Abstract
Purpose
The Hibiscus rosa-sinensis flower is widely used in Brazilian traditional medicine for the treatment of diabetes and has shown antifertility activity in female Wistar rats. However, there is no scientific confirmation of its effect on diabetes and pregnancy. The aim of this study was evaluate the effect of aqueous extract of H. rosa-sinensis flowers on maternal-fetal outcome in pregnant rats with diabetes.
Methods
Diabetes was induced by streptozotocin (STZ, 40 mg/kg) in virgin, adult, female Wistar rats. After diabetes induction, the rats were mated. The pregnant rats were distributed into four groups (n minimum = 11 animals/group): non-diabetic, non-diabetic treated, diabetic, and diabetic treated. Oral aqueous extract of Hibiscus rosa-sinensis was administered to rats in the treatment groups during pregnancy. At term pregnancy, maternal reproductive outcomes, fetal parameters, and biochemical parameters were analyzed.
Results
The non-diabetic treated group showed decreased high density lipoprotein cholesterol, increased atherogenic index (AI) and coronary artery risk index (CRI), and increased preimplantation loss rate compared to the non-diabetic group. Although treatment with H. rosa-sinensis led to no toxicity, it showed deleterious effects on cardiac and reproductive functions. However, the diabetic treated group showed increased maternal and fetal weights, reduced AI and CRI, and reduced preimplantation loss rate compared to the untreated diabetic group.
Conclusion
Our results demonstrate beneficial effects of this flower only in pregnant rats with diabetes and their offspring. Although these findings cannot be extrapolated to human clinical use, they show that the indiscriminate intake of H. rosa-sinensis may be harmful to healthy individuals and its use should be completely avoided in pregnancy.
Introduction
Several plant species are recognized in folk medicine to have antidiabetic properties [1]; however, few plants have received appropriate scientific investigation. The genus Hibiscus is used empirically due to possible hypoglycemic or antidiabetic effects. For this reason, many species of genus Hibiscus (Malvaceae) have gained the attention of researchers in recent years. H. rosa-sinensis Linn. is widely found in Brazil and is used for urban landscaping, as an ornamental plant and living fence; it is known as rose mallow, Chinese hibiscus, China rose, and shoe flower. Pharmacological studies showed that flowers of H. rosa-sinensis have numerous actions including antibacterial [2–3], wound healing [4–5], antidepressant [6–7], cardiac [8], and antioxidant [9] effects.
The usage of plant-derived antioxidants is considered as an alternative strategy for improving oxidative damage in diabetes. There is evidence that hyperglycemia-mediated oxidative stress induces free radical generation, which leads to maternal complications [10–12] and fetal distress [13–14]. The extract of flowers of H. rosa-sinensis contains phenolic compounds and flavonoids responsible for its antioxidant activity [15]. In addition, the flowers and leaves of this plant showed significant hypoglycemic effect in several studies [16–19]. However, there are few studies related to diabetes, pregnancy and treatment with medicinal plants [20], and no studies evaluating the effect of Hibiscus rosa-sinensis in diabetic pregnancy.
We hypothesized that H. rosa-sinensis would have a beneficial effect in diabetic pregnancy without deleterious effects on the mother or the fetus. Therefore, the aim of this study was to evaluate the effect of aqueous extract of H. rosa-sinensis flowers on pregnant rats with diabetes and to investigate maternal reproductive and fetal outcomes.
Materials and methods
Extraction of plant materials
Flowers of H. rosa-sinensis were collected from Barra do Garças, Mato Grosso State, Brazil, between May and June 2012, in the morning. The plant was identified and authenticated by the Instituto Nacional de Ciência e Tecnologia—Herbário Virtual da Flora e dos Fungos (INCT-HVFF), where a voucher specimen (157459) was left. The flowers of the plant were dried at 35 oC for a period of 24 h in an aerated stove, ground, and a powder was prepared. H. rosa-sinensis aqueous extract was prepared by boiling 20 g of the flower powder in 1 L of water for 5 min. The extract was agitated and covered until it reached room temperature. The residue was removed by filtration (1 mm pore size) and the extract was then adequately concentrated in a rotary evaporator (1 hour). A sample was separated for determination of the solid concentration, and the extract was divided into aliquots stored at -20°C until further use.
Experimental animals
Female Wistar rats (230–250 g) were obtained from the Federal University of Mato Grosso Vivarium and were maintained under standard laboratory conditions (22±3 oC, 12 h light/dark cycle), with pelleted food (Purina rat chow, Purina®, São Paulo, SP, Brazil) and tap water ad libitum. The procedures and animal handling were authorized by the Ethical Committee for Animal Research of the UFMT, Brazil (Protocol number 23108.001991/13-1).
After two weeks of acclimatization, diabetes was induced in rats with streptozotocin (STZ; Sigma Chemical Company®, St. Louis, Millstone). STZ was intravenously (i.v.) administered at a single dose of 40 mg/kg dissolved in citrate buffer (0.1 M, pH 6.5). Non-diabetic rats received i.v. citrate buffer. Tail blood glucose concentrations were measured using a One Touch Ultra glucometer (Johnson & Johnson®) 7 days after the STZ treatment and glucose concentrations exceeding 300 mg/dL confirmed the presence of diabetes [21].
Experimental groups
After one week of confirmation of diabetic state, virgin female Wistar rats were mated overnight with non-diabetic male Wistar rats. The morning on which spermatozoids were found in the vaginal smear (day 0 of pregnancy), the rats were divided at random into four experimental groups: non-diabetic (n = 12), non-diabetic treated with H. rosa-sinensis extract (n = 13), diabetic (n = 12), and diabetic treated with H. rosa-sinensis extract (n = 11). Treatments were given orally, by gavage, in the morning. An initial dose of 100 mg/kg/day of the H. rosa-sinensis extract was given from day 0 until the 7th day of pregnancy (implantation period). The dose was increased to 200 mg/kg/day from day 8 to 14 of pregnancy (embryonic period), and to 400 mg/kg/day from day 15 to 20 (fetal period).
Course of pregnancy
Glycemia was measured in tail blood every seven days up to the end of pregnancy, at approximately 9 a.m. At days 0 and 21 of pregnancy, body weight gain, food consumption, and water intake were measured. At day 21 of pregnancy, rats were anesthetized by sodium thiopental (Thiopentax®, São Paulo, Brazil—50 mg/kg body weight, intraperitonialy) and after decapitation, blood samples were collected from the neck wound for biochemical analysis. Liver tissue was collected and processed as described below to determine oxidative stress markers. The gravid uterus was weighed and dissected to count dead and live fetuses, resorption, implantation, and corpora lutea numbers. The number of implantation sites was determined by the Salewski method [22]. The rate of pre-implantation loss was calculated as: (number of corpora lutea—number of implantations) × 100 / number of corpora lutea. Post-implantation loss rate was calculated as: (number of implantations—number of live fetuses) ×100 / number of implantations [23].
The fetuses were weighed and classified as small (SPA), adequate (APA), or large (LPA) for pregnancy age [24], and evaluated microscopically to determine the presence of external anomalies [23]. After external analysis, half the fetuses were fixed in Bouin’s fluid and serial sections were prepared as described by Wilson [25] for visceral examination. The remaining fetuses were prepared for examination of the skeletons by the staining procedure of Staples and Schnell [26].
Biochemical profile analysis
The collected blood samples were were collected in dry tubes and maintained on ice for 30 min and then centrifuged at 1300 g for 10 min at 4°C. The serum supernatant was at -80°C for determination of biochemical parameters.
Serum concentrations of total cholesterol (CHO), triglycerides (TG), and high-density lipoprotein (HDL) concentrations were estimated by enzymatic methods [27] using Winner® assay kits (Rosário, Argentina). Very-low-density lipoprotein (VLDL) cholesterol values were calculated from the triglyceride concentrations [28]. The atherogenic index (AI) was calculated according to the method of Liu et al. [29] and expressed as: AI = (CHO-HDL)/HDL. Coronary artery risk index (CRI) was also calculated by the following formula: CHO/HDL [30]. A standard line were used the values of control group.
Oxidative stress markers in the liver
Collected liver samples were rapidly washed with phosphate buffer saline (0.01 M, NaCl 0.138 M, KCl 0.0027M, pH 7.4). Hepatic malondialdehyde (MDA) and total glutathione (GSH-t) level and superoxide dismutase (SOD) activity were determined using commercial kits (Cayman® Chemical Co., Ann Arbor, Michigan, U.S.A.).
Catalase (CAT) activity was determined following decreases in the initial H2O2 concentration (20 nM used as the initial substrate) at 240 nm and 25 oC, over a time frame of 120 seconds. Briefly, 1 μL of supernatant isolated from rat liver homogenates was diluted in phosphate-buffered saline (0.1 M, pH 7). H2O2 1 M was added to obtain a final volume of 1 mL with H2O2 2 mM in a cuvette. The decrease in absorption at 240 nm in 120 seconds was determined and CAT activity was calculated using a molar extinction coefficient (ɛ = 0.0436 nM/cm) and expressed as U/mg protein [31].
Reduced thiol group levels in liver homogenates were evaluated by the method described by Jollow et al. [32] based on the development of a yellow color when 5,5’-Dithiobis(2-nitrobenzoic acid) (DTNB) was added to compounds containing sulfhydryl groups. The absorbance was read at 412 nm.
Statistical evaluation
We acknowledge that the homogeneity among experimental units is one of the basics of experimental design, and considering that non-diabetic and diabetic treated rats are biologically different organisms. Thus, we performed the following comparisons: non-diabetic treated with H. rosa-sinensis vs. non-diabetic groups, non-diabetic vs. diabetic groups, diabetic treated with H. rosa-sinensis vs. non-diabetic treated groups, and diabetic treated vs. diabetic groups. Student's unpaired t-test for normal distribution and Mann-Whitney U test for abnormal distribution of data were used to compare two groups. The proportion data were analyzed by Fisher's exact test. p < 0.05 was considered statistically significant.
Results
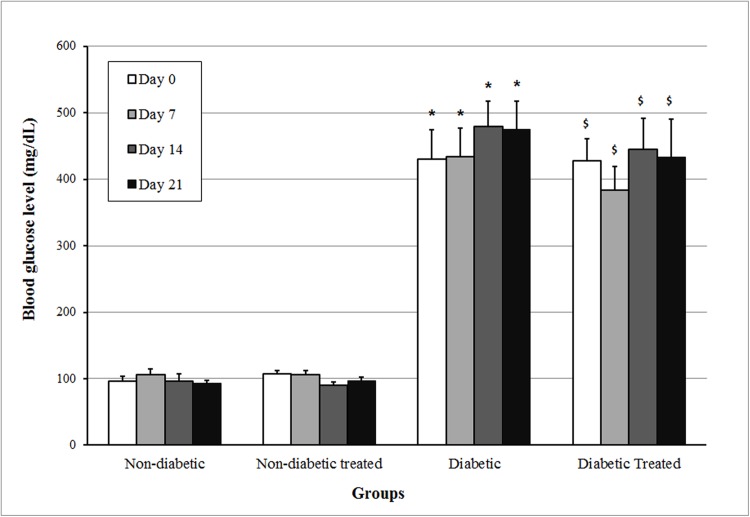
As shown in Fig 1, blood glucose levels of the rats of the two non-diabetic groups remained at approximately 100 mg/dL throughout the 21 days of pregnancy. In the diabetic groups, blood glucose levels were always above 300 mg/dL and treatment with the aqueous extract of Hibiscus rosa-sinensis did not modify blood glucose levels in non-diabetic or diabetic rats.
Fig 1. Blood glucose level on days 0, 7, 14, and 21 of non-diabetic and diabetic rats not treated or treated with Hibiscus rosa-sinensis aqueous extract during pregnancy.
N minimum = 11 animals/group. Data shown as mean ± standard error. *p < 0.05 compared to non-diabetic group (t test); $p < 0.05 compared to non-diabetic treated group (Mann-Whitney test).
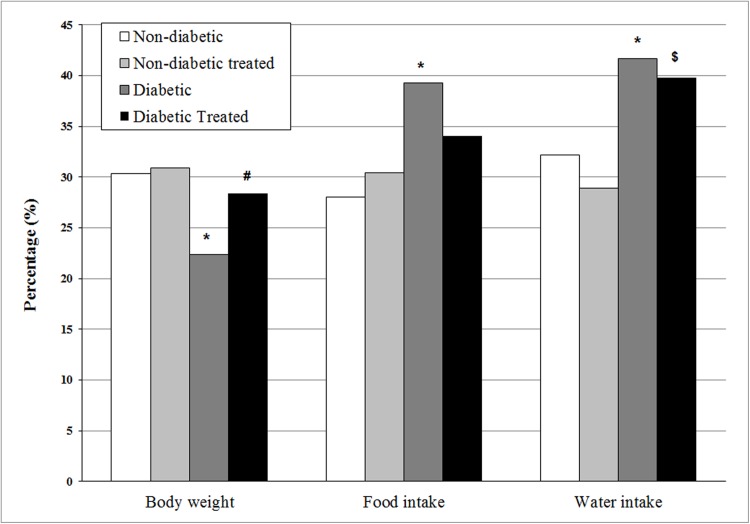
Treatment did not change body weight, water intake, or food intake in non-diabetic rats. The diabetic group not receiving treatment showed decreased body weight and increased food and water intake compared to the non-diabetic group. With the exception of the high water intake, the diabetic treated rats showed normalization of body weight and food intake to values that did not differ from those in the non-diabetic group (Fig 2).
Fig 2. Percentage of body weight gain, food intake, and water intake at 21 days of pregnancy as compared to onset of pregnancy in non-diabetic and diabetic rats not treated or treated with Hibiscus rosa-sinensis aqueous extract during pregnancy.
N minimum = 11 animals/group. *p < 0.05 compared to non-diabetic group; #p < 0.05 compared to diabetic group; $p < 0.05 compared to non-diabetic treated group (Fisher exact test).
Biochemical profiles are shown in Table 1. In non-diabetic rats, treatment decreased HDL concentration and increased both the AI and CRI compared to rats not receiving treatment. The diabetic rats not receiving treatment had higher TG, CHO, VLDL, and MDA concentrations, and higher AI and CRI compared to the non-diabetic rats. In addition, the HDL concentration was lower in diabetic rats than in non-diabetic rats. There was a significant increase in MDA concentration and SOD activity in diabetic treated rats compared to non-diabetic treated rats. Moreover, the diabetic treated rats showed decreased TG and VLDL concentrations, increased HDL concentration, and decreased AI and CRI compared to diabetic untreated animals.
Table 1. Biochemical profile at 21 days of pregnancy in non-diabetic and diabetic rats not treated or treated with Hibiscus rosa-sinensis aqueous extract during pregnancy.
| Groups | ||||
|---|---|---|---|---|
|
Non-diabetic (n = 12) |
Non-diabetic treated (n = 13) |
Diabetic (n = 12) |
Diabetic treated (n = 11) | |
| TG (mg/dL) | 136.1 ± 14.6 | 195.3 ± 33.6 | 591.3 ± 53.3* | 334.1 ± 104.1# |
| CHO (mg/dL) | 81.3 ± 1.8 | 114.6 ± 15.4 | 143.5 ± 12.3* | 111.6 ± 9.2 |
| HDL-c (mg/dL) | 43.7 ± 2.0 | 24.0 ± 2.4* | 15.3 ± 1.4* | 22.4 ± 1.2# |
| VLDL-c (mg/dL) | 27.2 ± 2.9 | 39.1 ± 6.7 | 118.3 ± 10.6* | 66.8 ± 20.7# |
| AI | 1.0 ± 0.1 | 4.3 ± 0.9* | 8.6 ± 1.4* | 4.6 ± 0.7# |
| CRI | 2.0 ± 0.1 | 5.3 ± 0.9* | 9.8 ± 1.5* | 5.6 ± 0.7# |
| MDA (nM/mg protein) | 92.2 ± 3.0 | 88.2 ± 10.3 | 214.5 ± 8.6* | 183.5 ± 13.4$ |
| SOD (U/mg protein) | 4.6 ± 0.3 | 4.5 ± 0.4 | 4.9 ± 0.4 | 6.4 ±0.6$ |
| GSH-t (U/mg protein) | 13.1 ± 0.5 | 16.6 ± 0.5 | 16.7 ± 1.2 | 15.9 ± 1.1 |
| Thiol groups (mM/mg protein) | 1.2 ± 0.1 | 1.4 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 |
| CAT (U/mg protein) | 6.5 ± 0.4 | 7.6 ± 1.3 | 4.5 ± 0.5 | 4.0 ± 0.7 |
Data shown as mean ± standard error (SD).
*p < 0.05 compared to non-diabetic group
#p < 0.05 compared to diabetic group
$p < 0.05 compared to non-diabetic treated group (t test).
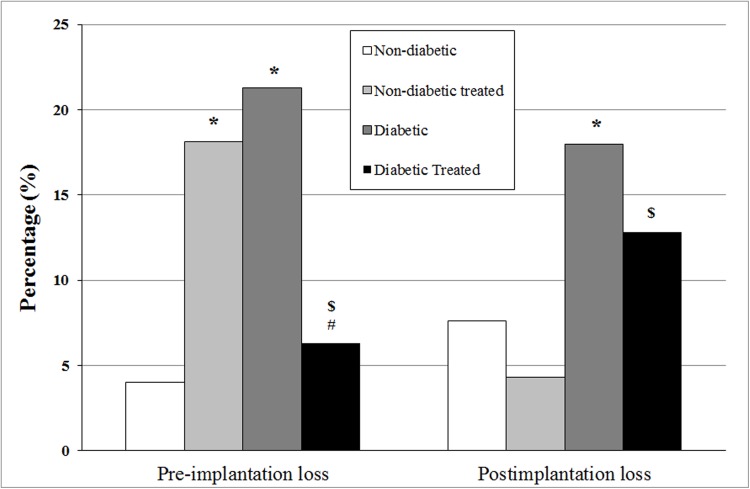
As shown in Fig 3, treatment increased pre-implantation losses in the non-diabetic treated group compared to the untreated group. The untreated diabetic rats showed a higher percentage of pre-implantation loses compared to non-diabetic rats. Treatment greatly decreased implantation loss in diabetic rats to values that did not differ from those in the non-diabetic group. Treatment did not modify postimplantation loss in non-diabetic rats. Untreated diabetic rats showed higher postimplantation loss than non-diabetic rats. Treatment decreased post-implantation losses in diabetics rats, although values remained higher than those in non-diabetic treated rats (Fig 3).
Fig 3. Percentage of pre- and postimplantation loss at 21 days of pregnancy in pregnant non-diabetic and diabetic rats not treated or treated with Hibiscus rosa-sinensis aqueous extract during pregnancy.
N minimum = 11 animals/group. *p < 0.05 compared to non-diabetic group; #p < 0.05 compared to diabetic group; $p < 0.05 compared to non-diabetic treated group (Fisher exact test).
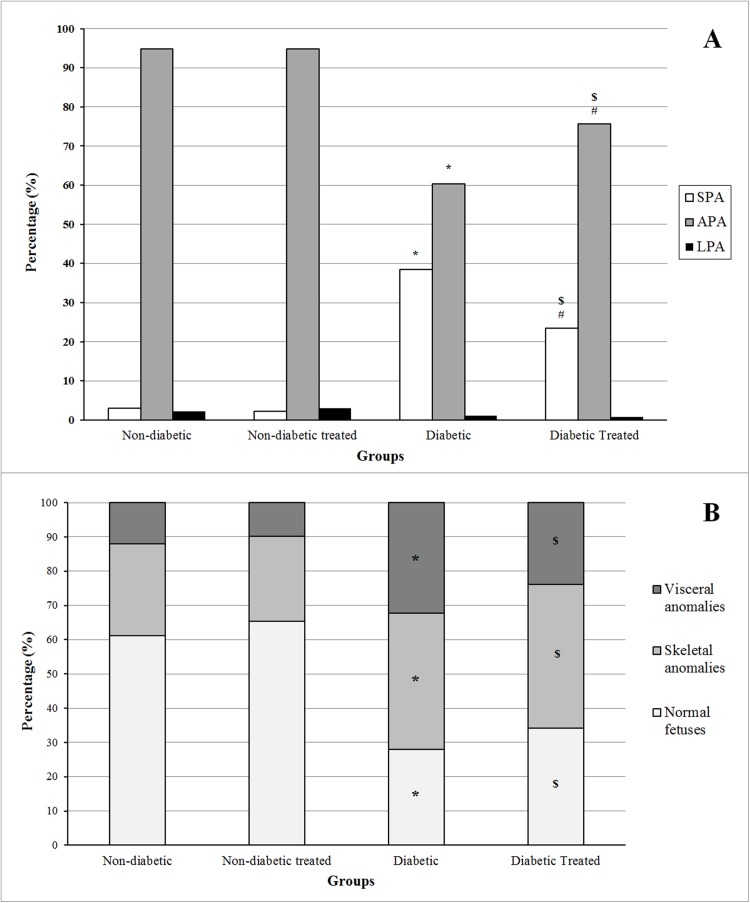
As shown in Fig 4A, treatment in non-diabetic rats did not modify the proportion of SPA, APA, or LPA. fetuses. The diabetic groups showed a decrease in the proportion of fetuses classified as APA and an increased proportion of fetuses classified as SPA compared to non-diabetic groups. The diabetic treated group showed an increase in the percentage of APA fetuses and a decrease in the percentage of SPA fetuses compared to the untreated diabetic group, although values did not reach those of either treated or untreated non-diabetic groups. As shown in Fig 4B, treatment did not affect the percentage of fetuses with skeletal and visceral anomalies, with a higher percentage of normal fetuses in the non-diabetic groups. However, in both the untreated and treated diabetic rats, the proportion of fetuses with visceral or skeletal anomalies was higher and the proportion of normal fetuses much lower compared to non-diabetic rats, without significant difference in values between the two diabetic groups.
Fig 4.
Percentage of fetuses classified as small (SPA), adequate (APA), or large (LPA) for pregnancy age (A) and percentage of fetal anomalies (B) at 21 days of pregnancy in pregnant non-diabetic and diabetic rats not treated or treated with H. rosa-sinensis aqueous extract during pregnancy. Non-diabetic (n fetuses = 134); Non-diabetic Treated (n fetuses = 134); Diabetic (n fetuses = 93); Diabetic Treated (n fetuses = 117). *p < 0.05 compared to non-diabetic group; #p < 0.05 compared to diabetic group; $p < 0.05 compared to non-diabetic treated group (Fisher exact test).
Discussion
Our results show that treatment with aqueous extract of H. rosa-sinensis flowers has no effect on blood glucose levels in non-diabetic and STZ-induced pregnant rats with severe diabetes. Others studies have shown a hypoglycemic effect of H. rosa-sinensis based on studies on traditional medicine, although these studies used a leaf aqueous extract [18–19] and flower ethanol extract [16–17,33] in non-pregnant rats. The absence of a hypoglycemic effect of H. rosa-sinensis in our study may be also related to the severity of diabetes. In fact, in the work by Venkatesh et al. [33], they used alloxan to induce diabetes, causing a more moderate increase in blood glucose levels than in our STZ-diabetic rats. Rats with severe diabetes induced by STZ in adult life reproduce blood glucose levels similar to that of humans with uncontrolled type 1 diabetes mellitus. The plant extract was insufficient to reduce persistent hyperglycemia in this study; this suggests that treatment in rats with moderate hyperglycemia might lead to beneficial results. Other factors that could explain the absence of a hypoglycemic effect in this study include the short treatment period, plant collect period, and the difference in sensitivity considering gender of animals tested.
Signs of uncontrolled hyperglycemia are polyphagia, polyuria, insulin deficiency, polydipsia, reduced anabolic processes, and accelerated catabolic processes. In addition, severe diabetes correlates directly with body weight loss, which is one of the most common signs of diabetes [34]; this is in agreement with our findings. We found that treatment of diabetic animals increased body weight when compared to diabetic rats, clearly showing a beneficial effect of H. rosa-sinensis extract.
H. rosa-sinensis treatment of non-diabetic pregnant rats led to reduced HDL levels and subsequent increased AI and CRI, contributing to an increased cardiovascular risk. This finding could be related to plant extract-derived nutrient overload and reticulum stress. Endoplasmic reticulum stress leads to a marked reduction of hepatic ATP-binding cassette transporter (ABCA1) expression, also known as the cholesterol efflux regulatory protein (CERP), which is a major regulator of cellular cholesterol and phospholipid homeostasis [35]. Subsequently, this reduction causes decreased HDL levels. The removal of excess cholesterol from macrophage foam cells by HDL and its principal apolipoprotein, apoA-1, is thought to be one of the key mechanisms underlying the atheroprotective properties of HDL [36–37]. Finally, this fact corroborates the negative correlation between HDL levels and AI and between HDL levels and CRI found in our study.
In contrast to non-diabetic rats, diabetic rats treated with the H. rosa-sinensis extract showed improvements in the lipid profile (reduced TG levels and increased HDL levels) and reduced cardiovascular risk, indicating a beneficial effect of the plant extract. The decrease in blood TG levels produced with treatment in the diabetic rats could contribute to this effect, since TG are known to play a regulatory role in lipoprotein interactions, rather than being an independent risk marker. This is supported by evidence that an increased plasma concentration of TG is associated with an elevated rate of coronary artery disease, increased low density lipoprotein (LDL) level, and increased cholesteryl ester transfer (HDL to apolipoprotein B) [38–39]. TG has also been proposed to be a major determinant of cholesterol esterification/transfer and HDL remodeling in human plasma [40–41]. The treatment with H. rosa-sinensis aqueous extract in diabetic male rats also showed an increased HDL-c level [4] as observed in our study. The main components of Hibiscus rosa-sinensis flowers extract related to antioxidant activities are tannin and anthocyanin [42]. The increase of HDL-c might be related to the anthocyanin action, since studies with anthocyanin supplementation in animals and humans showed similar results with improvement in the atherogenic activity, which was attributed to the action on reverse cholesterol transport [43–44]. However, the mechanism by which anthocyanins act is not well established.
There is evidence that STZ-induced diabetes promotes increased lipid peroxidation and decreased antioxidant enzymatic status, contributing to oxidative stress [45–47]. In our study, treatment with the plant extract in diabetic rats caused increased SOD activity, but this increase is not sufficient to influence non-diabetic oxidative stress induced by hyperglycemia, as shown by the unchanged elevated levels of MDA. Studies with Hibiscus sabdariffa aqueous extract showed anthocyanin-derived compounds presented high antioxidant capacity, with increased SOD activity in liver [48–49]. However, anthocyanin may have worse interaction with peroxyl radicals, the main radical species generate in the TBARS assay [50], and maybe this fact the MDA levels showed no difference in our study.
H. rosa-sinensis extract led to lipid metabolic perturbations in non-diabetic rats, which probably contributed to an inadequate intrauterine environment during embryonic implantation, making embryo attachment more difficult. This is characterized by an increased preimplantation loss rate, evident in our non-diabetic treated and untreated diabetic rats. Other researchers also found similar results after H. rosa-sinensis treatment, such as anti-implantation [51–52], abortion [53], and anti-fertility effects [54–55]. However, the implanted embryos from treated non-diabetic rats showed normal development. These findings contraindicate H. rosa-sinensis extract use during early pregnancy. In diabetic rats and their offspring, the plant extract showed beneficial effects on maternal reproductive performance. Our findings showed that STZ-induced diabetic rats had increases in both embryo loss and the incidence of fetuses classified as SPA, indicating intrauterine growth restriction, which is in agreement with the high proportion of fetal malformation due to hyperglycemia and/or oxidative stress previously reported [14,24]. After treatment with H. rosa-sinensis extract, there was a decreased rate of pre-implantation loss in diabetic rats and increased rate of fetuses classified as APA, demonstrating a beneficial effect of this plant extract that is potentially related to the improved lipid profile found in this specific group. However, treatment with H. rosa-sinensis did not reverse maternal diabetes-induced fetal abnormalities.
Conclusion
The present study demonstrates beneficial effects of this plant extract in diabetic pregnant rats, in both mothers and their offspring, and no benefits were identified in non-diabetic rats. However, although these findings cannot be extrapolated to humans, they show that the indiscriminate intake of H. rosa-sinensis extract may be harmful to healthy individuals and its use should be completely avoided in pregnancy.
Data Availability
All relevant data are within the paper.
Funding Statement
Ricardo T. Fujiwara and Débora C. Damasceno received support from Conselho Nacional de Desenvolvimento Científico e Tecnológico.
References
- 1.Damasceno DC, Volpato GT Antidiabetic botanical extracts In: Watson RR, Preedy VR editors. Botanical Medicine in Clinical Practice. CAB International, London; 2008. pp. 547–551. [Google Scholar]
- 2.Nayak D, Ashe S, Rauta PR, Nayak B. Biosynthesis, characterisation and antimicrobial activity of silver nanoparticles using Hibiscus rosa-sinensis petals extracts. IET Nanobiotechnol. 2015; 9: 288–293. doi: 10.1049/iet-nbt.2014.0047 [DOI] [PubMed] [Google Scholar]
- 3.Ruban P, Gajalakshmi K. In vitro antibacterial activity of Hibiscus rosa-sinensis flower extract against human pathogens. Asian Pac J Trop Biomed. 2012; 2: 399–403. doi: 10.1016/S2221-1691(12)60064-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhaskar A, Nithya V. Evaluation of the wound-healing activity of Hibiscus rosa sinensis L (Malvaceae) in Wistar albino rats. Indian J Pharmacol. 2012; 44: 694–698. doi: 10.4103/0253-7613.103252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shivananda Nayak B, Sivachandra Raju S, Orette FA, Chalapathi Rao AV. Effects of Hibiscus rosa sinensis L (Malvaceae) on wound healing activity: a preclinical study in a Sprague Dawley rat. Int J Low Extrem Wounds. 2007; 6: 76–81. doi: 10.1177/1534734607302840 [DOI] [PubMed] [Google Scholar]
- 6.Khalid L, Rizwani GH, Sultana V, Zahid H, Khursheed R, Shareef H. Antidepressant activity of ethanolic extract of Hibiscus rosa sinenesis Linn. Pak J Pharm Sci. 2014; 27: 1327–1331. [PubMed] [Google Scholar]
- 7.Shewale PB, Patil RA, Hiray YA. Antidepressant-like activity of anthocyanidins from Hibiscus rosa-sinensis flowers in tail suspension test and forced swim test. Indian J Pharmacol. 2012; 44: 454–457. doi: 10.4103/0253-7613.99303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gauthaman KK, Saleem MT, Thanislas PT, Prabhu V, Krishnamoorthy KK, Devaraj NS. Cardioprotective effect of the Hibiscus rosa sinensis flowers in an oxidative stress model of myocardial ischemic reperfusion injury in rat. BMC Complement Altern Med. 2006; 6: 32–39. doi: 10.1186/1472-6882-6-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masaki HS, Sakaki S, Atsumi T, Sakurai H. Active oxygen scavenging activity of plant extracts. Biol Pharm Bull. 1995; 18: 162–166. doi: 10.1248/bpb.18.162 [DOI] [PubMed] [Google Scholar]
- 10.Damasceno DC, Sinzato YK, Lima PH, Souza MSS, Volpato GT, Campos KE, et al. Effects of exposure to cigarette smoke prior to pregnancy in diabetic rats. Diabetol Metab Syndr. 2011; 3: 20 doi: 10.1186/1758-5996-3-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinzato YK, Volpato GT, Iessi IL, Bueno A, Calderon IM, Rudge MV, et al. Neonatally induced mild diabetes in rats and its effect on maternal, placental, and fetal parameters. Exp Diabetes Res. 2012; 2012: 108–163. doi: 10.1155/2012/108163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volpato GT, Damasceno DC, Sinzato YK, Ribeiro VM, Rudge MV, Calderon IM. Oxidative stress status and placental implications in diabetic rats undergoing swimming exercise after embryonic implantation. Reprod Sci. 2015; 22: 602–608. doi: 10.1177/1933719114556485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Consonni M, Damasceno DC, Kempinas WG, Nassr ACC, Volpato GT, Dallaqua B, et al. Effect of oral supplementation of the linoleic and gammalinolenic acids on the diabetic pregnant rats. Braz Arch Biol Technol. 2012; 55: 695–703. doi: 10.1590/S1516-89132012000500008 [Google Scholar]
- 14.Volpato GT, Damasceno DC, Rudge MVC, Padovani CR, Calderon IMP. Effect of Bauhinia forficata aqueous extract on the maternal-fetal outcome and oxidative stress biomarkers of streptozotocin-induced diabetic rats. J Ethnopharmacol. 2008; 116: 131–137. doi: 10.1016/j.jep.2007.11.013 [DOI] [PubMed] [Google Scholar]
- 15.Bhaskar A, Nithya V, Vidhya VG. Phytochemical screening and in vitro antioxidant activities of the ethanolic extract of Hibiscus rosa-sinensis. Ann Biol Res. 2011; 2: 653–661. [Google Scholar]
- 16.Moqbel FS, Naik PR, Najma HM, Selvaraj S. Antidiabetic properties of Hibiscus rosa sinensis L. leaf extract fractions on nonobese diabetic (NOD) mouse. Indian J Exp Biol. 2011; 49: 24–29. [PubMed] [Google Scholar]
- 17.Pillai SS, Mini S. Hibiscus rosa sinensis Linn. petals modulates glycogen metabolism and glucose homeostasis signalling pathway in streptozotocin-induced experimental diabetes. Plant Foods Hum Nutr. 2016; 71: 42–48. doi: 10.1007/s11130-015-0521-6 [DOI] [PubMed] [Google Scholar]
- 18.Sachdewa A, Khemani LD. Effect of Hibiscus rosa sinensis Linn. ethanol flower extract on blood glucose and lipid profile in streptozotocin induced diabetes in rats. J Ethnopharmacol. 2003; 89: 61–66. doi: 10.1016/S0378-8741(03)00230-7 [DOI] [PubMed] [Google Scholar]
- 19.Sachdewa A, Nigam R, Khemani LD. Hypoglycemic effect of Hibiscus rosa sinensis L. leaf extract in glucose and streptozotocin induced hyperglycemic rats. Indian J Exp Biol. 2001; 39: 284–286. [PubMed] [Google Scholar]
- 20.Volpato GT, Moraes-Souza RQ, Soares TS, Leal-Silva T, Damasceno DC. Medicinal plants for diabetes treatment during pregnancy. Curr Med Chem. 2017; 24: 404–410. doi: 10.2174/0929867323666161003122914 [DOI] [PubMed] [Google Scholar]
- 21.Pinheiro MS, Rodrigues LS, Neto LS, Moraes-Souza RQ, Soares TS, et al. Effect of Bauhinia holophylla treatment in Streptozotocin-induced diabetic rats. An Acad Bras Cienc. 2017; 89: 263–272. doi: 10.1590/0001-3765201720160050 [DOI] [PubMed] [Google Scholar]
- 22.Salewski E. Farbemethode zum markroskopishen nachweis von implantatconsstellen an uterus der ratter naunyn schmuderbergs. Arch. Pharmacol. (Weinheim). 1964; 247: 367 doi: 10.1007/BF02308461 [Google Scholar]
- 23.Damasceno DC, Kempinas WG, Volpato GT, Consoni M, Rudge MVC, Paumgartten FJR. Anomalias Congênitas: Estudos Experimentais. Coopmed, Belo Horizonte; 2008. [Google Scholar]
- 24.Damasceno DC, Silva HP, Vaz GF, Vasques-Silva FA, Calderon IM, Rudge MV, et al. Diabetic rats exercised prior to and during pregnancy: maternal reproductive outcome, biochemical profile, and frequency of fetal anomalies. Reprod Sci. 2013; 20: 730–738. doi: 10.1177/1933719112461186 [DOI] [PubMed] [Google Scholar]
- 25.Wilson JG. Methods for administering agents and detecting malformations in experimental animal In: Wilson JG, Warkany J editors. Teratology: Principles and Techniques. University of Chicago Press, Chicago; 1965. pp. 47–74. [Google Scholar]
- 26.Staples RE, Schnell VL. Refinements in rapid clearing technique in the KOH-alizarin red S method for fetal bone. Stain Technol. 1964; 39: 61–63. [PubMed] [Google Scholar]
- 27.Young D.S. Effects of drugs on clinical laboratory tests AACC Press, London; 2000. [Google Scholar]
- 28.Knopfholz J, Disserol CC, Pierin AJ, Schirr FL, Streisky L, Takito LL, et al. Validation of the friedewald formula in patients with metabolic syndrome. Cholesterol. 2014; 2014: 261–878. doi: 10.1155/2014/261878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu CS, Lin CC, Li TC. The relation of white blood cell count and atherogenic index ratio of LDL-cholesterol to HDL-cholesterol in Taiwan school children. Acta Paediatr Taiwan. 1999; 40: 319–324. [PubMed] [Google Scholar]
- 30.Boers M, Nurmohamed MT, Doelman CJ, Lard LR, Verhoeven AC, Voskuyl AE, et al. Influence of glucocorticoids and disease activity on total and high density lipoprotein cholesterol in patients with rheumatoid arthritis. Ann Rheumatic Dis. 2003; 62: 842–845. doi: 10.1136/ard.62.9.842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aebi H. Catalase in vitro. Meth Enzymol. 1984; 105: 121–126. doi: 10.1016/S0076-6879(84)05016-3 [DOI] [PubMed] [Google Scholar]
- 32.Jollow DJ, Mitchel JR, Zamppaglione Z, Gillette JR. Bromobenzene induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolites. Pharmacol. 1974; 11: 51–57. doi: 10.1159/000136485 [DOI] [PubMed] [Google Scholar]
- 33.Venkatesh S, Thilagavathi J, Shyam Sundar D. Antidiabetic activity of flowers of Hibiscus rosa sinensis. Fitoterapia. 2008; 79: 79–81. doi: 10.1016/j.fitote.2007.06.015 [DOI] [PubMed] [Google Scholar]
- 34.Kahn CR, Weir GC, King GL, Jacobson AM, Moses AC, Smith RT. Joslin’s Diabetes mellitus. Lippincott Williams & Wilkans, Philadelphia; 2004. [Google Scholar]
- 35.Luciani MF, Denizot F, Savary S, Mattei MG, Chimini G. Cloning of two novel ABC transporters mapping on human chromosome 9. Genomics. 1994; 21: 150–159. doi: 10.1006/geno.1994.1237 [DOI] [PubMed] [Google Scholar]
- 36.Rader DJ. Mechanisms of disease: HDL metabolism as a target for novel therapies. Nat Clin Pract Cardiovasc Med. 2007; 4:102–109. doi: 10.1038/ncpcardio0768 [DOI] [PubMed] [Google Scholar]
- 37.Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med. 2008; 263: 256–273. doi: 10.1111/j.1365-2796.2007.01898.x [DOI] [PubMed] [Google Scholar]
- 38.Guérin M, Le Goff W, Lassel TS, Van Tol A, Steiner G, Chapman MJ. Proatherogenic role of elevated CE transfer from HDL to VLDL1 and dense LDL in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2001; 21: 282–289. doi: 10.1161/01.ATV.21.2.282 [DOI] [PubMed] [Google Scholar]
- 39.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor to cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population based prospective studies. J Cardiovasc Risk. 1996; 3: 213–219. doi: 10.1177/174182679600300214 [PubMed] [Google Scholar]
- 40.Dobiásová M. Atherogenic index of plasma [log(triglycerides/HDL-cholesterol)]: theoretical and practical implications. Clin Chem. 2004; 50: 1113–1115. doi: 10.1373/clinchem.2004.033175 [DOI] [PubMed] [Google Scholar]
- 41.Murakami T, Michelagnoli S, Longhi R, Gianfranceschini G, Pazzucconi F, Calabresi L, et al. Triglycerides are major determinants of cholesterol esterification/transfer and HDL remodeling in human plasma. Arterioscler Thromb Vasc Biol. 1995; 15: 1819–1828. 0.1161/01.ATV.15.11.1819 [DOI] [PubMed] [Google Scholar]
- 42.Mak YW, Chuah LO, Ahmad R, Bhat R. Antioxidant and antibacterial activities of hibiscus (Hibiscus rosa-sinensis L.) and Cassia (Senna bicapsualris L.) flower extracts. J King Saud Univ Sci. 2013; 25: 275–282. doi: 10.1016/j.jksus.2012.003 [Google Scholar]
- 43.Zhu Y, Huang X, Zhang Y, Wang Y, Liu Y, Sun R, et al. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J Clin Endocrinol Metab. 2014; 99: 561–569. doi: 10.1210/jc.2013-2845 [DOI] [PubMed] [Google Scholar]
- 44.Qin Y, Xia M, Ma J, Hao YT, Liu J, Mou HY, et al. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr. 2009; 90: 485–492. doi: 10.3945/ajcn.2009.27814 [DOI] [PubMed] [Google Scholar]
- 45.Volpato GT, Calderon IMP, Sinzato S, Campos KE, Rudge MVC, Damasceno DC. Effect of Morus nigra aqueous extracttreatment on the maternal–fetal outcome, oxidative stress status and lipid profile of streptozotocin-induced diabetic rats. J Ethnopharmacol. 2011; 138: 691–696. doi: 10.1016/j.jep.2011.09.044 [DOI] [PubMed] [Google Scholar]
- 46.Damasceno DC, Volpato GT, Calderon IMP, Rudge MVC. Oxidative stress and diabetes in pregnant rats. Anim Reprod Sci. 2002; 72: 235–244. doi: 10.1016/S0378-4320(02)00094-5 [DOI] [PubMed] [Google Scholar]
- 47.Eriksson UJ, Cederberg J, Wentzel P. Congenital malformations in offspring of diabetic mothers-animal and human studies. Rev Endocr Metab Disord. 2003; 4: 79–93. doi: 10.1023/A:1021879504372 [DOI] [PubMed] [Google Scholar]
- 48.Ajiboye TO, Salawu NA, Yakubu MT, Oladiji AT, Akanji MA, Okogun JI. Antioxidant and drug detoxification potentials of Hibiscus sabdariffa anthocyanin extract. Drug Chem Toxicol. 2011; 34: 109–115. doi: 10.3109/01480545.2010.536767 [DOI] [PubMed] [Google Scholar]
- 49.Da-Costa-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M. Hibiscus sabdariffa L.—a phytochemical and pharmacological review. Food Chem. 2014; 165: 424–443. doi: 10.1016/j.foodchem.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 50.Fernández-Arroyo S, Rodríguez-Medina IC, Beltrán-Debón R, Pasini F, Joven J, Micol V, et al. Quantification of the polyphenolic fraction and in vitro antioxidant and in vivo anti-hyperlipemic activities of Hibiscus sabdariffa aqueous extract. Foof Res Int. 2011; 44: 1490–1495. doi: 10.2016/j.foodres.2011.03.040 [Google Scholar]
- 51.Kholkute SD, Udupa KN. Effects of Hibiscus rosa sinensis on pregnancy of rats. Planta Med. 1976; 26: 321–329. doi: 10.1055/s-0028-1097671 [DOI] [PubMed] [Google Scholar]
- 52.Pal AK, Bhattacharya K, Kabir SN, Pakrashi A. Flowers of Hibiscus rosa sinensis, a potential source of contragestive agent II. Possible mode of action with reference to antiimplantation effect of benzene extract. Contraception. 1985; 32: 517–529. doi: 10.1016/0010-7824(85)90021-6 [DOI] [PubMed] [Google Scholar]
- 53.Singh MP, Singh RH, Udupa KN. Antifertility activity of a benzene extract of Hibiscus rosa sinensis flowers in female albino rats. Planta Med. 1982; 44: 171–174. doi: 10.1055/s-2007-971433 [DOI] [PubMed] [Google Scholar]
- 54.Murthy DR, Reddy CM, Patil SB. Effect of benzene extract of Hibiscus rosa-sinensis on the estrous cycle and ovarian activity in albino mice. Biol Pharm Bull. 1997; 20: 756–758. doi: 10.1248/bpb.20.756 [DOI] [PubMed] [Google Scholar]
- 55.Vasudeva N, Sharma SK. Post-coital antifertility activity of Hibiscus rosa-sinensis Linn. roots. Evid Based Complement Alternat Med. 2008; 5: 91–94. doi: 10.1093/ecam/nem003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.