Abstract
Objective
To determine radiographic hand osteoarthritis (HOA) prevalence in patients with HIV-1 infection in comparison with the general population and to address whether metabolic syndrome (MetS) may increase the risk of HOA during HIV-1 infection.
Patients
Patients with HIV-1 infection and MetS (International Diabetes Federation, IDF criteria) aged 45–65 years were matched by age and gender to HIV-1-infected subjects without MetS and underwent hand radiographs. Framingham OA cohort was used as general population cohort.
Methods
Radiographic HOA was defined as Kellgren–Lawrence (KL) score ≥2 on more than one joint. Radiographic severity was assessed by global KL score and number of OA joints. HOA prevalence was compared with that found in the Framingham study, stratified by age and sex. Logistic and linear regression models were used to determine the risk factors of HOA in patients with HIV-1 infection.
Results
301 patients (88% male, mean age 53.4 ±5.0 years) were included, 152 with MetS and 149 without it. Overall, HOA prevalence was 55.5% and was higher for those with MetS than those without it (64.5% vs 46.3%, p=0.002). When considering men within each age group, HOA frequency was greater in patients with HIV-1 infection than the general population (all ages: 55.8% vs 38.7%; p<0.0001), due to the subgroup with MetS (64.9%; p<0.0001), as well as the subgroup without MetS, although not significant (46.6%; p=0.09). Risk of HOA was increased with MetS (OR 2.23, 95% 95% CI 1.26% to 3.96%) and age (OR 1.18, 95% CI 1.12 to 1.25). HOA severity was greater for patients with MetS than those without. HOA was not associated with previous or current exposure to protease inhibitors or HIV infection-related markers.
Conclusions
HOA frequency is greater in patients with HIV-1 infection, especially those with MetS, than the general population.
HIV-associated mortality is greatly reduced because of the widespread use of efficient antiretroviral therapy.1 Consequently, in the USA and Europe, patients with HIV-1 infection older than 50 years represent more than 50% of the follow-up population.2–4 Similarly, age-related health problems such as cardiovascular diseases have been increasing with increasing age in patients with HIV because of chronic inflammation, immune activation and immunosenescence, and lifelong antiretroviral therapy.5–7 These comorbidities, called non-AIDS-related comorbidities, are more prevalent in patients with HIV infection than people of the same age without HIV, which suggests an extra ‘hit’ of ageing related to HIV-1 infection and/or antiretroviral therapy, which can lead to geriatric syndromes with impairment and frailty.8–11
Osteoarthritis (OA) is the most common rheumatologic disease due to ageing, affecting about six million people with hip and/or knee OA in France12 and 27 million in the USA.13 The disease has never been studied in the setting of HIV comorbidities. Among all localisations, hand OA (HOA) is associated with pain, disability and deteriorated quality of life to the same extent as rheumatoid arthritis.14–16 However, to what extent HIV-1-positive patients have OA and HOA is unknown.
OA is a heterogeneous group of diseases that can be differentiated by the risk factors (ie, ageing, obesity, trauma). Each factor has specific pathophysiological pathways, all leading to joint destruction.17 18 Obesity-associated OA is one of the most-studied phenotypes characterised by the association of obesity or overweight with OA on weight-bearing joints due to mechanical overload.19 However, recently, the demonstration of an association between obesity and HOA has shed light on a potential role of systemic metabolic disturbances in the pathophysiology of OA.20 Indeed, several studies have raised the possibility of an association of metabolic syndrome (MetS) and OA, but which did not persist after adjustment on body mass index or weight in some of them.21–23 In addition to accelerated ageing, patients with HIV infection frequently have MetS because HIV infection, via chronic inflammation and immune activation, which contributes to dyslipidaemia and insulin resistance (IR).24 Moreover, antiretroviral therapy, especially protease inhibitors, can induce a lipodystrophic syndrome characterised by altered body fat composition, dyslipidaemia and IR25 and subsequent MetS development.26
Considering the increased life expectancy, accentuated ageing and high prevalence of MetS in patients with HIV infection, we hypothesised that HOA could represent a novel HIV-associated non-AIDS-related comorbidity. Similarly, we aimed to determine whether MetS might be associated with risk of radiographic HOA in patients with HIV infection and whether the prevalence of HOA in patients with HIV infection is greater than in the general population.
METHODS
Patients
The present study, called Metabolic Syndrome and Fibrosis-Osteoarthritis (METAFIB-OA), is an ancillary study of the METAFIB study (NCT02353767). METAFIB is a cross-sectional single-centre study that recruited 458 patients with HIV infection >18 years old from January 2011 to December 2012 from outpatient clinics. HIV-positive status for at least 5 years was confirmed by Western blot analysis or ELISA in patients without chronic viral hepatitis coinfection to investigate the impact of MetS on liver fibrosis. All patients were HIV-1-positive and were separated in two subgroups, with (HIV-1+MetS+) and without MetS (HIV-1+MetS−). MetS was defined by the International Diabetes Federation (IDF) criteria (see online supplementary table S1).27 Patients with MetS + were matched to those without MetS+ by sex and age (±5 years), with a ratio of 1:1.
For all patients, metabolic and HIV-1 infection clinical characteristics were recorded at the time of participation to METAFIB-OA cross-sectional study. A fasting blood sample was obtained from all subjects for assessing blood cell count, CD4+ and CD8+ T-cell count, ultrasensitive HIV-1 viral (usHIV-1) load, lipid levels, glycaemia and insulinaemia for homeostasis model assessment IR (HOMA-IR) calculation by the standard procedure of the hospital. The lowest count (nadir) for the CD4+ and CD8+ T cells was extracted from medical files.
Between September 2011 and April 2012, all patients between 45 and 64 years from the METAFIB were contacted for the ancillary METAFIB-OA study. Exclusion criteria were pregnancy, inflammatory rheumatic disease or Dupuytren’s disease. To compare the prevalence of HOA between METAFIB-OA patients and the general population, we used data from the Framingham cohort, a population-based cohort consisting of the Offspring and the Community cohorts, in which HOA prevalence had been previously examined.28 29
The study was approved by the Institutional Review Board (Comité de Protection des Personnes, Paris Ile de France V, Paris). All patients gave informed consent.
Radiographic definition of HOA and severity
For METAFIB-OA study (ie, between September 2011 and April 2012), all patients underwent bilateral postero-anterior hand radiography at 100% at AP-HP Saint-Antoine Hospital, and two trained assessors (A-LT and CR-J) scored an equal number of radiographs. The readers were blinded to clinical data and subgroup (MetS+ or MetS−). Hand radiographs were graded by the Kellgren–Lawrence (KL) grading scale,30 which assesses the distal interphalangeal (DIP), proximal interphalangeal (PIP), interphalangeal thumb (IP-1), metacarpal (MCP) and first carpometacarpal (CMC-1) joints with a grading system from 0 to 4 (0, no OA; 1, doubtful OA; 2, definite minimal OA; 3, moderate OA; 4, severe OA). Any patient with at least one finger joint scored at KL grade ≥2 was considered to have radiographic HOA. Thumb-base OA was also considered separately as unilateral or bilateral with KL score ≥2 on one or two CMC-1 joints31 because it is more likely related to loading than the other three joints (DIP, PIP, or MCP).32–35
Radiographic severity was assessed by (1) the global KL score (sum of the scores for all joints; range 0–128), (2) number of OA joints (KL ≥2; range 1–32), (3) number of patients with erosive HOA according to the Verbruggen–Veys (VV) anatomical phase score, consisting of five phases with a numerical value representing the evolution of HOA (N, normal joint; S, stationary OA with osteophytes and joint-space narrowing; J, complete loss of joint space in the whole or part of the joint; E, subchondral erosion; R, remodelling of subchondral plate).36 The DIP, PIP, IP-1 and MCP joints were assessed. Erosive HOA diagnosis was based by the presence of at least one erosive joint on bilateral HOA radiographs (E or R phase).
Before this scoring, both readers (A-LT and CR-J) underwent a training session to assess the interobserver and intraobserver reproducibility with 20 hand radiographs from routine practice. Reproducibility between the readers was estimated first by the Cohen κ coefficient for inter-reader concordance and then by the intraclass correlation coefficient (ICC) for intrareader concordance. For the total KL scale, the scores were 0.64 (95% 95% CI 0.61 to 0.67) and 0.95 (95% CI 0.80 to 0.98), respectively. For the VV score, the ICCs were 0.9 (95% CI 0.91 to 0.99) and 0.98 (95% CI 0.91 to 0.99), respectively.
HOA symptoms
Patients from METAFIB who participated to the ancillary METAFIB-OA study completed a brief standardised questionnaire at the time of radiography about their dominant hand, menopausal status for women and history of psoriasis. For joint pain assessment, the following binary question was asked: “On most days, do you have any pain, aching or stiffness in any of your joints?”
Biomarker assessment
A blood sample was performed at the time of the inclusion in the main METAFIB study, so some months before METAFIB-OA. High-sensitivity C reactive protein (hsCRP) was measured by nephelometry on an IMMAGE analyser (Beckman-Coulter, Villepinte, France). We measured plasma levels of high-sensitive interleukin 6 level, reflecting global inflammation and ageing, soluble CD14 (sCD14) and soluble CD163 (sCD163) (Quantikine ELISA Kit, R&D Systems, Oxford, UK), two markers of monocyte/macrophage activation involved in HIV-related chronic immune activation,37 and leptin (Quantikine; R&D Systems, Oxford, UK) and total adiponectin (ALPCO, EUROBIO, Les Ulis, France), two adipokines involved in MetS38 39 and OA.40
Statistical analysis
Descriptive data are presented as mean±SD, median (IQR) or number (%). HIV+MetS+ and HIV+MetS− patients and subjects with and without HOA were compared by χ2 test for categorical variables and Wilcoxon rank-sum test for continuous variables.
We compared HOA prevalence from the METAFIB-OA with the community-based cohort from the Framingham study that estimated HOA prevalence by a slightly modified version of the KL scale (ie, HOA diagnosis with ≥1 joint radiographic OA by a modified KL scale) by χ2 test by gender and age group.
To determine the factors associated with HOA or thumb-base OA, we calculated crude ORs with 95% CIs by univariate modelling in the entire population. We explored variables related to demographic characteristics, HIV-1 infection features and metabolic disturbances. Variables associated with HOA diagnosis on univariate analysis with p<0.2 were entered in a backward stepwise multivariate conditional logistic regression model. Multivariate linear regression was used to determine factors associated with structural radiographic severity, as defined above (three definitions). MetS was entered in the logistic model along with other variables and kept in the final model, whatever the level of significance. Results are presented as β regression coefficients. All measurements were log-transformed to remove positive skewness and were compared between patients with and without HOA by the Wilcoxon rank-sum test. Univariate and multivariate analyses with a logistic model were adjusted for MetS. All analyses involved use of STATA V.12.1 (StataCorp, College Station, TX, USA) and p<0.05 was considered statistically significant.
RESULTS
Population characteristics
The main METAFIB study included 222 HIV1+MetS+ and 222 HIV1+MetS− subjects; 173 patients with MetS+ and 166 without MetS− 45–64 years old were screened for the METAFIB-OA study (figure 1). Characteristics of the study population are given in table 1, with stratification by MetS status. The two groups did not differ except for all MetS characteristics (p<0.0001), as expected. HOA symptoms were reported by 27% of the population.
Figure 1.
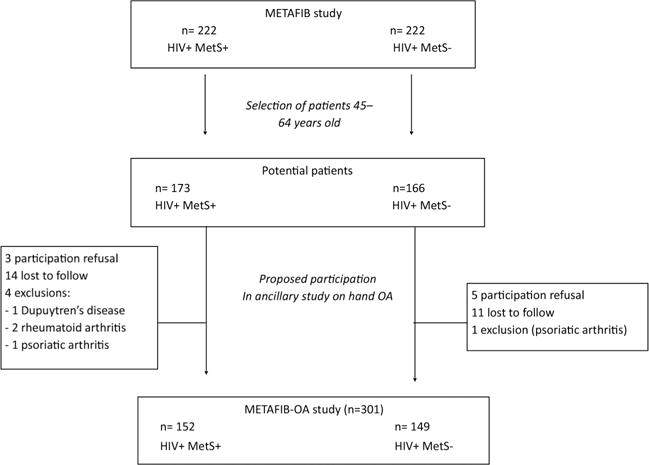
Flow chart of Metabolic Syndrome and Fibrosis (METAFIB)-OA. MetS+, presence of metabolic syndrome; MetS−, absence of metabolic syndrome; OA, osteoarthritis.
Table 1.
Baseline characteristics of the METAFIB-OA cohort
| Total (n=301) |
HIV-1 + MetS+ (n=152) |
HIV-1 + MetS− (n=149) |
p Value | |
|---|---|---|---|---|
|
| ||||
| Demographic features | ||||
|
| ||||
| Male gender, n (%) | 265 (88.0) | 134 (88.2) | 131 (87.9) | 0.9 |
|
| ||||
| Age (years), mean (SD) | 53.4 (5.0) | 53.5 (4.9) | 53.4 (5.1) | 0.7 |
|
| ||||
| Postmenopausal status (n=37), n (%) | 26 (70.3) | 14 (77.8) | 12 (63.2) | 0.5 |
|
| ||||
| Predominant side, n (%) | ||||
|
| ||||
| Right-handed | 250 (83.1) | 133 (87.5) | 117 (78.5) | |
|
| ||||
| Left-handed | 32 (10.6) | 13 (8.6) | 19 (12.8) | |
|
| ||||
| Mixed | 19 (6.3) | 6 (3.9) | 13 (8.7) | 0.1 |
|
| ||||
| Hand OA features | ||||
|
| ||||
| Hand trauma history, n (%) | 62 (20.6) | 26 (%) | 36 (%) | 0.2 |
|
| ||||
| Psoriasis, n (%) | 13 (4.3) | 4 (2.6) | 9 (6.0) | 0.2 |
|
| ||||
| Pain, n (%) | 80 (26.6) | 42 (27.6) | 38 (25.5) | 0.7 |
|
| ||||
| HIV features | ||||
|
| ||||
| Duration of HIV infection (years), mean (SD) | 17.7 (7.3) | 17.0 (7.1) | 18.3 (7.5) | 0.2 |
|
| ||||
| CDC-C stage, n (%) | 81 (26.9) | 43 (28.3) | 38 (25.5) | 0.8 |
|
| ||||
| CD4 level (/mm3), mean (SD) | 623 (265) | 625 (257) | 621 (274) | 0.9 |
|
| ||||
| CD4/CD8 ratio, mean (SD) | 0.87 (0.37) | 0.86 (0.4) | 0.87 (0.3) | 0.4 |
|
| ||||
| Undetectable usHIV viral load, n (%) | 240 (79.7) | 82 (53.9) | 90 (60.8) | 0.2 |
|
| ||||
| Duration of exposure to protease inhibitors (months), mean (SD) | 47.7 (29.2) | 26.9 (31.1) | 27.4 (33.6) | 0.8 |
|
| ||||
| Metabolic syndrome components | ||||
|
| ||||
| Waist circumference (cm), mean (SD) | 92.2 (10.8) | 98.2 (10.1) | 86.3 (8.1) | <0.0001 |
|
| ||||
| BMI (kg/m2), mean (SD) | 24.9 (5.8) | 26.4 (4.8) | 23.3 (6.3) | <0.0001 |
|
| ||||
| Obesity (BMI≥30), n (%) | 32 (10.6) | 26 (17.1) | 6 (4.0) | <0.0001 |
|
| ||||
| Hypertension, n (%) | 56 (18.6) | 40 (26.3) | 16 (10.7) | <0.0001 |
|
| ||||
| Triglycerides (mmol/L), mean (SD) | 1.95 (1.9) | 2.47 (1.7) | 1.42 (2.0) | <0.0001 |
|
| ||||
| HDL-cho (mmol/L), mean (SD) | 1.21 (0.4) | 1.05 (0.3) | 1.36 (0.4) | <0.0001 |
|
| ||||
| LDL-cho (mmol/L), mean (SD) | 2.9 (0.9) | 3.1 (0.8) | 2.8 (0.9) | 0.0003 |
|
| ||||
| Glycaemia (mmol/L), mean (SD) | 5.45 (1.08) | 5.85 (1.3) | 5.05 (0.64) | <0.0001 |
|
| ||||
| HOMA-IR score, mean (SD) | 2.42 (2.79) | 3.51 (3.50) | 1.31 (0.92) | <0.0001 |
|
| ||||
| Type 2 diabetes, n (%) | 65 (21.6) | 57 (37.5) | 8 (5.4) | <0.0001 |
Data are number (%) or mean (SD) for the total population, and number (%) and median (IQR) for cases and controls.
Hypertension was defined as systolic pressure ≥140 mm Hg and/or diastolic pressure ≥90 mm Hg. Diabetes was defined as glycaemia >6 mmol/L.
BMI, body mass index; CDC, Centers for Disease Control and Prevention; cho, cholesterol; HDL, high-density lipoprotein; HOMA-IR score, Homeostasis Model Assessment of Insulin Resistance; LDL, low-density lipoprotein; METAFIB, Metabolic Syndrome and Fibrosis; MetS−, absence of metabolic syndrome; MetS, metabolic syndrome; MetS+, presence of metabolic syndrome; OA, osteoarthritis; usHIV: ultrasensitive HIV.
HOA prevalence and structural severity in the METAFIB-OA group
Radiographic HOA prevalence and severity are stratified by gender in table 2. Overall, radiographic HOA was significantly more frequent in patients with MetS than those without it (64.5% vs 46.3%; p=0.002). Stratification by gender yielded similar results (64.9% in male with MetS vs 46.5% in male without MetS, p=0.002 and 61.1% in female with MetS vs 44.4% in without MetS, p=0.002). The same difference was observed for thumb-base OA.
Table 2.
Radiographic hand OA and severity in the whole METAFIB-OA cohort and in cases and controls
| Total (n=301) |
HIV-1 + MetS+ (n=152) |
HIV-1+ MetS− (n=149) |
p Value | |
|---|---|---|---|---|
|
| ||||
| OA | ||||
|
| ||||
| HOA ≥1 joint KL ≥2 | ||||
|
| ||||
| Men | 148 (49.2) | 87 (57.2) | 61 (40.9) | |
|
| ||||
| Women | 19 (6.3) | 11 (7.2) | 8 (5.3) | |
|
| ||||
| Total cohort | 167 (55.5) | 98 (64.5) | 69 (46.3) | 0.002 |
|
| ||||
| Thumb-base OA | ||||
|
| ||||
| KL ≥2 on 1 or 2 sides | ||||
|
| ||||
| Men | 55 (18.3) | 37 (24.3) | 18 (12.1) | |
|
| ||||
| Women | 6 (2.0) | 3 (2.0) | 3 (2.0) | |
|
| ||||
| Total cohort | 61 (20.3) | 40 (26.1) | 21 (14.1) | 0.01 |
|
| ||||
| Erosive OA | ||||
|
| ||||
| Men | 7 (2.3) | 5 (3.3) | 2 (1.3) | |
|
| ||||
| Women | 0 | 0 | 0 | |
|
| ||||
| Total cohort | 7 (2.3) | 5 (3.3) | 2 (1.3) | 0.5 |
|
| ||||
| Severity criteria | ||||
|
| ||||
| Sum of KL scores | 5.2±8.8 | 6.7±0.9 | 3.7±0.5 | 0.002 |
|
| ||||
| No. of joints with KL ≥2 | 2.5±4.1 | 3.2±0.4 | 1.8±0.2 | 0.002 |
Data are number (%) or mean±SD.
HOA, hand OA; KL, Kellgren–Lawrence score; METAFIB, Metabolic Syndrome and Fibrosis; MetS−, absence of metabolic syndrome; MetS+, presence of metabolic syndrome; OA, osteoarthritis.
Radiographic structural severity based on KL total score and number of OA joints was significantly more pronounced in MetS+ patients than those without it (p=0.002). Few cases of erosive HOA were observed in MetS+ subgroup (n=5, 3.3%) as well as in MetS− subgroup (n=2, 1.3%), with no significant difference between them.
Comparison of METAFIB-OA group with age-matched general population from the Framingham cohort
For patients 45–64 years old, mean HOA prevalence was greater in the METAFIB group than the Framingham population (55.5% vs 39.3%, p<0.0001) (table 3). This difference was mainly due to MetS+ patients in the METAFIB-OA cohort (64.5% in HIV+MetS+ vs 39.3% in Framingham, p<0.0001, for all ages), but there was also a numeric difference of HOA prevalence between Framingham cohort and HIV+MetS− patients (for all ages: 46.3% in HIV+MetS− vs 39.3% in Framingham p=0.09).
Table 3.
Prevalence of radiographic hand OA in the general population (Framingham cohort study) and in the METAFIB-OA study by age
| Age groups (years) | Framingham total population (n=1508) | METAFIB cohort (n=301) | METAFIB HIV-1+ MetS+ (n=152) | METAFIB HIV-1+ MetS− (n=149) | METAFIB vs Framingham | METAFIB cases vs Framingham | METAFIB controls vs Framingham |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Men+women (45–64) | 39.3 | 55.5 | 64.5 | 46.3 | <0.0001 | <0.0001 | 0.09 |
|
| |||||||
| Men | n=641 | n=265 | n=134 | n=131 | |||
|
| |||||||
| 45–49 | 10.3 | 28.6 | 32.1 | 25.7 | 0.004 | 0.006 | 0.03 |
|
| |||||||
| 50–54 | 31.9 | 50.0 | 57.1 | 41.5 | 0.003 | 0.001 | 0.23 |
|
| |||||||
| 55–59 | 43.9 | 68.6 | 80.6 | 55.9 | 0.0004 | <0.0001 | 0.20 |
|
| |||||||
| 60–64 | 56.4 | 88.1 | 100 | 76.2 | 0.0002 | 0.0001 | 0.08 |
|
| |||||||
| All ages (45–64) | 38.7 | 55.8 | 64.9 | 46.6 | <0.0001 | <0.0001 | 0.09 |
|
| |||||||
| Women | n=867 | n=36 | n=18 | n=18 | |||
|
| |||||||
| 45–49 | 13.5 | 38.5 | 50.0 | 20.0 | 0.02 | 0.006 | 0.68 |
|
| |||||||
| 50–54 | 26.0 | 46.7 | 57.1 | 37.5 | 0.08 | 0.07 | 0.47 |
|
| |||||||
| 55–59 | 45.8 | 100 | 100 | 100 | 0.005 | 0.06 | 0.03 |
|
| |||||||
| 60–64 | 63.2 | 0 | 0 | 0 | 0.19 | NA | 0.19 |
|
| |||||||
| All ages (45–64) | 39.7 | 52.8 | 61.1 | 44.4 | 0.12 | 0.07 | 0.68 |
Radiographic hand OA definition was presence of ≥1 affected joint with KL score ≥2.
KL, Kellgren-Lawrence score; METAFIB, Metabolic Syndrome and Fibrosis; MetS+, presence of metabolic syndrome; MetS−, absence of metabolic syndrome; OA, osteoarthritis.
Considering the differences in sex ratios between the two cohorts and the impact of ageing on OA development, we compared HOA prevalence stratified on sex and age. The prevalence of HOA was significantly higher for men in the METAFIB cohort than men in the Framingham study within each age group. Furthermore, a comparison of age-matched men from the Framingham and the METAFIB cohort by presence and absence of MetS showed that this difference was due to a higher prevalence of HOA in the METAFIB cases (for all ages: 64.9%) than in Framingham subjects (38.7%; p<0.0001). However, we also found greater prevalence of HOA in METAFIB male controls than men from the Framingham study, although not significantly (for all ages, 46.6%, vs 38.7%; p=0.09).
The METAFIB cohort contained few females (n=36), but prevalence of HOA was greater for the METAFIB women than Framingham women, although not significantly (table 3). Conversely, unilateral or bilateral thumb-base OA frequency did not differ between the METAFIB group and Framingham cohort (data not shown).
Determinants of HOA
In univariate analysis, age, CD4 T-cell count, detectable hsHIV-1 viral load, HOMA-IR, triglycerides level and presence of MetS were associated with HOA (table 4).
Table 4.
Univariate and multivariate analysis of associations between sociodemographic factors, HIV infection characteristics and metabolic variables and radiographic hand OA in the METAFIB-OA study population
| Variable | HOA diagnosis
|
Univariate analysis
|
Multivariate analysis
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| HOA+ (n=167) |
HOA− (n=134) |
p Value | OR | 95% CI | p Value | OR | 95% CI | p Value | |
|
| |||||||||
| Sociodemographic variables | |||||||||
|
| |||||||||
| Age (years), mean (SD) | 55.1 (5.0) | 51.4 (4.3) | 10−5 | 1.18 | 1.12 to 1.25 | 10−5 | 1.18 | 1.11 to 1.25 | 10−5 |
|
| |||||||||
| Male gender, n (%) | 148 | 117 | 0.5 | 0.88 | 0.44 to 1.78 | 0.5 | – | ||
|
| |||||||||
| Previous hand trauma, n (%) | |||||||||
|
| |||||||||
| HIV characteristics | |||||||||
|
| |||||||||
| Duration of HIV infection (years), mean (SD) | 18.0 (7.4) | 17.3 (7.1) | 0.5 | 1.01 | 0.98 to 1.04 | 0.5 | – | ||
|
| |||||||||
| CD4 level (/mm3), mean (SD) | 600 (251) | 652 (281) | 0.1 | 0.99 | 0.99 to 1.00 | 0.1 | 0.99 | 0.99 to 1.00 | 0.2 |
|
| |||||||||
| Undectable hsHIV viral load, n (%) (n=290) | 87/161 (29.0) | 85/129 (28.2) | 0.03 | 1.64 | 1.02 to 2.65 | 0.05 | 1.37 | 0.80 to 2.34 | 0.3 |
|
| |||||||||
| Duration of exposure to protease inhibitors (months), mean (SD) | 27.5 (32.6) | 26.6 (31.8) | 0.7 | 1.00 | 0.99 to 1.01 | 0.8 | – | ||
|
| |||||||||
| Metabolic variables | |||||||||
|
| |||||||||
| Waist circumference (cm), mean (SD) | 92.8 (10.5) | 92.0 (11.2) | 0.4 | 1.01 | 0.99 to 1.03 | 0.6 | – | ||
|
| |||||||||
| Obesity (BMI≥30), n (%) | 14 (4.7) | 18 (6.0) | 0.1 | 0.98 | 0.94 to 1.02 | 0.3 | – | ||
|
| |||||||||
| Hypertension, n (%) | 35 | 21 | 0.2 | 1.43 | 0.79 to 2.59 | 0.3 | – | ||
|
| |||||||||
| Triglycerides (mmol/L), mean (SD) | 2.14 (2.36) | 1.72 (1.1) | 0.07 | 1.19 | 0.99 to 1.43 | 0.07 | 1.06 | 0.9 to 1.25 | 0.5 |
|
| |||||||||
| HDL-chol (mmol/L), mean (SD) | 1.2 (0.4) | 1.2 (0.4) | 0.7 | 0.93 | 0.51 to 1.70 | 0.8 | – | ||
|
| |||||||||
| HOMA-IR score, mean (SD) (n=297) | 2.48 (2.78) | 2.36 (2.81) | 0.06 | 1.02 | 0.93 to 1.10 | 0.7 | – | ||
|
| |||||||||
| Diabetes, n (%) | 36 (%) | 29 (%) | 0.6 | 0.99 | 0.57 to 1.73 | 0.9 | – | ||
|
| |||||||||
| MetS, n (%) | 98 (32.6) | 54 (%) | 10−3 | 2.10 | 1.32 to 3.34 | 0.002 | 2.18 | 1.26 to 3.96 | 0.005 |
Radiographic hand OA definition was presence of ≥1 affected joint with a Kellgren–Lawrence score ≥2. Hypertension was defined as systolic pressure ≥140 mm Hg and/or diastolic pressure ≥90 mm Hg. Diabetes was defined as glycaemia >7 mmol/L.
BMI, body mass index; HDL, high-density lipoprotein; HOA−, hand OA absence; HOA+, hand OA presence; HOMA-IR score, Homeostasis Model Assessment of Insulin Resistance; METAFIB, Metabolic Syndrome and Fibrosis; MetS, metabolic syndrome; OA, osteoarthritis.
In multivariate analysis, only the presence of MetS (adjusted OR=2.23, 95% CI 1.26 to 3.96; p=0.002) and age (adjusted OR per year=1.18, 95% CI 1.12 to 1.25; p=0.00001) remained independently associated with HOA (see online supplementary figure S1). These two factors were associated with HOA severity (see online supplementary table S2).
Considering each metabolic component separately, only IR assessed by HOMA-IR and triglycerides level was associated but not significantly with HOA on univariate analysis (p=0.06 and 0.07, respectively) but not after adjustment (table 4).
For thumb-base OA, only age remained significantly associated with HOA (OR=1.10, 95% CI (1.04 to 1.17); p=0.001), with a nonsignificant association with MetS (OR=1.86, 95% CI 0.98 to 3.45; p=0.06) (see online supplementary table S3).
Of note, HOA diagnosis and severity were not associated with HIV-1 infection characteristics or HIV infection-related markers (table 4, see online supplementary tables S2 and S3).
Association between biological markers and HOA
To further elucidate the mechanism, MetS or HIV-related characteristics, that may favour HOA, we tested a set of metabolic or HIV-related biomarkers. Plasma sCD14 level was significantly higher in patients with HOA than without HOA (2203.8 vs 2010.8 pg/mL; p=0.02) (see online supplementary table S4). This finding was corroborated by univariate analysis finding log (sCD14) level associated with HOA in the whole study population (OR=4.9, 95% CI 1.1 to 21.6; p=0.03). However, after adjustment for MetS, this association became non-significant (OR=3.9, 95% CI 0.9 to 17.2; p=0.07). Plasma levels of adipokines, hsCRP and sCD163 did not differ by HOA diagnosis.
DISCUSSION
In this study, radiographic HOA prevalence was determined for the first time in patients with HIV infection and found to be higher than in the general population from the Framingham cohort. This frequency was further increased in patients with HIV infection with MetS. Age and MetS were associated with HOA during HIV-1 infection. Furthermore, these two factors were also associated with HOA radiographic severity.
Several diseases such as atherosclerosis complications and cancer have emerged as key points in the global therapeutic management of patients with HIV infection. Surprisingly, although OA represents the most common age-related joint disease and with the growing interest in the role of systemic cardiometabolic disturbances in OA pathophysiology, only one preliminary study showed that in 35 HIV-infected men, total body and android fat mass were inversely related to knee cartilage volume measured by MRI.41 Here, taking advantage of the unique METAFIB study including patients with HIV-1 infection with or without MetS, we observed a higher prevalence of radiographic HOA in patients with HIV infection as compared with the Framingham cohort, representing the general population. Differences between the two cohorts were obvious in men, but the small sample of women limits the power of the statistical analysis. In men, although the prevalence of HOA increased with age in both cohorts, HOA occurred more frequently in patients with HIV-1 infection, especially those with MetS. Interestingly, men from the METAFIB cohort without MetS showed a higher prevalence of HOA, although not significantly, than men from the Framingham cohort in each age group. Such a result emphasises the effect of ageing during HIV infection beyond that of MetS.
Several studies have suggested an association between obesity and HOA.20 With obesity, the conditions hypertension, dyslipidaemia and glucose intolerance alone or together could increase the risk of OA. In the Rotterdam cohort,42 the prevalence of HOA was higher in overweight patients with hypertension and diabetes than patients with only overweight. Here, the model of HIV-1 infection further supports the ‘metabolic’ OA phenotype because the prevalence of HOA was greater in patients with MetS than without MetS.17–19 Of note, obesity was not associated with HOA diagnosis in the METAFIB-OA study: such a result agrees with those from the population-based Netherlands Epidemiology of Obesity cohort, finding HOA linked more to metabolic systemic factors than weight itself.22 Radiographic severity was more severe in patients with MetS, so MetS is a risk factor and also an aggravating factor of radiographic HOA during HIV infection. Radiographic HOA could progress quickly in these patients because radiographic severity is associated with radiographic progression.43
The association between HIV and HOA complicated by MetS and ageing emphasises the involvement of systemic metabolic inflammatory mediators. Interestingly, plasma sCD14 level was associated with HOA but less so after adjustment for MetS, which suggests that sCD14 and thus macrophage activation induced by MetS could be a link between MetS and HOA and its severity. Of note, plasma sCD14 level is associated with symptoms and radiographic progression in knee OA, and synovial fluid sCD14 level is associated with activated macrophages infiltrating knee synovium.44 HOA in patients with MetS may have increased synovial inflammation, as is found in patients with knee OA and type 2 diabetes.45
Separate analyses of thumb-base OA showed that this localisation was not more prevalent in patients with HIV than the general population. Such a result was expected because mechanical factors and the morphology of the trapezo-MCP joint are crucial for this localisation. However, thumb-base OA was more prevalent in patients with HIV with MetS than without MetS. So, metabolic disturbances may represent an additional risk factor, focal mechanical injury being a precipitating event.46 47
This study has several limitations. First, hand radiography was performed 1 year after assessment of MetS and serum samples taken for examining exploratory markers. However, OA progression is a very slow process, so this bias may have minimal effect on our findings. Second, the Framingham study used the modified version of the KL scoring system, with HOA definition based on the presence of osteophytes and/or definite joint-space narrowing,29 whereas the original KL scale, used in METAFIB-OA, defined HOA exclusively by the presence of osteophytes. Furthermore, in the Framingham study, the evaluation of OA in the thumb base included assessment of both the CMC-1 and triscaphoid joints, whereas only the CMC-1 joints were assessed in METAFIB-OA. Hence, the scoring system of the Framingham study would classify more joints with definite OA. Consequently, the differences between the two cohorts may have been underestimated. Third, only 25% of patients reported hand pain. However, this frequency agrees with data from other cohorts and OA pain may fluctuate through time.14 29 Finally, to compare HOA between the French METAFIB-OA group and the general population, we have used the Framingham cohort although characteristics of general population in USA and France are certainly different. However, no HOA assessment in a population-based cohort is available in France until now.
In conclusion, HIV-1 infection represents a special setting in which the risk of radiographic HOA and its severity are increased, due to accelerated ageing and MetS. Beyond classical OA phenotypes, the ‘HIV-related OA’ subtype could be thus individualised.
Supplementary Material
Acknowledgments
Hayette Rougier (Infectious Diseases Department, AP-HP, Saint-Antoine Hospital, Paris, France) for logistics concerns in the METAFIB-OA substudy; Raphaèle Séror (Rheumatology Department, AP-HP, Bicêtre Hospital, Le Kremlin Bicêtre, France) for advice concerning reproducibility in the reading of hand radiographs; and Laura Smales (BioMedEditing, Toronto, Canada).
Funding NIH AR47785 for Framingham cohort access and Bristol Myer Squibb (BMS) that financially supported the assessment of biomarkers but was not involved in the analysis of the study or interpretation of the results. All coauthors are independent of these funding sources.
Footnotes
Handling editor Hans WJ Bijlsma
►Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/annrheumdis-2016-209262).
Trial registration number NCT02353767.
Correction notice This article has been corrected since it was published Online First. The title of the study in the first sentence of the Methods/Patients section has been corrected.
Contributors All authors fulfil the following four criteria: (1) substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; AND (2) drafting the work or revising it critically for important intellectual content; AND (3) final approval of the version to be published; AND (4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests None declared.
Patient consent Obtained.
Ethics approval Comité de Protection des Personnes, Paris Ile de France V, Paris.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Puhan MA, Van Natta ML, Palella FJ, et al. Excess mortality in patients with AIDS in the era of highly active antiretroviral therapy: temporal changes and risk factors. Clin Infect Dis. 2010;51:947–56. doi: 10.1086/656415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaboration ATC. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CfDCa. P. HIV/AIDS surveillance report among persons aged 50 or over. 2005 Also 347 available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/
- 4.Pharris A, Spiteri G, Noori T, et al. Ten years after Dublin: principal trends in HIV surveillance in the EU/EEA, 2004 to 2013. Euro Surveill. 2014;19:20968. doi: 10.2807/1560-7917.es2014.19.47.20968. [DOI] [PubMed] [Google Scholar]
- 5.Aberg JA. Aging, inflammation, and HIV infection. Top Antivir Med. 2012;20:101–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Capeau J. Premature aging in human immunodeficiency virus (HIV) infected patients: detection, pathophysiological mechanisms and management. Bull Acad Natl Med. 2011;195:2013–22. [PubMed] [Google Scholar]
- 7.Brothers TD, Rockwood K. Biologic aging, frailty, and age-related disease in chronic HIV infection. Curr Opin HIV AIDS. 2014;9:412–8. doi: 10.1097/COH.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 8.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 9.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infec Dis. 2008;47:542–53. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 11.High KP, Brennan-Ing M, Clifford DB, et al. HIV and aging: state of knowledge and areas of critical need for research. A Report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012;60(Suppl 1):S1–S18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guillemin F, Rat AC, Mazieres B, et al. Prevalence of symptomatic hip and knee osteoarthritis: a two-phase population-based survey. Osteoarthritis Cartilage. 2011;19:1314–22. doi: 10.1016/j.joca.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahaghin S, Bierma-Zeinstra SMA, Ginai AZ, et al. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study) Ann Rheum Dis. 2005;64:682–7. doi: 10.1136/ard.2004.023564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slatkowsky-Christensen B, Mowinckel P, Loge JH, et al. Health-related quality of life in women with symptomatic hand osteoarthritis: a comparison with rheumatoid arthritis patients, healthy controls, and normative data. Arthritis Rheum. 2007;57:1404–9. doi: 10.1002/art.23079. [DOI] [PubMed] [Google Scholar]
- 16.Michon M, Maheu E, Berenbaum F. Assessing health-related quality of life in hand osteoarthritis: a literature review. Ann Rheum Dis. 2011;70:921–8. doi: 10.1136/ard.2010.131151. [DOI] [PubMed] [Google Scholar]
- 17.Bijlsma JWJ, Berenbaum F, Lafeber FPJG. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–26. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 18.Grotle M, Hagen KB, Natvig B, et al. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Courties A, Gualillo O, Berenbaum F, et al. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage. 2015;23:1955–65. doi: 10.1016/j.joca.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf E, Nelissen RG, Ioan-Facsinay A, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis. 2010;69:761–5. doi: 10.1136/ard.2008.106930. [DOI] [PubMed] [Google Scholar]
- 21.Puenpatom RA, Victor TW. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad Med. 2009;121:9–20. doi: 10.3810/pgm.2009.11.2073. [DOI] [PubMed] [Google Scholar]
- 22.Visser AW, de Mutsert R, le Cessie S, et al. The relative contribution of mechanical stress and systemic processes in different types of osteoarthritis: the NEO study. Ann Rheum Dis. 2015;74:1842–7. doi: 10.1136/annrheumdis-2013-205012. [DOI] [PubMed] [Google Scholar]
- 23.Shin D. Association between metabolic syndrome, radiographic knee osteoarthritis, and intensity of knee pain: results of a national survey. J Clin Endocrinol Metab. 2014;99:3177–83. doi: 10.1210/jc.2014-1043. [DOI] [PubMed] [Google Scholar]
- 24.Alencastro PR, Wolff FH, Oliveira RR, et al. Metabolic syndrome and population attributable risk among HIV/AIDS patients: comparison between NCEP-ATPIII, IDF and AHA/NHLBI definitions. AIDS Res Ther. 2012;9:29. doi: 10.1186/1742-6405-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wand H, Calmy A, Carey DL, et al. Metabolic syndrome, cardiovascular disease and type 2 diabetes mellitus after initiation of antiretroviral therapy in HIV infection. AIDS. 2007;21:2445–53. doi: 10.1097/QAD.0b013e3282efad32. [DOI] [PubMed] [Google Scholar]
- 26.Mutimura E, Hoover DR, Shi Q, et al. Insulin resistance change and antiretroviral therapy exposure in HIV-infected and uninfected Rwandan women: a longitudinal analysis. PLoS ONE. 2015;10:e0123936. doi: 10.1371/journal.pone.0123936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 28.Feinleib M, Kannel WB, Garrison RJ, et al. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–25. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 29.Haugen IK, Englund M, Aliabadi P, et al. Prevalence, incidence and progression of hand osteoarthritis in the general population: the Framingham Osteoarthritis Study. Ann Rheum Dis. 2011;70:1581–6. doi: 10.1136/ard.2011.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kellgren JH, Lawrence JS. Radiological Assessment of Osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W, Doherty M, Leeb BF, et al. EULAR evidence-based recommendations for the diagnosis of hand osteoarthritis: report of a task force of ESCISIT. Ann Rheum Dis. 2009;68:8–17. doi: 10.1136/ard.2007.084772. [DOI] [PubMed] [Google Scholar]
- 32.Chaisson CE, Zhang Y, Sharma L, et al. Grip strength and the risk of developing radiographic hand osteoarthritis: results from the Framingham Study. Arthritis Rheum. 1999;42:33–8. doi: 10.1002/1529-0131(199901)42:1<33::AID-ANR4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 33.Goislard de Monsabert B, Vigouroux L, Bendahan D, et al. Quantification of finger joint loadings using musculoskeletal modelling clarifies mechanical risk factors of hand osteoarthritis. Med Eng Phys. 2014;36:177–84. doi: 10.1016/j.medengphy.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Hunter DJ, Zhang Y, Sokolove J, et al. Trapeziometacarpal subluxation predisposes to incident trapeziometacarpal osteoarthritis (OA): the Framingham Study. Osteoarthritis Cartilage. 2005;13:953–7. doi: 10.1016/j.joca.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Jónsson H, Elíasson GJ, Jónsson A, et al. High hand joint mobility is associated with radiological CMC1 osteoarthritis: the AGES-Reykjavik study. Osteoarthritis Cartilage. 2009;17:592–5. doi: 10.1016/j.joca.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verbruggen G, Veys EM. Numerical scoring systems for the anatomic evolution of osteoarthritis of the finger joints. Arthritis Rheum. 1996;39:308–20. doi: 10.1002/art.1780390221. [DOI] [PubMed] [Google Scholar]
- 37.Leeansyah E, Malone DF, Anthony DD, et al. Soluble biomarkers of HIV transmission, disease progression and comorbidities. Curr Opin HIV AIDS. 2013;8:117–24. doi: 10.1097/COH.0b013e32835c7134. [DOI] [PubMed] [Google Scholar]
- 38.Ma K, Jin X, Liang X, et al. Inflammatory mediators involved in the progression of the metabolic syndrome. Diabetes Metab Res Rev. 2012;28:388–94. doi: 10.1002/dmrr.2291. [DOI] [PubMed] [Google Scholar]
- 39.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 40.Abella V, Scotece M, Conde J, et al. Adipokines, metabolic syndrome and rheumatic diseases. J Immunol Res. 2014;2014:343746. doi: 10.1155/2014/343746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fillipas S, Tanamas SK, Davies-Tuck ML, et al. The relationship between body composition and knee structure in patients with human immunodeficiency virus. Int J STD AIDS. 2015;26:133–8. doi: 10.1177/0956462414531404. [DOI] [PubMed] [Google Scholar]
- 42.Dahaghin S, Bierma-Zeinstra SM, Koes BW, et al. Do metabolic factors add to the effect of overweight on hand osteoarthritis? The Rotterdam Study. Ann Rheum Dis. 2007;66:916–20. doi: 10.1136/ard.2005.045724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bijsterbosch J, Watt I, Meulenbelt I, et al. Clinical and radiographic disease course of hand osteoarthritis and determinants of outcome after 6 years. Ann Rheum Dis. 2011;70:68–73. doi: 10.1136/ard.2010.133017. [DOI] [PubMed] [Google Scholar]
- 44.Daghestani HN, Pieper CF, Kraus VB. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 2015;67:956–65. doi: 10.1002/art.39006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schett G, Kleyer A, Perricone C, et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care. 2013;36:403–9. doi: 10.2337/dc12-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anakwe RE, Middleton SD. Osteoarthritis at the base of the thumb. BMJ. 2011;343:d7122. doi: 10.1136/bmj.d7122. [DOI] [PubMed] [Google Scholar]
- 47.Radin EL, Paul IL, Rose RM. Role of mechanical factors in pathogenesis of primary osteoarthritis. Lancet. 1972;299:519–22. doi: 10.1016/s0140-6736(72)90179-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


