Abstract
Purpose
To compare CT-fluoroscopy guided manual and CT-guided robotic positioning system (RPS) assisted needle placement by experienced IR physicians to targets in swine liver.
Materials and Methods
Manual and RPS assisted needle placement was performed by six experienced IR physicians to four 5mm fiducial seeds placed in swine liver (n=6). Placement performance was assessed for placement accuracy, procedure time, number of confirmatory scans, needle manipulations, and procedure radiation dose. Intra-modality difference in performance for each physician was assessed using paired t-Test. Inter-physician performance variation for each modality was analyzed using Kruskal-Wallis test.
Results
Paired comparison of manual and RPS assisted placements to a target by the same physician indicated accuracy outcomes were not statistically different (manual: 4.53 mm; RPS: 4.66 mm; p=0.41), but manual placement resulted in higher total radiation dose (manual: 1075.77mGy/cm; RPS: 636.4 mGy/cm; p=0.03), required more confirmation scans (manual: 6.6; RPS: 1.6; p<0.0001) and needle manipulations (manual: 4.6; RPS: 0.4; p<0.0001). Procedure time for RPS was longer than manual placement (manual: 6.12mins; RPS: 9.7mins; p=0.0003). Comparison of inter-physician performance during manual placement indicated significant differences in the time taken to complete placements (p=0.008) and number of repositions (p=0.04) but not in other study measures (p>0.05). Comparison of inter-physician performance during RPS assisted placement suggested statistically significant differences in procedure time (p=0.02) and not in other study measures (p>0.05).
Conclusions
CT-guided RPS assisted needle placement reduced radiation dose, number of confirmatory scans and needle manipulations when compared to manual needle placement by experienced IR physicians, with equivalent accuracy.
Keywords: Image guided biopsy, Navigation system, Robotic guidance, Needle placement
INTRODUCTION
Different needle guidance and placement assistance systems have been developed for percutaneous image-guided procedures in the thorax and abdomen to improve targeting accuracy independent of physician experience, and to reduce radiation exposure to the physician and the patient. Such assistance systems include optical [1; 2], electromagnetic navigation [3–5], laser overlay [6], US guided [7], fluoroscopy-guided [8] and robotic systems [9–19]. The robotic approach offers several advantages over other assistance systems including: 1) lack of line of sight restrictions encountered in optical systems, 2) the ability to function unaffected by the presence of ferrous materials that may interfere with electromagnetic navigation systems and 3) the presence of a robust platform for guiding large diameter needles [20]. It has also been suggested that the use of robotic assistance during needle placement can help minimize the radiation exposure to operators and patients during CT or fluoroscopy guided interventional procedures [17; 20–27]. At the same time, the use of robotic guidance may also reduce the number of needle adjustments required to reach a target, thereby reducing patient complications such as bleeding[28].
The efficacy of robotic assistance for needle placements in the thorax or abdomen has been typically evaluated through experimental placements performed on phantoms [15], animals [29] or patients [26] without comparison to manual placements to similar targets by experienced IR physicians using standard technique. We address this knowledge gap by comparing CT-Fluoroscopy guided manual and CT-guided RPS assisted needle placement by experienced IR physician when targeting small in-vivo targets in liver.
MATERIAL AND METHODS
Animal Use
Six female swine (35–50 kg) were used in experiments following an experimental protocol approved by the Instituitional Animal Care and Use Committee. Animals were sedated with intravenous tiletamine hydrochloride and zolazepam hydrochloride (6 mg/kg; Telazol; Fort Dodge Animal Health, IA). General anesthesia was maintained with inhaled isoflurane (1.5–3% Aerrane; Baxter Healthcare, Round Lake, IL) after endotracheal intubation. The swine were positioned into the CT-scanner (Lightspeed A6, GE Healthcare, Princeton, NJ) in decubitus position. Prior to each study an experienced IR physician who was not the study participant placed four metal fiducial markers (5mm long, 18G in diameter) in each of the four major lobes of swine livers. The seeds were evenly spaced out in depths within the range of 50–120mm (4 seeds per animal, 24 seeds in total). Each IR physician participating in the study worked on a single animal. All RPS assisted needle placements were performed using breath holds during image acquisition for planning and during placement of the needle. The breath hold was facilitated using a muscle paralytic (Rocuronium; 1.2 mg/kg) administered intravenously before suspending breathing on the ventilator. All animals studied were euthanized after the procedure.
Experimental Methods for Manual Needle Placement
Six IR physicians with at least eight years of experience in independently performing image guided needle placement participated in this study. A baseline non-contrast CT was performed (LightSpeed 16; GE Healthcare, Milwaukee, Wis) and the study participant was allowed to view this scan to evaluate the seed locations. The IR physician was informed on the metrics that were being gathered for the study, and was asked to place the needle tip as close as possible to the seed being targeted, while treating each needle placement as a separate procedure. The procedures were timed starting with the acquisition of the planning scan and ending when the IR physician confirmed that they were satisfied with the needle location. CT-fluoroscopy guidance was used to manually target the markers using 100 or 150mm length coaxial needles (18G biopsy needles, E-Z-EM Inc, Westbury NY). The four manual placements were performed sequentially, and preceded the four RPS assisted placements.
Experimental Methods for RPS Assisted Placement
CT guided RPS assisted needle insertion were performed using a commercially available platform (Perfint Healthcare Inc., Chennai, India.). The RPS resides on a wheeled cart and consists of two components, a software module that assists in the planning of needle placements for biopsy and ablations, and an articulated 5 degrees of freedom robotic arm with a disposable needle guide mounted on its end effector. The cart is docked on a metal plate mounted on the floor beside the CT table, after which fresh CT-scan (1.25mm slice thickness, 20 images) acquisitions were performed with breath-hold to plan for each RPS assisted needle placement. The RPS’s onboard computer received the DICOM formatted images from the CT console via an ethernet cable, and was used for planning and navigation using the on-board software (Figure 1).
Figure 1. Robotic Positioning System.
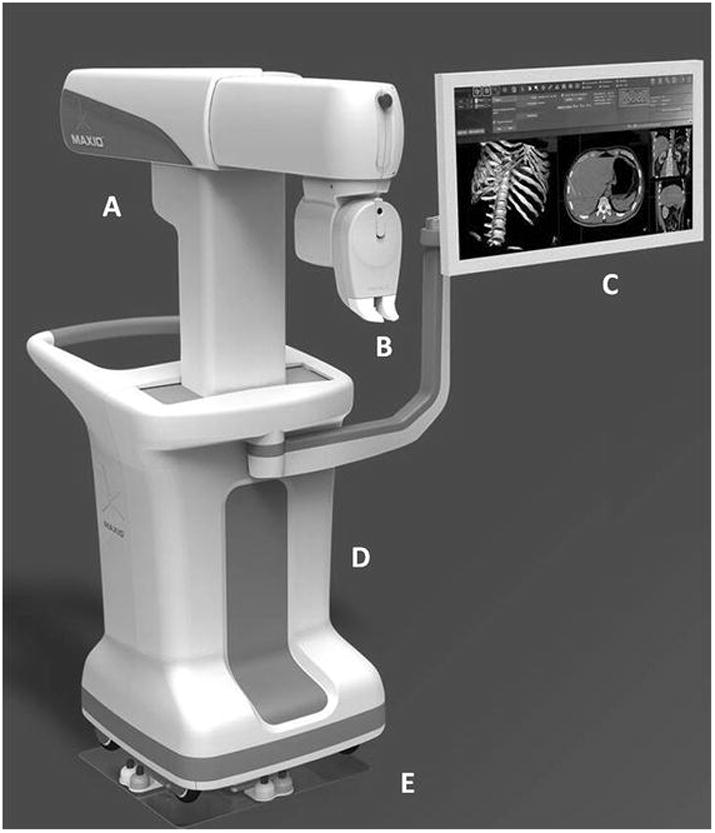
Overview of the Robotic Positioning System used for performing the experiments.
A: Articulated arm capable of motion along three separate axes.
B: End effector of the robotic arm with the needle guide. Capable of motion around two axes for orienting the needle towards the target.
C: Monitor showing view of the onboard software used for planning needle placement and operating the robot.
D: Cart enclosing computer, control equipment and the robotic arm.
E: Docking plate used to accurately position the cart adjacent to the CT table and register the robot with the CT imaging workspace.
Prior to commencing the RPS assisted needle placements, the IR study physician was oriented to the RPS system and given an overview of the workflow for planning and placement using this system. Without specific training or practice sessions for using the RPS, the participant was then asked to use planning sequence to determine an entry point (needle puncture site on skin surface) and the target point (center of the fiducial) for needle placement. The participant then used interactive drop down menus to select the appropriate needle length (100 or 150mm) for carrying out the plan. The angulations of the needle, the depth of the lesion as well as the needle trajectory path were calculated by the workstation and shown on the treatment plan (Figure 2a). The software onboard the RPS provided the physician visual feedback on critical structures within the vicinity of the planned trajectory, and the physician planning the placement was ultimately responsible for choosing path that avoided critical organs or bone across the needle trajectory.
Figure 2. Procedure Workflow.
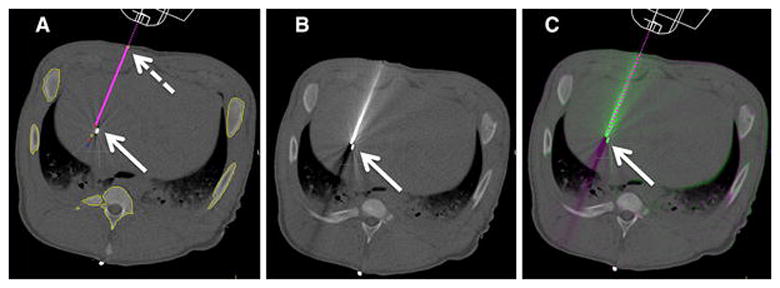
A: Planning image, magenta line shows planned needle trajectory from skin entry (dashed arrow) to target seed (arrow).
B: Post needle placement CT image.
C: Image overlay comparing planned trajectory (dashed line) and actual needle position.
Upon receiving confirmation that the physician was satisfied with the plan, it was sent to the robotic arm. The RPS repositioned its robotic arm such that the needle guide on the effector was positioned and oriented to carry out the planned needle placement. The physician then placed the needle in the needle guide at the end-effector of the arm (Figure 3). The onboard software computed the depth of needle insertion in accordance to the plan made by the physician. The breathing of the animal was placed on hold and the physician then inserted the needle through the skin and pushed the needle to the predetermined depth in a single action. Once the needle was in place, the physician used button controls to unclamp the end effector and withdraw the robotic arm from the entry site. A confirmatory scan was performed following needle placement to assess targeting adequacy (Figure 2b and 2c). In the event of poor targeting, the robot was repositioned according to the original plan and the procedure was repeated one more time.
Figure 3. RPS Needle Placement Process.
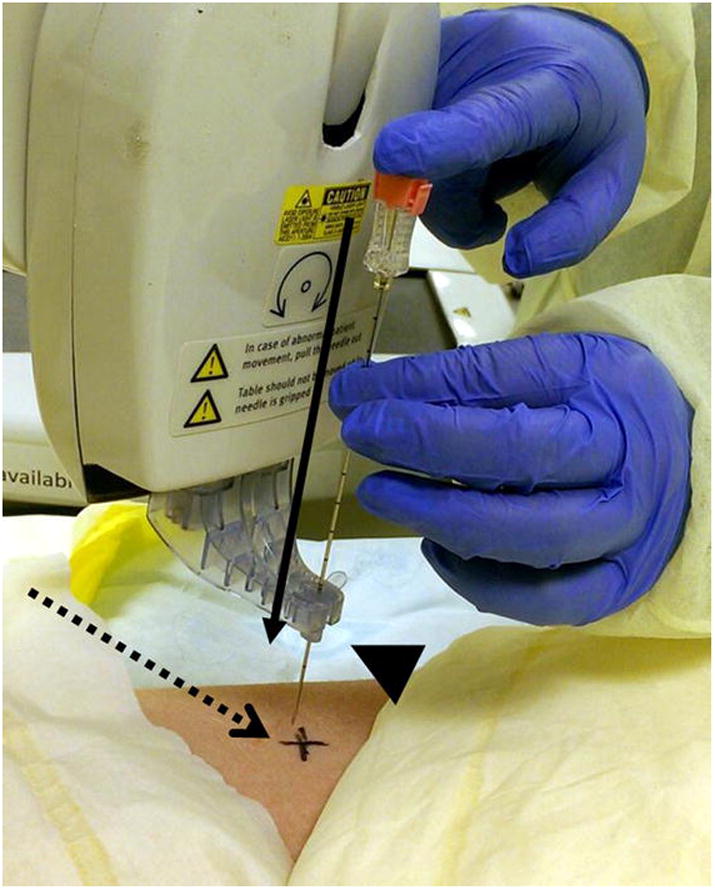
Insertion of needle through the disposable needle holder (arrowhead) mounted on the end effector of the robotic arm. The needle motion is constrained to linear motion along the preset path planned by the physician (solid arrow). The entry point on the skin is marked for easy reference (dashed arrow).
Statistical Analysis
Average, standard deviation and median calculations were performed on the data recorded on the number of needle manipulations required for reaching the target, the number of confirmatory scans taken to complete the procedure, the radiation dosage received by the animal, and the total time taken for each procedure. The final confirmatory scan images were analyzed to calculate the accuracy and precision of needle placement. The accuracy of targeting was measured as the shortest distance between the needle tip and the closest point on the targeted seed using an image-processing tool (Amira, FEI Visualization Sciences Group, Burlington MA) (Figure 4).
Figure 4. Representative Seed Location and Accuracy Measurement.
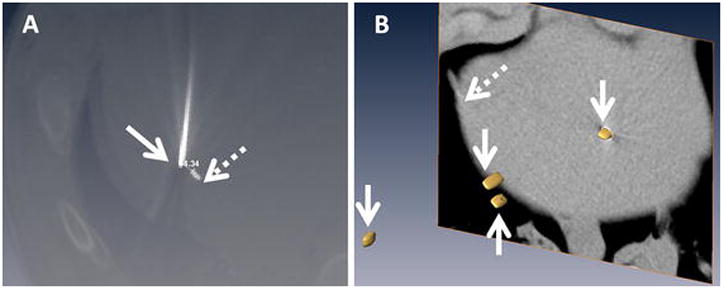
A: Screenshot showing needle placed (solid arrow) to a target (dashed arrow) in the liver. The image set used for treatment planning was registered with post-treatment confirmation scan to allow measurements.
B: Volumetric distribution of the targets extracted as isosurfaces (arrows) within the liver. CT slice showing on section of the liver is shown in the background (liver boundary marked with dashed arrow).
The data was analyzed for paired needle placements (manual and RPS) by each physician, and for inter-physician difference for each modality used for needle placement. Differences in outcomes following manual or RPS assisted needle placement for each physician were analyzed using student’s t-Test. For the latter, manual needle placement results from each physician were compared to outcomes from other participants performing manual needle placement, this analysis was repeated separately for needle placements in the RPS assisted group. Inter-physician variability in performance using different modalities of needle placement (manual or RPS) was assessed for each modality using the Kruskal-Wallis test. The total dose (DLP: dose length product) from the whole procedure received by the swine during the needle placement was recorded as the computed tomography dose index volume (CTDIvol). The radiation doses difference between manual and RPS needle placements was compared with the Wilcoxon sum rank test.
RESULTS
Forty-eight needle placements, 24 placed manually and 24 placed using RPS, were completed successfully. The needle entry point for robotic placement did not overlap the entry point for manual placement for any study participant. All robot-assisted needle placements required a separate skin nick to facilitate needle entry.
Inter-physician Outcomes Comparison Following Manual Needle Placement
Breath-hold was obtained for 5 punctures (20.8%, 5/24) during manual needle placement. Analysis of placement results from different physicians during manual needle placement suggested similar outcomes when considering accuracy, (mean=4.53 mm; range, 8.35–0.98 mm; SD= 1.24mm; p=0.198) and the number of confirmatory scans used to place the needle (mean= 6.6; range, 1–22; SD= 2.5; p=0.086). However, there was significant variation between physicians for the number of needle manipulations required to reach the target (mean= 4.6; range, 0–18; SD= 2.47; p=0.045) and for the time required to complete the procedure (mean= 6.12 minutes; range, 2–13; SD= 2.58; p=0.0082).
Inter-physician Outcomes Comparison Following RPS Assisted Needle Placement
Analysis comparing needle placements performed by different physicians using performing CT-guided RPS assistance suggested no significant differences between physicians regarding the accuracy of placement (mean= 4.66mm; range, 1.38–10.36; SD= 1.05; p=0.606), the number of confirmatory scans used to place and reposition the needle (mean= 1.6; range, 1–3; SD=0.34; p=0.276) and the number of needle manipulations required to reach target (mean= 0.41; range, 0–2; SD=0.34; p=0.131). Statistically significant variation was observed in the procedure time between different physicians (mean= 9.6 minutes; range, 4–22; SD=2.93; p=0.0202). Results are summarized in Table 1 and Figure 5.
Table 1.
Comparative results between manual and robotic positioning system assisted placements.
| Distance (mm) | Mean | SD |
Kruskal-Wallis test p |
T-test p |
|
|---|---|---|---|---|---|
| Distance (mm) | Manual | 4.53 | 1.24 | 0.198 | 0.417 |
| Robotic | 4.66 | 1.05 | 0.606 | ||
| No. of confirmatory scans | Manual | 6.6 | 2.5 | 0.086 | <0.0001 |
| Robotic | 1.6 | 0.34 | 0.276 | ||
| No. of needle manipulations | Manual | 4.6 | 2.47 | 0.045 | <0.0001 |
| Robotic | 0.41 | 0.34 | 0.131 | ||
| Time (min) | Manual | 6.12 | 2.58 | 0.008 | 0.0003 |
| Robotic | 9.6 | 2.93 | 0.02 | ||
| Radiation dose (DLP, mGy/cm) | Manual | 1075.77 | 717.74 | - | 0.03 |
| Robotic | 636.4 | 373.32 | - |
Figure 5. Overview of Results.
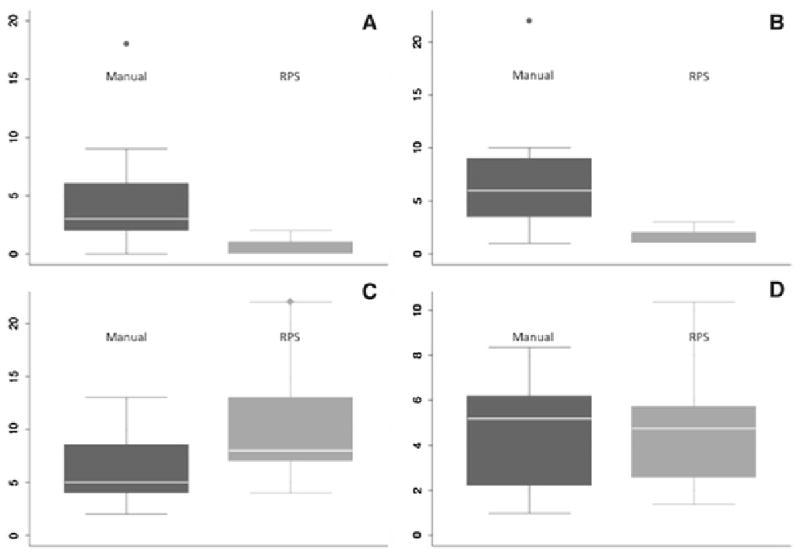
A: Number of confirmatory scans
B: Number of Needle Manipulations
C: Accuracy (mm)
D: Procedure time (minutes) for manual and robotic positioning system (RPS) assisted placement.
The whiskers show the upper and lower adjacent values, and the box covers the data in the 25th to 75th percentile. The line represents the median of the dataset.
Paired Comparison of Placement Outcomes for Each Physician
When comparing paired outcomes for each physician placing needles manually and using RPS to the same target, it was observed that the number of needle manipulations, and confirmatory scans required to reach a target were significantly lower when using RPS than manual placement (both p<0.0001). However, procedure time was significantly longer with RPS than manual placement (p=0.0003). Finally, no statistically significant difference was observed in the accuracy of placement between the two modalities (p=0.417).
Radiation Dose Comparison
The total DLP and CTDIvol dose for the entire procedure during manual placements was recorded at 1075.77 ± 717.74 mGy/cm and 179.58 ± 139.16 mGy respectively. For RPS procedures, total DLP and CTDIvol dose were 636.40 ± 373.32 mGy/cm and 128.33 ± 79.30 mGy respectively. A comparison of radiation dose received during placements with the two modalities indicated significant difference for DLP (P=0.03) but not for CTDIvol (P=0.43).
DISCUSSION
Our study demonstrates that RPS yields certain distinct benefits during needle placements to small targets when compared 1-to-1 to manual placement by experienced interventional radiologists. For any participant in the study, the use of RPS resulted in statistically significant reduction in the number of needle manipulations and total radiation dose received while targeting a specific target. This was achieved with no statistical difference in final accuracy of placement and with a limited increase in the time required to complete the needle placement. Most importantly, while experienced in manual placement of needles, the participants were essentially novice robot operators with limited experience or training in using the system. Despite this factor, no difference in performance was observed when outcomes were analyzed between all participants using a given modality (manual or RPS).
Procedures performed using sequential or fluoroscopy CT-guidance exposes both physicians and patients to radiation [30; 31]. Usually if performed under CT fluoroscopy, manual needle placement necessitates the physicians and their hands to remain in proximity to the CT-scan plan where radiation exposure can be maximal. Similar to other techniques proposed in literature [22; 30–34], needle placement with RPS can result in reduction of radiation exposure to both the patient and the physician. Moreover, by reducing the number of needle manipulations or repositioning required to reach a target, the use of RPS may also decrease the cumulative radiation exposure to patients when multiple needle placements are performed. Further reductions in radiation dosage could be achieved if the RPS could advance the needle to the target automatically based on a plan established by the physician. Additionally, RPS may prove a useful tool for relatively inexperienced physicians or physicians who occasionally perform procedures that require needle placement [20; 35; 36]. Our study has shown that despite limited training, all participants in the study were able to adequately use RPS to achieve outcomes that were comparable to manual placement by experienced physicians. The use of RPS may also limit the tremor of the freehand technique [37; 38]. Therefore, challenging biopsies may be performed more safely with RPS by limiting multiple needle adjustments and therefore procedural risk can be reduced in particularly when targeting lesions near vulnerable anatomy (bowel, nerves or vessels). Following this improvement of needle placement performance, limited complication rates and increased diagnostic yield of biopsies may be expected when using RPS to assist needle placements [39–41]. While reduction in number of needle manipulations could potentially reduce incidence of puncture related bleeding in patients, no such evidence was observed during this study. As the study was performed on healthy animals will no tumor burden, there was no clinically relevant bleeding in our experiments independent of the placement modality.
The use of RPS for needle placement requires additional time to allow for placement planning using the onboard software [21]. Indeed, this adds to the total procedure time for placements performed with RPS assistance. Procedures performed by experienced IR physicians were significantly shorter compared to the RPS approach. While significant, this difference in procedure time between the two modalities was approximately 3 minutes for RPS assisted placements and was not related with an increase in radiation dose received by the patient. While a limited amount of time is inherently required for transferring images and for performing needle placement planning on the robot, the increased time for RPS assisted procedures can also be attributed to the fact that operators using the system did not have specific training or practice sessions in using the system. Therefore one may conclude that total procedure time for using RPS will likely reduce with increased operator experience.
Our study has the following limitations. The study was performed using an animal model with artificial targets. Tumors may be more challenging to target due to their position, vascularization or surrounding anatomy. The use of general anesthesia and paralysis for inducing breath hold during RPS assisted needle placement is another limitation of the study. Local anesthesia cannot be performed in swine models and a paralytic is required to achieve and maintain an effective breath-hold in this animal model. Also, this study does not compare the cost-effectiveness of using RPS with manual needle placement. This may be the focus of a future clinical study using this system. Finally, in our experimental protocol manual placement always preceded RPS placement which could be interpreted to lead to potential learning effects, and consequentially better results for RPS placements. However, we would like to point out that all study metrics were independent of potential learning effects. Considering that skin entry point and targets remained the same for almost all placements, accuracy of targeting during RPS then became a function of how well the robot was able to position the needle guide and did not depend on the operator’s prior experience in placing a needle at that location. Likewise, RPS procedures consistently lasted longer than manual placement, independent of the physician performing the experiment. These considerations lead us to believe in the absence of any learning effects affecting our experimental findings.
In summary, CT-guided robotic positioning system needle placements significantly reduced the radiation exposure, the number of confirmatory scans and needle manipulations required to reach a target when compared to manual placements by an experienced IR physician without loss of placement accuracy. Additionally, the use of a standardized workflow, necessitated by the use of RPS, may reduce the inter-physician variability in performance during needle placement procedures. The RPS may become a valuable tool to assist experienced IR physicians but also train the less experienced or those in training in image guided needle placement interventions.
Acknowledgments
F. Cornelis would like to acknowledge support from CHU de Bordeaux, Université de Bordeaux, Canceropôle du Grand Sud-Ouest, Association de la Recherche contre le Cancer, Société Française de Radiologie, Société d’Imagerie Génito-Urinaire, Philippe Foundation and the Fulbright Scholarship Program.
Footnotes
Statement of human and animal rights:
The study has been approved by the appropriate institutional research ethics committee and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Conflicts of Interest statement:
The project was performed with support of a grant to the corresponding author (GS) from Perfint Healthcare Inc. The grant provided salary support for GS for performing the study. The others have no conflict of interest to report. Author #3 (ML) is a salaried employee of Perfint Healthcare Inc who provided technical support with the robot positioning system for the duration of the experiments. ML was not involved in the design of experiments, data collection, analysis or interpretation of presented results.
BIBLIOGRAPHY
- 1.Lu Y, Li C, Liu M, et al. MRI-guided stereotactic aspiration of brain abscesses by use of an optical tracking navigation system. Acta radiologica. 2013 doi: 10.1177/0284185113493272. [DOI] [PubMed] [Google Scholar]
- 2.Grasso RF, Cazzato RL, Luppi G, et al. Percutaneous lung biopsies: performance of an optical CT-based navigation system with a low-dose protocol. Eur Radiol. 2013;23(11):3071–3076. doi: 10.1007/s00330-013-2932-9. [DOI] [PubMed] [Google Scholar]
- 3.Ward TJ, Goldman RE, Weintraub JL. Electromagnetic navigation with multimodality image fusion for image-guided percutaneous interventions. Techniques in vascular and interventional radiology. 2013;16(3):177–181. doi: 10.1053/j.tvir.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Hakime A, Deschamps F, De Carvalho EG, Barah A, Auperin A, De Baere T. Electromagnetic-tracked biopsy under ultrasound guidance: preliminary results. Cardiovascular and interventional radiology. 2012;35(4):898–905. doi: 10.1007/s00270-011-0278-8. [DOI] [PubMed] [Google Scholar]
- 5.Banovac F, Cheng P, Campos-Nanez E, et al. Radiofrequency ablation of lung tumors in swine assisted by a navigation device with preprocedural volumetric planning. J Vasc Interv Radiol. 2010;21(1):122–129. doi: 10.1016/j.jvir.2009.09.012. 2818540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeo CT, Ungi T, PUT, Lasso A, McGraw RC, Fichtinger G. The effect of augmented reality training on percutaneous needle placement in spinal facet joint injections. IEEE transactions on bio-medical engineering. 2011;58(7):2031–2037. doi: 10.1109/TBME.2011.2132131. [DOI] [PubMed] [Google Scholar]
- 7.Neshat H, Cool DW, Barker K, Gardi L, Kakani N, Fenster A. A 3D ultrasound scanning system for image guided liver interventions. Medical physics. 2013;40(11):112903. doi: 10.1118/1.4824326. [DOI] [PubMed] [Google Scholar]
- 8.Gao H, Luo CF, Hu CF, Zhang CQ, Zeng BF. Minimally invasive fluoro-navigation screw fixation for the treatment of pelvic ring injuries. Surgical innovation. 2011;18(3):279–284. doi: 10.1177/1553350611399587. [DOI] [PubMed] [Google Scholar]
- 9.Cleary K, Melzer A, Watson V, Kronreif G, Stoianovici D. Interventional robotic systems: applications and technology state-of-the-art. Minim Invasive Ther Allied Technol. 2006;15(2):101–113. doi: 10.1080/13645700600674179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fichtinger G, Fiene JP, Kennedy CW, et al. Robotic assistance for ultrasound-guided prostate brachytherapy. Med Image Anal. 2008;12(5):535–545. doi: 10.1016/j.media.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onogi S, Morimoto K, Sakuma I, et al. Development of the needle insertion robot for percutaneous vertebroplasty. Med Image Comput Comput Assist Interv. 2005;8(Pt 2):105–113. doi: 10.1007/11566489_14. [DOI] [PubMed] [Google Scholar]
- 12.Rasmus M, Huegli RW, Bilecen D, Jacob AL. Robotically assisted CT-based procedures. Minim Invasive Ther Allied Technol. 2007;16(4):212–216. doi: 10.1080/13645700701520636. [DOI] [PubMed] [Google Scholar]
- 13.Zangos S, Melzer A, Eichler K, et al. MR-compatible assistance system for biopsy in a high-field-strength system: initial results in patients with suspicious prostate lesions. Radiology. 2011;259(3):903–910. doi: 10.1148/radiol.11101559. [DOI] [PubMed] [Google Scholar]
- 14.Hungr N, Fouard C, Robert A, Bricault I, Cinquin P. Interventional radiology robot for CT and MRI guided percutaneous interventions. Med Image Comput Comput Assist Interv. 2011;14(Pt 1):137–144. doi: 10.1007/978-3-642-23623-5_18. [DOI] [PubMed] [Google Scholar]
- 15.Koethe Y, Xu S, Velusamy G, Wood BJ, Venkatesan AM. Accuracy and efficacy of percutaneous biopsy and ablation using robotic assistance under computed tomography guidance: a phantom study. Eur Radiol. 2013 doi: 10.1007/s00330-013-3056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patriciu A, Awad M, Solomon SB, et al. Robotic assisted radio-frequency ablation of liver tumors--randomized patient study. Med Image Comput Comput Assist Interv. 2005;8(Pt 2):526–533. doi: 10.1007/11566489_65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoffner R, Augscholl C, Widmann G, Bohler D, Bale R. Accuracy and feasibility of frameless stereotactic and robot-assisted CT-based puncture in interventional radiology: a comparative phantom study. RoFo : Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 2009;181(9):851–858. doi: 10.1055/s-0028-1109380. [DOI] [PubMed] [Google Scholar]
- 18.Schulz B, Eichler K, Siebenhandl P, et al. Accuracy and speed of robotic assisted needle interventions using a modern cone beam computed tomography intervention suite: a phantom study. Eur Radiol. 2013;23(1):198–204. doi: 10.1007/s00330-012-2585-0. [DOI] [PubMed] [Google Scholar]
- 19.Schell B, Eichler K, Mack MG, et al. Robot-assisted biopsies in a high-field MRI system - first clinical results. RoFo : Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 2012;184(1):42–47. doi: 10.1055/s-0031-1281774. [DOI] [PubMed] [Google Scholar]
- 20.Mozer P, Troccaz J, Stoianovici D. Urologic robots and future directions. Curr Opin Urol. 2009;19(1):114–119. doi: 10.1097/MOU.0b013e32831cc1ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solomon SB, Patriciu A, Bohlman ME, Kavoussi LR, Stoianovici D. Robotically driven interventions: a method of using CT fluoroscopy without radiation exposure to the physician. Radiology. 2002;225(1):277–282. doi: 10.1148/radiol.2251011133. 3107539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gianfelice D, Lepanto L, Perreault P, Chartrand-Lefebvre C, Milette PC. Effect of the learning process on procedure times and radiation exposure for CT fluoroscopy-guided percutaneous biopsy procedures. J Vasc Interv Radiol. 2000;11(9):1217–1221. doi: 10.1016/s1051-0443(07)61367-0. [DOI] [PubMed] [Google Scholar]
- 23.Silverman SG, Tuncali K, Adams DF, Nawfel RD, Zou KH, Judy PF. CT fluoroscopy-guided abdominal interventions: techniques, results, and radiation exposure. Radiology. 1999;212(3):673–681. doi: 10.1148/radiology.212.3.r99se36673. [DOI] [PubMed] [Google Scholar]
- 24.Tovar-Arriaga S, Tita R, Pedraza-Ortega JC, Gorrostieta E, Kalender WA. Development of a robotic FD-CT-guided navigation system for needle placement-preliminary accuracy tests. Int J Med Robot. 2011;7(2):225–236. doi: 10.1002/rcs.393. [DOI] [PubMed] [Google Scholar]
- 25.Yanof J, Haaga J, Klahr P, et al. CT-integrated robot for interventional procedures: preliminary experiment and computer-human interfaces. Comput Aided Surg. 2001;6(6):352–359. doi: 10.1002/igs.10022. [DOI] [PubMed] [Google Scholar]
- 26.Abdullah BJ, Yeong CH, Goh KL, et al. Robot-assisted radiofrequency ablation of primary and secondary liver tumours: early experience. Eur Radiol. 2014;24(1):79–85. doi: 10.1007/s00330-013-2979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, Thiruvalluvan K, Krzeminski L, Moore WH, Xu Z, Liang Z. CT-guided robotic needle biopsy of lung nodules with respiratory motion - experimental system and preliminary test. Int J Med Robot. 2013;9(3):317–330. doi: 10.1002/rcs.1441. [DOI] [PubMed] [Google Scholar]
- 28.Pollock R, Mozer P, Guzzo TJ, et al. Prospects in percutaneous ablative targeting: comparison of a computer-assisted navigation system and the AcuBot Robotic System. J Endourol. 2010;24(8):1269–1272. doi: 10.1089/end.2009.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bax JS, Waring CS, Sherebrin S, et al. 3D image-guided robotic needle positioning system for small animal interventions. Med Phys. 2013;40(1):011909. doi: 10.1118/1.4771958. [DOI] [PubMed] [Google Scholar]
- 30.Nawfel RD, Judy PF, Silverman SG, Hooton S, Tuncali K, Adams DF. Patient and personnel exposure during CT fluoroscopy-guided interventional procedures. Radiology. 2000;216(1):180–184. doi: 10.1148/radiology.216.1.r00jl39180. [DOI] [PubMed] [Google Scholar]
- 31.Kato R, Katada K, Anno H, Suzuki S, Ida Y, Koga S. Radiation dosimetry at CT fluoroscopy: physician’s hand dose and development of needle holders. Radiology. 1996;201(2):576–578. doi: 10.1148/radiology.201.2.8888264. [DOI] [PubMed] [Google Scholar]
- 32.Nickoloff EL, Khandji A, Dutta A. Radiation doses during CT fluoroscopy. Health Phys. 2000;79(6):675–681. doi: 10.1097/00004032-200012000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Daly B, Krebs TL, Wong-You-Cheong JJ, Wang SS. Percutaneous abdominal and pelvic interventional procedures using CT fluoroscopy guidance. AJR Am J Roentgenol. 1999;173(3):637–644. doi: 10.2214/ajr.173.3.10470894. [DOI] [PubMed] [Google Scholar]
- 34.Paulson EK, Sheafor DH, Enterline DS, McAdams HP, Yoshizumi TT. CT fluoroscopy--guided interventional procedures: techniques and radiation dose to radiologists. Radiology. 2001;220(1):161–167. doi: 10.1148/radiology.220.1.r01jl29161. [DOI] [PubMed] [Google Scholar]
- 35.Baek SK, Carmichael JC, Pigazzi A. Robotic surgery: colon and rectum. Cancer journal. 2013;19(2):140–146. doi: 10.1097/PPO.0b013e31828ba0fd. [DOI] [PubMed] [Google Scholar]
- 36.Evans SM, Millar JL, Frydenberg M, et al. Positive surgical margins: rate, contributing factors and impact on further treatment: findings from the Prostate Cancer Registry. BJU international. 2013 doi: 10.1111/bju.12509. [DOI] [PubMed] [Google Scholar]
- 37.Oggero E, Pagnacco G, Morr DR, Barnes SZ, Berme N. The mechanics of drop landing on a flat surface--a preliminary study. Biomed Sci Instrum. 1997;33:53–58. [PubMed] [Google Scholar]
- 38.Baur C, Guzzoni D, Georg O. VIRGY: a virtual reality and force feedback based endoscopic surgery simulator. Stud Health Technol Inform. 1998;50:110–116. [PubMed] [Google Scholar]
- 39.Tiong L, Maddern GJ. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2011;98(9):1210–1224. doi: 10.1002/bjs.7669. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi K, Bhargava P, Raja S, et al. Image-guided biopsy: what the interventional radiologist needs to know about PET/CT. Radiographics. 2012;32(5):1483–1501. doi: 10.1148/rg.325115159. [DOI] [PubMed] [Google Scholar]
- 41.Hui GC, Tuncali K, Tatli S, Morrison PR, Silverman SG. Comparison of percutaneous and surgical approaches to renal tumor ablation: metaanalysis of effectiveness and complication rates. J Vasc Interv Radiol. 2008;19(9):1311–1320. doi: 10.1016/j.jvir.2008.05.014. [DOI] [PubMed] [Google Scholar]


