Abstract
We induced angiogenesis in the tibial medulla and cortex of rabbits by electrical and mechanical stimulation, with the aim of future application to ischemic disease.
Sixteen New Zealand rabbits were divided into 4 groups: in Group 1, a K wire was inserted into the medullary channel; in Group 2, a hole was drilled into the tibia; in Group 3, electrical stimulation was applied to the medullary channel; and in Group 4 (the control group), nothing was done. The interventions were applied during a 21-day period, after which all animals were evaluated scintigraphically and histopathologically.
All 3 interventional groups were significantly superior to the control group in regard to medullary and cortical vascularity: the P values were 0.021 in all comparisons to control. However, the most fibrotic changes in the medulla occurred in the group that had been treated with electricity (P = 0.008). Slight fibrotic changes occurred in the hole group (P = 0.040), and none occurred in the K-wire group.
In sum, all 3 interventions are capable of inducing medullary angiogenesis, but electricity is inferior in regard to fibrotic change. We believe that this present study can establish a baseline for further work that explores clinical applications to problematic ischemic conditions, including delayed sternal wound healing after cardiac surgery.
Key words: Angiogenesis; bone regeneration; electric stimulation therapy; neovascularization, physio-logic; sternum/blood supply; surgical wound dehiscence/prevention & control; wound healing/physiology
Several kinds of studies concerning angiogenesis and stem cells have been reported in the recent literature. According to these articles, stem cells can play a big role in the advent of angiogenesis.1,2 Of the many new and distinct methods of treating ischemic disease, a number are based on stem cell injections.2 Because such reports have demonstrated the importance of stem cells in the advent of angiogenesis, we decided to induce stem cell activity, but to use different methods. In our judgment, stimulating native angiogenic pathways via medullary irritation is easier and cheaper than preparing stem cells via sophisticated methods. Therefore, this study was designed to investigate the angiogenic effects of 3 different techniques on the tibial medulla.
To attain the best sternal healing or to resolve problems with unhealed sternum after sternotomy, many different interventions—both surgical3–6 and nonsurgical7 —have already been tested. However, problems with sternal healing are ongoing, because an ideal method to maintain good wound healing has not yet been described. We believe that the angiogenic activity of the medulla is vital, particularly for patients with unhealed sternal wounds, but also for patients with ischemic limbs or myocardium. This study has fairly simple clinical application, and we hope that its good results will inspire further research into the possibility of its use in patients with unhealed sternum after sternotomy.
Materials and Methods
This study was approved by the Ethics Committee of Animal Care of Inonu University. We obtained 16 New Zealand rabbits from a government institution and divided them equally into 4 groups:
Group 1: Wire Group
A K wire, an item used routinely in orthopedic surgery, was inserted into the intramedullary channel of the tibia of 1 leg (operated leg) of each subject. The untouched legs served as self controls.
Group 2: Hole Group
A hole was drilled on the lateral proximal region of the tibia of 1 leg (operated leg) of each subject. The untouched legs served as self controls.
Group 3: Electricity Group
Electrical stimulation was applied to the intramedullary channel of the tibia of 1 leg (operated leg) of each subject. The untouched legs served as self controls.
Group 4: Control Group
Nothing was done to any leg.
Subjects were placed on a normal diet.
The Outset of the Experiment
The subjects were operated upon after induction of anesthesia. Preoperatively, 35 mg/kg ketamine was administered intramuscularly; this was followed by 10 mg/kg ketamine and 3 mg/kg xylazine, injected into the ear veins.
The operative procedures were as follows:
Group 1: Wire Group
After a small incision was made in the proximal lateral region of the right hind tibia, the K wire was inserted into the intramedullary channel. The other limbs were not touched. Externally, the wire was affixed to the skin.
Group 2: Hole Group
In the same region, a hole was made in the right hind tibia with a surgical drill. Nothing was inserted into the medullary channel. The other limbs were not touched.
Group 3: Electricity Group
Two copper cables covered with plastic were inserted into the medullary channel though the hole created in the same region as in the other groups. The tips of the cables were covered with rhodium to prevent oxidization. Continuous direct electrical current (10 mA) was supplied by a battery, which was affixed to the skin. The other limbs were not touched.
Group 4: Control Group
Nothing was done.
At the end of the period of 21 days, animals were evaluated as described below.
Scintigraphic Procedure
To attain equal dispersion of scintigraphic material through every capillary of both hind limbs, a radionuclide was injected into the abdominal aorta, after a retroperitoneal surgical approach. This intervention was performed after proper anesthesia (as described above), while the subjects were alive. The rabbits were kept alive for 20 minutes after the injection, so that there would be enough perfusion time for optimum dispersion of the radionuclide, 4 mCi (148 MBq) of 99m technetium human albumin macroaggregates (99mTc-MAA)(Pulmocis® CIS Bio Int., Gif Sur Yvette, France). The number of particles administered to each subject was approximately 50,000, at least 80% of which were between 30 and 50 μm in diameter, with no 99mTc-MAA particle above 100 μm or under 10 μm.
Euthanasia was carried out via the injection of air into the pericardium. After performing euthanasia, we obtained anterior static planar images (Fig. 1) of both hind limbs (3 min and 256 × 256 image matrix) by means of a dual-head gamma camera with a low-energy, high-resolution, parallel-hole collimator (ADAC Vertex V60 digital gamma camera; Milpitas, Cali).

Fig. 1 Anterior static planar scintigraphy of a hind limb. Regions of interest (ROIs) are denoted by the rectangles.
Histologic Procedures
After scintigraphy, both tibias of the hind limbs (operated and untouched) were excised and placed in individual jars filled with 10% formaldehyde. After fixation, the bones were decalcified with 5% formic acid. Sections 5 μm thick were stained with either hematoxylin and eosin or periodic acid-Schiff (PAS). The PAS stain was preferred because of its affinity with the basal membrane of the vascular wall, which renders the vessels easier to distinguish.
Measurements
Scintigraphic Measurements
Rectangular regions of interest (ROIs) were drawn on the all images. Scintigraphic results were evaluated by 2 experienced observers.
Histologic Measurements
To calculate the total numbers of whole vessels in a tibial medulla, all vessels in the entire medulla were counted, one by one, under 400-fold magnification (CH30 Light Microscope, Olympus; Tokyo, Japan). In addition, all vessels in the cortical bone were counted one by one, under the same magnification. This counting was performed for each limb. Thereafter, all slides were evaluated pathologically. Granulations, edema, and fibrotic and necrotic changes were noted and evaluated. For the purposes of statistical analysis, each of these individual pathologic findings was assigned a value of 1 and its absence was assigned a value of zero. All histopathologic procedures were carried out by the same pathologist.
The pathologist was blinded to the codes and interventions pertaining to all subjects, as were the nuclear medicine specialists.
Statistical Calculations
The accumulated results were analyzed by a biostatistician. Wilcoxon signed rank, Kruskal-Wallis, Mann-Whitney U, and Pearson product moment correlation tests were performed with the aid of SPSS® 10.0 (SPSS, Inc., Chicago, Ill). Statistical significance was assumed when the P value was less than 0.05.
Results
The mean numbers of vessels in each group are summarized in the figures. Figure 2 indicates the numbers of medullary vessels, while Figure 3 indicates the numbers of the vessels in the cortical bone. The “self-control” results found in the unoperated hind legs of rabbits in the intervention groups yielded no statistically significant differences, whether compared with operated legs in those same animals or with unoperated legs of rabbits in the control group.
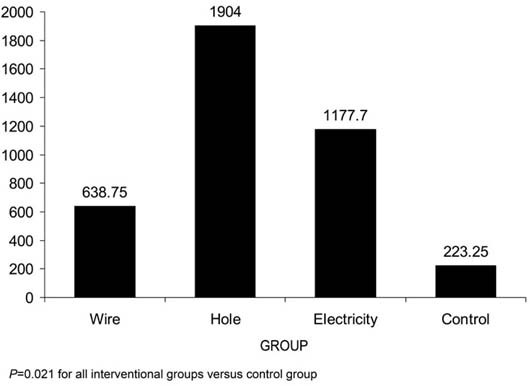
Fig. 2 The mean numbers of medullary vessels in each group as determined by histopathologic evaluation.
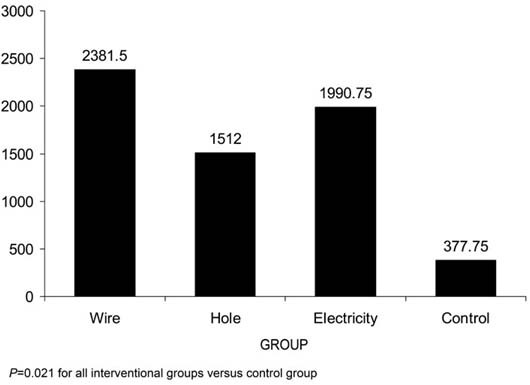
Fig. 3 The mean numbers of cortical vessels in each group as determined by histopathologic evaluation.
When we compared the results of the various interventions with those of the control group to discover the superior technique, all 3 displayed a statistically significant difference from the control group. In regard to medullary vessels, the hole group, the K-wire group, and the electricity group had the same significant P value, 0.021. Similarly, in regard to the numbers of vessels in the cortical bone, all comparisons to control yielded the same significant P value of 0.021.
Another important consideration was pathologic change. There was no great distinction between the groups in regard to granulation or edema formation. In contrast with other pathologic events, fibrotic changes were significantly different when some of the intervention groups were compared with the control group. The most fibrotic changes in the medulla occurred in the electricity group (P = 0.008). There were slight fibrotic changes in the hole group (P = 0.040), and none were detected in the K-wire group.
When we compared the interventions with each other, we observed only 2 significant superiorities in the groups. In regard to the numbers of the medullary vessels, the hole group was superior to the K-wire group (P = 0.043), but in regard to the numbers of the vessels in the cortical bone, the K-wire group had better results than the hole group (P = 0.021). The other comparisons between the groups did not yield any noteworthy results.
When we examined the scintigraphic results, no significant comparative outcome was obtained. The mean levels of activities in the ROIs were 1.53 in the K-wire group, 1.81 in the hole group, 1.55 in the electricity group, and 2.41 in the control group.
Discussion
In spite of advances in medicine, the most common cause of death in many countries is still atherosclerotic disease. Unfortunately, ischemic coronary artery heart disease remains at the top of the list, although there have been many technical refinements in treating this condition. Although ischemic peripheral arterial diseases do not always result in death, they cause socioeconomic loss due to the amputation of extremities. These unresolved problems explain why research in angiogenesis has attracted so much attention. Many alternative interventions—such as gene therapy, the injection of angiogenic factors, the injection of stem cells, and the stimulation of native angiogenic capacity—are being assessed in various research centers.1,2,8–10 Despite the huge number of investigations on this subject, the most suitable method has not yet been identified.
We believe that stimulation of the body's native angiogenic capacity is the cheapest and easiest of these techniques; therefore, it is the most readily available for use in every country. Moreover, the injection of angiogenic factors such as vascular endothelial growth factor (VEGF) has some disadvantages: systemic side-effects (like hypotension) and the stimulation of tumor growth.11
In setting out to design a study of angiogenesis by stimulation of an organism's native angiogenic capacity, we scanned the recent literature and saw that electrical or electromagnetic stimulation was one of the techniques most commonly used for that purpose. Because we were also interested in angiogenesis by stem cell injection, we planned to stimulate stem cells by means of electricity in their birthplace, the medulla.
To the best of our knowledge, this study is the first in the literature to report the angiogenic results of electrical stimulation upon the medulla. Furthermore, we decided to stimulate the medulla mechanically, to see how that compared with electrical stimulation. For this purpose, we used the K wire in one group and a simple hole in another.
All 3 interventions increased medullary vessel count, when results were compared with those of the control group (P = 0.021). Similarly, their angiogenic effects in the cortical bone were superior to those of the control group (P = 0.021). Despite showing the most fibrotic effects (P = 0.008), electricity was as capable of stimulating angiogenesis as were the other interventions. The most acceptable outcomes in regard to fibrotic change were seen in the K-wire group, which displayed no fibrotic change. The hole group manifested slight fibrosis (P = 0.040).
Unfortunately, we could establish no correlation between our histopathologic results and our scintigraphic results, and this we attribute to the large diameters of the 99mTc-MAA radionuclide beads that were in use. In obtaining our histopathologic results, we were able to count capillaries smaller than 10 μm in diameter, in addition to larger vessels in the medulla and the cortex. At least 80% of the 99mTc-MAA beads ranged in diameter from 30 to 50 μm, and no bead was under 10 μm. Moreover, our scintigraphic findings showed no increase in the number of arteries bigger than 10 μm in diameter.
Our failure to obtain early scintigraphic evidence of angiogenesis is not unique. Medalion and coworkers12 observed that the sternal blood supply 10 days after left internal thoracic artery (LITA) harvesting could not be observed by gamma camera. They found an increase in blood supply after nearly 18 months, but were unable to determine the point at which angiogenesis starts after LITA harvesting. Our histopathologic examinations have revealed that angiogenesis can be achieved in 3 weeks in rabbits, although this cannot be substantiated by scintigraphy.
Future Clinical Applications
This study was designed to determine the possibility of angiogenic stimulation of the tibial medulla by each of 3 interventions. To our knowledge, the placement of electrodes in the medulla and the use of a K wire for mechanical stimulation have not previously been reported. Because it has been demonstrated that a technique useful for inducing angiogenesis in ischemic limbs1 also promotes sternal healing,7 we believe that our observations can be applied to the treatment of both ischemic limbs and unhealed sternal wounds.
Ischemic Disease
We believe that in future both electrical and mechanical stimulation of the medulla can be applied to the treatment of patients with any ischemic disease. Just as factors like VEGF have systemic effects after they are released,11 so do stem cells. According to our results, stem cells can be stimulated in their native environment, the medulla. We believe that our results will form a good reference point for further study of applications to clinical ischemic events.
Sternal Wound Healing in Difficult Cases
Another hopeful result of this study is the prospect of angiogenesis in the cortical bone. Previous reports13–15 have revealed the considerable usefulness of electrical stimulation in patients with nonunion bone fractures. Although we did not investigate the healing of bone, it can be inferred that angiogenesis in problematic bone lays the groundwork for good healing. If bone is not supplied with enough blood, complete healing will not occur. This subject is of particular interest to thoracic surgeons. It is known that sternal wound complication is more likely when both internal thoracic arteries (ITA) have been used as grafts, and such complications tend to require extensive surgery. The takedown of even 1 ITA can badly devascularize the sternum.16 Although the overall rate of important wound complications after cardiac surgery is low (from 0.8%–1.5%),17 these complications usually take the form of mediastinitis and sternal dehiscence. Furthermore, the rate of such complications is as high as 8% when bilateral ITA-to-coronary artery bypass grafting is performed.17 The mortality rate associated with sternal dehiscence has been variously reported at 6% to 70%. With early effective treatment, it falls to 5% to 10%.17
It is possible that the induction of angiogenesis in the sternal medulla by electrical or mechanical means can lower the both the morbidity and mortality rates associated with unhealed sternum after cardiac surgery; similar benefits of electrical stimulation have been reported elsewhere.18,19 Iwakura and colleagues7 have demonstrated specifically, in rats, that angiogenic interventions can speed bone regeneration in the devascularized sternum, although they used a gelatin sheet that incorporated basic fibroblast growth factor. We hope that the results of our study will establish a baseline for further study of medullary angiogenesis in patients with diseased sternum after cardiac surgery. It is even conceivable that the limits of bilateral use of the ITA (in patients with diabetes, for example) can be expanded, should it become possible to improve the healing of devascularized sternum.
Conclusion
In summary, all 3 interventions are capable of inducing medullary angiogenesis, but electricity is inferior in regard to fibrotic change. We believe that these results present hope and that this study can establish a baseline for further work that explores clinical applications in problematic patients with any ischemic condition, including delayed sternal wound healing after cardiac surgery.
Footnotes
Address for reprints: Ilker Alat, MD, Inonu Universitesi Tip Fakultesi, Turgut Ozal Tip Merkezi, Kalp ve Damar Cerrahisi Anabilim Dali, 44069 Malatya, Turkey
E-mail: ilkeralat@hotmail.com
This study was presented at the 21st Gevher Nesibe Medicine Days (4th Experimental and Clinical Research Congress), Kayseri, Turkey, in May 2003, and was awarded 2nd prize.
References
- 1.Tomanek RJ, Schatteman GC. Angiogenesis: new insights and therapeutic potential. Anat Rec 2000;261:126–35. [DOI] [PubMed]
- 2.Hamano K, Li TS, Kobayashi T, Hirata K, Yano M, Kohno M, Matsuzaki M. Therapeutic angiogenesis induced by local autologous bone marrow cell implantation. Ann Thorac Surg 2000;73:1210–5. [DOI] [PubMed]
- 3.Zeitani J, Bertoldo F, Bassano C, Penta de Peppo A, Pellegrino A, El Fakhri FM, Chiariello L. Superficial wound dehiscence after median sternotomy: surgical treatment versus secondary wound healing. Ann Thorac Surg 2004;77:672–5. [DOI] [PubMed]
- 4.Centofanti P, La Torre M, Barbato L, Verzini A, Patane F, di Summa M. Sternal closure using semirigid fixation with thermoreactive clips. Ann Thorac Surg 2002;74:943–5. [DOI] [PubMed]
- 5.Szerafin T, Jaber O, Peterffy A. Reduction of wound healing problems after median sternotomy [letter]. Ann Thorac Surg 1999;68:2388–9. [DOI] [PubMed]
- 6.Pezzella AT. Reduction of wound healing problems after median sternotomy by use of retention sutures [letter]. Ann Thorac Surg 1999;68:1891–2. [DOI] [PubMed]
- 7.Iwakura A, Tabata Y, Nishimura K, Nakamura T, Shimizu Y, Fujita M, Komeda M. Basic fibroblast growth factor may improve devascularized sternal healing. Ann Thorac Surg 2000;70:824–8. [DOI] [PubMed]
- 8.Brown MD, Egginton S, Hudlicka O, Zhou AL. Appearance of the capillary endothelial glycocalyx in chronically stimulated rat skeletal muscles in relation to angiogenesis. Exp Physiol 1996;81:1043–6. [DOI] [PubMed]
- 9.Patterson C, Runge MS. Therapeutic angiogenesis: the new electrophysiology? Circulation 1999;99:2614–6. [DOI] [PubMed]
- 10.Kanno S, Oda N, Abe M, Saito S, Hori K, Handa Y, et al. Establishment of a simple and practical procedure applicable to therapeutic angiogenesis. Circulation 1999;99:2682–7. [DOI] [PubMed]
- 11.Freedman SB, Isner JM. Therapeutic angiogenesis for coronary artery disease. Ann Intern Med 2002;136:54–71. [DOI] [PubMed]
- 12.Medalion B, Katz MG, Lorberboym M, Bder O, Schachner A, Cohen AJ. Decreased sternal vascularity after internal thoracic artery harvesting resolves with time: an assessment with single photon emission computed tomography. J Thorac Cardiovasc Surg 2002;123:508–11. [DOI] [PubMed]
- 13.Brighton CT. The semi-invasive method of treating nonunion with direct current. Orthop Clin North Am 1984; 15:33–45. [PubMed]
- 14.Sharrard WJ. A double-blind trial of pulsed electromagnetic fields for delayed union of tibial fractures. J Bone Joint Surg Br 1990;72:347–55. [DOI] [PubMed]
- 15.Barutcu A, Kostem L. Treatment of infected nonunion of the tibia with electrical stimulation combined with local muscle flaps. Eur J Plast Surg 1987;10:164–7.
- 16.Kirklin JW, Barratt-Boyes BG. Cardiac surgery: morphology, diagnostic criteria, natural history, techniques, results, and indications. Vol 1. 2nd ed. New York: Churchill Livingstone; 1993. p. 285–381.
- 17.Kirklin JW, Barratt-Boyes BG. Cardiac surgery: morphology, diagnostic criteria, natural history, techniques, results, and indications. Vol 1. 2nd ed. New York: Churchill Livingstone; 1993. p. 195–247.
- 18.Stefanovska A, Vodovnik L, Benko H, Turk R. Treatment of chronic wounds by means of electric and electromagnetic fields. Med Biol Eng Comput 1993;31:213–20. [DOI] [PubMed]
- 19.Alvarez OM, Mertz PM, Smerbeck RV, Eaglstein WH. The healing of superficial skin wounds is stimulated by external electrical current. J Invest Dermatol 1983;81:144–8. [DOI] [PubMed]


