Abstract
Background:
Bone marrow mesenchymal stem cells (BM-MSCs) are multipotent adult stem cells and have become an important source of cells for engineering tissue repair and cell therapy. Vascular endothelial growth factor (VEGF) promotes angiogenesis and contributes fibrous integration between tendon and bone during the early postoperative stage. Both MSCs and VEGF can stimulate cell proliferation, differentiation, and matrix deposition by enhancing angiogenesis and osteogenesis of the graft in the tunnel.
Hypothesis:
Injection of intratunnel BM-MSCs and VEGF enhances the early healing process of a tendon graft.
Study Design:
Controlled laboratory study.
Methods:
In this controlled animal laboratory study, each of 4 groups of rabbits underwent unilateral anterior cruciate ligament (ACL) reconstruction with use of the ipsilateral semitendinosus tendon. The rabbits received intratunnel injection of BM-MSCs and VEGF with a fibrin glue seal covering the distal tunnel at the articular site. Evaluation using magnetic resonance imaging (MRI), collagen type III expression, and biomechanical analyses were performed at 3- and 6-week intervals.
Results:
All parameters using MRI, collagen type III expression, and biomechanical analysis of pullout strength of the graft showed that application of intratunnel BM-MSCs and VEGF enhanced tendon-to-bone healing after ACL reconstruction.
Conclusion:
Intratunnel injections of BM-MSCs and VEGF after ACL reconstruction enhanced graft tunnel healing. Overall, the femoral tunnel that received BM-MSCs and VEGF had better advanced healing with increased collagen type III fibers and better outcomes on MRI and biomechanical analysis. MRI is the most reliable tool for clinical use in evaluating stages of ACL healing after reconstruction, since biopsy is an invasive procedure.
Keywords: graft tunnel healing, bone marrow mesenchymal stem cell, vascular endothelial growth factor, anterior cruciate ligament
Anterior cruciate ligament (ACL) reconstruction is the treatment of choice for individuals with knee instability during daily activities, those in manual labor jobs, or those who wish to preform well in pivoting sports.9 Several graft options have been described for surgical reconstruction of the ACL, including the hamstring tendon and the bone–patellar tendon–bone (BPTB) graft. Hamstring tendon autografts are favored by some surgeons due to the risk of patellar tendinitis and anterior knee pain related to BPTB grafts.7,28,31 The success of ACL reconstruction depends in part on the healing process between the graft and bone tunnel. The weak link during the early healing process is the attachment site between the bone and tendon (bone-tendon interface), and the hypoxic condition of the graft may cause potential tunnel widening and graft laxity, limiting rehabilitation and return to activity.2 The early stages of tendon-bone healing play a crucial role in determining the integration process of the interface in ACL reconstruction. Therefore, it is important to determine how to stimulate graft and bone healing to gain early return to daily activities, functional exercises, and sports.
Many approaches to enhance tendon-to-bone healing have been introduced to accelerate graft tunnel healing. Acceleration and improvement of tendon-to-bone healing may reduce the risk of graft failure and enable early aggressive rehabilitation.13 Approaches to enhance tendon-to-bone healing include mechanical stimulation32 and tendon wrapping with periosteum,35 as well as interface filling with growth factors,2,19 bone marrow stromal cells,20 and mesenchymal stem cells (MSCs).12 Bone marrow mesenchymal stem cells (BM-MSCs) are multipotent adult stem cells and have become an important source of cells for engineering tissue repair and cell therapy. Their osteogenic differentiation potential has been characterized in several in vitro studies.4 In addition, small animal model–based studies have verified their bone formation capability in vivo when implanted in injured tissue, indicating great potential for therapeutic application.4 Vascular endothelial growth factor (VEGF) is an endothelial mitogen that promotes angiogenesis, increases capillary permeability, and contributes to fibrous integration between tendon and bone during the early postoperative stage.11,13
Both MSCs and VEGF can stimulate cell proliferation, differentiation, and matrix deposition by enhancing angiogenesis and osteogenesis of the graft in the tunnel. Research has found that healing is preceded by formation of a fibrovascular interface tissue between the tendon and bone,24 followed by progressive bone ingrowth into this interface tissue.23 Sakai et al29 found in their study that Sharpey-like fibers composed of collagen type III in the early phase were gradually replaced by collagen type I from 12 weeks onward, until continuity between the Sharpey-like fibers and inner fibers of the tendon was achieved by 26 weeks. Sharpey-like fibers are an important factor of biomechanical effect in the tendon graft on bone tunnel. Sharpey-like fibers inhibit tendon graft failure pulling force on the bone tunnel.32
In order to stimulate the graft and enhance bone healing, we introduced a modified method of tissue engineering after ACL reconstruction in a rabbit model by applying intratunnel BM-MSCs and VEGF. The purpose of the current study was to test the hypothesis that injection of intratunnel BM-MSC and VEGF enhances the early healing process of a tendon graft in the bone tunnel of rabbits after ACL reconstruction. Magnetic resonance imaging (MRI), collagen type III evaluation, and biomechanical analysis were used to examine outcomes.
Methods
Study Design
A controlled animal laboratory study between July 2015 and January 2016 was performed on New Zealand white rabbits (Oryctolagus cuniculus). The study protocol was approved by the Animal Care and Use Committee, Indonesia. All rabbits were housed in the animal care laboratory at our institution and were cared for according to the standards of the National Institutes of Health. Each of the 24 rabbits underwent unilateral ACL reconstruction of the right knee with use of the semitendinosus tendon. ACL reconstruction surgery was performed by 2 surgeons; the rabbits were under general anesthesia with intramuscular injection of ketamine (40 mg/kg) and xylazine (10 mg/kg) at the site of the quadriceps muscle using sterile techniques. The semitendinosus tendon was harvested from the ipsilateral knee and then transplanted into the knee joints as an ACL graft. The graft was placed into bone tunnels in the lateral femoral condyle and proximal tibia and secured to the surrounding periosteum outside the femoral tunnel, followed by the tibial tunnel, using 4-0 Ethibond (Ethicon) suture. One knee received intratunnel injection of allogeneic BM-MSC and VEGF with fibrin glue sealant covering the proximal aspect of the femoral tunnel as well as the distal tunnel in the articular site. We divided the animals into 4 groups as follows: (1) group I, control 3 weeks—ACL reconstruction of the left knee with semitendinosus autograft, evaluation after 3 weeks; (2) group II, control 6 weeks—ACL reconstruction of the left knee with semitendinosus autograft, evaluation after 6 weeks; (3) group III, treated 3 weeks—ACL reconstruction of the right knee with semitendinosus autograft with intratunnel injection of BM-MSC and VEGF, evaluation after 3 weeks; and (4) group IV, treated 6 weeks—ACL reconstruction of the right knee with semitendinosus autograft with intratunnel injection of BM-MSC and VEGF, evaluation after 6 weeks. The animals were allowed to move freely in their cages after the operation. Rabbits in each group were sacrificed at the time of evaluation, then each femoral tunnel was analyzed by using MRI, collagen type III expression, and biomechanical analysis.
Isolation, Culture, and Transplantation of Bone Marrow Stem Cells
Whole bone marrow was aspirated from the pelvic bone of other rabbits. Each aspirate was mixed and coated with Ficoll–phosphate-buffered saline (Ficoll-PBS) 0.077 density (Takara Bio) and then centrifugated at 1600 rpm for 15 minutes. The “buffy coat” located on Ficoll-PBS was collected with a Pasteur pipette. Cells were placed on 5- or 10-cm2 plates, followed by incubation at 37°C with humidity of 5% CO2 for 24 hours. Plates were microscopically examined daily. Every 3 days the cells were washed with 5- or 10-mL PBS, and 10-mL complete culture medium (CCM) was added until the cells became 60% to 80% confluent. In the fourth passage of MSCs, the phenotype was characterized by immunocytochemistry: about 89.1% of BM-MSCs showed positive expression of CD105 and were negative for hematopoietic surface markers of CD45.
For intratunnel application in the rabbit model, 2 × 106 BM-MSCs were used with 100 μg VEGF. A staining of 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) was used to mark and trace BM-MSCs in the femoral tunnel. The number of cells was confirmed by identifying the number of fluorescent cells and the fluorescence intensity.
Collagen Type III Evaluation
Immunostaining was performed using collagen type III alpha 1 monoclonal rabbit antibody (Rockland Immunochemicals). Antigens were activated in 1% trypsin solution (0.2 g trypsin, 0.2 g calcium chloride, 200 mL 0.05 M Tris–HCl buffer) at 37°C for 30 minutes. Endogenous peroxidase was then blocked by reacting with 0.3% H2O2 in absolute ethanol for 30 minutes at room temperature. The primary antibodies were diluted with antibody diluent (Dako S 3022). Each diluent solution was reacted overnight at 4°C. Secondary antibodies were reacted with rabbit antibodies on dextran polymer (Dako K 4000) for 30 minutes at room temperature. Diaminobenzidine (DAB; Dako) was used for staining. The washing solution in each process was 0.1% Tween 20 in 0.05 M Tris–HCl buffer with 0.3 M NaCl. Finally, hematoxylin was used to perform counterstaining, and the observations were made under an optical microscope. Collagen type III evaluated with semiquantitative analysis of the sections was done by light microscopy according to the immunoreactive score (IRS) by Remmele and Stegner.26 The IRS evaluation was based on a modification, which does not only evaluate the visualized grade of color intensity (staining), but also adds the fraction of cells in each intensity category.18 As suggested by Remmele and Stegner, the predominant grade of intensity was used and the IRS with points from 0 to 12.10
MRI Scan Evaluation
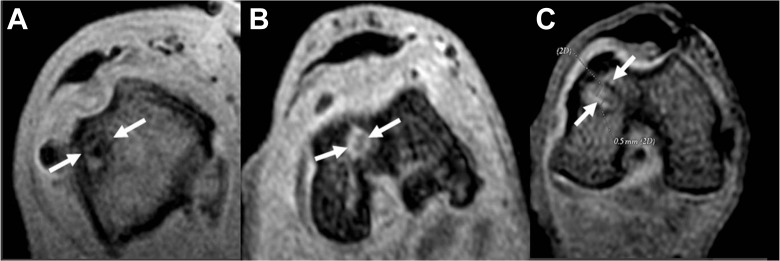
Rabbit knees were assessed on the basis of coronal and axial 5-T MRI scans (General Electric), which analyzed the femoral tunnel sections in 3-dimensional spoiled gradient–recalled echo (3D SPGR) fat saturation sequences (repetition time 2900 ms, echo time 22 ms, number of excitations 3, matrix 320 × 224, field of view 200, slice thickness 2 mm, no spacing) in order to image collagen-containing structures of graft tunnel healing by the identification of high- and low-intensity signals and presence of interface. This was performed at both 3 and 6 weeks postsurgery by a musculoskeletal radiologist who was blinded to the groups. The variables analyzed included graft signal intensity, tunnel widening, and bone-tendon interface diameter. Graft signal intensity was scored on a scale from 1 to 3: 1 indicates a high-intensity signal, similar to that of the synovial fluid; 2 indicates a moderate-intensity signal, similar to that of skeletal muscle; and 3 indicates a low-intensity signal, similar to that of the posterior cruciate ligament, shown in Figures 1 and 2. We also calculated all scores of graft signal intensity of each treated knee taken from 3 locations: the proximal, mid, and distal aspects of the tendon graft.
Figure 1.
Axial magnetic resonance 3-dimensional spoiled gradient–recalled echo (3D SPGR) images of the femoral tunnel. (A) Specimen demonstrating hypointense signal representing less vascularized tissue. (B) Specimen demonstrating moderate vascularized tissue. (C) Specimen demonstrating high vascularized tissue.
Figure 2.
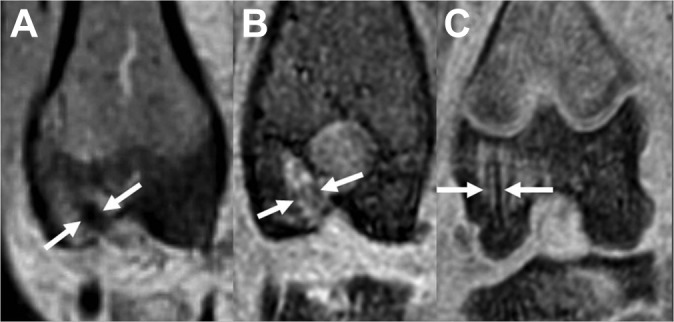
Coronal magnetic resonance 3-dimensional spoiled gradient–recalled echo (3D SPGR) images of the femoral tunnel. (A) Specimen demonstrating hypointense signal representing less vascularized tissue. (B) Specimen demonstrating moderate vascularized tissue. (C) Specimen demonstrating high vascularized tissue.
The tunnel diameter (the width of the femoral tunnel in millimeters) was measured in the coronal and axial sections at both 3 and 6 weeks postsurgery. The bone-tunnel interface diameter (the width of the interface between the adjacent bone tunnel and the tendon graft in millimeters) was also measured in the coronal and axial sections. The mean values of the tunnel diameter and interface measurements were calculated from both coronal and axial sections.
Biomechanical Analysis
The femur and tibia were sectioned 5 cm from the knee joint. All soft tissues around the knee were carefully removed except for the autograft connecting the 2 bones. The suture material used to secure the grafts was not disturbed. These samples were mounted onto a materials testing machine (Instron Corporation). The line of distraction was parallel to both the femoral and tibial tunnels. The specimens were subjected to clinical loading with a strain rate of 40 mm/s (100% per second). The grafts were then loaded to ultimate failure, and the load and the maximal stress at the point of failure were noted. The ultimate tensile strength data were tabulated.
Statistical Analysis
The data collected from the MRI, collagen type III, and biomechanical evaluations were analyzed using the Kolmogorov-Smirnov test, which verified the normal Gaussian distribution of the data sets. After that, comparison between the control and treatment groups at 3 and 6 weeks was performed using an independent t test. Then, the Pearson correlation coefficient was applied between the signal intensity scores, tunnel diameter, bone-tendon interface, collagen type III, and pullout strength data. All analyses were performed using the statistical software package SPSS version 21 (IBM Corp). An alpha value of 5% was considered significant. All data are presented as mean ± SD with 95% confidence intervals.
Results
DAPI Evaluation
To analyze MSC migration in the femoral tunnel after application of allogeneic BM-MSCs and VEGF, preparations were stained with DAPI (Figure 3).
Figure 3.
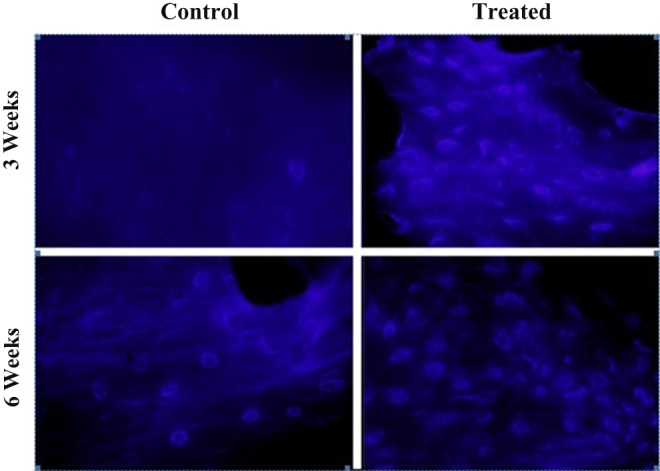
Mesenchymal stem cells stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI). The number of cells with a DAPI-marked nucleus in the (A) control and (B) treated groups at 3- and 6-week evaluation. The number of cells with a DAPI-marked nucleus increased in the treated group compared with the control group.
The mean score of collagen type III was significantly higher in the treated group than in the control group at both 3 weeks and 6 weeks (4.00 ± 2.13 vs 8.63 ± 2.48 and 5.32 ± 1.75 vs 10.10 ± 2.13, respectively). In the femoral tunnel of the treated groups, there was a rapid increase of collagen type III fibers at the 3-week follow-up, followed by a slight decrease in collagen type III expression at the 6-week evaluation.
At 3 weeks, the collagen type III expression was shown as brown fibers and created continuity in the bone tendon interface, while at 6 weeks, the collagen type III fibers appeared denser. Sharpey-like fibers increased gradually from 3 to 6 weeks. These fibers were mainly present in large numbers and more closely connected between the bone-tendon interface tissue at 6 weeks (Figure 4).
Figure 4.
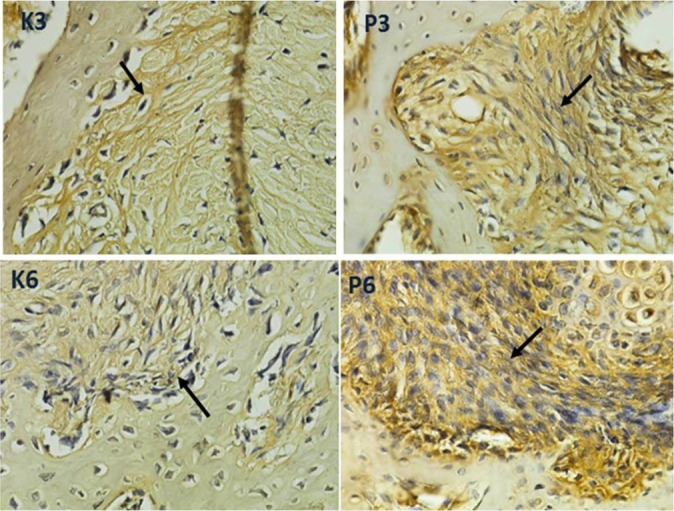
Histological images at 3 and 6 weeks postoperatively with immunostaining for collagen type III in the bone marrow mesenchymal stem cell and vascular endothelial growth factor–treated (P3, P6) and control specimens (K3, K6) showed differences in the expression of collagen type III in the form of brown fiber on the tendon-bone interface (black arrow) between the control and treatment groups at 3 and 6 weeks. Collagen type III expressions of P3 and P6 were more obvious than for K3 and K6 (immunohistochemical staining; magnification 1000×; H600L Nikon microscope; Fi2 camera DS 300 megapixel).
MRI Evaluation
MRI signal intensity correlated with graft maturation. The mean score of signal intensity was significantly higher in the treated group than in the control group at both 3 and 6 weeks (3.33 ± 0.52 vs 7.17 ± 0.75 and 3.50 ± 1.22 vs 8.83 ± 0.40, respectively). The graft signal intensity in the femoral tunnel showed a gradual increase during the 3- and 6-week follow-up in the treated groups compared with the control groups, corresponding with a gradual decrease in the interface and tunnel diameter. Tunnel and interface widening is considered to be associated with increased anteroposterior translation.21
Biomechanical Analysis
Biomechanical analysis correlated with ultimate tensile strength of tendon graft tunnel. The mean value of ultimate tensile strength was significantly higher in the treated group than in the control group at 3 and 6 weeks (0.99 ± 0.12 vs 1.35 ± 0.11 and 1.28 ± 0.04 vs 1.47 ± 0.12, respectively; P = .001 and P = .014). At the 3- and 6-week time points, the treated knee had on average 36% and 15% greater ultimate tensile strength than did the control knee, respectively.
A summary of collagen type III, MRI parameter findings, and biomechanical analysis is presented in Table 1.
TABLE 1.
Comparison of Tendon-to-Bone Healing of the Femoral Tunnel in Control and Treated Groupsa
| Group | MRI Evaluation | Collagen Type III | Biomechanical Analysis | ||
|---|---|---|---|---|---|
| Signal Intensity Score | Tunnel Diameter (mm) | Interface Diameter (mm) | Remmele score | Ultimate Tensile Strength (N/mm2) | |
| Control | |||||
| 3 wk | 3.33 ± 0.52 | 2.07 ± 0.04 | 0.61 ± 0.05 | 4.00 ± 2.13 | 0.99 ± 0.12 |
| 6 wk | 3.5 ± 1.22 | 2.14 ± 0.05 | 0.59 ± 0.02 | 5.32 ± 1.75 | 1.28 ± 0.04 |
| Treated | |||||
| 3 wk | 7.17 ± 0.75 | 1.97 ± 0.05 | 0.50 ± 0.03 | 8.63 ± 2.48 | 1.35 ± 0.11 |
| 6 wk | 8.83 ± 0.40 | 1.98 ± 0.03 | 0.48 ± 0.03 | 10.10 ± 2.13 | 1.47 ± 0.12 |
aValues are presented as mean ± SD. MRI, magnetic resonance imaging.
Correlation Between Collagen Type III, MRI Findings, and Biomechanical Analysis
In the present study, we found a strong correlation between decreased interface diameter and decreased tunnel diameter (P = .001, r = 0.676) as well as increased ultimate tensile strength (P = .007, r = −0.537). There was a strong correlation between increased signal intensity and decreased interface diameter, decreased tunnel diameter, and increased ultimate tensile strength in the treated groups (P = .001, r = −0.810; P = .001, r = −0.680, and P = .000, r = 0.713, respectively). A strong correlation was found between collagen type III expression and signal intensity, interface diameter, tunnel diameter, and increased pullout strength in the treated groups (P = .001, r = 0.793; P = .001, r = −0.673; P = .016, r = −0.488; and P = .002, r = 0.598, respectively) (Figures 5 –8). All parameters using collagen type III, MRI, and biomechanical tests showed that application of intratunnel BM-MSC and VEGF enhanced tendon-to-bone healing after ACL reconstruction.
Figure 5.
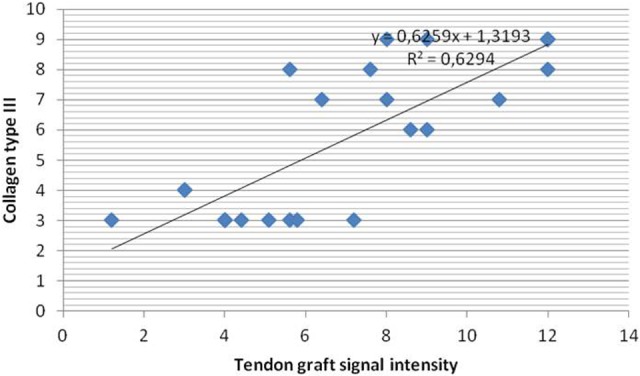
Correlation between collagen type III and tendon graft signal intensity the between the control and treated groups.
Figure 6.
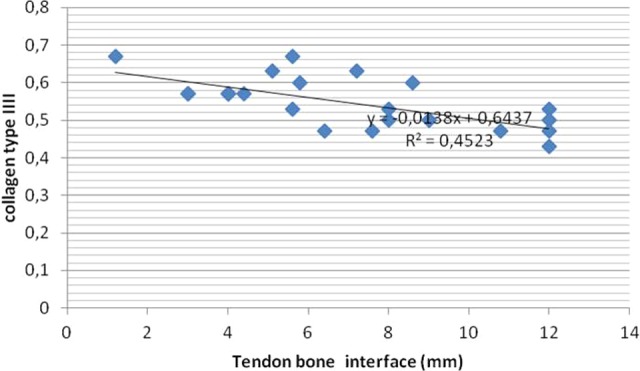
Correlation between collagen type III and interface diameter (mm) between the control and treated groups.
Figure 7.
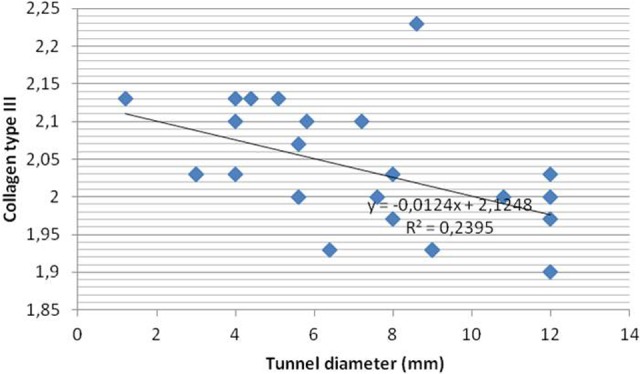
Correlation between collagen type III and tunnel diameter (mm) between the control and treated groups.
Figure 8.
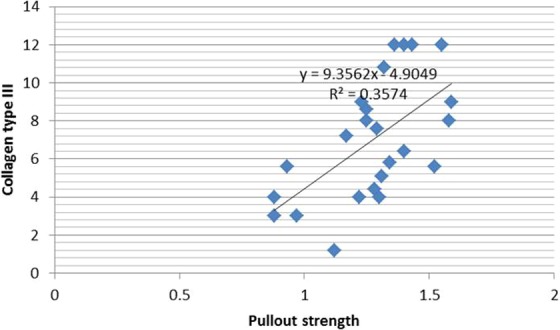
Correlation between collagen type III and pullout strength between the control and treated groups.
Discussion
The primary weakness of a tendon graft reconstruction during the early postoperative period is at the bone-tendon interface. The healing at the bone-tendon interface occurs initially by granulation tissue in the interface between the bone and the tendon along the bone tunnel. As time goes by, the progressive increase in strength is correlated with the degree of bone ingrowths, mineralization, and maturation of the healing tissue. Progressive re-establishment of collagen fiber continuity between the tendon and the bone facilitates re-establishment of a tendon osseous junction.6 In the present study, we showed in a rabbit model that application of intratunnel BM-MSC and VEGF improves tendon-to-bone healing after ACL reconstruction, as demonstrated by improvement in all parameters using collagen type III, MRI, and biomechanical tests. Immunostaining showed a significant increase of the formation of collagen type III fibers during the first 3 weeks after operation compared with the controls, which showed a gradual increase of collagen type III fibers, confirming that early healing proceeds at the interface 3 weeks after surgery. A strong adhesion of the tendon graft to the wall of the bone tunnel forms Sharpey-like fibers, which obliquely oriented from the wall of the tunnel to the tendon graft, composed of collagen type III.22 In the early healing phase, fibrovascular tissue is formed at the interface between the tendon graft and the inside wall of the bone tunnel.23 Collagen fibers are subsequently deposited at the tendon-bone interface, and the tendon and bone become directly joined by characteristic anchoring fibers, which resemble Sharpey fibers and firmly connect the periosteum to the bone.8,23 These fibers, called Sharpey-like fibers,11,23,36 are important structures for inhibition of pullout failures of tendon grafts from bone tunnels.23
These results are similar to previous studies that have examined the integration of tendon-bone healing. While differences exist in the animal models and experimental methods used in the various experiments, Sharpey-like fibers are generally observed through hematoxylin and eosin staining and polarized light microscopy at between 3 and 4 weeks postoperatively. Sharpey-like fibers increase gradually from 6 to 12 weeks, and during this period, mechanical testing showed that tendon midsubstance ruptures occur more frequently than pullout failures. Thus, Sharpey-like fibers have been considered to be an important structure from the viewpoint of mechanics.8,23,29 Liu et al15 observed that anchoring fibers resembling Sharpey-like fibers were composed of type III collagen fibers at 6 weeks after surgery in a rabbit model. Similarly, Kanazawa et al15 observed Sharpey-like fibers up to 8 weeks after surgery in a rabbit model and found that they were solely composed of collagen type III.
MRI is a reliable and noninvasive tool for diagnosing acute ACL injury and evaluating its reconstruction, particularly when clinical assessment is difficult. MRI has been used to evaluate stages of ACL healing after different grafting techniques.2,14 In this study, we found a gradual increase in MRI signal intensity during the 3- and 6-week follow-up in the treated group compared with the control group. It is assumed that during initial remodeling, the graft undergoes revascularization and resynovialization, resulting in increased signal intensity or hyperintensity on MRI, which may be used to evaluate the bone-tendon interface. Signal hyperintensity is thought to reflect newly formed tissue in the bone tunnel. After this initial period, the ACL graft will return to a hypointense signal, comparable to the native ACL.1,2
Weiler et al36 and Anderson et al2 showed that MRI signal intensity may be correlated with graft maturation. In animal models, the addition of growth factors (platelet-derived growth factor [PDGF], bone morphogenetic protein [BMP], and transforming growth factor–β [TGF-β]) improved the structural and mechanical properties of a free tendon graft in ACL reconstruction, as measured by MRI, histologic, and biomechanical testing.2,33
Tunnel widening is most likely related to issues arising from both mechanical and biological causes. Wilson et al in Wagner et al34 analyzed the mechanical and biological causes that can potentially produce tunnel widening. Synovial fluid in the graft-tunnel interface is rich in cytokines and is responsible for producing osteolysis. In the present study, the use of BM-MSC and VEGF improved the graft press-fit to the walls of the femoral tunnel and reduced tunnel widening; this was confirmed by the finding of a significant reduction in both the tunnel and interface diameter in the treated compared with control groups. The absence of an interface reflects major integration and maturation of the graft. In the intratunnel graft healing process, incorporation progresses through the formation of a new matrix at the tendon-bone interface. Proliferation of new trabecular bone along the edge of the tunnel, seen as early as 3 weeks after surgery, results in a tight connection between adjacent bone and tendon graft.
All MRI parameters in this study showed a good correlation with immunohistochemistry testing of collagen type III fiber formation. This emphasizes that MRI can be used as a reliable modality for measuring different stages of the graft healing process, based on graft signal intensity, bone-tendon interface diameter, and tunnel diameter and for making it possible to establish graft maturation by a noninvasive technique.3,5,30
The ultimate tensile strength test was used because the activity level of these animals postoperatively was not strictly limited. Each animal was allowed to move as tolerated. It is likely that the reconstructed ligaments were exposed to some level of repeated loading before sacrifice.2 In the present study, the ultimate tensile strength of ACL reconstructions with intratunnel BM-MSCs and VEGF injection was significantly greater than that in the control groups, averaged more than 15% of the tendon-bone complex strength, and also had significant correlation with collagen III and signal intensity as well as interface diameter on MRI evaluation. Overall, the treated knee had more advanced healing and had greater ultimate load to failure. Kanazawa et al11 studied the intra-articular injection of mesenchymal stromal cells in partially torn ACLs in a rat model and found that the ultimate failure load of the femur-ACL-tibia complex with MSC was significantly higher than in the control group without MSC at 4 weeks after surgery.4
A good combination of surgical, biological, and biomechanical enhancement may improve the surgical prognosis of ACL reconstruction. The application of cell therapy using mesenchymal progenitor cells or MSCs has been widely studied within the area of ACL reconstruction. MSCs harvested from mesenchymal tissues (ie, bone marrow) can differentiate into various cell types (ie, osteoblasts, fibroblasts) required to regenerate different tissues such as bone, cartilage, tendon, ligament, and fat. Growth factors also have shown to be able to regulate and improve the cellular activities and proliferation and extracellular matrix deposition and to influence the differentiation of MSCs into fibroblasts to repair the torn ligaments. Growth factors have exhibited positive effects on various biological processes needed to improve ACL healing.15–17,25,27 The proliferation of BM-MSCs, which targets cells by the autocrine or paracrine role, strengthens osteoinduction and ultimately promotes tendon-bone healing. BM-MSCs promote the integration of the tendon-bone interface by differentiating into bone and cartilage cells or enhancing collagen formation.4
One clinical study evaluated the effect of coating tendon grafts with MSCs on the rate and quality of graft osteointegration in ACL reconstruction.12 In the current study, the application of BM-MSCs and VEGF have significantly improved and stimulated cell proliferation, differentiation, and matrix deposition by enhancing angiogenesis and osteogenesis of the graft in the tunnel. The improvement of the cell has been reflected by the increase in collagen type III, graft signal intensity, and ultimate tensile strength, as well as the reduction of tunnel and bone-tunnel interface diameters. The results of the experiment can be seen 3 and 6 weeks after reconstruction. Our findings demonstrated the potential of BM-MSCs and VEGF to improve tendon-bone healing, which may be a novel method offering physiologically and biomechanically stronger ligament reconstructions in clinical practice. However, more animal and clinical studies are needed to confirm the effectiveness of the modality.
There are some limitations to this study. The evaluations were performed at 3 and 6 weeks instead of longer time periods. We did not demonstrate the final healing since the original intention of the experiment was to assess the early healing of the graft tunnel with the addition of MSCs. The tibial tunnel was not evaluated in this study because of the relative paucity of cancellous bone in the tibial metaphysis in rabbits, and hence a less vigorous healing process. Future studies are needed to show how this treatment may affect the recovery process, during a longer period of observation with controlled exercises incorporated into the protocol. Another limitation is that rabbit healing is much quicker than healing in humans; however, our results can still be interpreted as faster healing in treatment groups compared with controls.
Conclusion
Delivering of intratunnel BM-MSCs and VEGF after ACL reconstruction enhances the graft tunnel healing. Overall, the femoral tunnel that received BM-MSCs and VEGF had more advanced healing with greater collagen type III fibers, MRI scoring evaluation, and biomechanical strength. MRI is a reliable tool for clinical use in evaluating stages of ACL healing after reconstruction, since ligament biopsy is an invasive procedure.
Acknowledgment
The authors thank Helen Susilowati and Eryk Hendrianto for their technical assistance.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
Ethical approval for this study was obtained from Airlangga University (No. 510-KE).
References
- 1. Amiel D, Kleiner JB, Roux RD, Harwood FL, Akeson WH. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4:162–172. [DOI] [PubMed] [Google Scholar]
- 2. Anderson K, Seneviratne AM, Izawa K, Atkinson BL, Potter HG, Rodeo SA. Augmentation of tendon healing in an intraarticular bone tunnel with use of a bone growth factor. Am J Sports Med. 2001;29:689–698. [DOI] [PubMed] [Google Scholar]
- 3. Deehan DJ, Clawston TE. The biology of integration of the anterior cruciate ligament. J Bone Joint Surg Br. 2005;87:889–895. [DOI] [PubMed] [Google Scholar]
- 4. Dong Y, Zhang Q, Li Y, Jiang J, Chen S. Enhancement of tendon-bone healing for anterior cruciate ligament (ACL) reconstruction using bone marrow–derived mesenchymal stem cells infected with BMP-2. Int J Mol Sci. 2012;13:13605–13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fules PJ, Madhav RT, Goddard RK, Newman-Sanders A, Mowbray MA. Evaluation of tibial bone tunnel enlargement using MRI scan cross-sectional area measurement after autologous hamstring tendon ACL replacement. Knee. 2003;10:87–91. [DOI] [PubMed] [Google Scholar]
- 6. Goldblatt JP, Fitzsimmons SE, Balk E, Richmond JC. Reconstruction of the anterior cruciate ligament: meta-analysis of patellar tendon versus hamstring tendon autograft. Arthroscopy. 2005;21:791–803. [DOI] [PubMed] [Google Scholar]
- 7. Goradia VK, Rochat MC, Grana WA, Rohrer MD, Prasad HS. Tendon-to-bone healing of a semitendinosus tendon autograft used for ACL reconstruction in a sheep model. Am J Knee Surg. 2000;13:143–151. [PubMed] [Google Scholar]
- 8. Grana WA, Egle DM, Mahnken R, Goodhart CW. An analysis of autograft fixation after anterior cruciate ligament reconstruction in a rabbit model. Am J Sports Med. 1994;22:344–351. [DOI] [PubMed] [Google Scholar]
- 9. Ju YJ, Muneta T, Yoshimura H, Koga H, Sekiya I. Synovial mesenchymal stem cells accelerate early remodeling of tendon–bone healing. Cell Tissue Res. 2008;332:469–478. [DOI] [PubMed] [Google Scholar]
- 10. Kaemmerer D, Petre L, Lupp A, et al. Comparing of IRS and Her2 as immunohistochemical scoring schemes in gastroenteropancreatic neuroendocrine tumors. Int J Clin Exp Pathol. 2012;5(3):187–194. [PMC free article] [PubMed] [Google Scholar]
- 11. Kanazawa T, Soejima T, Murakami H, Inoue T, Katouda M, Nagata K. An immunohistological study of the integration at the bone-tendon interface after reconstruction of the anterior cruciate ligament in rabbits. J Bone Joint Surg Br. 2006;88:682–687. [DOI] [PubMed] [Google Scholar]
- 12. Kiapour AM, Murray MM. Basic science of anterior cruciate ligament injury and repair. Bone Joint Res. 2014;3:20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kohno T, Ishibashi Y, Tsuda E, Kusumi T, Tanaka M, Toh S. Immunohistochemical demonstration of growth factors at the tendon-bone interface in anterior cruciate ligament reconstruction using a rabbit model. J Orthop Sci. 2007;12:67–73. [DOI] [PubMed] [Google Scholar]
- 14. Lim JK, Hui J, Li L, Thambyah A, Goh J, Lee EH. Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy. 2004;20:899–910. [DOI] [PubMed] [Google Scholar]
- 15. Liu SH, Panossian V, al-Shaikh R, et al. Morphology and matrix composition during early tendon to bone healing. Clin Orthop Relat Res. 1997;339:253–260. [DOI] [PubMed] [Google Scholar]
- 16. Longo UG, Lamberti A, Maffulli N, Denaro V. Tissue engineered biological augmentation for tendon healing: a systematic review. Br Med Bull. 2011;98:31–59. [DOI] [PubMed] [Google Scholar]
- 17. Maywood RM, Murphy BJ, Uribe JW, Hechtman KS. Evaluation of arthroscopic anterior cruciate ligament reconstruction using magnetic resonance imaging. Am J Sports Med. 1993;21:523–527. [DOI] [PubMed] [Google Scholar]
- 18. McCarty KS, Jr, Miller LS, Cox EB, Konrath J, McCarty KS., Sr Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109:716–721. [PubMed] [Google Scholar]
- 19. Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Am J Sports Med. 2003;33:381–394. [DOI] [PubMed] [Google Scholar]
- 20. Moreau JE, Chen J, Bramono DS, et al. Growth factor induced fibroblast differentiation from human bone marrow stromal cells in vitro. J Orthop Res. 2005;23:164–174. [DOI] [PubMed] [Google Scholar]
- 21. Neddermann A, Willbold E, Witte F, et al. Tunnel widening after anterior cruciate ligament reconstruction: an experimental study in sheep. Am J Sports Med. 2009;37:1609–1617. [DOI] [PubMed] [Google Scholar]
- 22. Nagumo A, Yasuda K, Numazaki H, et al. Effects of separate application of three growth factors (TGF-β1, EGF, and PDGF-BB) on mechanical properties of the in situ frozen-thawed anterior cruciate ligament. Clin Biomech (Bristol, Avon). 2005;20:283–290. [DOI] [PubMed] [Google Scholar]
- 23. Nakase J, Kitaoka K, Matsumoto K, Tomita K. Facilitated tendon-bone healing by local delivery of recombinant hepatocyte growth factor in rabbits. Arthroscopy. 2010;26:84–90. [DOI] [PubMed] [Google Scholar]
- 24. Ouyang HW, Goh JC, Lee EH. Use of bone marrow stromal cells for tendon graft-to-bone healing: histological and immunohistochemical studies in a rabbit model. Am J Sports Med. 2004;32:321–327. [DOI] [PubMed] [Google Scholar]
- 25. Proffen BL, Haslauer CM, Harris CE, Murray MM. Mesenchymal stem cells from the retropatellar fat and peripheral blood stimulate ACL fibroblast migration, proliferation, and collagen gene expression. Connect Tissue Res. 2013;54:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 27. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel: a biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75-A:1795–1803. [DOI] [PubMed] [Google Scholar]
- 28. Rodeo SA, Kawamura S, Ma B. The effect of osteoclastic activity on tendon-to-bone healing: an experimental study in rabbits. J Bone Joint Surg Am. 2007;89:2250–2259. [DOI] [PubMed] [Google Scholar]
- 29. Sakai T, Yasuda K, Tohyama H, et al. Effects of combined administration of transforming growth factor-β1 and epidermal growth factor on properties of the in situ frozen anterior cruciate ligament in rabbits. J Orthop Res. 2002;20:1345–1351. [DOI] [PubMed] [Google Scholar]
- 30. Scherping SC, Jr, Schmidt CC, Georgescu HI, Kwoh CK, Evans CH, Woo SL. Effect of growth factors on the proliferation of ligament fibroblasts from skeletally mature rabbits. Connect Tissue Res. 1997;36:1–8. [DOI] [PubMed] [Google Scholar]
- 31. Sherman OH, Banffy MB. Anterior cruciate ligament reconstruction: which graft is best? Arthroscopy. 2004;20:974–980. [DOI] [PubMed] [Google Scholar]
- 32. Tabuchi K, Soejima T, Kanazawa T. Chronological changes in the collagen type composition at tendon-bone interface in rabbits. Bone Joint Res. 2012:1:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Uchio Y, Ochi M, Adachi N, Kawasaki K, Kuriwaka M. Determination of time of biologic fixation after anterior cruciate ligament reconstruction with hamstring tendons. Am J Sports Med. 2003;31:345–352. [DOI] [PubMed] [Google Scholar]
- 34. Wagner M, Kaab MJ, Schallock J, Haas NP, Weiler A. Hamstring tendon versus patellar tendon anterior cruciate ligament reconstruction using biodegradable interference fit fixation: a prospective matched-group analysis. Am J Sports Med. 2005;33:1327–1336. [DOI] [PubMed] [Google Scholar]
- 35. Wang CJ, Wang FS, Yang KD, Weng LH, Sun YC, Yang YJ. The effect of shock wave treatment at the tendon-bone interface—a histomorphological and biomechanical study in rabbits. J Orthop Res. 2005;23:274–280. [DOI] [PubMed] [Google Scholar]
- 36. Weiler A, Peters G, Maurer J, Unterhauser FN, Sudkamp NP. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging. A two-year study in sheep. Am J Sports Med. 2001;29:751–761. [DOI] [PubMed] [Google Scholar]