Abstract
Unlike many of the nontyphoidal Salmonella serovars such as S. Typhimurium that cause restricted gastroenteritis, Salmonella Typhi is unique in that it causes life-threatening typhoid fever in humans. Despite the vast difference in disease outcomes that S. Typhi and S. Typhimurium cause in humans, there are few genomic regions that are unique to S. Typhi. Of these regions, the most notable is the small locus encoding typhoid toxin, an AB toxin that has several distinct characteristics that contribute to S. Typhi’s pathogenicity. As a result, typhoid toxin and its role in S. Typhi virulence have been studied in an effort to gain insight into potential treatment and prevention strategies. Given the rise of multidrug-resistant strains, research in this area has become increasingly important. This article discusses the current understanding of typhoid toxin and potential directions for future research endeavors in order to better understand the contribution of typhoid toxin to S. Typhi virulence.
Keywords: Salmonella Typhi pathogenesis, typhoid fever, bacterial toxins, bacterial pathogenesis, host-pathogen interactions
Introduction
Salmonella enterica serovar Typhi (S. Typhi) is the cause of typhoid fever in humans. It remains a major global health concern due to the continuous, widespread outbreaks in Southeast Asia and sub-Saharan Africa [1-4]. When antibiotics are able to successfully control S. Typhi infections, typhoid fever mortality rate is estimated at approximately 1 percent – resulting in ~200,000 annual deaths mostly among children in developing countries [1-4]. However, multidrug-resistant strains of S. Typhi have emerged and become prevalent in typhoid outbreak areas, which suggests that the mortality rate will likely increase in the future if new treatment options are not developed. For instance, in 2010, only 7 percent of typhoid fever cases in Malawi were multidrug-resistant, compared to 2014, 97 percent of typhoid fever cases were resistant to antibiotics [5,6]. A better understanding of the pathogenic mechanisms of S. Typhi infections would provide critical insight into new opportunities to develop specific treatment and prevention strategies, which would be useful in treating against multidrug-resistant S. Typhi infections.
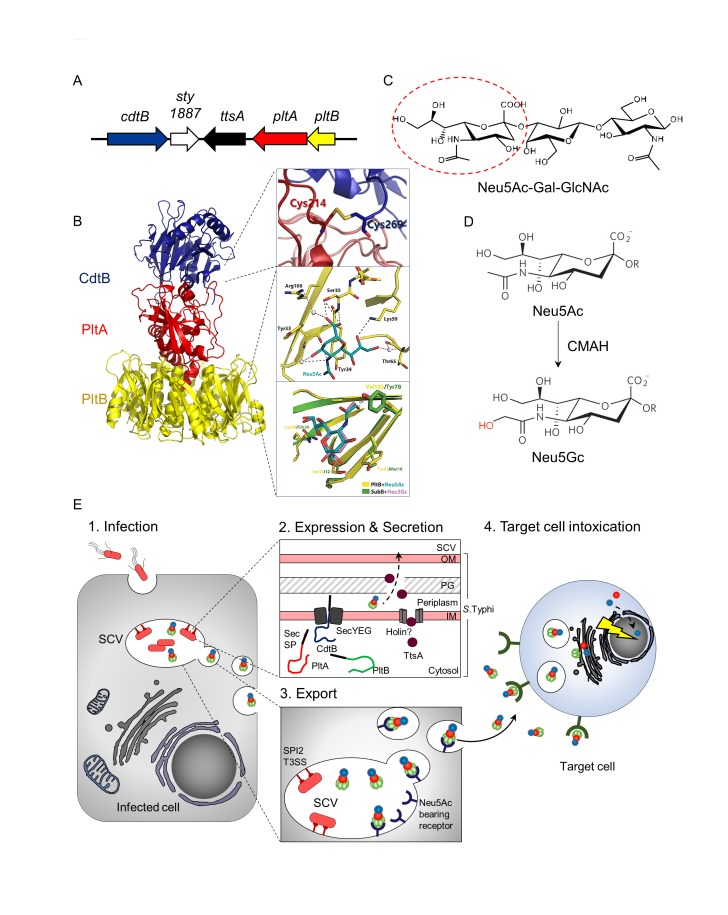
Typhoid toxin has been discovered via genome comparison analysis of S. Typhi to that of S. Typhimurium [7]. The small island encoding all three components of typhoid toxin, along with two other genes, one of which is required for typhoid toxin secretion, is present in the S. Typhi genome, but not in the counterpart of S. Typhimurium [8] (Figure 1A). S. Typhi expresses active typhoid toxin, as demonstrated by the observation that human cells treated with culture supernatants of S. Typhi-infected cells induced G2 cell cycle arrest that is a readout of the typhoid toxin intoxication process [7]. Furthermore, the in vivo function of typhoid toxin has been investigated in two types of the mouse model. In an acute-phase mouse model, systemic administration of recombinant typhoid toxin recapitulates many of the acute phase symptoms that are characteristics of typhoid fever in humans [9]. In a persistent infection mouse model, known as a humanized mouse model that is able to partially support S. Typhi infection, typhoid toxin involves in the establishment of S. Typhi persistent infection, presumably by altering the immune cell functions to its favor, although the underlying molecular mechanism(s) awaits to be understood [10]. In this model, it was shown that immunodeficient mice that were engrafted with human stem cells were able to support S. Typhi infection [10]. Recent human subject research implies the critical function of typhoid toxin in S. Typhi virulence. For example, convalescent typhoid fever patients showed high titers of antibodies specific to the components of typhoid toxin in their sera, which also indicates that typhoid toxin is produced during S. Typhi infection of humans [11,12].
Figure 1.
Typhoid toxin’s distinct molecular and cellular features contribute to its unique pathogenic mechanisms. (A) Schematic representation of the S. Typhi pathogenicity islet encoding typhoid toxin components, ttsA, and sty1887. (B) Distinct A2B5 typhoid toxin complex. Homopentameric receptor binding B subunit PltB (yellow or green in 1E) associates with a ADP-ribosyl transferase, PltA (red) via hydrophobic interactions. CdtB (blue), the A subunit of cytolethal distending toxin links to the PltB/PltA complex by a single disulfide bond through distinctly positioned extra cysteine residues in both PltA and CdtB (inset). The receptor-binding pocket of PltB is shown in detail, indicating the amino acid residues that are present (inset). Val 103 in the binding pocket is a nonpolar residue on PltB that allows it to interact with Neu5Ac-terminated glycans (inset). (C) Sialoglycan consensus sequence Neu5Acα2,3Galβ1,3/β1,4GlcNAc/Glc to which PltB binds. (D) Humans do not express functional CMAH enzyme, which converts Neu5Ac that binds toxin to Neu5Gc that does not bind toxin. Humans are therefore susceptible to typhoid toxin, while chimpanzees and other non-human primates are resistant. (E) Cartoon of typhoid toxin trafficking. Toxin is expressed by intracellular S. Typhi from infected cells (1. Infection and 2. Expression & Secretion), and the toxin is then ferried to the extracellular environment (3. Export) and enters into target cells to intoxicate them (4. Target cell intoxication). The toxin in the extracellular milieu binds to cells that can be remotely located, including immune cells in circulation and in the spleen as well as brain endothelial cells. Neu5Ac, N-acetylneuraminic acid, Gal, galactose, GlcNAc, N-acetylglucosamine, Neu5Gc, N-glycolylneuraminic acid, CMAH, cytidine monophosphate-N-acetylneuraminic acid hydroxylase, TTSS, type III secretion system, SPI2, Salmonella pathogenicity island 2, SCV, Salmonella containing vacuole, Sec SP, Sec-dependent signal peptide, IM, inner membrane, OM, outer membrane, PG, peptidoglycan.
Typhoid toxin belongs to the family of bacterial AB toxins consisting of an “A” enzymatic subunit and a “B” receptor binding subunit. Bacterial AB toxins are secreted virulence factors that often play an essential role in virulence to the related bacterial pathogens. However, typhoid toxin has several features that are different from that of other AB toxins. For instance, the organization of typhoid toxin is unique in that it has two enzymatic “A” subunits as opposed to just one. This highlights a remarkable molecular evolution as to how two separate toxins become one [9] and therefore implies the potentially distinct virulence contributions of typhoid toxin to S. Typhi infection. Anthrax toxin also has two enzymatic subunits, but its A subunits form two individual toxin complexes that have one A subunit at a time [13]. Therefore, the organization of typhoid toxin is the first of its kind to be discovered. The trafficking mechanism of typhoid toxin is another feature that differentiates it from other AB toxins. Since typhoid toxin is exclusively produced by intracellular S. Typhi in infected cells, it indicates that Salmonella-containing vacuole (SCV) is involved in export trafficking of typhoid toxin within infected cells to extracellular milieu. This mechanism helps typhoid toxin to be trafficked out of the infected host cell to find and intoxicate other target cells. The host adaptation of typhoid toxin is also notable. Typhoid toxin is adapted to one type of sialic acid that is abundantly expressed in humans. This phenomenon is in agreement with S. Typhi’s inability to cause typhoid fever in hosts other than humans. Finally, its tissue tropism is an important feature of this toxin. Even though the typhoid toxin receptor is rather ubiquitously expressed, when typhoid toxin is systemically administered into mice, typhoid toxin exclusively targets the immune system and the central nervous system, which corresponds to many symptoms of typhoid fever. The binding of typhoid toxin to immune cells also implies that there is an underlying mechanism of typhoid toxin that helps establish S. Typhi chronic infection. In this article, we will discuss the details of the previously mentioned discoveries and the current status and future direction of research on typhoid toxin to better understand its contribution to S. Typhi virulence.
Salmonella Typhi and Typhoid Fever
S. Typhi is the etiologic agent of the human systemic infectious disease typhoid fever. S. Paratyphi is the closely related serovar that causes a similar disease known as paratyphoid fever [14]. Both S. Typhi and S. Paratyphi infections are human-restricted, although some higher primates such as chimpanzees can be experimentally infected [2,15,16]. In contrast, nontyphoidal Salmonella serovars such as S. Typhimurium infect both humans and other animals, and cause gastroenteritis known as Salmonellosis in humans [17-19]. Approximately 90 percent of the S. Typhi and S. Typhimurium genomes are homologous, and few genes are unique to the genome of S. Typhi. A notable large region specific to S. Typhi is Salmonella pathogenicity island 7 (SPI-7) that encodes the viaB locus consisting of 10 genes related to the biosynthesis and export of Vi polysaccharide capsule, which involves in circumventing Toll-like receptor-mediated immune surveillance [20,21]. Another notable small island is the locus encoding five genes including three typhoid toxin components, a bacteriophage muramidase homolog controlling typhoid toxin secretion, and a small gene of unknown function (Figure 1A) [8,22]. The presence of over 200 putative pseudogenes in the S. Typhi genome, most of which are functional in S. Typhimurium is also a distinct feature, which is considered related to the narrow host adaptation of S. Typhi, compared to the broad host range serovar S. Typhimurium [23-25]. In agreement with this observation, S. Typhi expresses a significantly smaller number of effector proteins than most other serovars, although there are highly conserved virulence components among serovars, such as two type III secretion systems including their expression regulators that are essential in the interaction of Salmonella enterica with host cells, which delivers bacterial effector proteins into host cells to modulate cellular functions to their favor [17,26-39]. One of the missing effectors from S. Typhi is GtgE, which is involved in neutralizing a host restriction pathway that prevents its growth in macrophages of nonpermissive species [40,41].
Typhoid fever is accompanied by various acute-phase symptoms including fever and abdominal pain, immunologic symptoms such as leukopenia, and in some instances neurological complications [42]. Neuropsychiatric manifestations have been observed at a range of 5 percent to 84 percent [43-46]. Two distinct presentations of typhoid fever-associated neurological complications are reported: (i) the acute confusional state or delirium and (ii) delayed neurological complications that become apparent after the fever is subsidized [47]. The latter complication has long been reported, although not so frequently, but more frequently among patients resistant to treatment, which directly related to the S. Typhi infection and in some instances involves upper motor function defects, although the pathogenesis is not known [43-48]. A small portion (1 to 6 percent) of patients infected with S. Typhi can become asymptomatic carriers by establishing a persistent infection that is associated with gall bladder infection [49,50]. A recent study using mice whose immune system had been humanized by fetal human stem cell grafting demonstrated that typhoid toxin helped S. Typhi establish persistent infection [10], which supports the notion that typhoid toxin involves the transmission of the infection among people for its long-term existence.
Typhoid Toxin is an Atypical AB Toxin, Forming an A2B5 Organization
The atomic structure of typhoid toxin, having a length of approximately 90 Å and a width of approximately 60 Å, demonstrates an unprecedented A2B5 organization with two covalently linked A subunits, CdtB and PltA, non-covalently associated with a pentameric B subunit PltB (Figure 1B). The evolution of uniquely positioned Cys residues in both CdtB and PltA has resulted in the adaptation of CdtB for its tethering to the PltA-PltB complex (Figure 1B inset). The complex consists of a short helix at the carboxyl-terminal end of PltA that inserts into the hydrophobic lumen of the PltB channel and therefore stabilizes it. More specifically, the A subunits, CdtB and PltA, are positioned in a tandem linear arrangement, which indicates that CdtB does not interact with the PltB subunit (Figure 1B). A disulfide bond, linking CdtB and PltA, is important for the assembly of typhoid toxin holotoxin in the periplasm of S. Typhi (Figure 1B inset), which ensures that CdtB and PltA are trafficked to the same target cell simultaneously throughout the entire trafficking process from the secretion from S. Typhi to SCV in infected cells to extracellular milieu to target cells (Figure 1E). After endocytosis and retrograde transport in target cells, CdtB and PltA are likely separated due to the reduction of the disulfide bond that links these subunits, which allows the two A subunits to be translocated to the cytoplasm and delivered to their place of action (Figure 1E).
PltA is a ADP-ribosyl transferase, which has the amino acid sequence as well as structural similarities to that of the pertussis toxin S1. The positions of the conserved catalytic residues (Glu 133 in typhoid toxin and Glu 129 in pertussis toxin S1), as well as the disulfide bonds (Cys 56-Cys 207 in typhoid toxin and Cys 41-Cys 201 in pertussis toxin S1) overlap almost completely [9,51]. Like the pertussis toxin activation process, the intra-subunit disulfide bond is anticipated to be reduced to allow the access of NAD and its putative substrates to the active site, and consequently, a reducing activating step is likely a required step before contacting its host cell target(s). CdtB, the A subunit of the cytolethal distending toxin (CDT) has a nuclease activity that induces cell-cycle arrest and/or cell death by damaging the DNA of the intoxicated cell [7,8]. Typhoid toxin CdtB aligns very well to the Haemophilus ducreyi CdtB [9,52]. The positions of the conserved catalytic residues in typhoid toxin’s CdtB, including CdtB His 160, overlap almost completely with those of its homolog in H. ducreyi. The receptor-binding B subunit PltB recognizes the specific sialoglycan consensus sequence, N-acetylneuraminic acid (Neu5Ac)α2,3Galactose (Gal)β1,3/β1,4Glucose (Glc)/N-acetylglucosamine (GlcNAc), which is rather abundantly expressed in humans [9] (Figure 1C and 1D). Among the trisaccharide-consensus sequence, terminal Neu5Ac glycan is the most important glycan in this binding, which directly fits in the binding pocket of PltB [53]. PltB Ser 35 is the most important residue for interaction with the typhoid-toxin glycan receptor (Figure 1B insert) [9]. The sialoglycan consensus sequence, Neu5Acα2,3Galβ1,3/β1,4GlcNAc/Glc, is displayed on ganglioside GD2, as well as on complex type N-linked glycoproteins. Typhoid toxin binds to ganglioside GD2, and N-glycans printed on glycan microarray chips, as well as naturally displayed on cells and tissues, although typhoid toxin preferentially binds to N-linked glycoproteins over ganglioside GD2, due to the laterally positioned PltB binding pocket [9,53,54] (Figure 1B).
Typhoid Toxin Secretion and Export Trafficking
Typhoid toxin’s secretion mechanism within intracellular S. Typhi in infected cells has been better understood. Typhoid toxin components, CdtB, PltA, and PltB, have an N-terminal Sec signal peptide, which mediates their trafficking from the cytoplasm to the periplasm through the Sec machinery in S. Typhi (Figure 1E). The genomic island that encodes typhoid toxin also contains two other genes, sty1887 and sty1889 (a.k.a. ttsA). The latter gene has been recently determined to be necessary for toxin secretion from the periplasm of S. Typhi to the SCV in infected cells, and was renamed as ttsA (for typhoid toxin secretion A) [22]. TtsA belongs to a group of bacteriophage muramidases that hydrolyze N-acetylmuramic residues of peptidoglycan, so that it assists typhoid toxin secretion from the periplasm to the SCV (Figure 1E). However, how TtsA protein is exported from the bacterial cytoplasm to the periplasm remains to be understood, as it lacks an identifiable N-terminal sec-dependent secretion signal. There are few examples of other muramidases that are transported to the periplasm by holins that are often encoded directly next to muramidases, which suggests the role of holins in TtsA delivery to the periplasm [22] (Figure 1E).
After its secretion to the SCV in infected cells, typhoid toxin has a rather unusual trafficking mechanism that may be linked to S. Typhi’s host specificity. Unlike many other bacterial toxins, typhoid toxin is exclusively produced by intracellular S. Typhi, indicating the specialized export trafficking mechanism, which has been better characterized in [55]. Typhoid toxin is secreted into the lumen of the Salmonella-containing vacuole (SCV) within the infected cells, where it is packaged into vesicle carrier intermediates that transport it to the extracellular milieu [8]. More specifically, typhoid toxin is sorted from the SCV into the vesicle carrier intermediates by the interaction of its B subunit PltB with specific sialylated glycan packaging receptors [55] (Figure 1E). Toxin packaging was impeded in CRISPR/Cas-9 generated cells deficient in N-linked glycoprotein glycosylation or the synthesis of specific gangliosides GD2, or from cells displaying N-glycolylneuraminic acid (Neu5Gc)-terminated glycans, which are incompatible with PltB-receptor binding. Exocytic sorting of typhoid toxin requires a specific intracellular compartment since the toxin was not exported when expressed by a S. Typhi mutant that is unable to traffic to SCV (Figure 1E). Following export, the toxin reaches target cells by interacting with human-specific Neu5Ac-terminated glycan receptors, as explained above and in Figure 1C-1E [9,53]. The cellular targets for typhoid toxin may not necessarily be cells that contain the bacteria in the SCV, but could also be other uninfected cells like immune cells including neutrophils and lymphocytes, as well as brain endothelial cells.
Host Adaptation of Typhoid Toxin
Several factors contribute to S. Typhi’s host specificity as indicated by the lack of typical symptomatology of typhoid fever expressed by chimpanzees. Typhoid toxin shows strong selectivity for Neu5Ac-terminated glycans that are predominantly expressed in human cells, as opposed to Neu5Gc-terminated glycans that are predominantly expressed in other mammals such as chimpanzees [53]. The abundance of Neu5Gc on the surface glycans render cells not permissive for toxin binding and therefore resistant to typhoid toxin.
More specifically, human glycans are unusual because they predominantly carry Neu5Ac due to the lack of a functional enzyme, termed cytidine monophosphate-N-acetylneuraminic acid hydroxylase, (CMAH, a pseudogene in humans), which in other mammals converts Neu5Ac to Neu5Gc [56] (Figure 1D). CMAH transgenic mice, constitutively expressing CMAH and thus displaying Neu5Gc and not Neu5Ac in all tissues, are resistant to typhoid toxin [53]. The atomic structure of typhoid toxin bound to Neu5Ac reveals the structural basis for its binding specificity [53]. Although the PltB subunit shares its oligosaccharide-binding fold with the B subunits of other AB5 toxins [57], it lacks a residue that is required to form a hydrogen bond with the extra hydroxyl group in Neu5Gc-terminated glycans. Instead, PltB has a nonpolar residue Val 103 that allows it to interact with Neu5Ac-terminated glycans [53] (Figure 1B inset). It is worthwhile to note that C57Bl/6 mice naturally express typhoid toxin receptor Neu5Ac, while 100 percent of the sialoglycans expressed in CMAH null mice is the same as those in humans [53]. Furthermore, although there might be a yet unidentified difference, both C57Bl/6 and CMAH null mice developed comparable toxin binding tropism and clinical manifestations upon typhoid toxin challenge. These results demonstrated the remarkable molecular evolution of bacterial toxins produced by the exclusive human pathogen Salmonella Typhi, resulting in its host adaptation.
Typhoid Toxin Tissue Tropism and Typhoid Fever
The sialoglycan consensus sequence Neu5Acα2,3Galβ1,3/β1,4GlcNAc/Glc (typhoid toxin receptor) is rather common among surface glycoproteins, but an expression of the related glycoproteins, as well as their glycosylation levels and their further modifications, may be different in various cell types [58-62]. This concept implies that different glycoproteins on different target cells can act as receptors for typhoid toxin. In support of this idea, it was recently demonstrated that Podocalyxin-like 1 protein is the receptor for typhoid toxin in epithelial cells, while CD45 serves as the typhoid toxin receptor in immune cells, including monocytes and T and B cells.
Typhoid toxin administered intravenously into mice was primarily found in two organs: the spleen, together with its effect on other immune cells in circulation, explaining the acute phase symptomes of typhoid fever associated to immune cells, and the brain, most typhoid toxin found on/around the Blood-Brain Barrier (BBB) (unpublished observation). Strikingly, typhoid toxin did not localize in the endothelium of blood vessels of other organs (e.g. lung, liver, and kidney), which strongly supports a yet unidentified in vivo mechanism resulting in the specific tissue tropism of typhoid toxin (unpublished observation). In support of this specificity, other controls including PltB binding defective mutant toxin, and other lectins such as Ricinus communis agglutinin I that binds to galactose [63,64], both of which we did not find on/in the brain. Typhoid toxin’s tropism to immune cells is also distinct. Although the details of these mechanisms have yet to be fully understood, the effect of typhoid toxin on immune cells is presumably concentration-dependent. In low concentrations, typhoid toxin binding to immune cells, including neutrophils, lymphocytes, monocytes, and macrophages, appears to help S. Typhi to establish persistent infection, presumably by altering both innate immune responses and adaptive immune responses ([10] and unpublished observation). On the other hand, in high concentrations, the toxin is able to cause the death of immune cells [9]. Although this evidence is convincing, further investigations are required in order to fully explain this underlying mechanism.
The cdt Locus in Nontyphoidal Salmonella Serovars
den Bakker et al. recently reported that a few nontyphoidal Salmonella enterica serovars such as S. Javiana have adapted the cdt locus into their genome [65]. As the Javiana cdt locus encodes a similar but modified toxin component, it will be called “Javiana toxin” in this article. In a follow-up study, Rodriquez-Rivera et. al. showed that S. Javiana that infected Henle-407 cells produced functional Javiana toxin, as measured by G2 cell cycle arrest of infected cells three days post-infection [66]. Similar to other nontyphoidal Salmonella infection, S. Javiana infection in most cases is asymptomatic or restricted to the gastrointestinal (GI) tract ([67] and unpublished observation). However, in rare instances, particularly among immunocompromised individuals, S. Javiana was found in the blood. For instance, a clinical report of S. Javiana small outbreak in New Mexico in 1942 described that a few patients developed an alarming, atypical symptom complex of nontyphoidal Salmonella infection, and some cases even led to the death [68]. These results imply that S. Javiana genetically engineered to express typhoid toxin might be served as a mouse model to study typhoid toxin in the context of natural infection by using a condition causing systemic infection. It was previously proposed that Toll-like receptor 11 (TLR11) was responsible for the clearance of S. Typhi infections in mice, thus Tlr11-/- mice was a prospective animal model for studying these infections [69]. However, these results could not be reproduced by other laboratories and were therefore considered to be unreliable [70,71]. These support that further development of animal model will be critical to better understand the role of typhoid toxin in S. Typhi virulence.
Conclusions and Outlook
Unlike many other bacterial pathogens that can infect both humans and other animals, S. Typhi is human-restricted. As humans are essential for the survival of S. Typhi, it is reasonable for S. Typhi to have developed multiple means to sustain its population to avoid its potential extinction. In addition to the asymptomatic carrier, it is intriguing to speculate that the acute symptomatic phase of S. Typhi infection could also be an indirect means to rapidly spread S. Typhi among people, although in both cases only a small portion of S. Typhi likely finds its next host. Based on the findings discussed in this article, we speculate that typhoid toxin helps maintain S. Typhi’s long-term survival and transmission by targeting the immune system and the central nervous system. Current animal models that are being used to study the role of typhoid toxin during the acute phase as well as the chronic phase are useful, but both have limitations. The development of an optimal animal model would facilitate the advancement of knowledge of the typhoid toxin biology in the context of natural infection.
Acknowledgments
Ongoing work relevant to this article is supported by Cornell President’s Council of Cornell Women Affinito-Stewart Award, and the USDA National Institute of Food and Agriculture, Hatch project 1010701.
Glossary
- SCV
Salmonella-containing vacuole
- CDT
cytolethal distending toxin
- Neu5Ac
N-acetylneuraminic acid
- Neu5Gc
N-glycolylneuraminic acid
- SPI-7
Salmonella pathogenicity island 7
- Glc
Glucose/ GlcNAc, N-acetylglucosamine
- CMAH
cytidine monophosphate-N-acetylneuraminic acid hydroxylase
- BBB
Blood-Brain Barrier
- GI
gastrointestinal
- TLR11
Toll-like receptor 11
References
- Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bulletin of the World Health Organization. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- Crump JA, Mintz ED. Global trends in typhoid and paratyphoid Fever. Clin Infect Dis. 2010;50(2):241–246. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry CM, Hien TT, Dougan G. et al. Typhoid fever. N Engl J Med. 2002;347(22):1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- Parry CM, Threlfall EJ. Antimicrobial resistance in typhoidal and nontyphoidal salmonellae. Curr Opin Infect Dis. 2008;21(5):531–538. doi: 10.1097/QCO.0b013e32830f453a. [DOI] [PubMed] [Google Scholar]
- Feasey NA, Gaskell K, Wong V. et al. Rapid emergence of multidrug resistant, H58-lineage Salmonella typhi in Blantyre, Malawi. PLoS Negl Trop Dis. 2015;9(4):e0003748. doi: 10.1371/journal.pntd.0003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong VK, Baker S, Pickard DJ. et al. Phylogeographical analysis of the dominant multidrug-resistant H58 clade of Salmonella Typhi identifies inter- and intracontinental transmission events. Nat Genet. 2015;47(6):632–639. doi: 10.1038/ng.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghjoo E, Galan JE. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc Natl Acad Sci U S A. 2004;101(13):4614–4619. doi: 10.1073/pnas.0400932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano S, Ugalde JE, Galan JE. Delivery of a Salmonella Typhi exotoxin from a host intracellular compartment. Cell Host Microbe. 2008;3(1):30–38. doi: 10.1016/j.chom.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Song J, Gao X, Galan JE. Structure and function of the Salmonella Typhi chimaeric A(2)B(5) typhoid toxin. Nature. 2013;499(7458):350–354. doi: 10.1038/nature12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Willinger T, Rongvaux A. et al. A mouse model for the human pathogen Salmonella typhi. Cell Host Microbe. 2010;8(4):369–376. doi: 10.1016/j.chom.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Juarez S, Nga TV. et al. Immune profiling with a Salmonella Typhi antigen microarray identifies new diagnostic biomarkers of human typhoid. Sci Rep. 2013;3:1043. doi: 10.1038/srep01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles RC, Sheikh A, Krastins B. et al. Characterization of anti-Salmonella enterica serotype Typhi antibody responses in bacteremic Bangladeshi patients by an immunoaffinity proteomics-based technology. Clin Vaccine Immunol. 2010;17(8):1188–1195. doi: 10.1128/CVI.00104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JA, Collier RJ. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
- Buckle GC, Walker CL, Black RE. Typhoid fever and paratyphoid fever: Systematic review to estimate global morbidity and mortality for 2010. J Glob Health. 2012;2(1):010401. doi: 10.7189/jogh.02.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darton TC, Blohmke CJ, Pollard AJ. Typhoid epidemiology, diagnostics and the human challenge model. Curr Opin Gastroenterol. 2014;30(1):7–17. doi: 10.1097/MOG.0000000000000021. [DOI] [PubMed] [Google Scholar]
- Edsall G, Gaines S, Landy M. et al. Studies on infection and immunity in experimental typhoid fever. I. Typhoid fever in chimpanzees orally infected with Salmonella typhosa. J Exp Med. 1960;112:143–166. doi: 10.1084/jem.112.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRock DL, Chaudhary A, Miller SI. Salmonellae interactions with host processes. Nat Rev Microbiol. 2015;13(4):191–205. doi: 10.1038/nrmicro3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl ME, Miller SI. Salmonella: a model for bacterial pathogenesis. Annu Rev Med. 2001;52:259–274. doi: 10.1146/annurev.med.52.1.259. [DOI] [PubMed] [Google Scholar]
- Dougan G, Baker S. Salmonella enterica serovar Typhi and the pathogenesis of typhoid fever. Annu Rev Microbiol. 2014;68:317–336. doi: 10.1146/annurev-micro-091313-103739. [DOI] [PubMed] [Google Scholar]
- Raffatellu M, Chessa D, Wilson RP. et al. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect Immun. 2005;73(6):3367–3374. doi: 10.1128/IAI.73.6.3367-3374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangdi T, Lee CY, Spees AM. et al. The Vi capsular polysaccharide enables Salmonella enterica serovar typhi to evade microbe-guided neutrophil chemotaxis. PLoS Pathog. 2014;10(8):e1004306. doi: 10.1371/journal.ppat.1004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodak H, Galan JE. A Salmonella Typhi homologue of bacteriophage muramidases controls typhoid toxin secretion. EMBO Rep. 2013;14(1):95–102. doi: 10.1038/embor.2012.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhill J, Dougan G, James KD. et al. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature. 2001;413(6858):848–852. doi: 10.1038/35101607. [DOI] [PubMed] [Google Scholar]
- McClelland M, Sanderson KE, Clifton SW. et al. Comparison of genome degradation in Paratyphi A and Typhi, human-restricted serovars of Salmonella enterica that cause typhoid. Nat Genet. 2004;36(12):1268–1274. doi: 10.1038/ng1470. [DOI] [PubMed] [Google Scholar]
- Sabbagh SC, Forest CG, Lepage C. et al. So similar, yet so different: uncovering distinctive features in the genomes of Salmonella enterica serovars Typhimurium and Typhi. FEMS Microbiol Lett. 2010;305(1):1–13. doi: 10.1111/j.1574-6968.2010.01904.x. [DOI] [PubMed] [Google Scholar]
- Hung CC, Garner CD, Slauch JM. et al. The intestinal fatty acid propionate inhibits Salmonella invasion through the post-translational control of HilD. Mol Microbiol. 2013;87(5):1045–1060. doi: 10.1111/mmi.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermeier JR, Slauch JM. Adaptation to the host environment: regulation of the SPI1 type III secretion system in Salmonella enterica serovar Typhimurium. Curr Opin Microbiol. 2007;10(1):24–29. doi: 10.1016/j.mib.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Figueira R, Holden DW. Functions of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system effectors. Microbiology. 2012;158(Pt 5):1147–1161. doi: 10.1099/mic.0.058115-0. [DOI] [PubMed] [Google Scholar]
- van der Heijden J, Finlay BB. Type III effector-mediated processes in Salmonella infection. Future Microbiol. 2012;7(6):685–703. doi: 10.2217/fmb.12.49. [DOI] [PubMed] [Google Scholar]
- Agbor TA, McCormick BA. Salmonella effectors: important players modulating host cell function during infection. Cell Microbiol. 2011;13(12):1858–1869. doi: 10.1111/j.1462-5822.2011.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman EA. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol. 2001;183(6):1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman EA, Ochman H. How Salmonella became a pathogen. Trends Microbiol. 1997;5(9):343–349. doi: 10.1016/S0966-842X(97)01099-8. [DOI] [PubMed] [Google Scholar]
- Miller SI, Kukral AM, Mekalanos JJ. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989;86(13):5054–5458. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan JE, Curtiss R, 3rd. Virulence and vaccine potential of phoP mutants of Salmonella typhimurium. Microb Pathog. 1989;6(6):433–443. doi: 10.1016/0882-4010(89)90085-5. [DOI] [PubMed] [Google Scholar]
- Galan JE, Curtiss R, 3rd. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci U S A. 1989;86(16):6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 1989;86(16):6383–8387. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- Knodler LA, Steele-Mortimer O. Taking possession: biogenesis of the Salmonella-containing vacuole. Traffic. 2003;4(9):587–599. doi: 10.1034/j.1600-0854.2003.00118.x. [DOI] [PubMed] [Google Scholar]
- Ehrbar K, Mirold S, Friebel A. et al. Characterization of effector proteins translocated via the SPI1 type III secretion system of Salmonella typhimurium. Int J Med Microbiol. 2002;291(6-7):479–485. doi: 10.1078/1438-4221-00156. [DOI] [PubMed] [Google Scholar]
- Colgan AM, Kroger C, Diard M. et al. The Impact of 18 Ancestral and Horizontally-Acquired Regulatory Proteins upon the Transcriptome and sRNA Landscape of Salmonella enterica serovar Typhimurium. PLoS Genet. 2016;12(8):e1006258. doi: 10.1371/journal.pgen.1006258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano S, Galan JE. A Rab32-dependent pathway contributes to Salmonella typhi host restriction. Science. 2012;338(6109):960–963. doi: 10.1126/science.1229224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano S, Liu X, Galan JE. Proteolytic targeting of Rab29 by an effector protein distinguishes the intracellular compartments of human-adapted and broad-host Salmonella. Proc Natl Acad Sci U S A. 2011;108(45):18418–18423. doi: 10.1073/pnas.1111959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump JA, Sjolund-Karlsson M, Gordon MA. et al. Epidemiology, Clinical Presentation, Laboratory Diagnosis, Antimicrobial Resistance, and Antimicrobial Management of Invasive Salmonella Infections. Clin Microbiol Rev. 2015;28(4):901–937. doi: 10.1128/CMR.00002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali G, Rashid S, Kamli MA. et al. Spectrum of neuropsychiatric complications in 791 cases of typhoid fever. Trop Med Int Health. 1997;2(4):314–318. doi: 10.1111/j.1365-3156.1997.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Lutterloh E, Likaka A, Sejvar J. et al. Multidrug-resistant typhoid fever with neurologic findings on the Malawi-Mozambique border. Clin Infect Dis. 2012;54(8):1100–1106. doi: 10.1093/cid/cis012. [DOI] [PubMed] [Google Scholar]
- Sejvar J, Lutterloh E, Naiene J. et al. Neurologic manifestations associated with an outbreak of typhoid fever, Malawi--Mozambique, 2009: an epidemiologic investigation. PLoS One. 2012;7(12):e46099. doi: 10.1371/journal.pone.0046099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Kulkova N, Sokolova J. et al. Neurologic complications and sequellae of infectious diseases in Uganda and Kenya: Analysis of 288 cases from two rural hospitals. Neuro Endocrinol Lett. 2013;34(Suppl 1):28–31. [PubMed] [Google Scholar]
- Wadia RS, Ichaporia NR, Kiwalkar RS. et al. Cerebellar ataxia in enteric fever. J Neurol Neurosurg Psychiatry. 1985;48(7):695–697. doi: 10.1136/jnnp.48.7.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuntokun BO, Bademosi O, Ogunremi K. et al. Neuropsychiatric manifestations of typhoid fever in 959 patients. Arch Neurol. 1972;27(1):7–13. doi: 10.1001/archneur.1972.00490130009002. [DOI] [PubMed] [Google Scholar]
- Crawford RW, Rosales-Reyes R, Ramirez-Aguilar Mde L. et al. Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc Natl Acad Sci U S A. 2010;107(9):4353. doi: 10.1073/pnas.1000862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack DM. Salmonella persistence and transmission strategies. Curr Opin Microbiol. 2012;15(1):100–107. doi: 10.1016/j.mib.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Stein PE, Boodhoo A, Armstrong GD. et al. The crystal structure of pertussis toxin. Structure. 1994;2(1):45–57. doi: 10.1016/s0969-2126(00)00007-1. [DOI] [PubMed] [Google Scholar]
- Nesic D, Hsu Y, Stebbins CE. Assembly and function of a bacterial genotoxin. Nature. 2004;429(6990):429–433. doi: 10.1038/nature02532. [DOI] [PubMed] [Google Scholar]
- Deng L, Song J, Gao X. et al. Host adaptation of a bacterial toxin from the human pathogen salmonella typhi. Cell. 2014;159(6):1290–1299. doi: 10.1016/j.cell.2014.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Lasanajak Y, Xia B. et al. Shotgun glycomics: a microarray strategy for functional glycomics. Nat Methods. 2011;8(1):85–90. doi: 10.1038/nmeth.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SJ, Song J, Galan JE. Receptor-Mediated Sorting of Typhoid Toxin during Its Export from Salmonella Typhi-Infected Cells. Cell Host Microbe. 2016;20(5):682–689. doi: 10.1016/j.chom.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou HH, Hayakawa T, Diaz S. et al. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc Natl Acad Sci U S A. 2002;99(18):11736–11741. doi: 10.1073/pnas.182257399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byres E, Paton AW, Paton JC. et al. Incorporation of a non-human glycan mediates human susceptibility to a bacterial toxin. Nature. 2008;456(7222):648–652. doi: 10.1038/nature07428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Evolutionary forces shaping the Golgi glycosylation machinery: why cell surface glycans are universal to living cells. Cold Spring Harb Perspect Biol. 2011;3(6) doi: 10.1101/cshperspect.a005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. Golgi glycosylation. Cold Spring Harb Perspect Biol. 2011;3(4) doi: 10.1101/cshperspect.a005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasik BR, Barnard KN, Parrish CR. Effects of Sialic Acid Modifications on Virus Binding and Infection. Trends Microbiol. 2016;24(12):991–1001. doi: 10.1016/j.tim.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Multiple changes in sialic acid biology during human evolution. Glycoconj J. 2009;26(3):231–245. doi: 10.1007/s10719-008-9183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti E, Miyagi T. Structure and Function of Mammalian Sialidases. Top Curr Chem. 2015;366:183–208. doi: 10.1007/128_2012_328. [DOI] [PubMed] [Google Scholar]
- McGuinness CR, Mantis NJ. Characterization of a novel high-affinity monoclonal immunoglobulin G antibody against the ricin B subunit. Infect Immun. 2006;74(6):3463–3470. doi: 10.1128/IAI.00324-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutenber E, Ready M, Robertus JD. Structure and evolution of ricin B chain. Nature. 1987;326(6113):624–626. doi: 10.1038/326624a0. [DOI] [PubMed] [Google Scholar]
- den Bakker HC, Moreno Switt AI, Govoni G. et al. Genome sequencing reveals diversification of virulence factor content and possible host adaptation in distinct subpopulations of Salmonella enterica. BMC Genomics. 2011;12:425. doi: 10.1186/1471-2164-12-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Rivera LD, Bowen BM, den Bakker HC. et al. Characterization of the cytolethal distending toxin (typhoid toxin) in non-typhoidal Salmonella serovars. Gut Pathog. 2015;7:19. doi: 10.1186/s13099-015-0065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iveson JB, Bradshaw SD. Salmonella javiana infection in an infant associated with a marsupial, the quokka, Setonix brachyurus, in Western Australia. J Hyg (Lond) 1973;71(3):423–432. doi: 10.1017/s0022172400046404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alley RD, Pijoan M. Salmonella Javiana Food Infection. Yale J Biol Med. 1942;15(2):229–239. [PMC free article] [PubMed] [Google Scholar]
- Mathur R, Oh H, Zhang D. et al. A mouse model of Salmonella typhi infection. Cell. 2012;151(3):590–602. doi: 10.1016/j.cell.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Wilhelm CL, Wangdi T. et al. Absence of TLR11 in Mice Does Not Confer Susceptibility to Salmonella Typhi. Cell. 2016;164(5):827–828. doi: 10.1016/j.cell.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur R, Zeng W, Hayden MS. et al. Mice Lacking TLR11 Exhibit Variable Salmonella typhi Susceptibility. Cell. 2016;164(5):829–830. doi: 10.1016/j.cell.2016.02.020. [DOI] [PubMed] [Google Scholar]