initial step of adhesion to mannosylated glycoproteins induces the release of a 133 kDa protein referred to as the mannose-induced protease 133 (MIP-133) [7]. The serine protease MIP-133 induces apoptosis of corneal epithelial cells through a cytosolic phospholipase A2α pathway and facilitates trophozoite penetration into the corneal stroma [8]. MIP-133 also produces “stromal melting” or enzymatic degradation of the collagen matrix that forms the middle layer of the cornea. Acanthamoeba trophozoites secrete other proteases including a plasminogen activator (Acanthamoeba plasminogen activator or aPA) that produce similar degradation of the corneal stroma [6,9].
Figure 1.
Clinical features of Acanthamoeba keratitis. A) Normal human eye (reprinted with permission of National Eye Institute) and B) Eye with Acanthamoeba keratitis. The ring-like stromal infiltrate (arrow) is a characteristic feature of Acanthameoba keratitis [6].
Anti-disease Versus Anti-microbial Vaccines
A compelling body of evidence indicates that elements of the adaptive immune response, including complement fixing antibodies, are incapable of damaging either Acanthamoeba trophozoites or cysts [10]. However, mucosal immunization using killed trophozoites stimulates the production of secretory IgA antibodies in the tears that block adhesion of the trophozoites to the ocular surface but have no effect on the disease course if immunization is initiated after the trophozoites have bound to the corneal epithelium [10]. Thus, the adaptive immune response can prevent the initial first step in the infectious process, but fails to affect the viability once the amoebae have entered the cornea [10].
Acanthamoeba-borne protease, MIP-133, plays a crucial role in the pathogenesis of AK and has served as a target for vaccine development. Mucosal immunization with MIP-133 leads to the development of secretory IgA anti-MIP-133 antibodies that appear in the tears and block the enzymatic degradation of the corneas in Chinese hamsters [9]. This immunization strategy demonstrates that an “anti-disease” vaccine can mitigate crucial pathogenic components of AK without affecting the viability of the trophozoites. Thus, blocking the pathogenic proteases elaborated by Acanthamoeba trophozoites can be an effective strategy for mitigating corneal disease, although by itself, it is ineffectual in eliminating the infectious organisms.
Bacterial Flora of Ocular Surface and Risk for Acanthamoeba Keratitis
Recent years have witnessed an explosion in interest and research on the role of the microbiome in a variety of infectious and autoimmune diseases. Long before the microbiome was in vogue investigators observed a curious association between certain bacterial species in the flora of the ocular surface and the development of AK. In particular, Corynebacterium xerosis was implicated as a “co-factor” in AK. Using a rat model of AK, Badenoch et al. [11] demonstrated that the severity of AK was exacerbated if Acanthamoeba trophozoites were fed C. xerosis prior to injecting them into the cornea. However, subsequent studies revealed that another explanation for the exacerbation of AK by C. xerosis was due to the induction of MIP-133 by mannose that is present in the cell walls of C. xerosis. Of the bacterial species found in the ocular surface flora, C. xerosis stands apart from the rest by having the highest content of mannose in its cell wall [12]. Moreover, exposing trophozoites to C. xerosis in vitro induces the production of MIP-133 and produces a dramatic enhancement of in vivo pathogenicity of Acanthamoeba trophozoites in experimental hosts [12].
Endosymbionts and Pathogenesis of Acanthamoeba
Acanthamoeba trophozoites are metabolically active and use a wide variety of bacteria, fungi, and organic matter as a food source. Certain bacterial species have developed strategies to avoid lysis by the amoeba and survive as intracellular endosymbionts within Acanthamoeba. The association between the bacteria with the amoebae can be transient as in facultative intracellular bacteria or stable as in the case of obligate intracellular bacteria. These bacterial endosymbionts cannot survive outside of the amoebae and efforts to culture them axenically have proved to be extraordinarily difficult. Some of the most common endosymbionts identified in Acanthamoeba spp. are Mycobacteria, Legionella, Pseudomonas, and Chlamydia [13]. These bacterial endosymbionts influence the pathogenicity, virulence, and susceptibility of Acanthamoeba to therapeutic agents. In an attempt to understand the role of endosymbionts in promoting pathogenicity, Iovieno et al. [14] conducted a study using clinical isolates of Acanthamoeba from corneal scrapings, corneal biopsies, corneal buttons, contact lenses, and lens cases of patients presenting with AK. An intriguing aspect of this study was the observation that Acanthamoeba isolates collected at different time points from the same patient harbored different bacterial endosymbionts while Acanthamoeba isolated from different patients had similar endosymbionts. The clinical isolates in this study harbored bacterial endosymbionts including Pseudomonas and Mycobacterium inside both trophozoites and cysts. The Acanthamoeba isolates harboring endosymbionts had significantly higher in vitro cytopathic effects (CPE) against corneal epithelial cells compared to isolates without any endosymbionts [14]. Even though the exact mechanisms involved in the endosymbiont-associated pathogenicity by Acanthamoeba trophozoites are not fully understood, one can speculate that the mannosylated glycoproteins in the bacteria cell wall might act as a stimulus for the increased secretion of proteases by Acanthamoeba trophozoites. In turn, these proteases might contribute to the pathogenicity and severity of the disease. These pathogenic effects are most likely mediated at the corneal epithelial surface. Since it is widely viewed as a symbiotic relationship, Acanthamoeba might also be protecting these intracellular bacteria from therapeutic agents and the hostile host environment [15].
Innate Immunity and AK
More than 30 million Americans wear contact lenses, yet the incidence of AK in contact lens wearers in the U.S. is less than 33 cases per million contact lens wearers [16]. This raises the question as to why AK is not more prevalent. The low incidence of AK suggests that the host’s immune system might influence its development and severity. Serological analysis of the serum IgG and tear IgA levels in a cohort of healthy individuals with no history of AK revealed that 50 to 100 percent of these individuals possessed antibodies against Acanthamoeba antigens [10]. Interestingly, the serum IgG and tear IgA levels were significantly lower in patients with AK compared to the cohort of normal individuals with no history of AK. These findings suggest a significant role of the mucosal immune system in preventing AK [17,18].
This hypothesis was tested in the Chinese hamster and pig models of AK. Both the pig and the Chinese hamster develop a self-limiting disease that resolves within 3 to 4 weeks of infection. However, in both animal models, resolution of ocular infections does not provide resistance to reinfection with Acanthamoeba trophozoites. This strongly suggests that the adaptive immune response is either not induced by the corneal infections or is generated but is ineffectual in eliminating infectious Acanthamoebae [19].
In contrast to the adaptive immune response, a significant body of evidence suggests that cells of the innate immune apparatus, namely neutrophils and macrophages, play a significant role in the resolution of AK in Chinese hamsters. Elimination of conjunctival macrophages with liposomes containing the macrophagicidal drug, clodronate (dichloromethylene diphosphonate), prevents resolution of AK [10,19]. A similar exacerbation of AK occurs if neutrophils are eliminated by the in vivo administration of an antibody that depletes neutrophils. Importantly, in vitro studies have shown that both neutrophils and macrophages are capable of killing trophozoites [20].
A puzzling feature of AK is the almost complete absence of intraocular infections in Acanthamoeba patients. Of the hundreds of cases of AK that have been reported since the original description of this ocular disease in 1975, less than six cases culminated in the penetration of Acanthamoeba trophozoites into the posterior portion of the eye [21-23]. Studies in Chinese hamster model of AK have shown that the trophozoites are indeed capable of penetrating the cornea and entering the anterior chamber of the eye. Moreover, injection of as many as one million trophozoites into the anterior chamber of the Chinese hamster eye fails to produce infections in the retina or any portion of the posterior segment of the eye [24]. Within 24 hours of intraocular injection, trophozoites are incarcerated by neutrophils and are totally eliminated from the eye 15 days after injection. Importantly, there was no evidence of trophozoite invasion of the posterior regions of the eye and no discernible injury to the retina [24]. Collectively, these studies suggest that neutrophils act as an important barrier in preventing the progression of AK to endophthalmitis or retinitis.
Complement and Acanthamoeba Keratitis
The complement system is an important barrier to many microbial infections and acts in a cascade-like manner to kill bacterial and protozoal pathogens. The complement cascade can be activated by bacterial products through the alternative pathway or by complement-fixing antibodies through the classical pathway. Thus, the complement system straddles the innate and adaptive immune systems [25]. Activation of the complement pathway during AK can occur through the classical pathway by specific antibodies directed against antigens on the surface of Acanthamoeba trophozoites. In addition, mannosylated glycoproteins on the surface of Acanthamoeba might activate the alternative pathway of the complement cascade. The role of complement in the pathogenesis of AK is unresolved. In vitro studies using normal human serum have demonstrated that Acanthamoeba trophozoites are susceptible to complement-mediated lysis either in the presence or absence of antibody [26]. By contrast, studies using pathogenic strains of Acanthamoeba have demonstrated that the Acanthamoeba trophozoites are resistant to complement-mediated lysis due to their expression of complement regulatory proteins that disable the complement cascade [27]. These latter studies further demonstrated that treatment of the pathogenic strains of Acanthamoeba with metabolic inhibitors such as cytochalasin D, or serine protease inhibitor such as PMSF, which prevent the production of complement regulatory proteins, leads to increased susceptibility of pathogenic trophozoites to complement-mediated lysis. These observations are further supported by animal studies which have demonstrated that systemic immunization or generation of complement fixing antibodies does not influence the severity or duration of the disease [10].
Toll-like Receptors in Acanthamoeba Keratitis
In addition to neutrophils and macrophages, other important components of the innate immune system are the Toll-like receptors. The human cornea is exposed to a wide variety of ubiquitous pathogenic microorganisms. The ability of the cornea to recognize these pathogens and initiate an appropriate immune defense mechanism is crucial for maintaining corneal clarity and vision. The innate immune apparatus of the cornea consists of cell surface molecules that detect pathogen-associated molecular patterns (PAMP) that are expressed by molecules that are frequently expressed on microbial pathogens. Toll-like receptors (TLRs) often exist as transmembrane proteins that are expressed on various cells and serve to detect pathogens in the external environment. TLRs have extracellular and cytoplasmic domains that respond to PAMPs, which are expressed by many pathogens but are not displayed on host cells. This enables the innate immune system to distinguish between self and non-self. Several in vitro and in vivo studies have demonstrated that TLR-4 is upregulated during AK. Activation of the TLR-4 pathway stimulates the MyD88/ERK pathway leading to release of the proinflammatory cytokines such as IL-8, CXCL2 and TNF-α. An increased level of CXCL2 in animal models of AK also correlates with the recruitment of neutrophils. The presence of neutrophils complicates AK by acting as a double-edged sword. Neutrophils provide protection against the pathogen but if unbridled, they inflict significant damage to innocent bystander cells in the cornea [28].
Adaptive Immune Responses in AK
As mentioned earlier, Acanthamoeba spp. are ubiquitously distributed in a wide range of environments. Exposure to Acanthamoeba is commonplace as 90 to 100 percent of the adult population tested with no previous history of Acanthamoeba infections expressed serum antibodies specific for Acanthamoeba antigens. These finding suggest that exposure to Acanthamoeba is common, yet the disease is remarkably rare.
Animal studies have demonstrated that subcutaneous immunization with Acanthamoeba trophozoites and cysts induces a robust adaptive immune response in the form of delayed-type hypersensitivity (DTH) and IgG antibodies, yet fails to protect against ocular infection. Although mucosal immunization with Acanthamoeba antigens elicits secretory IgA antibodies in the tears, which prevent the initial binding of the trophozoites to the corneal epithelium, IgA antibodies cannot eliminate the amoebae once they have entered the corneal stroma [10]. Recent studies in a mouse model of AK have demonstrated a role for adaptive immune responses in the form of Th17 cells [29]. IL-17A plays a crucial role in the migration and activation of neutrophils. The predominance of IL-17A levels and neutrophils in the mouse model of AK reiterates the importance of neutrophils in mitigating AK. Even though IL-17A has been shown to promote inflammation and tissue damage in HSV and Pseudomonas keratitis, increased levels of IL-17A have the opposite effect and mitigate the severity and symptoms of AK [29].
Mucosal Immunity in AK
Mild corneal trauma produced by contact lens wear leads to upregulation of mannosylated glycoproteins on corneal surface. The pathogenic cascade of AK begins with the adhesion of the trophozoites on mannosylated corneal glycoproteins. Importantly, corneal trauma or abrasion is an important prerequisite for the development of AK in animal models. However, the mucus produced by the healthy conjunctiva along with the naturally anti-microbial components in the tear film reduces the likelihood of trophozoite binding to the corneal epithelium. The importance of mucosal immunity was firmly established in animal studies in which oral immunization with Acanthamoeba antigens along with a mucosal adjuvant (neutralized cholera toxin) protected hosts from corneal infections with Acanthamoeba trophozoites. However, the IgA antibodies in the tears of these hosts were not able to affect the viability of the trophozoites and oral immunizations failed to affect the disease course if it was initiated after trophozoites had penetrated the corneal surface. These observations suggest that mucosal immunity in the form of secretory IgA antibodies plays an important role only during the initial stages of the pathogenesis by preventing the adhesion of the trophozoites on to the corneal surface [30].
A recent study using a rabbit model of AK bypassed the initial adhesion step and produced corneal infections by intrastromal injections of Acanthamoeba trophozoites [31]. In this model, oral immunization via the mucosal route induced an IgA response which mitigated corneal disease. These observations contradict the previous observations as to how secretory IgA antibodies in the tears offer protection by preventing the adhesion of the trophozoites. Upon interaction with the pathogens, IgA can have diverse effector functions including prevention of adhesion of trophozoites, interactions with the Fcα receptors of neutrophils or activation of the complement. IgA has been shown to activate complement cascade via the alternate/lectin pathway. In the case of Acanthamoeba keratitis, IgA antibodies may be playing a role in activating the neutrophils, which in turn trigger the complement alternate pathway which further enhances the neutrophil responses and helps in mitigating the disease [31].
Is there an Immune-mediated Component of Acanthamoeba Keratitis?
It is noteworthy that two major causes of infectious keratitis, Pseudomonas keratitis and herpes simplex virus (HSV) stromal keratitis, are immune-mediated diseases. In the case of HSV keratitis the blinding lesions of the cornea are believed to be produced by the T cell responses to HSV antigens [32,33]. Importantly, corneal infections with HSV do not produce blinding keratitis in T cell-deficient nude mice. Although the T cell-deficient mice do not develop keratitis, they ultimately die from viral encephalitis. A body of clinical evidence hints that a similar condition might occur in AK. Chronic corneal inflammation and scleritis are common features of AK that often occur in the absence of detectable Acanthamoeba trophozoites or cysts in the inflamed lesions [34]. Scleritis is an especially perplexing problem that occurs in approximately 10 percent of the cases of AK and often requires the aggressive use of anti-inflammatory drugs [34-37]. Histological examination of scleral biopsies has revealed the presence of degenerated and necrotic cysts, yet viable organisms could not be cultured from the scleral lesions, which has led some to conclude that Acanthamoeba scleritis is an immune-mediated process that is initiated by a T cell-dependent immune response to Acanthamoeba antigens in the ocular lesions that persists in the absence of Acanthamoeba cysts or trophozoites [34]. One plausible explanation to account for the persistence of inflammation occurring in the absence of detectable Acanthamoeba trophozoites or cysts could be through the development of molecular mimicry in which Acanthamoeba antigens elicit an immune response that leads to the generation of T cell clones that cross-react with antigens expressed in the normal eye, which leads to the generation of additional T cell clones by a process called “epitope spreading”. Epitope spreading is a condition in which the adaptive immune response leads to the emergence of T and B cell subpopulations that have broader antigen specificity than those induced by the initial antigen exposure [38]. A possible example of this was described in case reports of ischemic inflammation that occurred in the retinas of four patients who were diagnosed with persistent AK [39]. Although histopathologic examination revealed Acanthamoeba cysts in the corneas of the patients, neither cysts nor trophozoites could be detected in the posterior regions of the eye. This is consistent with the typical clinical course of ocular Acanthamoeba infections in which trophozoites do not penetrate or invade the poster regions of the infected eye. Over the past 30 years there have been only 8 cases of extra corneal invasion of Acanthamoeba [21-23,40,41]. In the aforementioned report of severe ischemic inflammation that occurred in the retinas of four patients, histopathological analysis revealed that the retinal lesions were characterized by a perivascular lymphocytic infiltration, thrombosis of the retinal arteries, and ischemia. Although perivascular cuffing, thrombosis, and ischemia are generalized inflammatory findings in a number of maladies, they are a consistent feature of classic DTH lesions. Moreover, the possible existence of antigenic mimicry between Acanthamoeba and elements of the central nervous system (which includes the retina) is supported by a recent report indicating that mice infected with A. castellanii develop a central nervous system autoimmune disease through the generation of cross-reactive T cells that recognize myelin antigens [42]. Thus, what begins as an infection at the ocular surface can end in an immune-mediated disease of the retina and possibly the brain [39,42].
Conundrums and Unresolved Issues
In 1939, Winston Churchill referred to Russia as “… a riddle, wrapped in a mystery, inside an enigma”. One might characterize Acanthamoeba and the infections that it produces in much the same way. Acanthamoeba species are found in virtually every environmental niche on our planet ranging from thermal springs to beneath frozen ice. Acanthamoeba cysts can withstand remarkably high levels of ultraviolet and gamma irradiation that are toxic to all non-malignant mammalian cells, yet the cysts remain viable and pathogenic. Cysts are long-lived and retain their viability for over two decades under desiccating conditions. Acanthamoeba spp. can be isolated from the nasopharyngeal washes from almost a third of the healthy asymptomatic individuals sampled. Exposure to Acanthamoeba is common, with some serological surveys reporting the presence of serum antibodies specific for Acanthamoeba antigens in > 90 percent of asymptomatic individuals with no history of Acanthamoeba keratitis. Over 30 million people in the United States wear contact lenses, which places them in the group with the highest risk for developing AK, yet less than 150 of these will go on to develop AK > in a given year. Trophozoites ravage the cornea leaving dead and dying cells in their wake, yet the amoebae do not proceed beyond the cornea and of the hundreds of cases of AK reported, less than six have gone on to affect the retina.
Both cysts and trophozoites are strongly immunogenic and can induce robust DTH and serum IgG antibody responses, yet these adaptive immune elements do not protect against corneal infections nor are they necessary for the resolution of AK in animal models. Although granulomatous amoebic encephalitis is associated with immunocompromised patients, there is no evidence that the incidence of AK is higher in immunocompromised transplant recipients or AIDS patients.
We tend to think of the adaptive immune response as being designed to kill microorganisms, yet studies on AK have demonstrated that the mucosal immune system can be a highly effective barrier that prevents the trophozoites from gaining a foothold in the cornea without directly killing the microorganisms. The combined use of in vitro and in vivo experiments with Acanthamoeba trophozoites have revealed insights that could not have been realized otherwise [6,10]. For example, in vitro assays revealed that trophozoites from soil isolates of Acanthamoeba produced extensive cytolysis of corneal cells, which on first blush would suggest that these environmental strains would be highly pathogenic in vivo. However, in vivo experiments revealed that soil isolates of Acanthamoeba failed to produce corneal infections. Further examination revealed that soil isolates of Acanthamoeba had a profoundly reduced capacity to bind to corneal epithelial cells compared to clinical isolates from AK patients. Thus, relying on a single criterion (i.e, in vitro cytopathogenicity) to define pathogenicity is flawed and reminds us of the importance of verifying results in vivo or “in vivo veritas” (roughly translated from Latin, “in a living thing there is truth”). The reader is referred to several excellent review papers that discuss the importance of in vivo corroboration of in vitro findings [43-47].
Future Directions for AK Research
Despite a better understanding of the pathogenesis of Acanthamoeba keratitis, much remains to be learned for improving current forms of therapy, especially for Acanthamoeba scleritis. One of the most challenging features of AK is the emergence of infectious trophozoites from dormant cysts. Cysts can remain dormant for several years and trauma to the cornea or the therapeutic use of topical corticosteroids for unrelated ocular inflammation can trigger their excystment and increase the chances for arousing dormant cysts to active infectious trophozoites. It is still unclear as to the minimal number of cysts needed to develop a recurrent infection or how to rid the eye of dormant cysts. The development of novel contact lens care solutions and better diagnosis, may lead to decreased incidence of AK. However, the small percentage of people who are susceptible to this disease will benefit from a better treatment regimen that will prevent the need for corneal transplants to restore vision. Therapies or vaccines targeting the disease and blocking the pathogenic cascade of Acanthamoeba might be important adjuncts to antimicrobial therapy. Future studies to improve our understanding of the pathogenesis and immunology of AK may provide better insights as to why healthy immunocompetent individuals develop this disease, why it never progresses beyond cornea, and why the adaptive immune response fails to protect against future bouts of AK.
Acknowledgments
Supported in part by an unrestricted grant from the Research to Prevent Blindness.
Glossary
- AK
Acanthamoeba keratitis
- GAE
granulomatous amoebic encephalitis
- PHMB
polyhexamethylene biguanide
- DTH
Delayed type hypersensitivity
- IgA
Immunoglobulin A
- IgG
Immunoglobulin G
- MIP-133
Mannose induced protein
References
- Marciano-Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev. 2003;16(2):273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015;22:10. doi: 10.1051/parasite/2015010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awwad ST, Parmar DN, Heilman M. et al. Results of penetrating keratoplasty for visual rehabilitation after Acanthamoeba keratitis. Am J Ophthalmol. 2005;140(6):1080–1084. doi: 10.1016/j.ajo.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Mathers WD. Acanthamoeba: a difficult pathogen to evaluate and treat. Cornea. 2004;23(4):325. doi: 10.1097/00003226-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Panjwani N. Pathogenesis of acanthamoeba keratitis. Ocul Surf. 2010;8(2):70–79. doi: 10.1016/s1542-0124(12)70071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DW, Niederkorn JY. The pathophysiology of Acanthamoeba keratitis. Trends Parasitol. 2006;22(4):175–180. doi: 10.1016/j.pt.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Hurt M, Neelam S, Niederkorn J. et al. Pathogenic Acanthamoeba spp secrete a mannose-induced cytolytic protein that correlates with the ability to cause disease. Infect Immun. 2003;71(11):6243–6255. doi: 10.1128/IAI.71.11.6243-6255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi T, Abdi M, Alizadeh H. Role of phospholipase A(2) (PLA(2)) inhibitors in attenuating apoptosis of the corneal epithelial cells and mitigation of Acanthamoeba keratitis. Exp Eye Res. 2013;113(182):191. doi: 10.1016/j.exer.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh H, Neelam S, Niederkorn JY. Effect of immunization with the mannose-induced Acanthamoeba protein and Acanthamoeba plasminogen activator in mitigating Acanthamoeba keratitis. Invest Ophthalmol Vis Sci. 2007;48(12):5597–5604. doi: 10.1167/iovs.07-0407. [DOI] [PubMed] [Google Scholar]
- Clarke DW, Niederkorn JY. The immunobiology of Acanthamoeba keratitis. Microbes Infect. 2006;8(5):1400–1405. doi: 10.1016/j.micinf.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Badenoch PR, Johnson AM, Christy PE. et al. Pathogenicity of Acanthamoeba and a Corynebacterium in the rat cornea. Arch Ophthalmol. 1990;108(1):107–112. doi: 10.1001/archopht.1990.01070030113040. [DOI] [PubMed] [Google Scholar]
- Alizadeh H, Neelam S, Hurt M. et al. Role of contact lens wear, bacterial flora, and mannose-induced pathogenic protease in the pathogenesis of amoebic keratitis. Infect Immun. 2005;73(2):1061–1068. doi: 10.1128/IAI.73.2.1061-1068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche TR, Gautom RK, Seyedirashti S. et al. Occurrence of bacterial endosymbionts in Acanthamoeba spp. isolated from corneal and environmental specimens and contact lenses. J Clin Microbiol. 1993;31(5):1122–1126. doi: 10.1128/jcm.31.5.1122-1126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovieno A, Ledee DR, Miller D. et al. Detection of bacterial endosymbionts in clinical acanthamoeba isolates. Ophthalmology. 2010;117(3):445–452. doi: 10.1016/j.ophtha.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Esser S, Toenshoff ER, Haider S. et al. Diversity of bacterial endosymbionts of environmental acanthamoeba isolates. Appl Environ Microbiol. 2008;74(18):5822–5831. doi: 10.1128/AEM.01093-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehr-Green JK, Bailey TM, Visvesvara GS. The epidemiology of Acanthamoeba keratitis in the United States. Am J Ophthalmol. 1989;107(4):331–336. doi: 10.1016/0002-9394(89)90654-5. [DOI] [PubMed] [Google Scholar]
- Niederkorn JY. The role of the innate and adaptive immune responses in Acanthamoeba keratitis. Arch Immunol Ther Exp (Warsz) 2002;50(1):53–59. [PubMed] [Google Scholar]
- Niederkorn JY, Alizadeh H, Leher HF. et al. The immunobiology of Acanthamoeba keratitis. Springer Semin Immunopathol. 1999;21(2):147–160. doi: 10.1007/BF00810247. [DOI] [PubMed] [Google Scholar]
- Van Klink F, Leher H, Jager MJ. et al. Systemic immune response to Acanthamoeba keratitis in the Chinese hamster. Ocul Immunol Inflamm. 1997;5(4):235–244. doi: 10.3109/09273949709085064. [DOI] [PubMed] [Google Scholar]
- Hurt M, Apte S, Leher H. et al. Exacerbation of Acanthamoeba keratitis in animals treated with anti-macrophage inflammatory protein 2 or antineutrophil antibodies. Infect Immun. 2001;69(5):2988–2995. doi: 10.1128/IAI.69.5.2988-2995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffler KF, Eckhardt TJ, Reboli AC. et al. Acanthamoeba endophthalmitis in acquired immunodeficiency syndrome. Am J Ophthalmol. 1996;122(4):584–586. doi: 10.1016/s0002-9394(14)72126-9. [DOI] [PubMed] [Google Scholar]
- Jones DB, Visvesvara GS, Robinson NM. Acanthamoeba polyphaga keratitis and Acenthamoeba uveitis associated with fatal meningoencephalitis. Trans Ophthalmol Soc U K. 1975;95(2):221–232. [PubMed] [Google Scholar]
- Moshari A, McLean IW, Dodds MT. et al. Chorioretinitis after keratitis caused by Acanthamoeba: case report and review of the literature. Ophthalmology. 2001;108(12):2232–2236. doi: 10.1016/s0161-6420(01)00765-5. [DOI] [PubMed] [Google Scholar]
- Clarke DW, Alizadeh H, Niederkorn JY. et al. Failure of Acanthamoeba castellanii to produce intraocular infections. Invest Ophthalmol Vis Sci. 2005;46(7):2472–2478. doi: 10.1167/iovs.05-0140. [DOI] [PubMed] [Google Scholar]
- Rus H, Cudrici C, Niculescu F. The role of the complement system in innate immunity. Immunol Res. 2005;33(2):103–112. doi: 10.1385/IR:33:2:103. [DOI] [PubMed] [Google Scholar]
- Ferrante A, Rowan-Kelly B. Activation of the alternative pathway of complement by Acanthamoeba culbertsoni. Clin Exp Immunol. 1983;54(2):477–485. [PMC free article] [PubMed] [Google Scholar]
- Toney DM, Marciano-Cabral F. Resistance of Acanthamoeba species to complement lysis. J Parasitol. 1998;84(2):338–344. [PubMed] [Google Scholar]
- Alizadeh H, Tripathi T, Abdi M. et al. Pathogenic strains of Acanthamoeba are recognized by TLR4 and initiated inflammatory responses in the cornea. PLoS One. 2014;9(3):e92375. doi: 10.1371/journal.pone.0092375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawanshi A, Cao Z, Sampson JF. et al. IL-17A-mediated protection against Acanthamoeba keratitis. J Immunol. 2015;194(2):650–663. doi: 10.4049/jimmunol.1302707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh H, Apte S, El-Agha MS. et al. Tear IgA and serum IgG antibodies against Acanthamoeba in patients with Acanthamoeba keratitis. Cornea. 2001;20(6):622–627. doi: 10.1097/00003226-200108000-00013. [DOI] [PubMed] [Google Scholar]
- Feng X, Zheng W, Wang Y. et al. A Rabbit Model of Acanthamoeba Keratitis That Better Reflects the Natural Human Infection. Anat Rec (Hoboken) 2015;298(8):1509–1517. doi: 10.1002/ar.23154. [DOI] [PubMed] [Google Scholar]
- Hazlett LD, McClellan SA, Barrett RP. et al. Spantide I decreases type I cytokines, enhances IL-10, and reduces corneal perforation in susceptible mice after Pseudomonas aeruginosa infection. Invest Ophthalmol Vis Sci. 2007;48(2):797–807. doi: 10.1167/iovs.06-0882. [DOI] [PubMed] [Google Scholar]
- Metcalf JF, Hamilton DS, Reichert RW. Herpetic keratitis in athymic (nude) mice. Infect Immun. 1979;26(3):1164–1171. doi: 10.1128/iai.26.3.1164-1171.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovieno A, Gore DM. Acanthamoeba sclerokeratitis: epidemiology, clinical features, and treatment outcomes. Ophthalmology. 2014;121(12):2340–2347. doi: 10.1016/j.ophtha.2014.06.033. [DOI] [PubMed] [Google Scholar]
- Bacon AS, Frazer DG, Dart JK. et al. A review of 72 consecutive cases of Acanthamoeba keratitis, 1984-1992. Eye (Lond) 1993;7(Pt 6):719–725. doi: 10.1038/eye.1993.168. [DOI] [PubMed] [Google Scholar]
- Lee GA, Gray TB, Dart JK. et al. Acanthamoeba sclerokeratitis: treatment with systemic immunosuppression. Ophthalmology. 2002;109(6):1178–1182. doi: 10.1016/s0161-6420(02)01039-4. [DOI] [PubMed] [Google Scholar]
- Mannis MJ, Tamaru R, Roth AM. et al. Acanthamoeba sclerokeratitis. Determining diagnostic criteria. Arch Ophthalmol. 1986;104(9):1313–1317. doi: 10.1001/archopht.1986.01050210067027. [DOI] [PubMed] [Google Scholar]
- Cornaby C, Gibbons L, Mayhew V. et al. B cell epitope spreading: mechanisms and contribution to autoimmune diseases. Immunol Lett. 2015;163(1):56–68. doi: 10.1016/j.imlet.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Awwad ST, Heilman M, Hogan RN. et al. Severe reactive ischemic posterior segment inflammation in acanthamoeba keratitis: a new potentially blinding syndrome. Ophthalmology. 2007;114(2):313–320. doi: 10.1016/j.ophtha.2006.07.038. [DOI] [PubMed] [Google Scholar]
- Arnalich-Montiel F, Jaumandreu L, Leal M. et al. Scleral and intraocular amoebic dissemination in Acanthamoeba keratitis. Cornea. 2013;32(12):1625–1627. doi: 10.1097/ICO.0b013e31829ded51. [DOI] [PubMed] [Google Scholar]
- Ebrahimi KB, Green WR, Grebe R. et al. Acanthamoeba sclerokeratitis. Graefes Arch Clin Exp Ophthalmol. 2009;247(2):283–286. doi: 10.1007/s00417-008-0955-8. [DOI] [PubMed] [Google Scholar]
- Massilamany C, Marciano-Cabral F, Rocha-Azevedo B. et al. SJL mice infected with Acanthamoeba castellanii develop central nervous system autoimmunity through the generation of cross-reactive T cells for myelin antigens. PLoS One. 2014;9(5):e98506. doi: 10.1371/journal.pone.0098506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosco-Vilbois MH, Imhof BA. In vivo veritas. Immunol Today. 2000;21(2):64–65. doi: 10.1016/s0167-5699(99)01563-7. [DOI] [PubMed] [Google Scholar]
- Lotze MT. In vivo veritas. Clin Immunol. 1999;93(1):1–4. doi: 10.1006/clim.1999.4786. [DOI] [PubMed] [Google Scholar]
- Matarese G, La Cava A, Horvath TL. In vivo veritas, in vitro artificia. Trends Mol Med. 2012;18(8):439–442. doi: 10.1016/j.molmed.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin HW. In vivo veritas: pathogenesis of infection as it actually happens. Nat Immunol. 2007;8(11):1143–1147. doi: 10.1038/ni1529. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Tursz T. In vivo veritas. Nat Biotechnol. 2005;23(11):1372–1374. doi: 10.1038/nbt1105-1372. [DOI] [PubMed] [Google Scholar]