Abstract
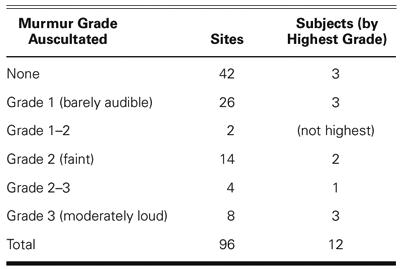
A pilot study was conducted to ascertain the level of agreement between auscultatory findings derived from heart sound recordings by a cardiologist and the results of a computer-based heart sound analysis algorithm. Heart sound recordings were obtained from volunteer subjects previously diagnosed with hypertrophic cardiomyopathy. Twenty-second recordings were obtained at each of 4 standard auscultatory locations on the precordium in 2 postures: standing and reclining. Detailed auscultatory findings were derived by a cardiologist, who listened to the heart sound recordings and was blinded to the study design. The recordings were analyzed by an algorithm that detects heart sounds and murmurs, and derives associated timing and energy parameters. The algorithm results were compared with the auscultatory findings provided by the cardiologist and correlated with the medical histories provided by the volunteer subjects.
A high degree of concordance between the medical histories, auscultatory findings, and computer analyses was obtained. The 1st and 2nd heart sounds were detected with high sensitivity (90.7%) and positive predictivity (93.0%). Systolic murmurs were detected with a sensitivity that increased from 50% for grade 1 to 100% for grades 2–3 and 3. The signal energy in the mid-frequency range correlated well with murmur grade judgments, and also agreed well with the cardiologist's judgments of the relative loudness of murmurs in standing versus reclining postures. The computer analysis algorithm thus instantiates the objective detection and identification of apical systolic murmurs that are louder in standing than in reclining postures; such murmurs are a cardinal sign of hypertrophic obstructive cardiomyopathy.
Key words: Athletics; cardiomyopathy, hypertrophic; death, sudden, cardiac; diagnosis, computer-assisted; heart auscultation; heart murmurs; human; screening
There is a clear and immediate need for a cost-effective screening method to identify young athletes who are at risk of sudden cardiac death due to hypertrophic cardiomyopathy (HCM). Hypertrophic cardiomyopathy is the most common cardiovascular cause of sudden death in this population, accounting for about one third of such events.1
Hypertrophic cardiomyopathy is a relatively common autosomal dominant genetic anomaly with heterogenous expression that is characterized by myocardial cellular disarray in various locations of the ventricles. In its obstructive form (HOCM), comprising about 40% of cases, there is systolic obstruction to aortic outflow due to the proximity of the anterior leaflet of the mitral valve and the ventricular septum, enlarged and distorted by the cellular disarray. This obstruction produces a systolic murmur, audible on auscultation, which diminishes when the patient squats from a standing position and increases in intensity when the patient performs the Valsalva maneuver or performs an isometric hand grip.
The nonobstructive form of HCM, comprising about 60% of cases, is characterized by myocardial cellular disarray in myocardial locations that do not produce obstruction and, therefore, do not produce a murmur. However, the alteration of myocardial ventricular structure frequently produces an atrial diastolic gallop (S4 sound) and a ventricular diastolic gallop (S3 sound).2
Other features of HCM that are potential flags in screening situations are a history of sudden death in close relatives, a history of syncope or near-syncope in the patient, and an abnormal electrocardiogram, which is found in an extremely high percentage of asymptomatic patients. A medical and family history and physical examination, including auscultation of the heart, are recommended by the American Heart Association (AHA) for pre-participation screening of competitive athletes. Although auscultation by a competent examiner using suitable maneuvers would be sufficient to detect a murmur associated with HCM, the variability of clinical skills and uneven compliance with AHA guidelines has created a situation in which young athletes with HCM are frequently not flagged for further study before they engage in competitive sports.3–5
This study was designed to assess patients with previously diagnosed HCM (both obstructive and nonobstructive) for the presence of heart murmur, murmur characteristics, change in murmur loudness with postural change, and detectability of murmurs by computer algorithm.
Patients and Methods
Those in attendence at the 2003 Annual Meeting of the Hypertrophic Cardiomyopathy Association in Morristown, New Jersey, were invited to participate in the study. Volunteers were enrolled with informed consent under an institutional review board-approved protocol and completed a brief medical history form. Heart sound recordings were subsequently obtained from the volunteer subjects, with use of an electronic stethoscope (E-Scope® II, Cardionics, Inc.; Webster, Tex) connected to a laptop computer. The subjects were auscultated in 2 postures, standing and reclining, by a registered nurse in a quiet, private room. During this procedure, heart sounds were recorded for each posture at 4 standard locations on the precordium at the point of palpated maximum impulse: the 2nd intercostal space at the right (2R) and left (2L) sternal borders, the 4th intercostal space at the left sternal border (4L), and the apex. In the reclining posture, the apex recording was obtained in the left-lateral decubitus position. At each auscultatory site, a 20-second recording of the subject's heart sounds was acquired.
The auscultatory recordings were made under computer control, using a voice-guided protocol and graphical user interface, and were evaluated automatically for data quality. Signals that were clipped, or were too loud or too faint, were discarded. Each auscultatory site was recorded until an acceptable signal quality was obtained.
The auscultatory recordings were meticulously audited by a cardiologist blinded to the study design (JB), who has long-term experience in listening to heart sound recordings. Each recording was played back through high-fidelity headphones and listened to repeatedly until the cardiologist was confident of the precise auscultatory findings. Grades were assigned to the murmurs in accordance with the following guidelines: Grade 1 is a barely perceptible murmur that requires listening carefully to several heartbeats before it is discerned; grade 2 is easily audible; and grade 3 is loud. Murmurs that are grade 4 or higher are defined as being palpable. Since heart sound recordings alone were available for consideration, no murmurs were assigned a grade higher than 3.
Spectrograms of each auscultatory recording were computed and examined independently of the cardiologist. The spectrogram of heart sound recordings can be used generally to determine the location of the 1st and 2nd heart sounds, level of background noise, presence or absence of murmur, timing, and spectral properties.6 Acoustic characteristics evident in the spectrogram were noted for each heart sound recording.
Each heart sound recording was also processed by a computer heart sound analysis and murmur detection algorithm. The computer algorithm identifies the 1st and 2nd heart sounds,7,8 resolves the recording into a sequence of systolic and diastolic intervals, and assesses the presence or absence of murmurs in each systolic and diastolic interval. The algorithm computes and reports the median and standard deviation of the instantaneous heart rate, along with the percentage of analyzed beats that contain murmurs. The algorithm also displays the recorded signal, and highlights on the displayed signal the duration and amplitude of murmurs detected in the signal (Fig. 1). The algorithm computes and displays the median normalized mid-range signal energy for each third of the systolic and diastolic intervals (Fig. 2).
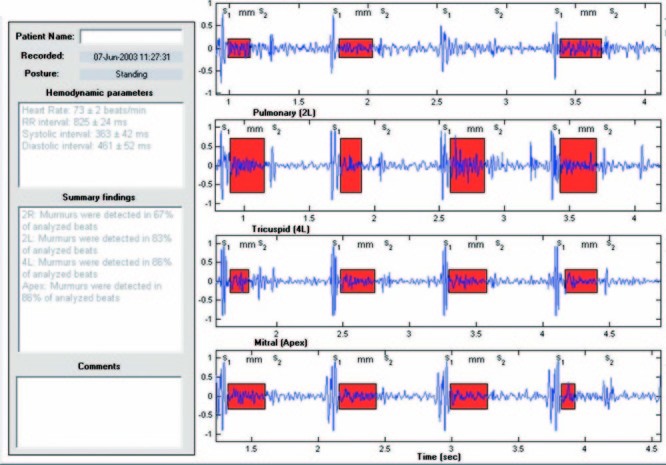
Fig. 1 Results of analysis of heart sounds for 1 subject (case 6) show summary hemodynamic and auscultatory findings, a selected segment of each heart sound recording, annotations of S1 and S2 events, and red shading to highlight murmur timing and relative amplitude.
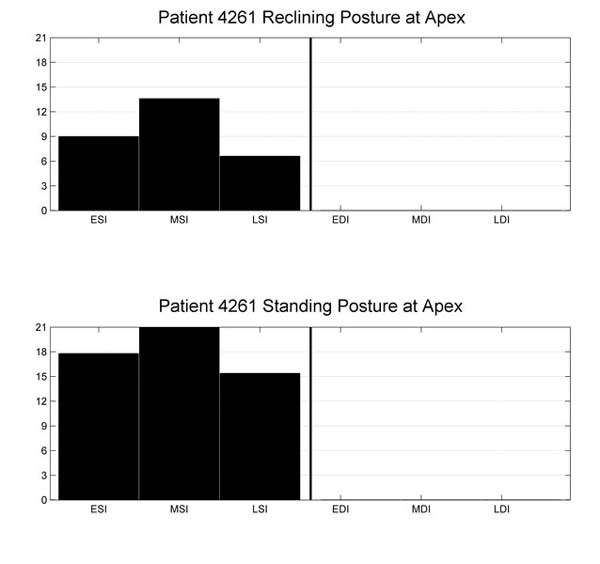
Fig. 2 Example display of normalized mid-range energy for 1 subject (case 2) in the reclining (top) and standing (bottom) postures.
EDI = early diastolic interval; ESI = early systolic interval; LDI = late diastolic interval; LSI = late systolic interval; MDI = mid-diastolic interval; MSI = mid-systolic interval
The auscultatory findings derived by the cardiologist from the heart sound recordings were then compared with the medical histories, in order to correlate auscultatory findings with physiological conditions and to confirm or disconfirm expected auscultato-ry consequences of the reported medical conditions. Next, the spectral properties of the signals were compared with the auscultatory findings to identify and confirm spectral properties associated with the various auscultatory findings. Finally, the murmur detection algorithm results were compared with the auscultatory findings, spectral properties, and medical history information to evaluate the accuracy of the murmur detection algorithm, and to correlate the murmur results (presence or absence) with auscultatory characteristics, spectral properties, and medical conditions. These comparisons were made in order to evaluate the automatic detection of systolic murmurs associated with HOCM.
Results
Twelve subjects were enrolled in the study and completed the protocol: 9 men and 3 women, whose ages ranged from 30 to 71 years (mean, 53 years). The interval since diagnosis with HCM or HOCM, when reported, ranged from 3 months to more than 15 years.
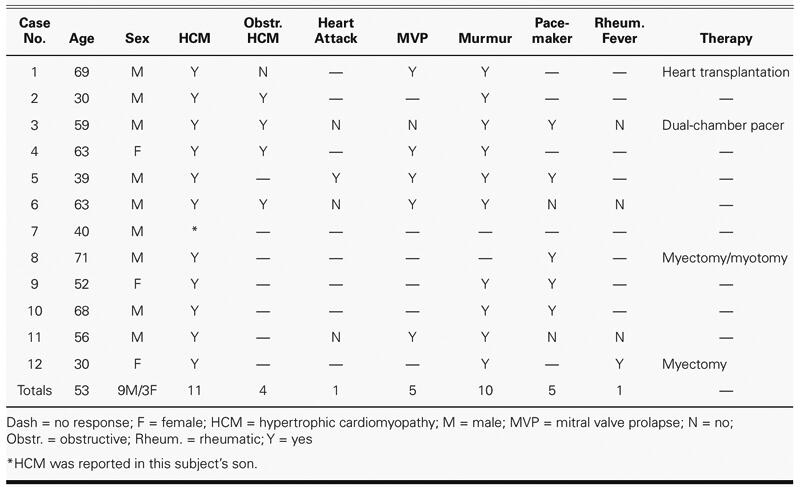
Eleven subjects reported a diagnosis of hypertrophic cardiomyopathy; the remaining subject reported HCM in the family (a son). Four of the subjects reported having HOCM: none of these reported surgical repair, but one reported having an implanted dual-chamber pacemaker.
Ten subjects reported a history of a heart murmur, which included all 4 who had HOCM. One subject reported having had rheumatic fever, 1 reported a heart attack, 5 reported mitral valve prolapse (MVP), and 5 reported having cardiac pacing devices implanted. Two of the 5 subjects with MVP also reported HOCM. Various corrective surgeries were also reported, including heart transplantation and myectomy (Table I).
TABLE I. Summary of Medical History Responses
The study resulted in 96 auscultatory recordings (12 subjects × 4 sites × 2 postures). Auscultatory findings were obtained from all recordings.
Auscultatory Findings
The auscultatory findings are summarized in Table II. Of the 4 reported cases of HOCM (cases 2, 3, 4, and 6), the cardiologist detected systolic murmurs in all 4 cases, at all 4 sites; the murmurs were loudest at the apex (in 3 subjects) and at 2L (in 1 subject). The murmurs were “louder on standing” (in 2) and “slightly louder on standing” (in 1). No comment about postural differences in loudness was made for the 4th HOCM subject, who was judged to have faint murmurs (grade 1) at all sites except 2L, where a grade 2 murmur was heard. This subject also had a dual-chamber pacemaker; it may be that the ventricular pacing for this patient was adjusted to resolve the obstruction and thus largely extinguished the murmur.
TABLE II. Summary of Auscultatory Findings
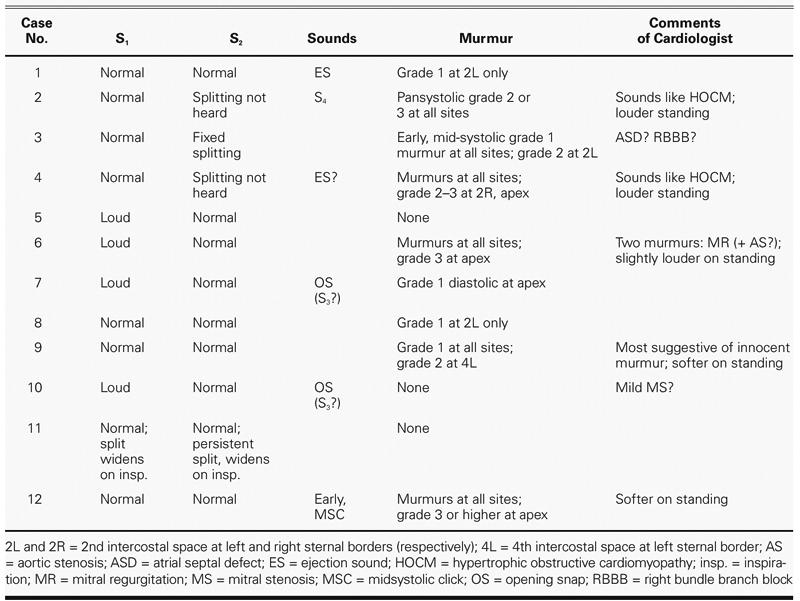
An S4 was heard in 1 subject (case 2); in that subject and another (case 4), HOCM was suspected. In a 3rd subject (case 6), 2 murmurs were audible; one was typical of mitral regurgitation, and the second, possibly of aortic stenosis. A fixed-split S2 was heard in 1 subject (case 3), consistent with dual-chamber pacing. There was thus good agreement between auscultatory findings and medical history, particularly with regard to the presence of murmurs and the suspicion of HOCM.
Of the 7 remaining cases of HCM reported as nonobstructive, murmurs were self-reported in 6. Of these 6, the cardiologist discerned murmurs in only 3 (cases 1, 9, and 12). One of these 3 had undergone heart transplantation; in this subject, a grade 1 murmur was detected at 2L only. Another of these 3 subjects had undergone a septal myectomy (case 12), and murmurs were found at all sites, with grade 3 or higher at the apex. There were no surgical procedures reported for the other 3 subjects reporting murmurs, in whom no murmurs were detected.
Of the 2 subjects not reporting murmurs (cases 7 and 8), murmurs were discerned by the cardiologist in both cases: a grade 1 apical diastolic mitral obstructive murmur was found in the subject with no reported HCM (case 7), and a grade 1 murmur was found at 2L only, in 1 subject (case 8), who reported a left ventricular myectomy/myotomy. Table III presents a summary of the murmurs heard, according to grade.
TABLE III. Summary of Murmurs and Grades Determined by Listening
No systolic clicks were detected in any of the 5 subjects reporting MVP; of these subjects, an ejection sound was heard in 1 (case 1) and was suspected in another (case 4). A systolic click was detected in 1 patient not reporting MVP (case 12).
Spectral Analysis Results
Spectrograms of the heart sound recordings were computed using a standard signal processing toolbox and were compared with the time-domain heart sound signals by a scientist (TO) highly experienced in signal processing and analysis. This analysis of the recorded signals and their spectra, independent of the auscultatory findings provided by the cardiologist, led to the identification of all systolic murmurs except for 2: the grade 1 diastolic murmur auscultated at the apex in case 7, and the grade 1 systolic murmur auscultated at 2L in case 8. Thus, spectral and signal analysis achieved an 80% sensitivity to grade 1 murmurs and a 100% sensitivity to murmurs above grade 1. There were no false-positive murmur detections by analysis of the spectra. No systolic clicks were identified by spectral analysis.
Murmur Detection Algorithm Results
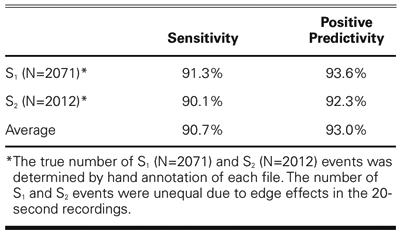
The computer heart sound analysis program was evaluated by comparing the S1 and S2 time-indices generated by the computer algorithm against the set of reference annotations, using a window of 100 ms around the fiducial points. The computer algorithm was found to have good sensitivity (90.7%) and positive predictivity (93.0%) for these data (see Table IV).
TABLE IV. Sensitivity and Positive Predictivity for S1 and S2 Detection
As depicted in Figure 1, the computer algorithm reports the summary hemodynamic and auscultatory findings in text format and displays a selected segment of the recorded signal for each auscultatory site, with annotations of the detected S1 and S2 events, and indications of the presence of murmurs using shaded boxes to indicate the relative murmur amplitude and duration.
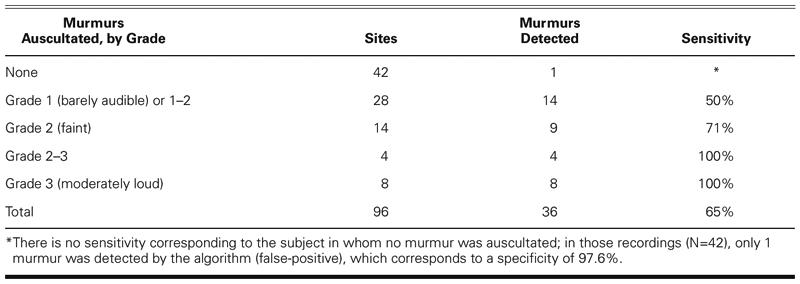
The computer algorithm detected murmurs in 36 of the 96 recordings, as summarized in Table V. When compared against the murmur judgments and murmur grades determined by the reference cardiologist, the algorithm generated 1 false-positive finding (specificity = 97.6%) and 35 true positives (sensitivity = 64.8%). When the murmur grade was taken into account, the algorithm detected 50% of grade 1 or grade 1–2 murmurs, 71% of grade 2 murmurs, and 100% of grade 2–3 and grade 3 murmurs.
TABLE V. Sensitivity of Murmur Detection Algorithm by Murmur Grade
As noted above, 1 subject was found to have a grade 1 apical diastolic murmur; this murmur was correctly detected and reported by the algorithm (Fig. 3).
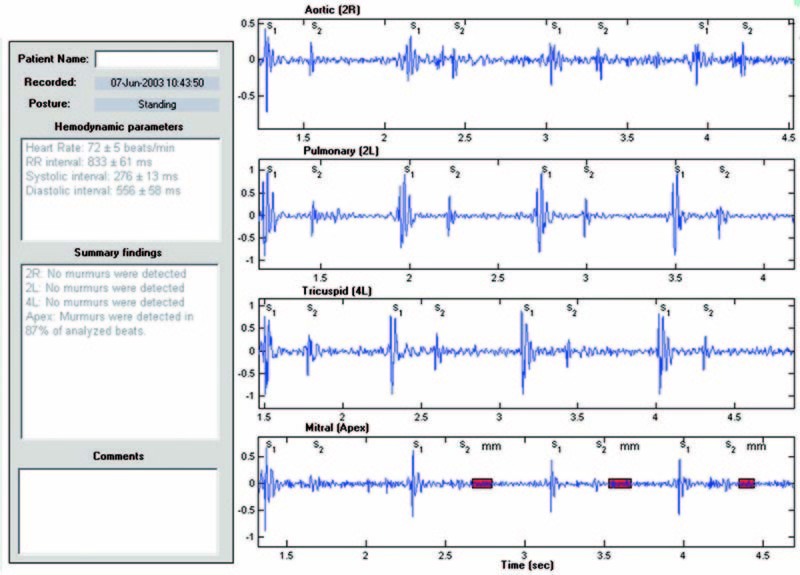
Fig. 3 Detection by algorithm of grade 1 apical diastolic murmur unreported by the subject (case 7). Red shading highlights murmur timing and relative amplitude.
Signal Energy Results
The computer algorithm also computes and reports a measure of the normalized signal energy in the mid-frequency range during systole and diastole. For each recording, the average mid-range signal energy is computed for each third of the systolic and diastolic intervals, for each beat, normalized by the total mid-range energy in the recording. This method is similar to one described for computing mid-systolic energies with reference to a synchronous electrocardiographic recording.9 The median value of each of these normalized energy levels is computed and displayed in the form of a bar graph (Fig. 2).
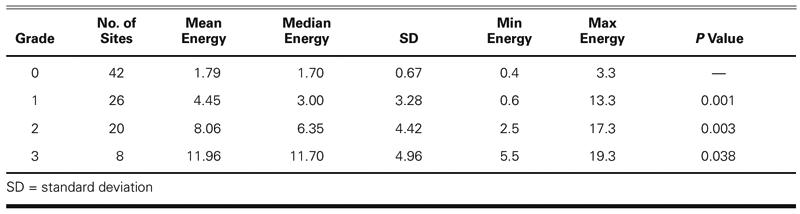
The median systolic signal energies were stratified by the cardiologist's judgments of the presence or absence of murmurs, and by grade, for detected murmurs. Since the murmurs detected were almost all systolic, only systolic energy values were evaluated. The systolic energy measure was found to correspond well to murmur grade judgments: there were 4 subjects with systolic energies that were clearly higher at all sites than those of the other subjects; these subjects were those identified with grade 2 or higher murmurs at more than 1 site. The remaining 8 subjects were those with grade 1 murmurs or no murmurs, or a grade 2 murmur at only 1 site. More precisely, the mean systolic energies for grades 0, 1, and 2 were determined to be distinct using a 1-tailed, 2-sample, unequal variance Student's t-test, with P < 0.05 considered significant (Table VI). (We treated the absence of murmur as grade 0.) The mean murmur energy for grade 3 murmurs was found to be distinct, at P = 0.038.
TABLE VI. Comparison of Energy Measure with Murmur Grade
The mid-frequency systolic energy was also compared, by auscultatory site, for each subject, for both the standing and the reclining postures. Because the systolic energy measure is normalized by total signal energy, it supports comparison of relative murmur energies between sites and postures. For reference, the systolic energies for the apical site are depicted in Figure 4. The systolic energy measure was markedly higher in the standing posture than in the reclining posture for 3 subjects (cases 2, 4, and 6). These 3 subjects were the ones identified by the cardiologist as having murmurs that were either “louder on standing” or “slightly louder on standing.” The energy difference between the standing and reclining postures was greater for the 2 “louder on standing” subjects than for the “slightly louder on standing” subject. Moreover, the systolic energy measure was markedly lower in the standing posture in case 12; the cardiologist's judgment for this subject was, correspondingly, “softer on standing.” Apical signal energy measures for these 4 subjects were relatively high, and murmurs for these subjects were judged by the cardiologist to be grade 2 or 3.
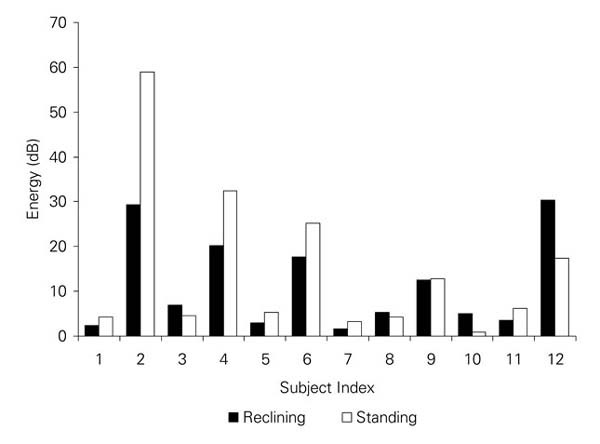
Fig. 4 Median, normalized, apical systolic mid-frequency energies for each subject in the reclining and standing postures.
The algorithm correctly identified apical systolic murmurs for these 4 subjects (cases 2, 4, 6, and 12) in both the standing and the reclining postures. The 3 subjects who had murmurs that were identified as having higher apical systolic energy in the standing posture were subjects who reported having HOCM. Thus, exactly those 3 reported HOCM cases that were flagged by the cardiologist as having apical systolic murmurs “louder on standing” were identified by the algorithm as having apical systolic murmurs with higher systolic energies in the standing posture.
Discussion
We have described a method for automated acquisition and analysis of heart sounds; the method automates a standard auscultatory examination using a voice-guided protocol in combination with a dynamic graphical user interface. The auscultatory data acquisition process includes immediate data quality assessment and feedback, is intuitive and easy to follow, can be efficiently and quickly completed, and provides a consistent and repeatable procedure.
The signal processing algorithms provide an analysis of the auscultatory data that is objective and stable; it removes intra- and interobserver variability and provides an archival record of acoustic signals and auscultatory findings. The acquisition protocol can be administered by someone less highly trained than a physician, and the results of the analysis can be reviewed by a cardiologist or other physician.
The results of the computer analysis of heart sounds obtained from a small population of subjects with previously diagnosed hypertrophic cardiomyopathy show accurate detection of the S1 and S2 events on the basis of the acoustic signal alone, and sensitive and specific detection of heart murmurs of grade 2 and higher. The algorithm identified 3 subjects with apical systolic murmurs with high mid-frequency signal energy and with higher energy in the standing posture; these subjects reported HOCM. In addition, the algorithm correctly identified a previously unreported grade 1 apical diastolic murmur.
The complex computer algorithm used for this study, if clinically validated on a larger scale, could be used to assist professionals in pre-participation sports screening in order to flag HOCM, the serious condition that is most frequently implicated in sudden cardiac death in young athletes. The algorithm is not designed to identify auscultatory concomitants of nonobstructive HCM; this is an area for future work.
Acknowledgments
We gratefully acknowledge Lisa Salberg, President, Hypertrophic Cardiomyopathy Association, for her support, and for the support of her organization in conducting this pilot study. We also acknowledge with thanks the Zargis staff who helped organize and conduct the pilot study protocol: Stephanie Chiang, Don Brooks, and Gayle Mineo.
Footnotes
Address for reprints: Raymond L. Watrous, PhD, Zargis Medical Corporation, 755 College Road East, Princeton, NJ 08540
E-mail: watrous@zargis.com
This research was sponsored by Zargis Medical Corp., where Drs. Watrous, Oskiper, and Grove are members of the research staff; Dr. Bedynek's collaboration was acknowledged by an honorarium from Zargis.
References
- 1.Maron BJ. Sudden death in young athletes. N Engl J Med 2003;349:1064–75. [DOI] [PubMed]
- 2.Epstein EJ, editor. Cardiac auscultation. Boston: Butterworth Heinemann; 1991.
- 3.Waller BF, Harvey WP, editors. Cardiovascular evaluation of athletes: toward recognizing young athletes at risk of sudden death. Newton (NJ): Laennec Publishing; 1993.
- 4.Chizner M, editor. Classic teachings in clinical cardiology. A tribute to W. Proctor Harvey. Fairfield (NJ): Laennec Publishing; 2003.
- 5.Cannon RO 3rd. Assessing risk in hypertrophic cardiomyopathy. N Engl J Med 2003;349:1016–8. [DOI] [PubMed]
- 6.McKusick VA. Cardiovascular sound in health and disease: being a comprehensive treatise, introduced by a historical survey, illustrated mainly by sound spectrograms (spectral phonocardiograms) and supplemented by an extensive bibliography; with a section on respiratory sound. Baltimore: Williams & Wilkins; 1958.
- 7.Oskiper T, Watrous RL. Detection of the first heart sound using a time-delay neural network. Comput Cardiol 2002: 29:537–40.
- 8.Gamero LG, Watrous RL. Detection of the first and second heart sound using probabilistic models. International Conference of the IEEE Engineering in Medicne and Biology Society 2003;25:2877–80.
- 9.Thompson WR, Hayek CS, Tuchinda C, Telford JK, Lombardo JS. Automated cardiac auscultation for detection of pathologic heart murmurs. Pediatr Cardiol 2001;22:373–9. [DOI] [PubMed]


