Abstract
To reveal the effects of ploidy level and haplotype on photosynthetic traits, we chose 175 genotypes of wild strawberries belonging to two haplotypes at two types of ploidy levels (diploidy and tetraploidy) and measured photosynthetic traits. Our results revealed that ploidy significantly affected the characteristics of light-response curves, CO2-response curves, and leaf gas exchange parameters, except intercellular CO2 concentration (Ci). Tetraploid species had a lower light saturation point (LSP) and CO2 saturation point (CSP), higher light compensation point (LCP), dark respiration (Rd), and CO2 compensation point (CCP) than diploid species. Furthermore, tetraploid species have lower photosynthetic capacity than diploid species, including net photosynthetic rate (Pn), stomatal conductivity (Gs), and transpiration rate (Tr). In addition, haplotype had a significant effect on LSP, CSP, Tr, and Ci as well as a significant interactive effect between ploidy and haplotype on the maximal photosynethic rate of the light-response curve and Rd. Most of the variance existed within haplotypes among individuals. These results suggest that polyploidization was the main driver for the evolution of photosynthesis with increasing ploidy level (i.e. from diploidy to tetraploidy in Fragaria species), while the origin of a chromosome could also affect the photosynthetic traits and the polyploidization effect on photosynthetic traits.
Introduction
Polyploidy is a prevalent biological phenomenon of the chromosomal evolution of extant species and genera, including major crop plants such as rice, maize, wheat, soybean, and cotton [1, 2]. Most, if not all plant species have a polyploidy ancestry [3], and polyploidy may have been critically important for flowering plant diversification and the development of cytological, morphological, chemical, physiological, and molecular characteristics [4–7], and subsequently the interactions with other members of the biotic community [5, 8]. Exploring the ecological effects of polyploidy for key traits of plant species will help to elucidate the evolutionary significance of polyploidization and potential for its occurrence [5].
Most of the previously published ecological studies focused on the effects of different cytotypes on plants traits, i.e. the number of chromosomes [9, 10]. Among these studies, advantages and disadvantages of allopolyploids as well as autoployploids have often been compared [9–11]. Photosynthesis is a central metabolic process in plants [12, 13] and photosynthetic traits can be used as indicators to judge the adaptability and resistance of plants [14]. The photosynthetic rate is sensitive to the ploidy level, which correlates with the amount of DNA per cell [15, 16] and has been well documented in numerous species [17, 18]. Both positive and negative correlations with ploidy level have been reported [16, 19, 20], and the anatomy structure as well as biochemical pathways have been well studied [15]. Recently, following the realization that genotypes may affect the resulting gene expression after polyploidization, Oates et al. reported that the effect of induced polyploidy on fertility and morphology of allotetraploids and autotetraploids of Rudbeckia spp. were variable both among and within genotypes [21]. The genotype is part (DNA sequence) of the genetic makeup of a cell), and therefore of an organism or individual, thus determining a specific characteristic (phenotype) of that cell/organism/individual. Polyploidization can result in structural changes of the genome, increase the genetic diversity by producing new genotypes through recombination, and enhance the colonization ability in new environments [11]. A haplotype (haploid genotype) is a group of genes in an organism that have been inherited together from a single parent, thus determining the parent-of-origin effect [22]. Furthermore, some haplotypes were verified to be very stable during evolution from diploid to tetraploid and hexaploid, such as in wheat species; consequently, polyploidization had no detectable consequences on the structure and evolution of both haplotypes [23]. We hypothesized that if the haplotype of a plant with different ploidy level were identical (i.e. sharing the same ancestry and no genome exchanges after polyploidization), then the effect of polyploidization should be the main driver of specialization of plants. However, to our knowledge, no other study focused on how haplotypes affect the effect of polyploidization on phenotypic traits and their interactive effect.
Fragaria species are stoloniferous perennial herbs that share the same basic architecture and life history with ploidy levels varying from diploidy to decaploidy [24]. As two representative species of strawberry plants, the diploid Fragaria pentaphylla and the tetraploid Fragaria moupinensis sharing similar haplotypes have a wide and overlapping area of distribution and similar habitats [25]. They provide a good model to test the effect of ploidy and haplotype on the variation of phenotypic traits. For this study, we chose 175 genotypes of wild strawberries that belong to two haplotypes at two levels of ploidy (diploidy and tetraploidy) and we obtained photosynthetic parameters including light-response and CO2-response curves as well as gas exchange parameters. We thus revealed the effects of the ploidy and haplotype on the photosynthetic traits and also their interactive effects.
Materials and methods
Plant species
Fragaria pentaphylla is a wild and diploid (2n = 14) species from the Fragaria genus, belonging to the Rosaceae family [26]. It is a perennial herb with a monopodial form of growth. Two small accessory leaflets are often present and plants feature a total of five leaflets. The leaflets are typically not overlapping when pressed and the sepals are spreading. The plant is a hermaphrodite and the fruits can be either red or white. F. pentaphylla is native to the Chinese provinces of Sichuan Qinghai, Gansu Shanxi, and Henan. This species is most often found in forests, forest clearings, scrub, mountain meadows, and open gravel at elevations between 1000 and 2700 m [26].
Fragaria moupinensis is a wild and tetraploid (2n = 28) species from the Fragaria genus, belonging to the Rosaceae family [26]. It is also a perennial herb with a monopodial form of growth. Two small accessory leaflets are either present or absent and the Leaflets are typically not overlapping when pressed and sepals always clasp the mature fruit. It is a dioecious plant with red fruits. F. moupinensis is native to the Chinese provinces of Sichuan, Gansu, Shanxi, Yunnan, and Xizang and is most often found in forest undergrowth, mountain meadows, and grasslands at elevations between 1400 and 4000 m [26].
Sample collection
During July 2013, we collected seeds and leaves of F. pentaphylla and F. moupinensis populations in Gansu, Qinhai, Yunnan, Sichuan and Shanxi Province and Xizang (Tibet) Autonomous Region. All the information for the sampling sites was listed on S1 Table. The field sites did not involve any endangered or protected species, and none of the populations were privately owned or under nature protection. No specific permissions were required for these locations. One to eight (with the distance at least 10 m apart) fruiting plants were randomly selected in every population. In totally, 175 plants from 65 populations were collected (S1 Table). Leaves were quickly dried via silica gel before returning them to the laboratory. Each fruit was squeezed on a filter paper to remove the flesh. Seeds were air-dried before returning to the laboratory and subsequently stored at -20°C.
Identification of haplotype
During November 2013, we extracted total genomic DNA from dried leaves via a plant total genomic DNA extraction kit (Dingguo Inc, Shang, China). Having examined the published Fragaria chloroplast genomes, we found that the NADH dehydrogenase subunit A (ndhA) intron was the most variable non-coding plastid sequence, which was verified to effectively discriminate the haplotype of plants [27]. Primers were designed according to published the ndhA intron sequence of Fragaria chloroplast genomes and PCR was conducted according to regular protocols. BioSune Biotechnology Co. Ltd., Shanghai, China, conducted PCR product sequencing. The haplotypes of the individuals were determined via alignment of the ndhA sequence by Aaron Liston from the Oregon State University. We selected haplotype A and haplotype B for the relative population number. The ndhA sequence of haplotype A and haplotype B is presented in the supplemental S1 Fig.
Identification of ploidy level
We utilized dried leaf tissue (approximately 20 mg) to determine the ploidy level via flow cytometry, using a modified method of Suda and Trávníček [28]: We suspended leaf tissue in 1 mL ice-cold extraction buffer and co-chopped the leaf with a plastic petri dish over a chilled brick with a fresh razor blade for 2 min until arriving at a fine slurry. Large leaf debris was removed via a 600-mesh nylon filter (Shanghai Aoran Hardware market, Shanghai, China). We added 1 ml of pre-cooled extraction buffer to the dish to rush the chopped tissue and we repeated the whole procedure with remaining tissue. We gathered both parts of the supernatant and placed them into a 5 ml centrifuge tube, adding RNaseA solution and incubating at 4°C for 10 min. We then added propidium iodide staining solution to the tube and incubated at 4°C for further 30 min in dark. We utilized an Attune flow cytometer (ThermoFisher Scientific Inc. Waltham, MA, USA) to analyze the stained solutions until 20,000 events were captured. We used the cultivated octoploidy strawberry line ‘Hongyan’ (obtained from Shaojiadu Cooperation of Linhai City, Zhejiang Province, China) as an external standard and inferred the ploidy level from the relative PI value compared to the external standard.
Seeds germination and transplanting
In total, we chose 97 diploidy genotypes (F. penthaphylla) and 20 tetraploidy genotypes (F. moupinensis) that belong to haplotype A, 28 diploidy genotypes (F. penthaphylla) and 30 tetraploidy genotypes (F. moupinensis) that belong to hayplotype B for this experiment. During November 2014, four seeds from every Fragaria individual were germinated in a walk-in growth chamber with conditions of 22°C during 16 hours of daytime and 15°C during 8 hours of nighttime, with a relative humidity of 90%. 30 days after germination, one seedling (genotype) per haplotype was transplanted and moved to a greenhouse with identical conditions. We randomly selected 20% seedlings and individuals with ambiguous ploidy level to double check the ploidy level by measuring the karyotype via the root-tip squashing method, modifying the method by Nathewet et al. [29]. We harvested approximately 2 cm root tips and pretreated these with 2 mM 8-hydroxyquinoline at room temperature (22°C) for 1 h, followed by keeping them at 4°C for more than 15 h, fixing them in Farmer’s solution for 2 h, soaking them in 1N HCl at 22°C for 1 h, macerating them in 1 N HCl at 60°C for 11 min, and staining them with Carbol fuchsin staining solution for 15 min. Then, we detected the number of chromosomes number using a BA310 digital microscope (Motic Instruments Inc., Xiamen, Fujian, China) at 100 × magnification.
Measurement of photosynthetic traits
During August 2015, we conducted in situ photosynthetic trait measurements on a sunny day on mature middle leaflet at the same position, using the Li-6400XT portable photosynthesis system (LI-COR Biosciences Inc., Lincoln, NE, USA). We used a red-blue LED light source attached to the system to produce steady photosynthetic active radiation (PAR).
To construct the light response curves, we obtained all photosynthesis measurements between 09:30 and 11:00 h (Beijing time) on mature middle leaflet from each plant with a leaf temperature of 25°C, a CO2 concentration of 400 ppm and a relative humidity of 70%. A photosynthetically active radiation lamp provided the light source. Prior to the measurements, we allowed the mature middle leaflet to acclimate under a PAR of 2,000 μmol m-2 s-1 for 30 min to avoid photo-inhibition. As soon as the value stabilized, we exposed the leaves to a series of PAR values for 3 min or so in the following order: 2,000, 1,500, 1,200, 1,000, 800, 600, 400, 200, 100, 50, 20, and 0 μmol m-2 s-1. The temporal interval between each concentration was 3 min. We fitted the entire photosynthetic light response curves in Origin 8.0 as a binary linear equation [30] calculating the maximum of the net photosynthetic rate (Pn) as Pmax. We utilized the following definitions: the light intensity that leads to maximum Pn was defined as the light saturation point (LSP), the light intensity that leads to zero Pn was defined as the light compensation point (LCP), and the Pn that leads to maximum a PAR of zero was defined as the dark respiration point (Rd). We measured three seedlings per haplotype.
To construct the CO2 response curves, we obtained all photosynthesis measurements between 09:30 and 11:00 h (Beijing time) on mature middle leaflet from each plant with a leaf temperature of 25°C, a PAR of 1,000 μmol m-2 s-1, and a relative humidity of 70%. A small portable cylinder that was filled to a specified CO2 pressure supplied the CO2. Prior to the measurements, we allowed the mature middle leaflet to acclimate under a PAR of 1,500 μmol m-2 s-1 for 30 min to avoid photo-inhibition. As soon as the value stabilized, we exposed the mature middle leaflet to a series of CO2 concentrations for 5 min or so in the following order: 1,500, 1,200, 1,000, 800, 600, 400, 200, 150, 120, 100, 80, and 50 μmol mol- 1. The temporal interval between each concentration was 5 min. We fitted the entire photosynthetic CO2-response curves in Origin 8.0 as a binary linear equation and calculated the maximum net photosynthetic rate (Pn) as Pmax [30]. The CO2 concentration that leads to maximum Pn was defined as the CO2 saturation point (CSP). The CO2 concentration that leads to zero Pn was defined as the CO2 compensation point (CCP). We measured three seedlings per haplotype.
After obtaining LSP of the strawberry (1000 μmol photons·m–2·s–1 or so), we measured the leaf gas exchange parameters of all 175 genotypes between 08:30 am and 11:30 am. We recorded Pn, stomatal conductance (Gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci) at two-hour intervals, choosing three matured leaves per plant (genotype), and performing six consecutive measurements [31].
Statistical analyses
The data are shown as mean ± standard deviation. We utilized two-way ANOVA to test the effect of ploidy and haplotype on the photosynthetic characteristics of plants, using ploidy and haplotype as the fixed factor. Levene's test was used to evaluate the homogeneity of variance. Reciprocal transform of Pn, Gs, and Tr were used for ANOVAs analysis, for the variance is not homogeneous. Linear contrasts after ANOVAs were used to analyze the significance of the differences of photosynthetic characteristics between diploidy and tetraploidy in the same haplotype. The existence of variance among ploidies and haplotypes were estimated via VARCOMP analysis [32, 33]. We conducted all these analyses via SPSS 19.0 software and used the software Origin 8.0 for mapping.
Results
Characteristics of light-response curves
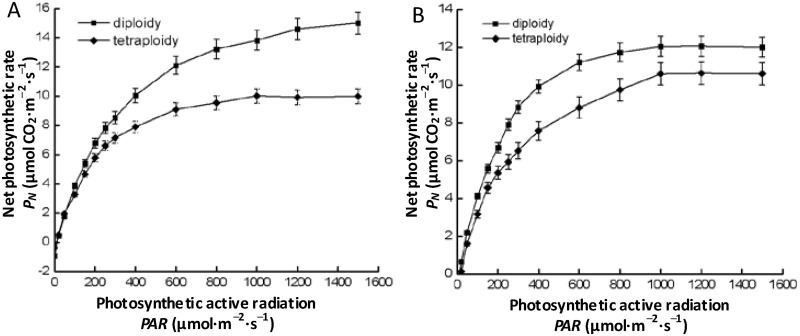
The patterns of light-response curves differed among different ploidy levels as well as among different haplotypes (Fig 1A and 1B, S2 Table). For identical haplotypes, tetraploid species had lower LSP, Pmax, and AQY, but higher LCP and Rd compared to diploid species (Table 1). Two-way ANOVA results revealed that ploidy posed extremely significant effects on LSP, Pmax, LCP, AQY, and Rd of Fragaria species, while haplotype posed extremely significant effects on LSP, and the interactive effect between ploidy and haplotype had extremely significant effect on Pmax, and a significant effect on Rd (Table 2).
Fig 1. Light response curve of Fragaria plants with different ploidy levels of haplotype A (A) and haplotype B (B).
Table 1. Characteristics of light-response curves of Fragaria plants with different ploidy levels and haplotypes.
The data are presented as mean ± standard deviation. Different lower case letters indicate significant differences between diploid and tetraploid level in the same haplotype at a p < 0.05 level.
| Haplotype | Ploidy | Light saturation point (μmol·m–2·s–1) | Pmax (μmol CO2·m–2·s–1) | Light compensation point (μmol·m–2·s–1) | Apparent quantum efficiency | Dark respiration rate (μmol CO2·m–2·s–1) |
|---|---|---|---|---|---|---|
| A | 2 | 1122±26a | 14.81±0.53a | 9.47±1.03b | 0.0377±0.0027a | 0.88±0.07b |
| A | 4 | 1048±22b | 9.70±0.25b | 13.92±1.67a | 0.0303±0.0015b | 1.04±0.05a |
| B | 2 | 1070±32a | 12.52±0.70a | 8.56±0.96b | 0.0365±0.0025a | 0.69±0.05b |
| B | 4 | 994±24b | 10.85±0.36b | 12.32±1.25a | 0.0316±0.0011b | 1.07±0.11a |
Table 2. Effects of ploidy, haplotype, population (nested to haplotype), and interactive ploidy × haplotype on the characteristics of light-response curves of Fragaria.
Values in bold indicate significant effect at a p < 0.05 level.
| Factor | Light saturation point | Pmax | Light compensation point | Apparent quantum yield | Dark respiration rate | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | |
| Ploidy | 26.269 | 0.001 | 141.948 | <0.001 | 35.851 | <0.001 | 11.057 | 0.009 | 36.860 | <0.001 |
| Haplotype | 12.917 | 0.006 | 4.071 | 0.074 | 3.339 | 0.101 | 2.567 | 0.144 | 3.282 | 0.103 |
| Ploidy × Haplotype | 0.002 | 0.967 | 36.625 | <0.001 | 0.251 | 0.628 | 0.513 | 0.492 | 6.850 | 0.028 |
Characteristics of CO2-response curves
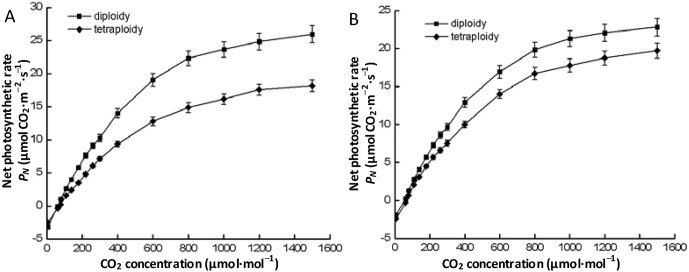
The patterns of CO2-response curves differed among different ploidy levels (species) as well as among different haplotypes (Fig 2A and 2B, S3 Table). For identical haplotypes, tetraploid species had lower CSP, Pmax, and ACE, but higher CCP compared to diploid species (Table 3). Two-way ANOVAs results revealed that ploidy posed extremely significant effects on CSP, Pmax, and ACE of Fragaria species, and haplotype posed significant effects on CSP, while the interactive effect between ploidy and haplotype had no significant effect on these traits (Table 4).
Fig 2. CO2-response curve of Fragaria plants with different ploidy level of haplotype A (A) and haplotype B (B).
Table 3. Characteristics of CO2-response curves of Fragaria with different ploidy levels in haplotype A and haplotype B.
Different lower case letters indicate significant differences between diploid and tetraploid level in the same haplotype at a p < 0.05 level.
| Haplotype | Ploidy | CO2 saturation point (μmol·mol–1) | CO2 compensation point (μmol·mol–1) | Pmax (μmol CO2·m–2·s–1) | Apparent carboxylation efficiency |
|---|---|---|---|---|---|
| A | 2 | 2253±114a | 64.63±5.95b | 27.04±1.95a | 0.0522±0.0039a |
| A | 4 | 1799±78b | 78.12±6.92a | 23.23±1.62b | 0.0371±0.0031b |
| B | 2 | 2015±99a | 69.30±6.05b | 25.96±0.70a | 0.0503±0.0023a |
| B | 4 | 1738±84b | 76.75±8.67a | 22.52±0.74b | 0.0361±0.0034b |
Table 4. Effects of ploidy, haplotype, population (nested to haplotype), and interactive ploidy × haplotype on the characteristics of CO2-response curves of Fragaria.
Values in bold indicate significant effect at a p < 0.05 level.
| Factor | CO2 saturation point | CO2 compensation point | Pmax | Apparent carboxylation efficiency | ||||
|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | |
| Ploidy | 47.964 | <0.001 | 3.812 | 0.083 | 21.772 | 0.001 | 11.287 | 0.008 |
| Haplotype | 7.981 | 0.020 | 0.095 | 0.765 | 0.807 | 0.392 | 0.073 | 0.794 |
| Ploidy × Haplotype | 2.806 | 0.128 | 0.318 | 0.587 | 0.001 | 0.979 | 0.000 | 0.986 |
Characteristics of leaf gas exchange
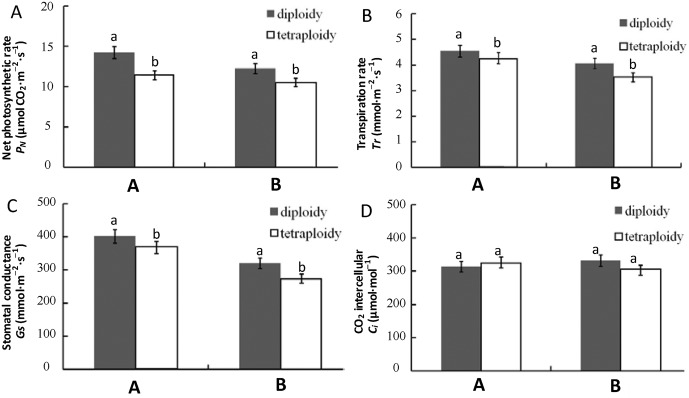
For identical haplotypes, mean Pn, Tr, and Gs of tetraploid species were significantly higher than for diploid species (Fig 3A, 3B and 3C, S4 Table). There was no significant difference in the intercellular CO2 concentration between tetraploid and diploid species (Fig 3D, S4 Table). Two-way ANOVA results revealed that ploidy posed significant effects on Pn, Tr, and Gs of Fragaria species, while haplotype posed a significant effect on Tr, Gs, and Ci, and no significant interactive effect was detected (Table 5). Most of the variance existed among individuals within haplotypes, while little variance existed among ploidy levels and haplotypes (Table 6).
Fig 3. Net photosynthetic rate (A), transpiration rate (B), stomatal conductance (C), and intercellular CO2 concentration (D) of Fragaria plants with different ploidy levels and haplotypes.
Table 5. Two-way ANOVA analysis of ploidy, haplotype, and interactive ploidy and haplotype on the photosynthetic characteristics of Fragaria.
Values in bold indicate significant effect at a p < 0.05 level.
| Factor | Net photosynthetic rate | Transpiration rate | Stomatal conductance | Intercellular CO2 concentration | ||||
|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | |
| Ploidy | 7.627 | 0.006 | 14.262 | <0.001 | 19.917 | <0.001 | 0.241 | 0.624 |
| Haplotype | 0.959 | 0.329 | 10.287 | 0.002 | 4.345 | 0.039 | 5.734 | 0.018 |
| Ploidy × Haplotype | 0.102 | 0.750 | 0.354 | 0.553 | 0.058 | 0.811 | 0.046 | 0.830 |
Table 6. Components of the variance of photosynthetic characteristics of Fragaria plants among ploidy levels (species), among haplotypes and within haplotypes.
| Net photosynthetic rate | Transpiration rate | Stomatal conductance | Intercellular CO2 concentration | |
|---|---|---|---|---|
| Among ploidy levels | 6.0% | 9.0% | 8.0% | 0.0% |
| Among haplotypes | 1.0% | 6.0% | 3.0% | 3.0% |
| Within haplotypes | 93.0% | 85.0% | 89.0% | 97.0% |
Discussion
Polyploidy has major evolutionary significance, especially in plants [34]. Our results revealed that ploidy had significant effects on the characteristics of light-response curves, CO2-response curves, and leaf gas exchange parameters, with the exception of the intercellular CO2 concentration. Similar results have also been reported for the Triticum genus [16]. These results suggest polyploidization might play an important role in the evolution of photosynthesis of Fragaria species with increasing ploidy level, i.e. from diploid to tetraploid Fragaria species. Our results positively supported the previous studies showing that polyploidy has distinct effects on photosynthesis [15, 35].
Polyploidy caused unique photosynthetic characteristics that evidently differ from those of diploid varieties due to complex changes in anatomical traits and biochemical responses [13, 36, 37]. Both LSP and LCP of plants reflect the requirement of the light condition of the plant and are taken as the indicators to acquiring light energy [38]. Our results revealed that diploid species had higher LSP, but lower LCP compared to tetraploid species, indicating that diploid F. pentaphylla could utilize a higher level of PAR and thus adapt to higher light illumination intensities than tetraploid F. moupinensis [39]. This lower LSP and higher LCP might reveal an adaptive evolutionary trait of plants under higher irradiance [40]. Based on Maxent modeling, we found that the niche breadth of diploid Fragaria plants was considerably larger than that of tetraploid Fragaria plants and that the tetraploid plants were likely to grow in regions of higher altitude (unpublished data). Furthermore, F. moupinensis is most often found in the undergrowth of forests, mountain meadows, and grasslands at higher elevations [26]. Thus, the lower LSP and higher LCP might be an important trait of tetraploid F. moupinensis to adapt to shady light environments of high altitude. These results also indicated that polyploidization shrinks the adaptive region of Fragaria to light intensities. The higher LCP and Rd of tetraploid species compared to dipoloid species also indicated that tetraploid Fragaria species consumed more photosynthetic accumulations to maintain a normal physiological metabolism, adapted to shady light environments at high altitude.
CO2 is the most important substrate within photosynthesis [41]. The polyploidization effect on CSP has been found for tetraploid poplars, which were evidently lower than those of diploid poplar clones [42]. Similarly, in our study, we found tetraploid species to have lower CSP, Pmax, and ACE than diploid species, but higher CCP, indicating tetraploid Fragaria have a smaller adaptive region to CO2 concentration and a weak ability to utilize low CO2 concentrations [39]. The CCP ranges from 30 to 70 μmol·mol–1 in common plants [42]. In this study, the CCP of both tetraploid and diploid species varied from 53 to 86 μmol·mol–1, indicating that the ability of both Fragaria species to use CO2 were relatively low. The lower LSP and higher CCP values might be an important trait of F. moupinensis to adapt to lowered CO2 concentrations at high altitude.
Our results revealed significantly higher mean Pn, Tr, and Gs of diploid species compared to tetraploid species, indicating polypoidy had a negative effect on the photosynthetic capacity of Fragaria species. Several studies on the photosynthetic capacity of various ploidy levels illustrated that the Pn per unit cell basis decreased along with an increase in ploidy level [15, 16, 19, 43, 44]; e.g., in citrus trees, the net CO2 assimilation rates based on leaves in autotetraploids were 24–35% lower than for their diploid counterparts [19]. Srivalli and Khanna-Chopra reported that polyploidy enlarged the mesophylle cells of leaves, thus reducing the surface area to volume ratio of the cells, providing increased resistance for CO2 exchange [43]. Li et al. reported that polyploidy enlarged the stomata and decreased stomata density, resulting in decreasing Gs, followed by Tr, which consequently slowed the photosynthetic system, leading to slower Ci absorption and consequently less production [44]. Srivalli and Khanna-Chopra also reported that polyploidy reduced the ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBPCO) content, thus reducing the rate of N mobilization, leading to lower Pn [43]. On the contrary, Austin et al. found that polyploidy can increased the ratio of chlorophyll a/b, i.e. led to a higher concentration of photosystems per chlorophyll, thus resulting in a higher photosynthetic rate per cell [45]. The conflicts indicated that more studies on more species are required to draw a general rule of the effect of polyploidization. In addition, it is important to understand the effect of polyploidy on morphological and physiological traits of plants, which can explain the mechanisms and ecological consequences that polyploidy offers [35, 46]. Further comparative studies should be conducted to concern these traits between diploidy and tetraploidy Fragaria species with same haplotypes in order to explore the adaptive mechanisms underlying the polyploidization.
Recently, the origin of whole genome was verified to play important roles in determining the effect of polyploidization [47]. Bilgrami et al. found that the whole chromosomes (genome) origin had a significant effect on Pn, Tr, Gs, and Ci of wheat during developmental phases [16]. Coate et al. found that Glycine dolichocarpa accessions with different origins indicated with different plastid types responded differently to plolyploidization [35]. In this study, chroloplast haplotypes were used to indicate the origins of the maternal ancestry [48], and we found significant effects of haplotype on LSP, CSP, Tr, and Ci, as well as a significant interactive effect of ploidy and haplotype on Pmax and Rd of the light-response curve. These results indicated that the origin of the chromosome could also affect the photosynthetic characteristics of Fragaria species and even the polyploidization effect of photosynthetic traits. Our results further confirmed previous report that synthetic strawberry (Fragaria) allo-octoploids show varying photosynthetic responses depending on parental species combinations [49]. Furthermore, we found that most of the variances existed within haplotypes among individuals (genotypes), which distributed in different geographical sites, indicating a large local evolutionary adaptive potential of the photosynthetic traits in adaptation to heterogeous environments of Fragaria species [50]. Geographical and climatic factors have been recognized to be the most important selective force driving for the variation of phenotypic traits [51]. Several studies verified strong similarities in bioclimatic niches and functional traits of Leucadendron L. [52] and Protea L. [53] based on niche modeling. The present distribution patterns of both Fragaria species might be a result of long-term expansion after polyploidization [54].
In summary, the lower LSP and CSP, but higher LCP, Rd, and CCP of tetraploid Fragaria species compared to diploid species evolved as long-term adaption to the shady light environment of high altitude after the polyploidization. Such polyploidization might be the main driver for the evolution of photosynthetic characteristics of Fragaria species. Except for chromosome number, the origin of the whole chromosomes could also affect the photosynthetic characteristics of Fragaria species. Future studies should be aimed at discriminating the effect of the origin of the whole chromosomes, genotypes, the geographical factors, and climatic factors on phenotypic and functional traits of Fragaria.
Supporting information
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Aaron Liston for the kind help in haplotype identification. We thank Jingsong Chen, Minghua Song, Tong Chen, Xiaoyan Wang, Beifen Yang, Jinzhi Li, Zhaokui Du for samples collection.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was financially supported by the National Natural Science Foundation of China (no. 31261120580) for MD and JL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Otto SP, Whitton J. Polyploid incidence and evolution. Ann Rev Gen. 2000; 34: 401–437. [DOI] [PubMed] [Google Scholar]
- 2.Mayrose I, Zhan SH, Rothfels CJ, Magnusonford K, Barker MS, Rieseberg LH, et al. Recently formed polyploid plants diversify at lower rates. Science. 2011; 333: 1257–1257. doi: 10.1126/science.1207205 [DOI] [PubMed] [Google Scholar]
- 3.Wood TE, Takebayashi N, Barker MS, Mayrose I, Greenspoon PB, Rieseberg LH. The frequency of polyploid speciation in vascular plants. Proc Nat Acad Sci. 2009; 106: 13875–13879. doi: 10.1073/pnas.0811575106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin DA. The timetable for allopolyploidy in flowering plants. Ann Bot. 2013; 112: 1201–1208. doi: 10.1093/aob/mct194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hull-Sanders HM, Johnson RH, Owen HA, Meyer GA. Effects of polyploidy on secondary chemistry, physiology, and performance on native and invasive genotypes of Solidago gigantea (Asteraceae). Am J Bot. 2009; 96: 762–770. doi: 10.3732/ajb.0800200 [DOI] [PubMed] [Google Scholar]
- 6.Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, et al. Ancestral polyploidy in seed plants and angiosperms. Nature. 2011; 473: 97–100. doi: 10.1038/nature09916 [DOI] [PubMed] [Google Scholar]
- 7.Miller M, Zhang C, Chen ZJ. Ploidy and hybridity effects on growth vigor and gene expression in arabidopsis thaliana hybrids and their parents. G3-Genes Genom Gen. 2012; 2: 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segraves KA, Thompson JN, Soltis PS, Soltis DE. Multiple origins of polyploidy and the geographic structure of heuchera grossulariifolia. Mol Ecol. 1999; 8: 253–262. [Google Scholar]
- 9.Romero-Aranda R, Bondada BR, Syvertsen JP, Grosser JW. Leaf characteristics and net gas exchange of diploid and autotetraploid citrus. Ann Bot. 1997; 79: 153–160. [Google Scholar]
- 10.Soltis DE, Soltis PS. What we still don’t know about polyploidy. Taxon. 2010: 59: 1387–1403. [Google Scholar]
- 11.Parisod C, Holderegger R, Brochmann C, Ainouche M L, Jenczewski E. Evolutionary consequences of autopolyploidy. New Phytol. 2010; 186: 5–17. doi: 10.1111/j.1469-8137.2009.03142.x [DOI] [PubMed] [Google Scholar]
- 12.Caemmerer SV, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981; 153: 376–387. doi: 10.1007/BF00384257 [DOI] [PubMed] [Google Scholar]
- 13.Evans JR. Improving photosynthesis. Plant Physiol. 2013; 162: 1780–1793. doi: 10.1104/pp.113.219006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fullana-Pericàs M, Conesa MÀ, Soler S, Ribas-Carbó M, Granell A, Galmés J. Variations of leaf morphology, photosynthetic traits and water-use efficiency in Western-mediterranean tomato landraces. Photosynthetica. 2017; 55(1): 121–133. [Google Scholar]
- 15.Warner DA, Edwards GE. Effects of polyploidy on photosynthesis. Photosynth Res. 1993; 35: 135–147. doi: 10.1007/BF00014744 [DOI] [PubMed] [Google Scholar]
- 16.Bilgrami SS, Houshmand SA, Khodambashi M, Zandi P, Siavoshi M, Khademi S, et al. Photosynthetic performance in ploidy levels and amphyploids of wheat during developmental stages. J Anim Plant Sci. 2015; 25: 1633–1643. [Google Scholar]
- 17.Warner DA, Edwards GE. C4 photosynthesis and leaf anatomy in diploid and autotetraploid Pennisetum americanum (pearl millet). Plant Science. 1988; 56: 85–92. [Google Scholar]
- 18.Vyas P, Bisht MS, Miyazawa SI, Yano S, Noguchi K, Terashima I, et al. Effect of polyploidy on photosynthetic properties and anatomy in leaves of phlox drummondii. Funct Plant Biol. 2007; 34: 673–682. [DOI] [PubMed] [Google Scholar]
- 19.Romero-Aranda R, Bondada BR, Syvertsen JP, Grosser JW. Leaf characteristics and net gas exchange of diploid and autotetraploid citrus. Ann Bot. 1997; 79: 153–160. [Google Scholar]
- 20.Kaminski A, Austin RB, Ford MA, Morgan CL. Flag leaf anatomy of Triticum and Aegilops species in relation to photosynthetic rate. Ann Bot. 1990; 66: 359–365. [Google Scholar]
- 21.Oates KM, Ranney TG, Touchell DH. Influence of induced polyploidy on fertility and morphology of rudbeckia species and hybrids. Hortsci. 2012; 47: 1217–1221. [Google Scholar]
- 22.Howey R, Mamasoula C, Töpf A, Nudel R, Goodship JA, Keavney BD, et al. Increased power for detection of parent-of-origin effects via the use of haplotype estimation. Am J Hum Gen. 2015; 97: 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isidore E, Scherrer B, Chalhoub B, Feuillet C, Keller B. Ancient haplotype resulting from extensive molecular rearrangements in the wheat A genome have been maintained in species of three different ploidy levels. Genom Res. 2005; 15: 526–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alpert P, Slominski C. Differences in performance between genotypes of Fragaria chiloensis, with different degrees of resource sharing. J Ecol. 2003; 91(1): 27–35. [Google Scholar]
- 25.Lei J, Dai H, Tan C, Deng M, Zhao M, Yaming AQ. Studies on the taxonomy of the strawberry (Fragaria) species distributed in China. Acta Horticulturae Sinica. 2006; 33: 1–5. [Google Scholar]
- 26.Yu DJ. Rosaceae In Flora of China. Vol 37 Beijing: Science Press; 1974. [Google Scholar]
- 27.Hinchliff CE, Smith SA. Some limitations of public sequence data for phylogenetic inference (in Plants). PLoS ONE. 2014; 9(7): e98986 doi: 10.1371/journal.pone.0098986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suda J, Trávníček P. Reliable DNA ploidy determination in dehydrated tissues of vascular plants by DAPI flow cytometry——New prospects for plant research. Cytometry Part A J Int Soc Anal Cytol. 2006; 69(4): 273–280. [DOI] [PubMed] [Google Scholar]
- 29.Nathewet P, Yanagi T, Iwastubo Y, Sone K, Takamura T, Okuda N. Improvement of staining method for observation of mitotic chromosomes in octoploid strawberry plants. Sci Hortic. 2009; 120: 431–435. [Google Scholar]
- 30.Ye ZP, Robakowski P, Suggett DJ. A mechanistic model for the light response of photosynthetic electron transport rate based on light harvesting properties of photosynthetic pigment molecules. Planta. 2013; 237: 837–847. doi: 10.1007/s00425-012-1790-z [DOI] [PubMed] [Google Scholar]
- 31.Hamid A, Agata W, Kawamitsu Y. Photosynthesis, transpiration and water use efficiency in four cultivars of mungbean, Vigna radiata (L.) Wilczek. Photosynthetica. 1990; 24: 96–101. [Google Scholar]
- 32.Kozlov MV. How reproducible are the measurements of leaf fluctuating asymmetry? Peerj. 2015; 3: e1027 doi: 10.7717/peerj.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burdick RK, Borror CM, Montgomery DC. A review of methods for measurement systems capability analysis. J Qual Technol. 2003; 35: 342–354. [Google Scholar]
- 34.Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, et al. Ancestral polyploidy in seed plants and angiosperms. Nature. 2010; 473(7345): 97–100. [DOI] [PubMed] [Google Scholar]
- 35.Coate JE, Luciano AK, Seralathan V, Minchew KJ, Owens TG, Doyle JJ. Anatomical, biochemical, and photosynthetic responses to recent allopolyploidy in Glycine dolichocarpa (Fabaceae). Am J Bot. 2012; 99: 55–67. doi: 10.3732/ajb.1100465 [DOI] [PubMed] [Google Scholar]
- 36.Parisod C. Polyploids integrate genomic changes and ecological shifts. New Phytol. 2012; 193: 297–300. doi: 10.1111/j.1469-8137.2011.04008.x [DOI] [PubMed] [Google Scholar]
- 37.Offermann S, Peterhansel C. Can we learn from heterosis and epigenetics to improve photosynthesis? Curr Opin Plant Biol. 2014; 19: 105–110. doi: 10.1016/j.pbi.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 38.Luo WC, Zeng FJ, Liu B, Zhang LG, Liu Z, Song C, et al. Photosynthetic and physiological characteristics of introduced plants at the desert-oasis ecotone. Acta Prataculturae Sinica. 2013; 22: 273–280. [Google Scholar]
- 39.Ning H, Luo Q, Ji X, Zhu Y, Sun H, Zhu L. Physiological responses of the photosynthetic carbon assimilation to environmental factors in three sandy plants. Scientia Silvae Sinicae. 2014; 50: 173–179. [Google Scholar]
- 40.Li QS, Deng M, Xiong YS, Coombes A, Zhao W. Morphological and photosynthetic response to high and low irradiance of Aeschynanthus longicaulis. Sci World J. 2014; 2014: 347461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao X, Li Y, Zheng M, Bian X, Liu M, Sun Y, et al. Comparative analysis of growth and photosynthetic characteristics of (Populus simonii × P. nigra) × (P. nigra × P. simonii) hybrid clones of different ploidides. PLoS ONE. 2015; 10(4): e0119259 doi: 10.1371/journal.pone.0119259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan RZ. Plant Physiology. Beijing: High Education Press; 2004; 80–81. [Google Scholar]
- 43.Srivalli B, Khanna-Chopra R. Ribulose-1,5-bisphosphate carboxylase/oxygenase content and degradation in diploid, tetraploid, and hexaploid wheat species during monocarpic senescence. Photosynthetica. 2004; 42: 393–398. [Google Scholar]
- 44.Li M, Wang C, Song J, Chi Y, Wang X, Wu Y. Evolutional trends of leaf stomatal and photosynthetic characteristics in wheat evolutions. Acta Ecologica Sinica. 2008; 28: 5385–5391. [Google Scholar]
- 45.Austin RB, Morgan CL, Ford MA, Bhagwat SG. Flag leaf photosynthesis of Triticum aestivum and related diploid and tetraploid species. Ann Bot. 1982; 49(2): 177–189. [Google Scholar]
- 46.Warner DA, Edwards GE. Effects of polyploidy on photosynthetic rates, photosynthetic enzymes, contents of DNA, chlorolphyll, and sizes and numbers of photosynthetic cells in the C4 dicot Atriplex confertifolia. Plant Physiol. 1989; 91: 1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wan CY, Li MS, Song JQ, Chi YG, Wang XF, Wu YF. Differences in stomatal and photosynthetic characteristics of five diploidy wheat species. Acta Ecologica Sinica. 2008; 28: 3277–3283. [Google Scholar]
- 48.Agrawal R, Agrawal N, Tandon R, Raina SN. Chloroplast genes as genetic markers for inferring patterns of change, maternal ancestry and phylogenetic relationships among Eleusine species. AoB PLANTS. 2014; 6: plt056 doi: 10.1093/aobpla/plt056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harbut RM, Sullivan JA, Proctor JTA. Temperature affects dry matter production and net carbon exchange rate of lower-ploidy Fragaria species and species hybrids. Can J Plant Sci. 2010; 90: 885–892. [Google Scholar]
- 50.Du LS, Yang BF, Guan WB, Li JM. Phenotypic variation and water selection potential in the stem structure of invasive alligator weed. Acta Oecologica. 2016; 71: 22–30. [Google Scholar]
- 51.Mclean N, Lawson CR, Leech DI, Van DPM. Predicting when climate-driven phenotypic change affects population dynamics. Ecol Lett. 2016; 19: 595–608. doi: 10.1111/ele.12599 [DOI] [PubMed] [Google Scholar]
- 52.Thuiller W, Rebelo T. Relating plant traits and species distributions along bioclimatic gradients for 88 Leucadendron taxa. Ecology. 2004; 85: 1688–1699. [Google Scholar]
- 53.Steenhuisenl SL, Johnson SD. Evidence for beetle pollination in the African grassland sugarbushes (Protea: Proteaceae). Plant Syst Evol. 2012; 298: 857–869. [Google Scholar]
- 54.Ohi T, KajitA T, Murata J. Distinct geographic structure as evidenced by chloroplast DNA haplotypes and ploidy level in Japanese Aucuba (Aucubaceae). Am J Bot. 2003; 90: 1645–1652. doi: 10.3732/ajb.90.11.1645 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.