Abstract
The relationship between skeletal muscle volume and the prognosis of patients with inflammatory bowel disease (IBD) remains undetermined. We conducted a retrospective study of 72 IBD patients who were admitted to the hospital due to disease exacerbation. We enrolled IBD patients who had undergone abdominal computed tomography and assessed the nutritional indices, such as the Onodera’s prognostic nutritional index (O-PNI) and the controlling nutritional status (CONUT) index. The L3 skeletal muscle index (SMI), which is the ratio of the cross-sectional area of skeletal muscles at the level of the third lumbar (L3) vertebra to the height squared, was used to identify sarcopenia. Sarcopenia, defined as a low SMI, was observed in 42% of all IBD patients (37% with Crohn’s disease (CD) and 48% with ulcerative colitis (UC)). In UC patients, the O-PNI and CONUT values, height, and albumin levels were significantly lower than in CD patients. The SMI strongly correlated with sex, body weight, albumin level, and O-PNI in IBD patients. Multivariate analysis using the Cox regression model demonstrated that the presence of sarcopenia (P = 0.015) and disease type (CD or UC) (P = 0.007) were significant factors predicting intestinal resection. The cumulative operation-free survival rate was significantly lower for sarcopenic patients than in all IBD patients (P = 0.003) and a stratified analysis of CD patients (P = 0.001) using the Kaplan–Meier method and log-rank test. The L3 skeletal muscle area is a prognostic factor for intestinal resection in patients with CD.
Introduction
Inflammatory bowel diseases (IBD), such as ulcerative colitis (UC) and Crohn’s disease (CD), are chronic gastrointestinal diseases that are associated with protein-energy malnutrition (PEM) [1, 2]. PEM is caused by low dietary intake, enhanced energy expenditure due to inflammation, impaired digestion and absorption, and protein leakage from ulcerative lesions [2–6]; it leads to decreases in skeletal muscle and adipose tissue volumes. Altered body composition, such as reduced fat-free mass, has been reported in IBD patients [7, 8]. Additionally, decreased skeletal muscle volume has been observed in 60% of CD patients in clinical remission [9]. A recent report demonstrated decreased phosphorylated:total Akt ratio in muscle biopsy obtained from CD patients, suggesting impaired activation of muscle protein synthesis pathways, notably the IGF-1-Akt pathway [10]. The involvement of upstream proinflammatory cytokines, such as interleukin-6 or tumor necrosis factor-α, was suspected.
There are several reports concerning altered body composition and clinical outcomes of IBD. Zhang et al. reported the relationships between decreased skeletal muscle volume and postoperative complications [11]. Furthermore, Holt et al. reported that visceral adiposity but not low skeletal muscle predicted endoscopic recurrence after surgery in CD [12]. However, the relationship between reductions in skeletal muscle volume and prognoses of IBD patients has yet to be elucidated.
The description of sarcopenia was first made in 1989 by Rosenberg to describe changes in body composition and related functions, such as age-related loss of muscle mass and function [13]. Sarcopenia can be considered primary (or age-related) when no other cause is evident except for aging itself or as secondary when causes apart from aging are evident [14, 15]. Sarcopenia is associated with age, activity, disease and nutritional statuses [14]. Recently, an association between disease status and prognosis was reported in the field of malignant neoplasms and chronic diseases, such as heart failure, obstructive pulmonary disease, diabetes mellitus, kidney disease, connective tissue disease, tuberculosis, and other wasting conditions [16–23]. Recently, there has been increased interest in the clinical importance of secondary sarcopenia [15].
According to the European Working Group on Sarcopenia in Older People (EWGSOP), the definition of sarcopenia is low muscle mass and low muscle strength or low physical performance [14]. Skeletal muscle volume is reported to have a strong correlation with physical performance, such as gait speed, as well as with muscle strength, such as grip strength or hip flexion force [24, 25]. Furthermore, the cross-sectional area of skeletal muscles at the level of the third lumbar (L3) vertebra is considered a surrogate marker of total skeletal muscle volume [26]. The L3 skeletal muscle index (SMI), which is the L3 skeletal muscle area divided by the height squared, is used to assess skeletal muscle volume [16, 18, 20]. Herein, we performed a retrospective study to investigate the relationship between the SMI and the prognosis of IBD.
Materials and methods
Study population and clinical data
This retrospective study was conducted with the approval of the Ethics Committee of the Shiga University of Medial Science (28–058). The Ethics Committee of the Shiga University of Medial Science waived the need for consent from individuals. The opt-out information was published in the following URL (http://www.shiga-med.ac.jp/hospital/doc/department/department/digestive_int/index.html). The clinical data was collected from medical records and the data was anonymized for analysis. The subjects were a consecutive series of IBD patients admitted to the Shiga University of Medical Science Hospital due to disease exacerbation and were assessed by the nutrition support team (NST) of the Shiga University of Medical Science Hospital between January 2011 and December 2016. A total 72 IBD patients had undergone abdominal computed tomography (CT) two weeks before or after the admission; they had also been assessed by the NST for their nutritional status and resting energy expenditure (REE) using indirect calorimetry within one week after admission. The REE and respiratory quotient (RQ) were measured via a computed open-circuit IC (AE-300S, Minato Medical Science Corporation, Osaka, Japan), as previously described [5, 6, 27–30]. The basal energy expenditure (BEE) was calculated using the Harris-Benedict equation [31]. For nutritional assessments, the subjective global assessment (SGA) [32], controlling nutritional status (CONUT) [33], malnutrition universal screening tool (MUST) [34], nutritional risk screening 2002 (NRS-2002) [35], and Onodera’s prognostic nutritional index (O-PNI) [36, 37] were used. O-PNI was calculated based on the serum albumin level and total lymphocyte count, using the following equation: O-PNI = 10 × [serum albumin (g/dL) + 0.005 × total lymphocyte count (/mL)]. Assessments of nutritional status and REE were performed after admission. Clinical backgrounds, laboratory data, clinical symptoms, endoscopic findings, and other clinical data related to intestinal resection, were collected retrospectively.
Assessment of muscle volume and definition of sarcopenia
The cross-sectional area of skeletal muscles at the level of the L3 vertebra was measured on CT images using image analysis software (OsiriX MD version 7.5.1, Pixmeo, Geneva, Switzerland), which enabled the identification of a specific tissue demarcation by the Hounsfield unit from -29 to +150 [38]. The skeletal muscles at the L3 level include the psoas, erector spinae, quadratus lumborum, transversus abdominis, internal oblique, external oblique, and rectus abdominis. The L3 skeletal muscle index (SMI) which is the L3 skeletal muscle area divided by the height squared is used to assess the skeletal muscle volume. The cut-off values of the SMI for identifying sarcopenia in liver disease are 42 cm2/m2 for men and 38 cm2/m2 for women [39]. Patients with an SMI below the cut-off value were defined as sarcopenia.
Statistical analyses
All statistical analyses were performed using Prism, version 6.05 (GraphPad, San Diego, CA), and IBM SPSS Statics, version 20.0 (IBM Corporation, Armonk, NY, USA). The χ2–test and Mann–Whitney U test were used appropriately. Spearman’s rank correlation coefficients were used to calculate the correlations between the SMI and clinical variables. For categorical or ordinal variables, logistic regression analysis was performed. Cox regression analysis was performed to estimate the risk of intestinal resection. After univariate analysis, all variables with P values less than 0.15 were considered in subsequent multivariate analyses. Cumulative operation-free survival rate was calculated using the Kaplan–Meier method. The log-rank test was used to determine the statistical difference between groups. P values were two-sided, and those less than 0.05 were considered statistically significant.
Results
The study participants included total 72 IBD patients (CD: 43 cases; UC: 29 cases). The clinical backgrounds of the patients are shown in Table 1. Sarcopenia, defined as an SMI below the cut-off value, was observed in 42% (30/72) of the IBD patients. A poor nutritional status, as assessed by O-PNI and CONUT, was significant in UC patients. Height and serum albumin levels were significantly lower in UC patients than in CD patients. Conversely, age was significantly higher in UC patients than in CD patients.
Table 1. Clinical backgrounds and outcomes of the patients.
| Crohn’s disease | Ulcerative colitis | P value | ||
|---|---|---|---|---|
| Male/female | 35/8 | 18/11 | 0.121a | |
| Age (years), median (IQR) | 29.0 (25.0–37.0) | 39 (28–55) | 0.015b | |
| Body mass index, median (IQR) | 19.4 (17.6–21.9) | 18.9 (17.3–20.1) | 0.424b | |
| Body weight (kg), median (IQR) | 55.2 (51.0–59.5) | 51.5 (44.0–57.8) | 0.052b | |
| Height (cm), median (IQR) | 170 (166–174) | 165 (156–169) | 0.002b | |
| Skeletal muscle index (cm2/m2), median (IQR) | 45.1 (40.4–52.5) | 40.7 (35.7–47.1) | 0.059b | |
| Sarcopenia/non-sarcopenia | 16/27 | 14/15 | 0.489a | |
| Days of admission, median (IQR) | 28.0 (15.5–54.0) | 30.0 (25.0–40.0) | 0.854b | |
| Intestinal resection (yes/no) | 19/24 | 6/23 | 0.071a | |
| Disease type | Ileal/colonic/ileocolonic | 13/4/26 | - | |
| Pancolitis / left-sided | - | 22/7 | ||
| Disease activity | CDAI, median (IQR) | 227 (159–298) | - | |
| Lichtiger score, median (IQR) | - | 12 (10–14) | ||
| Endoscopic activity | SES-CD, median (IQR) | 13 (8–16) | - | |
| UCEIS, median (IQR) | - | 6 (5–7) | ||
| Onodera’s prognostic nutritional index, median (IQR) | 37.2 (32.1–42.3) | 31.2 (27.0–34.7) | 0.005b | |
| Controlling nutritional status, median (IQR) | 6 (3–9) | 8 (6–10) | 0.020b | |
| Subjective global assessment, median (IQR) | 2 (2–3) | 2 (2–3) | 1.000b | |
| Malnutrition universal screening tool, median (IQR) | 2 (1–4) | 3 (2–3) | 0.166b | |
| Nutritional risk screening 2002, median (IQR) | 3 (2–4) | 4 (3–4) | 0.218b | |
| BEE (kcal), median (IQR) | 1417 (1331–1527) | 1394 (1241–1489) | 0.181b | |
| BEE / BW (kcal/kg), median (IQR) | 27.1 (25.3–27.7) | 26.6 (24.5–28.0) | 0.709b | |
| REE (kcal), median (IQR) | 1339 (1246–1590) | 1358 (1275–1486) | 0.865b | |
| REE / BW (kcal/kg), median (IQR) | 26.3 (24.5–28.9) | 28.0 (24.3–29.7) | 0.476b | |
| Respiratory quotient, median (IQR) | 0.80 (0.78–0.85) | 0.74 (0.72–0.83) | 0.082b | |
| Hematocrit (%), median (IQR) | 35.1 (29.7–39.8) | 30.8 (27.8–36.0) | 0.059b | |
| White blood cell count (/μL), median (IQR) | 7,500 (5,700–9,850) | 9,000 (7,500–12,000) | 0.055b | |
| Total lymphocyte count (/μL), median (IQR) | 1,295 (826–1,619) | 1,068 (884–1,527) | 0.584b | |
| Neutrophil / Lymphocyte ratio, median (IQR) | 4.41 (2.72–8.68) | 6.14 (4.54–8.71) | 0.054b | |
| Albumin (g/dL), median (IQR) | 3.0 (2.7–3.4) | 2.6 (2.1–2.9) | 0.002b | |
| Total cholesterol (mg/dL), median (IQR) | 130 (116–151) | 120 (102–147) | 0.502b | |
| C-reactive protein (mg/dL), median (IQR) | 2.82 (0.57–9.49) | 4.30 (2.05–8.39) | 0.165b | |
BW: body weight, CDAI: Crohn’s disease activity index, SES-CD: simple endoscopic score for Crohn’s disease, UCEIS: ulcerative colitis endoscopic index of severity, BEE: basal energy expenditure, REE: resting energy expenditure, IQR: interquartile range. Bold items indicate statistically significant results.
aχ2–test
bMann–Whitney U test.
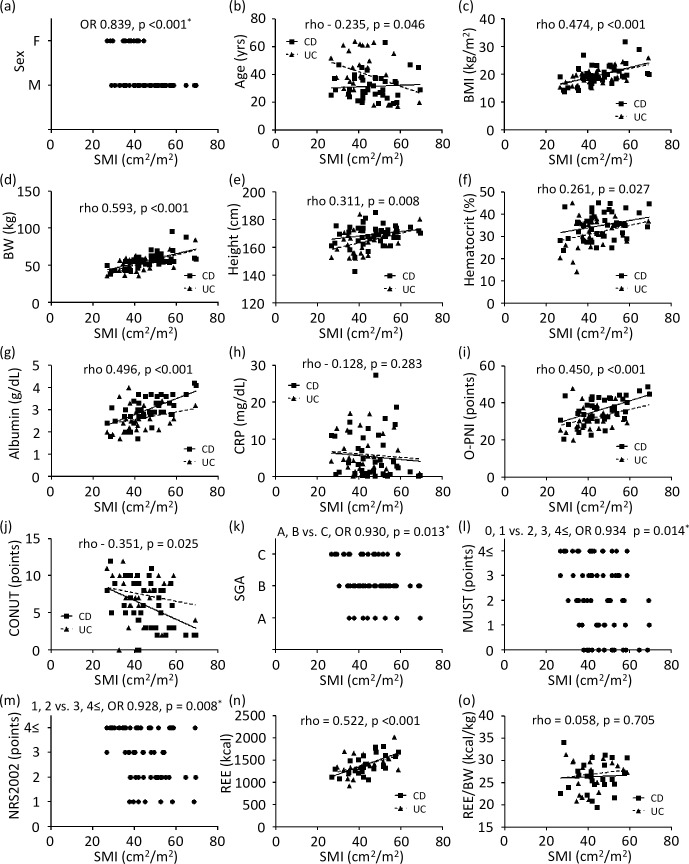
Correlations between the SMI and clinical variables were assessed (Fig 1). In IBD patients, the variables that were significantly correlated with the SMI were sex, age, body mass index, body weight, height, hematocrit, albumin level, O-PNI, CONUT, SGA, MUST, NRS-2002, and REE. To assess the correlation between the nutritional indices and SMI, SGA, MUST, and NRS-2002 were divided into two groups and evaluated by logistic regression analysis (shown in Fig 1). SGA, MUST, and NRS-2002 showed significant relationships with the SMI. Although REE was significantly correlated with the SMI, the significance was diminished as REE was normalized by body weight. Of all variables, the SMI has a strong correlation to sex, body weight, albumin level, and O-PNI.
Fig 1.
Dispersion graphs depicting correlations between SMI and sex (a), age (b), BMI (c), BW (d), height (e), hematocrit (f), albumin (g), CRP (h), O-PNI (i), CONUT (j), SGA (k), MUST (l), NRS-2002 (m), REE (n), and REE/BW (o). P values on each graph were calculated for all IBD patients. Rho indicates Spearman’s rank correlation coefficient. *Logistic regression analysis. OR: odds ratio, SMI: skeletal muscle index, BMI: body mass index, BW: body weight, CRP: C-reactive protein, O-PNI: Onodera’s prognostic nutritional index, CONUT: controlling nutritional status, SGA: subjective global assessment, MUST: malnutritional universal screening tool, NRS-2002: nutritional risk screening-2002, REE: resting energy expenditure.
Factors associated with intestinal resection were assessed by Cox regression analysis (Table 2). Univariate analysis revealed the presence of sarcopenia, disease type (CD or UC), and length of hospital stay as the most significant factors predicting surgery. Multivariate analysis using variables obtained at the time of hospitalization identified the presence of sarcopenia and disease type (CD or UC) as the most significant factors predicting surgery.
Table 2. Factors associated with intestinal resection for all IBD patients.
| All (n = 60) | Univariate HR (95% CI) | Multivariate HR (95% CI) |
|---|---|---|
| Age | 0.982 (0.951–1.014), 0.263 | - |
| Sex (Male/Female) | 0.890 (0.355–2.233), 0.804 | - |
| Body mass index | 1.006 (0.873–1.159), 0.935 | - |
| Skeletal muscle index | 0.959 (0.918–1.003), 0.067 | - |
| Sarcopenia/Non-sarcopenia | 0.313 (0.138–0.712), 0.006 | 0.318 (0.126–0.802), 0.015 |
| Disease (CD/UC) | 0.382 (0.152–0.959), 0.040 | 0.278 (0.108–0.714), 0.007 |
| O-PNI | 0.957 (0.903–1.015), 0.142 | - |
| Hemoglobin | 0.970 (0.876–1.076), 0.573 | - |
| Total lymphocyte count | 0.999 (0.999–1.001), 0.728 | - |
| Neutrophil lymphocyte count | 1.037 (0.967–1.112), 0.302 | - |
| Albumin | 0.592 (0.302–1.156), 0.124 | 0.782 (0.354–1.726), 0.542 |
| C-reactive protein | 1.062 (0.994–1.134), 0.073 | 1.059 (0.985–1.137), 0.119 |
| Length of stay | 1.058 (1.039–1.078), <0.001 | - |
Surgery was required 21 for patients (35%). Univariate and multivariate analysis were conducted by Cox regression analysis. Bold numbers indicate statistically significant results. CD: Crohn’s disease, UC: ulcerative colitis, CI: confidence interval
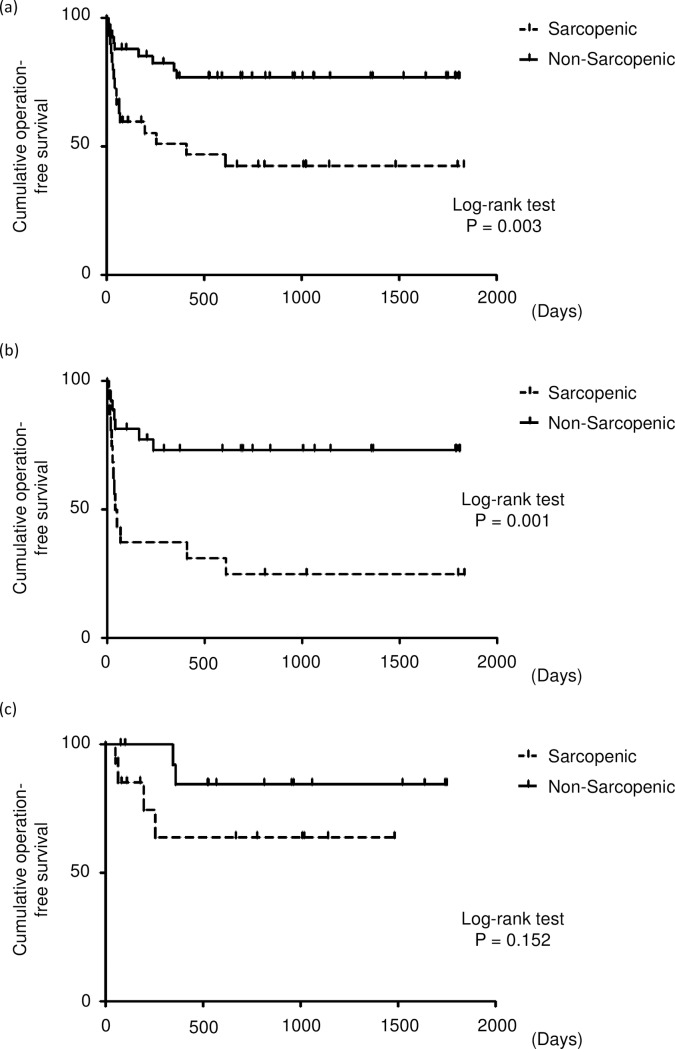
A Kaplan–Meier analysis was used to calculate the cumulative operation-free survival rate (Fig 2). Among all IBD patients, those with sarcopenia had a significantly lower cumulative operation-free rate than those without. A significant association between intestinal resection and sarcopenia was observed in CD patients. In UC patients, there was no significant difference between patients with and without sarcopenia in the cumulative operation-free survival rate.
Fig 2.
Cumulative operation-free survival rate for all patients (a), patients with Crohn’s disease (b), patients with ulcerative colitis (c).
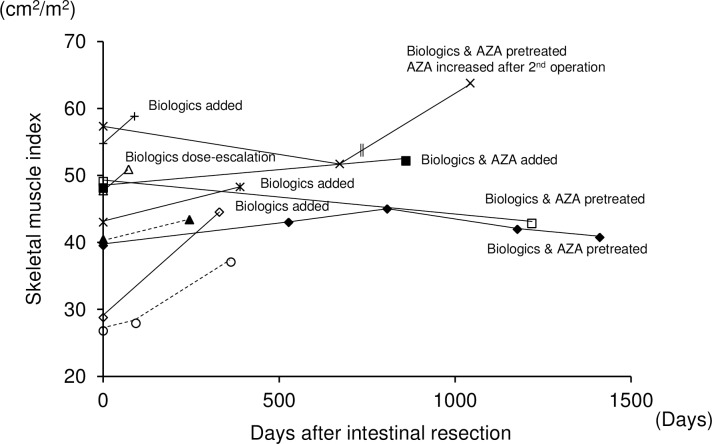
Post-operative course and the changes of SMI were summarized in Fig 3. Follow-up CT was obtained from eleven patients (CD: 9 cases; UC: 2 cases). The SMI was increased after intestinal resection. However, the recovery of SMI was not observed in patients with CD who had intestinal resection although treated with biologics and azathioprine.
Fig 3. Changes of SMI after intestinal resection.
Solid and dashed line indicates patients with CD and UC, respectively. Double vertical lines indicate intestinal resection. AZA: azathioprine.
Discussion
In this study, we enrolled IBD patients who were admitted to our hospital for disease exacerbation, and we demonstrated that a low SMI was a strong predictor of intestinal resection in CD patients. Previous reports have shown that altered body composition or decreased skeletal muscle volume was frequently observed in IBD patients [7–9]. Using dual-energy X-ray absorptiometry, Schneider et al. revealed that 60% of CD patients in clinical remission had sarcopenia [9]. Zhang et al. also reported that 61.4% of CD patients had sarcopenia assessed by cross-sectional images at the L3 level to assess skeletal muscle volume in CD patients with the cut-off value of SMI <55 cm2/m2 for men and <39 cm2/m2 for women [11]. Although this percentage is higher than that seen in our results, the frequency of aberrations in IBD patients varies by the population, assessment tools, and cut-off values. Appropriate cut-off values for IBD patients have not yet been determined. Therefore, the study of additional cases is necessary to determine the appropriate cut-off values for the SMI in IBD patients.
The nutritional status of UC patients was poorer than that of CD patients. UC can present as an acute severe extensive disease at the time of exacerbation [40] and can cause a rapid decrease in serum albumin levels. The nutritional indices O-PNI and CONUT showed a significant decrease in UC patients; notably, both indices include measurements of serum albumin level as a component of their respective calculations.
Previous reports demonstrated that skeletal muscle volume is strongly correlated with sex, age, body weight, and serum albumin levels [16–18, 20]. Our results showed no significant relationship between the SMI and serum albumin levels in UC patients. These results suggest that decreases in the skeletal muscle volume occur more slowly than decreases in serum albumin levels during an acute severe extensive disease. Conversely, there is a strong correlation between the SMI and serum albumin level in CD patients. In CD, serum albumin levels are considered to reflect chronic and persistent inflammation or intestinal deformities, such as stenosis and fistula.
Our results revealed a strong association between the SMI and intestinal resection in CD patients. In previous reports, a strong association between sarcopenia and prognosis has been reported in the field of malignant neoplasms and chronic diseases [16–22], most of which present irreversible and progressive clinical courses. Indications for intestinal resection differ between CD and UC. CD requires intestinal resection due to intestinal deformities, such as stenosis and fistula, and UC warrants resection for uncontrollable intestinal inflammation, such as bleeding or perforation. CD is often accompanied by irreversible complications, such as strictures or fistula, as shown by the Lémann score [41]. Therefore, an intestinal deformity may lead to PEM and result in a decreased SMI. In contrast, medical treatment sometimes brings about dramatic outcomes in UC patients. Therefore, the association between the SMI and intestinal resection is considered weak in UC.
Recovery of skeletal muscle volume was reported after induction of infliximab in CD patients [42] or after colectomy in UC patients [43]. We have demonstrated that post-operative changes of the SMI in 11 patients. Although the number of the patients in our cohort is limited, our results confirmed the same trend as the previous reports.
Our study has limitations by its single center and retrospective nature. First of all, the number of patients enrolled in this study is limited, hence the significance of each parameter should be confirmed in the prospective multicenter study. Secondly, there are some missing data in endoscopic assessment and indirect calorimetry. Endoscopic evaluation was not performed in 2 out of 43 CD patients. As for indirect calorimetry, the data was available in 42 out of 72 IBD patients. These parameters are not strongly related to the SMI (data not shown). Therefore, the missing data do not affect the conclusions. Thirdly, this study is observational study. It is necessary to consider whether intervention such as rehabilitation and nutritional therapy is related to prognosis and the SMI in the future study.
In conclusion, L3 skeletal muscle area can serve as a prognostic factor for intestinal resection in IBD patients, especially in those with CD. These results may reflect the fact that CD presents as a gastrointestinal disease arising from intestinal deformities.
Supporting information
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Krok KL, Lichtenstein GR. Nutrition in Crohn disease. Curr Opin Gastroenterol. 2003;19(2):148–153. [DOI] [PubMed] [Google Scholar]
- 2.Gassull MA, Abad A, Cabre E, Gonzalez-Huix F, Gine JJ, Dolz C. Enteral nutrition in inflammatory bowel disease. Gut. 1986;27 Suppl 1:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rigaud D, Angel LA, Cerf M, Carduner MJ, Melchior JC, Sautier C, et al. Mechanisms of decreased food intake during weight loss in adult Crohn's disease patients without obvious malabsorption. Am J Clin Nutr. 1994;60(5):775–781. [DOI] [PubMed] [Google Scholar]
- 4.Al-Jaouni R, Schneider SM, Piche T, Rampal P, Hebuterne X. Effect of steroids on energy expenditure and substrate oxidation in women with Crohn's disease. Am J Gastroenterol. 2002;97(11):2843–2849. doi: 10.1111/j.1572-0241.2002.07032.x [DOI] [PubMed] [Google Scholar]
- 5.Sasaki M, Johtatsu T, Kurihara M, Iwakawa H, Tanaka T, Bamba S, et al. Energy expenditure in Japanese patients with severe or moderate ulcerative colitis. J Clin Biochem Nutr. 2010;47(1):32–36. doi: 10.3164/jcbn.10-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasaki M, Johtatsu T, Kurihara M, Iwakawa H, Tanaka T, Tsujikawa T, et al. Energy metabolism in Japanese patients with Crohn's disease. J Clin Biochem Nutr. 2010;46(1):68–72. doi: 10.3164/jcbn.09-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryant RV, Trott MJ, Bartholomeusz FD, Andrews JM. Systematic review: body composition in adults with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38(3):213–225. doi: 10.1111/apt.12372 [DOI] [PubMed] [Google Scholar]
- 8.Valentini L, Schaper L, Buning C, Hengstermann S, Koernicke T, Tillinger W, et al. Malnutrition and impaired muscle strength in patients with Crohn's disease and ulcerative colitis in remission. Nutrition. 2008;24(7–8):694–702. doi: 10.1016/j.nut.2008.03.018 [DOI] [PubMed] [Google Scholar]
- 9.Schneider SM, Al-Jaouni R, Filippi J, Wiroth JB, Zeanandin G, Arab K, et al. Sarcopenia is prevalent in patients with Crohn's disease in clinical remission. Inflamm Bowel Dis. 2008;14(11):1562–1568. doi: 10.1002/ibd.20504 [DOI] [PubMed] [Google Scholar]
- 10.van Langenberg DR, Della Gatta P, Hill B, Zacharewicz E, Gibson PR, Russell AP. Delving into disability in Crohn's disease: dysregulation of molecular pathways may explain skeletal muscle loss in Crohn's disease. J Crohns Colitis. 2014;8(7):626–634. doi: 10.1016/j.crohns.2013.11.024 [DOI] [PubMed] [Google Scholar]
- 11.Zhang T, Cao L, Cao T, Yang J, Gong J, Zhu W, et al. Prevalence of Sarcopenia and Its Impact on Postoperative Outcome in Patients With Crohn's Disease Undergoing Bowel Resection. JPEN J Parenter Enteral Nutr. 2017;41(4):592–600. doi: 10.1177/0148607115612054 [DOI] [PubMed] [Google Scholar]
- 12.Holt DQ, Moore GT, Strauss BJ, Hamilton AL, De Cruz P, Kamm MA. Visceral adiposity predicts post-operative Crohn's disease recurrence. Aliment Pharmacol Ther. 2017;45(9):1255–1264. doi: 10.1111/apt.14018 [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 Suppl):990S–991S. [DOI] [PubMed] [Google Scholar]
- 14.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11(3):177–180. [PMC free article] [PubMed] [Google Scholar]
- 16.Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A, et al. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31(1):193–199. doi: 10.1016/j.nut.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 17.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–635. doi: 10.1016/S1470-2045(08)70153-0 [DOI] [PubMed] [Google Scholar]
- 18.Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10(2):166–173, 173 e161. doi: 10.1016/j.cgh.2011.08.028 [DOI] [PubMed] [Google Scholar]
- 19.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(2):95–101. doi: 10.1016/j.jamda.2013.11.025 [DOI] [PubMed] [Google Scholar]
- 20.Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, et al. Sarcopenia is an Independent Predictor of Severe Postoperative Complications and Long-Term Survival After Radical Gastrectomy for Gastric Cancer: Analysis from a Large-Scale Cohort. Medicine (Baltimore). 2016;95(13):e3164 doi: 10.1097/MD.0000000000003164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okumura S, Kaido T, Hamaguchi Y, Fujimoto Y, Kobayashi A, Iida T, et al. Impact of the preoperative quantity and quality of skeletal muscle on outcomes after resection of extrahepatic biliary malignancies. Surgery. 2016;159(3):821–833. doi: 10.1016/j.surg.2015.08.047 [DOI] [PubMed] [Google Scholar]
- 22.Hiraoka A, Aibiki T, Okudaira T, Toshimori A, Kawamura T, Nakahara H, et al. Muscle atrophy as pre-sarcopenia in Japanese patients with chronic liver disease: computed tomography is useful for evaluation. J Gastroenterol. 2015;50(12):1206–1213. doi: 10.1007/s00535-015-1068-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iritani S, Imai K, Takai K, Hanai T, Ideta T, Miyazaki T, et al. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J Gastroenterol. 2015;50(3):323–332. doi: 10.1007/s00535-014-0964-9 [DOI] [PubMed] [Google Scholar]
- 24.Hoshikawa Y, Iida T, Ii N, Muramatsu M, Nakajima Y, Chumank K, et al. Cross-sectional area of psoas major muscle and hip flexion strength in youth soccer players. Eur J Appl Physiol. 2012;112(10):3487–3494. doi: 10.1007/s00421-012-2335-7 [DOI] [PubMed] [Google Scholar]
- 25.Itoh S, Shirabe K, Yoshizumi T, Takeishi K, Harimoto N, Ikegami T, et al. Skeletal muscle mass assessed by computed tomography correlates to muscle strength and physical performance at a liver-related hospital experience. Hepatol Res. 2016;46(4):292–297. doi: 10.1111/hepr.12537 [DOI] [PubMed] [Google Scholar]
- 26.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985). 2004;97(6):2333–2338. doi: 10.1152/japplphysiol.00744.2004 [DOI] [PubMed] [Google Scholar]
- 27.Sasaki M, Okamoto H, Johtatsu T, Kurihara M, Iwakawa H, Tanaka T, et al. Resting energy expenditure in patients undergoing pylorus preserving pancreatoduodenectomies for bile duct cancer or pancreatic tumors. J Clin Biochem Nutr. 2011;48(3):183–186. doi: 10.3164/jcbn.10-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamoto H, Sasaki M, Johtatsu T, Kurihara M, Iwakawa H, Akabane M, et al. Resting energy expenditure and nutritional status in patients undergoing transthoracic esophagectomy for esophageal cancer. J Clin Biochem Nutr. 2011;49(3):169–173. doi: 10.3164/jcbn.11-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishida N, Sasaki M, Kurihara M, Ichimaru S, Wakita M, Bamba S, et al. Changes of energy metabolism, nutritional status and serum cytokine levels in patients with Crohn's disease after anti-tumor necrosis factor-alpha therapy. J Clin Biochem Nutr. 2013;53(2):122–127. doi: 10.3164/jcbn.13-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue M, Sasaki M, Takaoka A, Kurihara M, Iwakawa H, Bamba S, et al. Changes in energy metabolism after induction therapy in patients with severe or moderate ulcerative colitis. J Clin Biochem Nutr. 2015;56(3):215–219. doi: 10.3164/jcbn.14-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris JA, Benedict FG. A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci U S A. 1918;4(12):370–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11(1):8–13. doi: 10.1177/014860718701100108 [DOI] [PubMed] [Google Scholar]
- 33.Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, Gonzalez P, Gonzalez B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20(1):38–45. [PubMed] [Google Scholar]
- 34.Elia M. Screening for malnutrition: A multidisciplinary responsibility. Development and Use of the Malnutrition Universal Screening Tool ('MUST') for Adults. Redditch: BAPEN. 2003.
- 35.Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22(3):321–336. [DOI] [PubMed] [Google Scholar]
- 36.Onodera T, Goseki N, Nosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–1005. [PubMed] [Google Scholar]
- 37.Ban H, Bamba S, Nishida A, Inatomi O, Shioya M, Takahashi KI, et al. Prognostic factors affecting early colectomy in patients with moderate to severe ulcerative colitis treated with calcineurin inhibitors. Exp Ther Med. 2016;12(2):829–834. doi: 10.3892/etm.2016.3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985). 1998;85(1):115–122. [DOI] [PubMed] [Google Scholar]
- 39.Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. JSH guidelines for sarcopenia in liver disease (first edition): Recommendation from the working group for creation of sarcopenia assessment criteria in the JSH. Hepatol Res. 2016. doi: 10.1111/hepr.12774 [DOI] [PubMed] [Google Scholar]
- 40.Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53 Suppl 5:V1–16. doi: 10.1136/gut.2004.043372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pariente B, Cosnes J, Danese S, Sandborn WJ, Lewin M, Fletcher JG, et al. Development of the Crohn's disease digestive damage score, the Lemann score. Inflamm Bowel Dis. 2011;17(6):1415–1422. doi: 10.1002/ibd.21506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subramaniam K, Fallon K, Ruut T, Lane D, McKay R, Shadbolt B, et al. Infliximab reverses inflammatory muscle wasting (sarcopenia) in Crohn's disease. Aliment Pharmacol Ther. 2015;41(5):419–428. doi: 10.1111/apt.13058 [DOI] [PubMed] [Google Scholar]
- 43.Zhang T, Ding C, Xie T, Yang J, Dai X, Lv T, et al. Skeletal muscle depletion correlates with disease activity in ulcerative colitis and is reversed after colectomy. Clin Nutr. 2016. doi: 10.1016/j.clnu.2016.10.004 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.