Abstract
Anxiety is differentially expressed across a continuum of stressful/fearful intensity, influenced endocannabinoid systems and receptors. The hippocampus plays important roles in the regulation of affective behavior, emotion, and anxiety, as well as memory. Location of Cb1/Cb2 receptor action could be important in determining emotional valence, because while the dorsal hippocampus is involved in spatial memory and cognition, the ventral hippocampus has projections to the PFC, BNST, amygdala, and HPA axis, and is important for emotional responses to stress. During repeated social defeat in a Stress-Alternatives Model arena (SAM; an oval open field with escape portals only large enough for smaller mice), smaller C57BL6/N mice are subject to fear conditioning (tone = CS), and attacked by novel larger aggressive CD1 mice (US) over four daily (5 min) trials. Each SAM trial presents an opportunity for escape or submission, with stable behavioral responses established by the second day of interaction. Additional groups had access to a running wheel. Social aggression plus fear conditioning stimulates enhanced Cb2 receptor gene expression in the dorsal CA1, dorsal and ventral dentate gyrus subregions in animals displaying a submissive behavioral phenotype. Escape behavior is associated with reduced Cb2 expression in the dorsal CA1 region, with freezing and escape latency correlated with mRNA levels. Escaping and submitting animals with access to running wheels had increased Cb2 mRNA in dorsal DG/CA1. These results suggest that the Cb2 receptor system is rapidly induced during anxiogenic social interactions plus fear conditioning or exercise; with responses potentially adaptive for coping mechanisms.
Keywords: anxiety, Cb2, endocannabinoid, exercise, induction, hippocampus
INTRODUCTION
Anxious behaviors are widely expressed across vertebrate species (Kandel, 1983), with anxiety disorders being the most prevalent mental illness (affecting as much as 25% of the human population), and highly comorbid with other conditions such as Post-Traumatic Stress Disorder (PTSD) and depression (Kessler et al., 2010). Intensity of anxiety is expressed along a continuum, influenced by experience and a dynamic neurocircuitry (Robertson et al., 2015; Smith et al., 2016). Although treatable, only one in three people suffering from an anxiety disorder will receive effective medical treatment (Young et al., 2001).
Hippocampal regulation of affective states appears to occur in concert with its role in spatial learning; multiple functions arising from anatomical segregation (Bannerman et al., 2004; Fanselow and Dong, 2010). Dorsal hippocampal involvement in spatial cognition and memory contrasts with ventral hippocampal regulation of affective states associated with stress and emotion (Bannerman et al., 2003; McHugh et al., 2004; Fanselow and Dong, 2010; Schoenfeld et al., 2013). A dual purpose hippocampus may facilitate acquisition of conditioned fear by linking emotional valence with environmental cues (Maren and Fanselow, 1995; Anagnostaras et al., 2002). Modulatory regulation in the hippocampus may mitigate emotional and environmental components during the induction of anxiety (Martinowich et al., 2007; Flandreau et al., 2012; O’Loughlin et al., 2014; Dine et al., 2015). A recent and promising target lies with the endocannabinoid system (Hill et al., 2012; Hillard, 2014; Morena et al., 2016).
Phytocannabinoids, long used for self-medication of anxiety disorders, make use of endogenous cannabinoid circuits and receptors (Hill and Patel, 2013). Endocannabinoids are bioactive lipids, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), produced from postsynaptic terminals by increased neuronal activity or from microglia, then act via stimulation of presynaptic or microglial cannabinoid type 1 (Cb1) and 2 (Cb2) receptors, regulating GABAergic and/or glutamatergic transmission or glial function (Howlett et al., 2004; Mecha et al., 2015; Li and Kim, 2016b; Ronan et al., 2016; Stempel et al., 2016; Li et al., 2017). Endocannabinoids are thought to exert their effect on affective outcomes through the action of Cb1 receptors in the limbic system, but recent work suggests that Cb2 receptors also regulate anxious behavior (Garcia-Gutierrez and Manzanares, 2011).
Traditionally thought to modulate peripheral immune function, and therefore commensurately in the brain most studies have found Cb2 receptors primarily on microglia (Nunez et al., 2004; Cabral et al., 2008; Concannon et al., 2016), but there is also emerging evidence indicates that Cb2 receptors may also be located on neurons (Van Sickle et al., 2005; Atwood and Mackie, 2010; Ronan et al., 2016). Recent work suggests that Cb2 receptors are differentially expressed in reactive microglia, perivascular microglia, oligodendrocytes, activated astrocytes, neural progenitor cells, and specific neuronal subsets, and are upregulated by neuroinflammation, potentially linking the two systems (Navarro et al., 2016). They are found throughout the central nervous system, including amygdala and hippocampus (Gong et al., 2006; Onaivi et al., 2006; Garcia-Gutierrez et al., 2010). In hippocampus, where Cb2 synthesis pyramidal cell has been described (Brusco et al., 2008), the lack of reliable antibodies and controls have made immunohistochemical evidence suspect. Using Cb2 knock-out mice as controls, it has recently been reported that hippocampal expression of Cb2 mRNA appears to be mostly neuronal, and unusually, only rarely in microglia, with the Cb2 receptors functionally influencing excitatory synaptic transmission, plasticity, and long-term potentiation (Kim and Li, 2015; Li and Kim, 2015, 2016b; Stempel et al., 2016). In addition, Cb2 receptors appear to be volatile, rapidly increasing in expression in microglia in response to trauma (Maresz et al., 2005; Atwood and Mackie, 2010). Transgenic mice overexpressing the Cb2 receptors were resilient to anxiety and depression following chronic mild stress (Garcia-Gutierrez et al., 2010; Garcia-Gutierrez and Manzanares, 2011). Interestingly, acute blockade of Cb2 receptors in wild-type mice increases anxiety, whereas Cb2 stimulation decreases anxiety (Busquets-Garcia et al., 2011; Garcia-Gutierrez et al., 2012). Taken together, the evidence suggests, and we hypothesize, that Cb2 receptors may be rapidly mobilized to ameliorate highly traumatic and anxiogenic conditions.
The Stress Alternatives Model (SAM) allows for active measurements of the intensity of anxious behavior (Robertson et al., 2015; Smith et al., 2016). An oval open field (OF) arena with restricted-size portals, this apparatus allows for retreat of smaller subjects during aggressive bouts with larger mice over four daily (5 min) trials (Smith et al., 2014). In SAM social interactions an even division of escaping and submissive responses occur, with stable behavioral phenotypes established by the second day of interaction (Smith et al., 2014; Robertson et al., 2015). The addition of a conditioned stimulus (CS, tone) during the period of isolation prior to aggressive social interaction results in fear conditioning, but only for submissive (social defeat) animals (Carpenter and Summers, 2009; Smith et al., 2014). Escape clearly alleviates stress and anxiety associated with OF and aggression (Robertson et al., 2015). However, it is important to note that both escaping and submissive animals receive significant levels of aggression, and that neither the amount nor the intensity of aggression determines the which behavioral phenotype is adopted, nor gene expression of those groups (Prince et al., 2015).
We sought to test whether exposure to anxiogenic social interaction was sufficient stimulus to increase Cb2 gene expression in the hippocampus, and whether this expression would be modified by fear conditioning or running. We similarly examined expression of Cb1 receptors to determine which endocannabinoid receptor system was more plastic and inducible. We hypothesized that anxiety from social aggression would induce elevated Cb2 gene expression only in the ventral hippocampus. Secondarily, we hypothesized that the addition of a conditioning stimulus would elevate receptor levels in the both ventral and dorsal areas.
EXPERIMENTAL PROCEDURES
Animals
Adult (8 weeks) male C57BL6/N mice weighing ~23–24g (Harlan, Indianapolis; N = 63) were group housed, 4 animals per cage, during seven days of acclimation; before being housed singly for the duration of the experiment. For non-control treatments, a separate group of retired Hsd:ICR (CD1) male breeders weighing ~53g were used to provide aggression during the behavioral experiments (Harlan, Indianapolis; N = 78). Food and water were provided ad libitum and mice were on a 12:12 reversed light-dark cycle, with lights off at 10AM. The room was kept at room temperature (22°C). All experiments were performed in a manner that minimized suffering and the number of animals used, in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23), and approved by the Institutional Animal Care and Use Committee of the University of South Dakota.
Experimental design
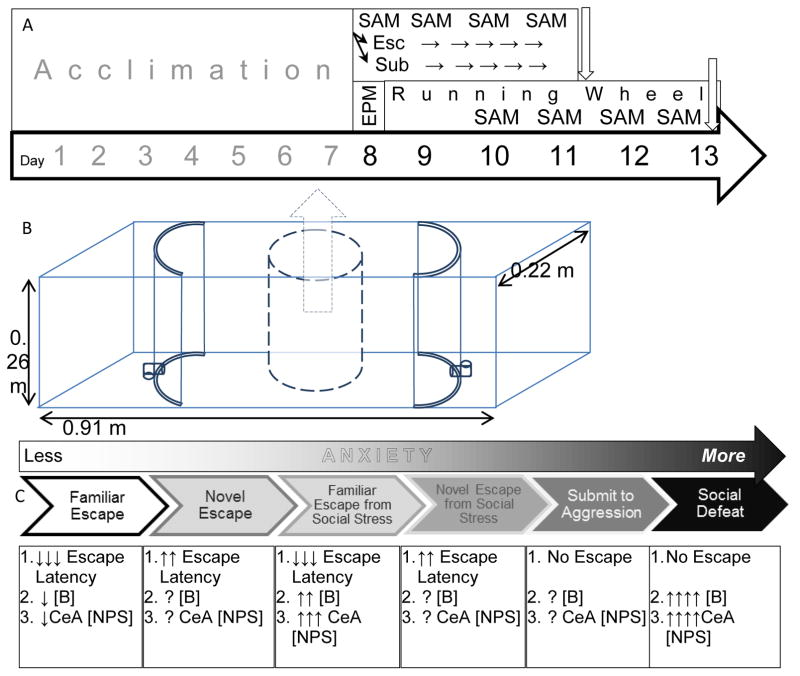
The SAM apparatus is used to create a gradient of anxiety based on four repeated socially aggressive interactions in an oval (directional) open field (OF) arena that also provides the opportunity for the test animal to escape without the aggressor being able to follow (Fig. 1). The lowest intensity anxious behavior in SAM apparatus accrues to the OF in the absence of aggressive interaction, and is not made use of in this work. When the OF is combined with social aggression, two behavioral phenotypes emerge that represent medium and high intensity anxious behavior. The fundamental elements of the experimental design are to make use of self-selection of escape and submission to compare medium and high intensity anxious behaviors respectively. In addition to this basic design we have separately added two elements to modify the intrinsic SAM anxiety intensity gradient, fear conditioning protocol (FC) within in the SAM apparatus (Total N = 24; FC training N = 17) and exercise wheels (RW) in the home cage (Total N = 39; RW exposure N = 17). Fear conditioning is associative learning that predicts the coming social anxiety (unconditioned stimulus; US) with an innocuous tone (conditioned stimulus; CS). Exercise has been shown to reduce anxiety and depression in humans and animal models, and elicits synthesis and release of endogenous anxiolytics and antidepressant neuromodulators. Therefore, in one experiment, all test mice were presented with a CS, followed by the social aggression from a larger mouse during the SAM interaction, which constitutes the US, and during the social interaction submissive (N = 6) and escaping (N = 11) phenotypes emerged (control N = 7). In another experiment, some animals had access to running wheels when not in the SAM and others did not. The SAM interaction for these animals did not include a CS prior to the SAM interaction US; again during the SAM interaction submissive (SAM only N = 7; RW + SAM N = 5) and escaping phenotypes (SAM only N = 6; RW + SAM N = 5) emerged (cage control N = 7; RW control N = 7).
Figure 1.
Experimental Design, SAM model, and previous results. A) Timeline and schematic of experimental design. The first (above) includes daily fear conditioning at the start of SAM aggressive interactions. The second (below) begins with elevated plus maze (EPM) on day 8 and access to running wheel. The running wheel is continued for six days, interrupted for the last four by 15 minute SAM aggressive interactions, but this time without fear conditioning. B) A blueprint of the SAM apparatus; a clear rectangular box 91×22×26 cm, divided into 3 sections by curved opaque barriers that include L-shaped tunnels for egress that are only large enough for the test mice (not large enough for the aggressors). An opaque cylinder sits in the central open field, for placement of the small mouse and fear conditioning when appropriate. It is removed at the beginning of the social interaction. C) A SAM derived gradient of anxious behavior intensity
Elevated plus maze
The elevated plus maze (EPM) was used prior to social interaction in the SAM (Fig. 1A) to determine predisposition to anxious behavior. Behavior on the EPM (height off floor: 1 meter, closed/open arm length: 0.3m, open junction of four arms: 0.065m × .0065m) was digitally recorded over a single 5 min bout. Elevated plus maze testing took place between 10AM and 5PM. Animals were gently placed in the intersection of the four arms, facing an open arm and released. Two independent scorers, unaware of treatment, performed scoring of open arm/closed arm entries and duration (s).
Running wheel
Immediately following exposure to the EPM, C57BL6/N mice were housed singly (transparent plastic cages 43×27×15cm) and separated into experimental groups: cage control- neither running wheel nor SAM exposure (N = 7); running wheel control- running wheel in cage, no SAM exposure (N = 7); SAM- exposure to SAM but no running wheel (N = 13); and Running Wheel SAM- exposure to both SAM and running wheel (N = 10). Mice in groups with a running wheel had ad libitum access to a 1.08m circumference wheel (NalgeneTM Activity Wheels). All running wheels used a magnetic sensor to record wheel movement (MinimitterTM) and this system was connected to a computer using VitalViewTM software to record running data in 15 second bins over a total of 8 days. Animals with running wheels in their home cages had twenty-four hours access, except during EPM and SAM testing.
Stress-Alternatives Model (SAM)
In SAM experiments, focal animals are subject to aggression (see Behavioral procedures below) and respond either by escaping (~50%) or remaining submissively (also ~50%) in a 5 min bout, and based on previous results a priori and hypotheses, are used to constitute experimental groups. The SAM (Smith et al., 2014) is a rectangular box (Length: 91cm, Width: 22cm, Height: 26cm) with a cover (L: 91cm, W: 25cm). The interior of the SAM box contains two movable semicircular polyvinyl chloride sections (diameter: 22cm, height: 26cm) each with a hole constructed from 1.9 cm diameter ninety degree polyvinyl chloride plumbing tubes placed just off the bottom portion of each section that allow for C57BL6/N mice to pass through but not CD1s. Also inside the SAM is a removable opaque divider cylinder (diameter 16.5cm, height 22cm) to separate the animals before the start of the behavioral interaction.
SAM behavioral procedures
Behavioral observations were recorded manually and digitally on video. Behavioral testing in the SAM took place between 10AM and 6PM under red light. The CD1 aggressor was first placed into the SAM inside the oval area but outside the cylindrical divider, and then the C57BL6/N test mouse was placed inside the cylindrical divider and allowed 30 seconds to acclimate. After acclimation the cylindrical divider separating the two animals was removed allowing the animals to interact for a maximum of 5 min, to minimize injury to the test mouse. A novel CD1 is used for each interaction (used once per day), to limit habituation; mice often display more interest in novel compared to familiar conspecifics (Young, 2002; Toth and Neumann, 2013). Behavioral SAM testing took place once per day for four consecutive days; allowing both the C57BL6/N test mice and CD1 aggressors 24hrs rest between trials. The duration of the interactions varied, because some animals escaped and some did not, and among those that did there were also differences in individual escape latency. Latency to escape was measured from the time the divider was removed (exposure to an aggressor) to the moment at which the animal passed through the escape portal. Duration of interaction was defined as the period from lifting of the divider to the moment that the animal exited, using one of the two available escape holes, or 5 min of submission. Interactions were scored (two naive independent trained scorers) for latency to escape. Once a test animal utilized an escape hole, a cover was placed over the hole for the remainder of the allotted 5 min.
Quantitative rtPCR
Brains were collected after decapitation and frozen at −80°C. Frozen brains were sliced coronally (200 μm), and the dorsal and ventral areas of the Dentate Gyrus (DG) and CA1 regions of the Hippocampus were microdissected following coordinates (Dorsal DG −0.94 to −1.94 from Bregma, Dorsal CA1 −1.22 to −1.94 from Bregma; Ventral DG and CA1 −3.40 to −3.88 from Bregma) based on a mouse brain atlas, using a blunt tip of a 26 gauge needle on a freezing block (−30°C). Samples were directly injected into lysis buffer (RNAqueous-Micro Kit, Life Technologies Corp) before homogenization with a pestle. Total RNA was extracted from microdissected samples using RNAqueous-Micro kit (Life Technologies Corp) and quantified using Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). Purified RNA was then used for complementary DNA (cDNA) synthesis in 20 μl reactions using the High Capacity cDNA archive kit (Life Technologies Corp). For all qPCR reactions 2 μl (3.3 ng) of total cDNA product was utilized in 20 μl reactions. Step One Plus Real-Time PCR System (Life Technologies Corp) was employed to perform all qrtPCR reactions using Taq-man Assay On Demand primer/probe sets (Life Technologies Corp) Transferrin receptor protein 1 (TFRC; Mm00441941_m1), Cb1 receptor gene CNR1 (Mm01212171_s1), and Cb2 receptor gene CNR2 (Mm00438286_m1). Each sample was normalized to the expression of housekeeping gene, GAPDH and run in duplicate. The TaqMan qPCR was performed at 50°C for 2 min and 95°C for 10 min, followed by 50 cycles at 95°C for 15 s and 60°C for 1 min. The mean critical threshold (CT) for combined hippocampal subregions is 28.76 ± 0.25 for Cb1 receptor mRNA, and 38.24 ± 0.14 for Cb2 receptor mRNA. Animals in each group were considered biological replicates, and changes in gene expression were either represented individually (regressions) or averaged (group means). The qPCR reactions for each sample were repeated twice and results from individual reactions were averaged. Changes in gene expression were quantified by real-time qPCR and analyzed using the 2−ΔΔCT method, comparing all samples to the average ΔCT value of the control animals (not exposed to the SAM apparatus). Values for qPCR data were expressed as mean fold change ± standard error of the mean.
Statistics
Gene expression results were compared across groups (Cage control, Escape, Submission) using one-way ANOVA for fear conditioning experiments, and two-way ANOVA (Exercise by Behavioral Phenotype design: including Cage control, Running wheel control, Escape, Running wheel plus escape, Submission, and Running wheel plus submission) for running wheel experiments and behavioral measures of fear conditioning (Conditioned Stimulus by Behavioral Phenotype design: including Freezing before tone, Submission, Before tone Escape, After Tone Submission, After Tone Escape). Linear regression analysis compared relationships between behavioral and gene expression results. For all analyses, each animal provided only a singular datum. During behavioral analyses or qPCR low cDNA quantity or lost tissue/samples resulted in some data being omitted from analyses. The data have been tested for the five assumptions of parametric statistics and transformed when necessary. The data are analyzed both non-parametrically and using the parametric statistics previously mentioned, and for multiple comparisons using the Holm-Sidak method; when the statistical analyses match, as they do for the data reported herein, we report the parametric results without α adjustment (Rothman, 1990; Perneger, 1998; Feise, 2002; Jennions and Moller, 2003; Moran, 2003; Nakagawa, 2004). Significant effects between groups for one-way analyses were examined with Student–Newman–Keuls post hoc analyses (to minimize Type I error) and Duncan’s Multiple Range Test (to minimize Type II error).
RESULTS
Elevated plus maze
The purpose of the EPM testing prior to SAM social interactions was to discover whether mice which would be eventually divided groups of escaping and submissive animals were innately disposed to anxious behavior. As in previous experiments, time spent in the open or closed arms were not significantly different (F2,40 = 0.0097, p ≥ 0.99; F2,40 = 0.34, p ≥ 0.71; data not shown) among control, submissive or escaping groups prior to the development of those phenotypes in the SAM (Smith et al., 2014). As before, the results suggest that the submissive and escape behavioral phenotypes expressed in the SAM apparatus were not based on groups with distinctively different innately anxious responses. The socially interacting mice eventually demonstrated self-selected escape or submissive behaviors.
Social interaction with fear conditioning
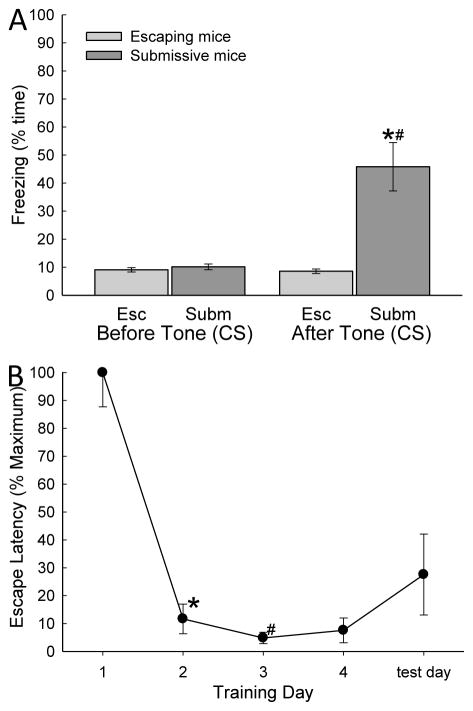
During the 30 seconds just prior to removal of the opaque cylindrical divider that allows the commencement of aggressive social interaction (and leads to social submission and defeat or escape) presentation of a conditioned stimulus (tone: 2500 Hz, 75 dB) produces significantly enhanced freezing responses (conditioned stimulus effect: F1,93 = 68.22, p ≤ 0.001; behavioral phenotype effect: F1,93 = 80.98, p ≤ 0.001; interaction effects: F1,93 = 72.25, p ≤ 0.001; Fig. 2A). Freezing is elicited by the CS on the fourth day of training, when the unconditioned stimulus was present (larger CD1 aggressor), and on test day (day 5) when no aggressor was present (conditioned response), but only in submissive mice (Smith 2014). Animals that escape did not show behavioral fear conditioning, at least with respect to freezing, during training or on test day.
Figure 2.
A) Fear conditioning was produced by pairing of a conditioned stimulus (CS = tone) and aggressive social interaction (unconditioned stimulus = US). The CS was presented during four days of training and on test day for 15s, followed by a 15s trace, after which the opaque cylindrical divider was removed and social interaction commenced. Increased percent time freezing was significant on day 4 and test day (* indicates statistical significance compared with days 1–3, p < 0.05; N=5–9) only in mice that chose to remain submissively (Non-Escapers, gray bars). Escaping mice (white bars) do not exhibit Pavlovian conditioning to the auditory cue (CS = tone; # indicates significant differences between Submissive mice and Escapers on that day, p < 0.05). B) Percent mean (± SEM) latency to escape for the animals exposed to social aggression (as a proportion of the time of first escape) significantly (* indicates statistical significance p < 0.05; N = 11) decreased after training day 1 (~236s) and they continued to escape faster (#, under ~45s) for the duration of the experiment.
For escaping animals, a significantly (F4,21 = 30.18, p ≤ 0.001; Fig. 2B) decreased escape latency was evident after training day 1 (~236s) and they continued to escape significantly faster (under ~45s) thereafter. Mice escaping on days 1 through 4, also escaped from the open field portion of the SAM on test day (day 5) with CS alone (tone) and no US (novel CD1 mice), with a latency that was not different from days 2–4. Submissive animals did not make use of the escape holes on test day, as they had not on training days.
Patterns of Cb receptor gene expression following SAM + FC
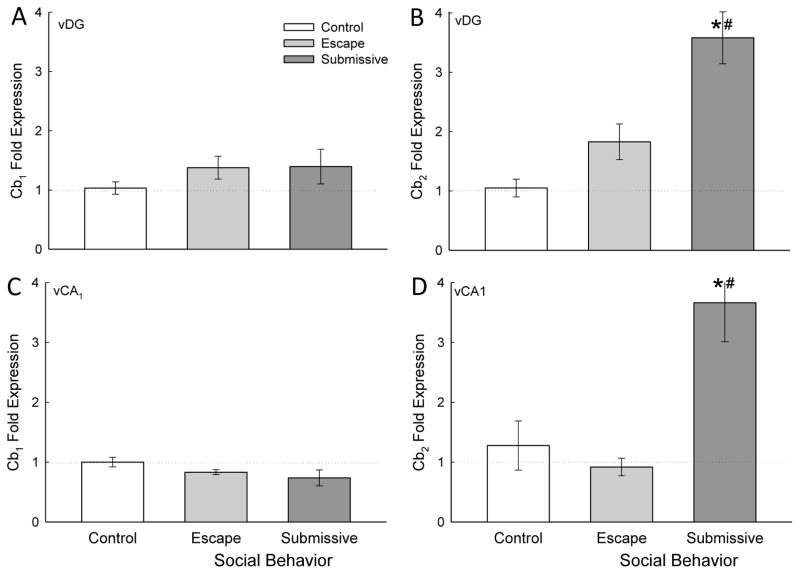
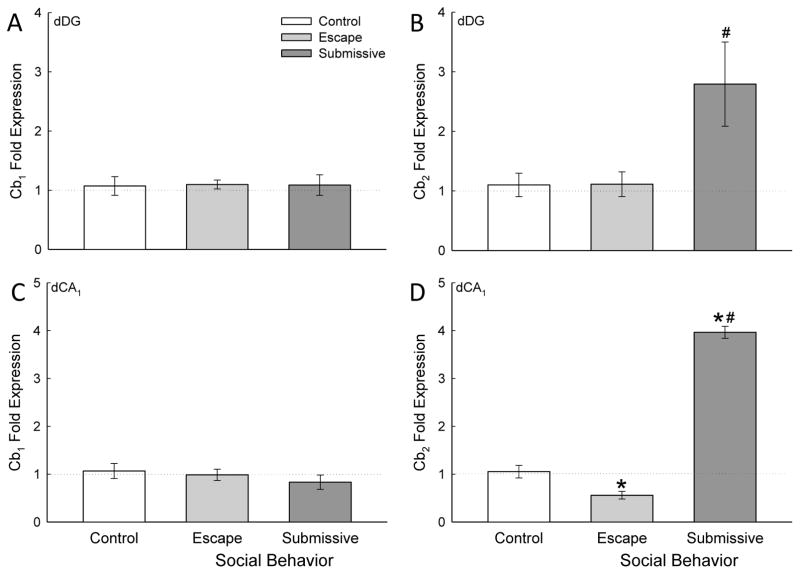
In the ventral hippocampus (Fig. 3) Cb2 receptor gene expression was uniquely enhanced in submissive animals in both DG and CA1, with no change in Cb1 receptor mRNA in response to fear conditioning plus SAM social anxiety. In dorsal hippocampus (Fig. 4), this pattern was repeated, with the exception that in dCA1, escaping mice exhibited diminished Cb2 gene expression, at the same time that submissive mice showed enhanced Cb2 mRNA (Fig. 4D). With this one exception, the gene expression patterns were also the same for DG as well as CA1 subregions, regardless of dorsal or ventral orientation. There was no clear effect of hippocampal region on expression of Cb1 or Cb2 cannabinoid receptors following fear conditioning and SAM social interaction (compare Fig. 3 with Fig. 4).
Figure 3.
Social stress modifies Cb2 receptor gene expression in the ventral hippocampus. A) Socially aggressive interactions plus fear conditioning in the SAM significantly (* compared with cage controls [white bar], # compared with escape [gray bar]) increased ventral dentate gyrus (vDG) Cb2 receptor mRNA (mean ± SEM) in submissive (dark gray bar) mice. B) In the vCA1 the Cb2 receptor mRNA is significantly (# compared with escape) increased in submissive animals (no change from control animals).
Figure 4.
Social stress modifies Cb2 receptor gene expression in the dorsal hippocampus. A) Socially aggressive interactions plus fear conditioning in the SAM significantly (* compared with cage controls [white bar], # compared with escape [gray bar]) increased dorsal dentate gyrus (dDG) Cb2 receptor mRNA (mean ± SEM) in submissive (dark gray bar). B) In the dCA1 the Cb2 receptor mRNA is significantly (* compared with cage controls) decreased in escaping animals, and significantly (* compared with cage controls, # compared with escape) increased in submissive mice.
SAM + FC gene expression of Cb1 receptors
There were no significant effects stimulated by SAM social interaction with fear conditioning on Cb1 mRNA fold expression (combined mean hippocampal Cb1 CT = 28.76 ± 0.25) in ventral regions of the hippocampus (vDG, F2,19 = 1.885, p ≥ 0.39; vCA1, F2,19 = 0.209, p ≥ 0.129; Fig. 3A, C).
Similarly, in dorsal regions (dDG, F2,19 = 0.01, p ≥ 0.99; dCA1 F2,19 = 0.632, p ≥ 0.542; Fig. 4A, C) of the hippocampus, fear conditioning just prior to SAM social interaction produced no significant changes in Cb1 mRNA fold expression.
SAM + FC gene expression of Cb2 receptors
Chronic submission and social defeat during SAM interactions paired with fear conditioning significantly increased Cb2 receptor gene expression (combined mean hippocampal Cb2 CT = 38.24 ± 0.14) compared to controls and escaping mice. Enhanced Cb2 receptor mRNA was evident in both the DG and the CA1.
In the ventral hippocampus, gene expression of Cb2 receptor was significantly increased compared to escaping mice and controls in the vDG (F2,16 = 12.74, p ≤ 0.001; Fig. 3B) as well as the vCA1 (F2,14 = 14.78, p ≤ 0.001; Fig. 3D).
In dDG, however, submissive Cb2 receptor mRNA was similarly elevated (F2,19 = 5.98, p ≤ 0.01; Fig. 4B) compared to both controls and escaping animals, in dCA1 Cb2 receptor expression was bi-directionally regulated for escaping versus submissive phenotypes (F2,19 = 18.75, p ≤ 0.001; Fig. 4D). Submissive mice exhibited significantly increased Cb2 receptor expression following fear conditioning and SAM interaction compared to controls (p < 0.05; Fig. 4B), while escaping mice exhibited significantly decreased Cb2 receptor mRNA compared to controls (p < 0.05; Fig. 4D).
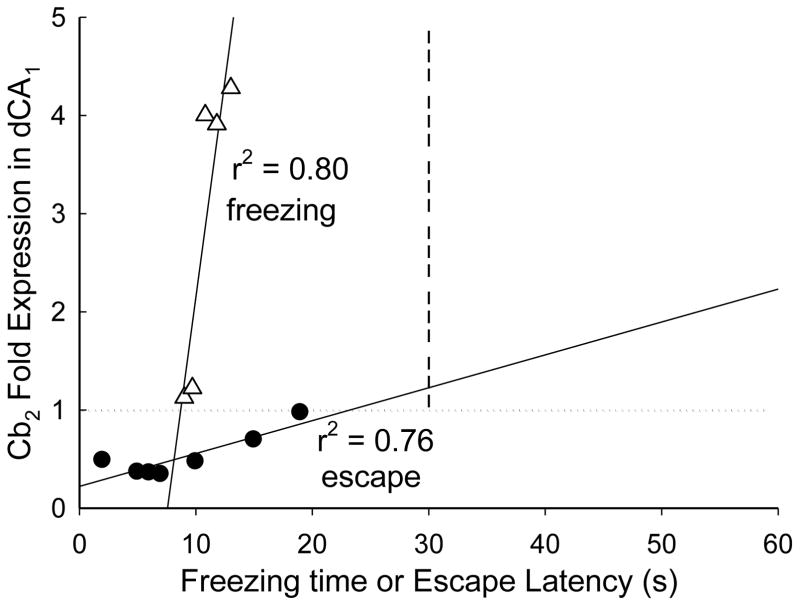
The relationship between Cb2 gene expression and freezing behavior of submissive animals and egress latency of escaping animals was suggestive of a functional impact. Surprisingly, the data reflect a significant linear regression between Cb2 gene expression in dCA1 and latency to escape on day 4 (F1,6 = 15.66, r2 = 0.76, p ≤ 0.011; Fig. 5) among animals showing reduced receptor mRNA. When examining freezing time during fear conditioning on day 4, there was also a potential functional relationship with Cb2 receptor gene expression. A significant positive regression between the duration of conditioned freezing and Cb2 expression was measured in submissive mice expressing elevated Cb2 mRNA (F1,4 = 15.66, r2 = 0.80, p ≤ 0.042; Fig. 5). Although Cb2 gene expression was elevated in other hippocampal regions, there were no other significant correlations with behavioral data; likely due to low power of the statistical comparisons.
Figure 5.
Gene expression for Cb2 receptors is positively related to anxiogenic and negatively related to anxiolytic behaviors. Freezing behavior in response to a CS (tone; open triangles) was exhibited a significant positive regression with Cb2 mRNA fold expression (r2 = 0.80). Escape latency during socially aggressive interactions (with a CD1 mouse; closed circles) exhibited a significant positive regression with Cb2 gene expression. Therefore, the rapidity of escape is negatively correlated with Cb2 mRNA.
Exercise and Social Interaction (without fear conditioning)
Gene expression of Cb1 receptors
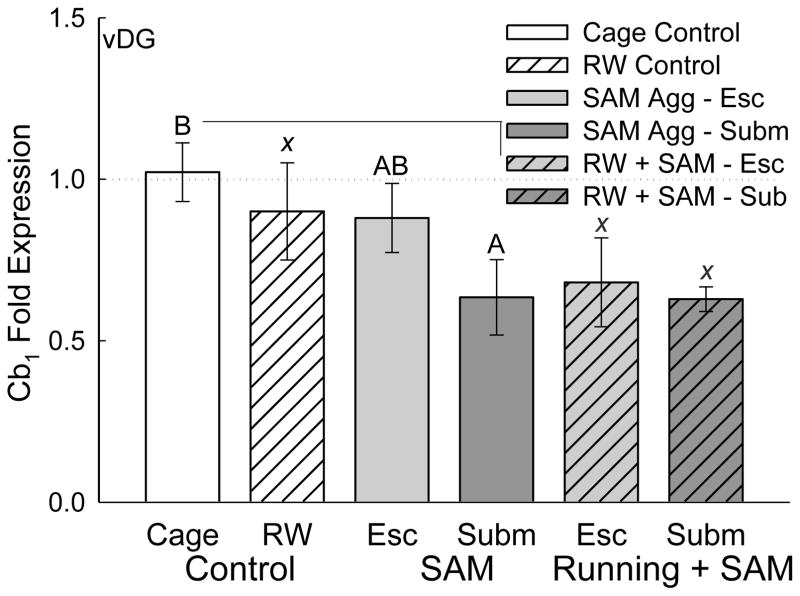
Chronic social defeat, without fear conditioning, significantly decreased Cb1 receptor mRNA expression in the vDG of the submissive group (Phenotype effect: F2,27 = 4.778, p ≤ 0.017; Fig. 6). Voluntary exercise did not significantly impact Cb1 receptor mRNA in the vDG of control, escaping or submissive animals (Exercise effect: F2,27 = 0.995, p ≥ 0.383), although Cb1 gene expression was lower in exercising escape and submissive groups than in cage controls (F2,14 = 5.202, p ≤ 0.02).
Figure 6.
Social stress reduces Cb1 receptor gene expression in the ventral dentate gyrus (vDG) of the hippocampus. Submissive mice (without access to a running wheel; solid dark gray bar) following socially aggressive interactions had significantly reduced Cb1 mRNA gene expression in vDG, compared with cage controls (solid white bar). Exercise alone (hatched white bar) did not affect Cb1 gene expression; however, escaping and submissive mice with running wheels were not different from submissive mice with reduced Cb1 expression, but exhibited significantly reduced Cb1 mRNA compared to cage controls (statistical comparison not marked). Significant comparisons are denoted by bars topped with differing letters (A vs. B) but not by bars with any similar letter (such as AB vs. B or χ vs χ).
In addition, there were no changes in Cb1 receptor gene expression in dDG (Exercise effect: F2,28 = 2.726, p ≥ 0.083; Phenotype effect: F2,28 = 0.646, p ≥ 0.532; Interaction effect: F4,28 = 1.497, p ≥ 0.23), dCA1 (Exercise effect: F2,28 = 0.194, p ≥ 0.825; Phenotype effect: F2,28 = 0.285, p ≥ 0.754; Interaction effect: F4,28 = 0.277, p ≥ 0.891), or vCA1 (Exercise effect: F2,26 = 0.167, p ≥ 0.847; Phenotype effect: F2,26 = 0.416, p ≥ 0.664; Interaction effect: F4,26 = 0.0931, p ≥ 0.984).
Gene expression of Cb2 receptors
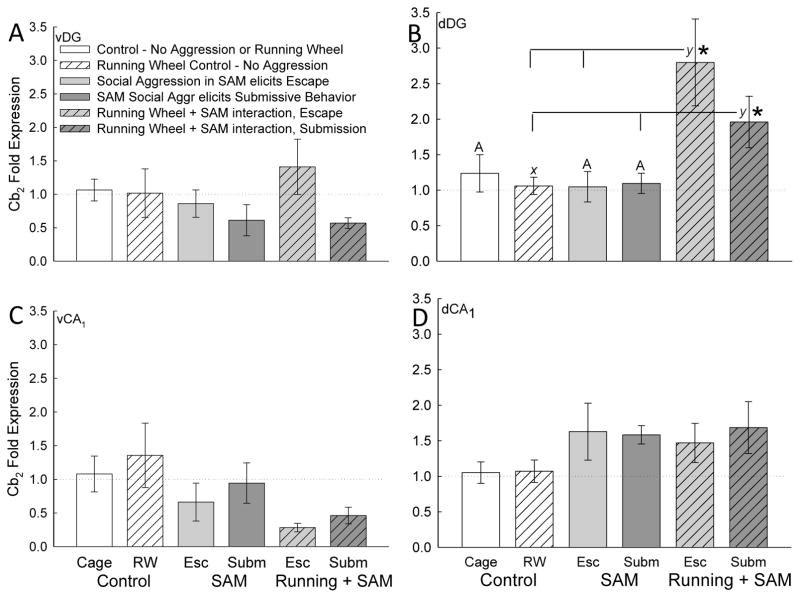
Gene expression of Cb2 receptor in the dDG was dynamically upregulated by the addition of exercise to escape and submissive phenotypes (Exercise effect: F2,28 = 6.237, p ≤ 0.006; Interaction effect: F4,28 = 3.264, p ≤ 0.026; Fig. 7B), but was unchanged by the anxiety produced SAM exposure alone (compare gray bars to gray hatched bars). There were no reportable effects on Cb2 receptor mRNA in vDG (Exercise effect: F2,21 = 0.243, p ≥ 0.786; Phenotype effect: F2,21 = 0. 892, p ≥ 0.425; Interaction effect: F4,21 = 0.841, p ≥ 0.514; Fig. 7A), vCA1 (Exercise effect: F2,22 = 0.309, p ≥ 0.737; Phenotype effect: F2,22 = 2.548, p ≥ 0.101; Fig. 7B), or dCA1 (Exercise effect: F2,25 = 0.653, p ≥ 0.529; Phenotype effect: F2,25 = 1.928, p ≥ 0.166; Interaction effect: F4,25 = 0.177, p ≥ 0.948; Fig. 7C).
Figure 7.
Social stress and exercise increases Cb2 receptor gene expression in the dorsal dentate gyrus (dDG) of the hippocampus. A, C, D) Social aggression in the SAM, whether it elicited Escape (light gray bars) or submission (dark gray bars), was not enough to induce Cb2 gene expression. Access to a running wheel (hatched bars), with or without SAM social aggression, also did not stimulate Cb2 mRNA transcription in ventral dentate (vDG), vCA1 or dCA1 regions. B) However, socially aggressive interactions in conjunction with exercise (shaded hatched bars) significantly increase Cb2 receptor gene expression of escaping (light gray hatched bar) and submissive (dark hatched bar) mice compared with running wheel controls (hatched white bar). Animals given access exercise in addition to socially aggressive interactions had significantly elevated Cb2 mRNA (*) compared with animals exposed to aggression without exercise (light [Escape] and dark [Submission] gray bars). Significant comparisons are denoted by bars topped with an asterisk (*) or differing letters (χ vs. y), but not by bars with any similar letter (such as A vs. A or χ vs χ).
DISCUSSION
While anxious behavior elicited during SAM social interactions alone has been demonstrated to influence neuromodulator and neurotrophin gene expression (such as NPS, Orx1 receptor, and BDNF) in amygdala and hippocampus (Smith et al., 2014; Robertson et al., 2015; Smith et al., 2016), an additional stimulus was needed to impact gene expression of cannabinoid receptors. Results from previous studies suggest that endocannabinoids are physiologically released in limbic brain regions only under conditions of high arousal (Morena and Campolongo, 2014), and induce different effects depending on aversiveness of environmental conditions and stress level (Campolongo et al., 2013). Endocannabinoids have been shown to play a role in neurogenesis while promoting synaptic plasticity within the hippocampus (Aguado et al., 2005; Aguado et al., 2006; Zhu, 2006). Anandamide exhibits low-efficacy agonism of both Cb1 and Cb2 receptors, with greater affinity for Cb1 receptors (Parsons and Hurd, 2015). The most abundant endocannabinoid, 2-AG, binds with equal affinity at both receptors, and has recently been shown to be important for extinction learning in hippocampal-dependent tasks (Kishimoto et al., 2015). In Cb2 receptor knockout mice, contextual fear memory was impaired (Li and Kim, 2016a), which appears to be modulated through Cb2-NMDA interactions in dorsal hippocampus (Nasehi et al., 2017). In the hippocampi of transgenic mice overexpressing Cb2 receptors, there was an increase in GABAAα2 and GABAAγ2 gene expression, indicating an increase in inhibitory output from the area (Garcia-Gutierrez and Manzanares, 2011). In this way, Cb2 activation and overexpression in response to chronic stress may be acting to mitigate the induction of anxiety at the presentation of stressful stimuli, possibly by facilitating extinction learning. Our results suggest that fear conditioning treatment, and perhaps exercise as well, may serve as the additional arousal necessary for generating increased gene expression of the Cb2 receptor in hippocampus. This stands in stark contrast with Cb1 receptor gene expression, which has extremely dense central distribution, higher than most other G-protein coupled receptors, highest in hippocampus and motor areas (Breivogel and Childers, 1998), and clearly demonstrated associations with anxiety and depression. In our experiments Cb1 mRNA only showed minor reduction in mRNA in ventral dentate gyrus. The contrast between these two cannabinoid receptor systems suggests that Cb2 receptors, although low in initial density, are highly sensitive to conditions that promote induction of gene expression, and that anxious behavior, when combined with fear conditioning or exercise, may be enough arousal to stimulate this induction. The bidirectional relationships between Cb2 gene expression and submissive freezing or escape latency (Fig. 4) suggest important functional attributes for the receptor in hippocampus. These results support previous studies that suggest Cb2 receptors may be a valuable target for therapeutic actions (Onaivi et al., 2008a; Onaivi et al., 2008b; Atwood and Mackie, 2010; Garcia-Gutierrez et al., 2010; Garcia-Gutierrez and Manzanares, 2011; Ortega-Alvaro et al., 2011; Garcia-Gutierrez et al., 2012; Garcia-Gutierrez et al., 2013; Navarrete et al., 2013; Ortega-Alvaro et al., 2015).
Our results demonstrating enhanced Cb2 receptor gene expression should be interpreted within the scope of the SAM’s capacity for generating and revealing affective responses. Previous results using this apparatus have revealed anxious behavioral responses that vary in intensity along a continuum or gradient (Robertson et al., 2015; Smith et al., 2016). As the intensity of anxious behavioral responses grows, plasma corticosterone and the anxiety-responsive neuropeptide S (NPS) in the central amygdala (CeA) rise commensurately (Fig. 1C). The continuum of corticosterone concentration and CeA NPS expression is evident in escaping and submissive behavioral phenotypes, and while elevated in both groups, significantly greater in submissive animals. Interestingly, though both escaping and submissive animals receive significant levels of aggression, neither the quantity nor the intensity of aggression determines which behavioral phenotype is adopted, and therefore differences in hormone and gene expression in those groups are determined by the choice of behavioral response (Prince et al., 2015). This gradient is amenable to treatment with anxiolytic treatments (such as exercise on the running wheel, familiarity with escape, NPS, the CRF1 antagonist antalarmin), which reverses highly anxious submissive behaviors and allows for escape. Anxiogenic treatments (The noradrenergic α2A antagonist yohimbine, aggression) block escape behavior, and promote submission (Robertson et al., 2015; Smith et al., 2016). The results presented here for Cb2 expression in dorsal and ventral hippocampal DG and CA1 regions are a comparison of animals expressing the highest level of anxiety (following social defeat) with animals that have significantly less anxiety (exhibited during escape from aggression; see Fig. 1C). Animals showing the highest level of anxiety also develop behavioral fear conditioning when paired with a conditioned stimulus, like a tone (Smith et al., 2014). The freezing behavior in response to the conditioning was positively correlated with Cb2 gene expression in dCA1. Conversely, escape celerity was negatively correlated (i.e. latency was positively correlated, Fig. 4) with dCA1 Cb2 mRNA levels. In addition to unique behavioral responses, the gradient of anxiety produced by the SAM is represented by progressively elevated levels of corticosterone and CeA neuropeptide S gene expression. Orexin and its receptor (Orx1 and Orx2), as well as BDNF and TrKB receptor, gene expression are also modified in the hippocampus (Arendt et al., 2012; Arendt et al., 2013; Prince et al., 2015; Summers et al., 2015). Behavioral, hormonal, and gene expression changes are upregulated by anxiogenic drugs and ameliorated by anxiolytic drugs (Robertson et al., 2015; Smith et al., 2016). Similar to our results in hippocampus, gene expression for BDNF in the BLA was elevated, not with high level anxiety alone, but only when fear conditioning was combined with high level anxiety (Smith et al., 2014; Smith et al., 2016).
Cannabinoid receptors are clearly linked with diminished anxiety and anxious behavior, demonstrated by anxiogenic effects in Cb1 knockouts and anxiolytic effects of Cb2 overexpression (Garcia-Gutierrez and Manzanares, 2011; Hill and Patel, 2013). Deletion of glutamatergic Cb1 receptors enhance anxious behavior, and modulate low dose cannabinoid anxiolysis, whereas Cb1 receptors on GABAergic cells produce anxiogenic responses in response to elevated agonist concentrations. However, while Cb1 receptors are important for ameliorating the effects of anxiety and anxious behavior, when considering our data they do not appear to be highly modifiable by induction of anxiety. Cannabinoid type-2 receptors also exhibit powerful influence over anxiety, seen in studies using overexpression and knockouts of the gene, both suggesting a Cb2 anxiolytic response (Garcia-Gutierrez et al., 2010; Garcia-Gutierrez and Manzanares, 2011; Ortega-Alvaro et al., 2011). Our data, demonstrating positive regressions of Cb2 mRNA with freezing time, suggests that rapid induction of Cb2 gene expression is promoted by anxious behavior plus fear conditioning (Fig. 5). On the other hand, the anxiolytic escape response also changed Cb2 gene expression activity, diminishing Cb2 mRNA in a manner dependent on the celerity of escape. The data suggest that reduced anxiety limits the need for elevated Cb2 expression (Fig. 5). Chronic blockade of the Cb2 receptor, however, has been demonstrated to elicit an anxiolytic phenotype, which suggests that the Cb2 receptor is anxiogenic (Garcia-Gutierrez et al., 2012). Considering its rapid upregulation of gene expression (Figures 3 and 4), chronic stimulation of the Cb2 receptor may not be its normal physiological mode, given that Cb2 receptors have very low density are highly inducible (Maresz et al., 2005). Although we cannot identify which cell types express Cb2 receptors, our results suggest that they can be induced rapidly by anxiety combined with fear conditioning (Figures 3 and 4).
As peripheral Cb2 receptor gene and protein expression may be induced by disease (Julien et al., 2005), and dramatically enhanced brain Cb2 receptor expression (up to 100 fold) may be triggered by peripheral and central inflammatory responses (Maresz et al., 2005; Bouchard et al., 2012), Cb2 receptors appear to respond to traumatic events by repopulating tissues such as liver and brain that normally do not express many of these receptors, if any at all. It has been well established that peripheral and central Cb2 receptors are dramatically upregulated by traumatic disease states such as liver cirrhosis, Huntington’s Disease, or encephalomyelitis (Julien et al., 2005; Maresz et al., 2005; Bouchard et al., 2012). Anxiety represents emotional trauma, which has been demonstrated to be modified by Cb2 activity (Garcia-Gutierrez et al., 2010; Garcia-Gutierrez and Manzanares, 2011; Ortega-Alvaro et al., 2011; Garcia-Gutierrez et al., 2012).
Here we demonstrate for the first time that affective trauma, induced by anxiety and Pavlovian fear conditioning, is also associated with rapidly enhanced Cb2 receptor gene expression in the hippocampus, although in unknown cell types. As inflammation is typically an important part of Cb2 inducibility, and inflammation in the hippocampus is linked with anxious behavior (Fan et al., 2016), it is possible that our results are also derived from anxiety-induced inflammatory responses. Social defeat has also been demonstrated to produce neuroimmune responses, including activation of microglia (Wohleb et al., 2011; Wohleb et al., 2014; Brachman et al., 2015; Lehmann et al., 2016; McKim et al., 2016), one of the potential sites of Cb2 induction. Social defeat-induced neuroinflammation occurs in hippocampus as well as other limbic structures, and influences gene expression therein (McKim et al., 2016). In our model, social defeat is one of the elements involved in stimulation of Cb2 induction, but has no effect on its own (Fig. 7A–C), only producing Cb2 induction when paired with fear conditioning (Fig. 3B, D; 4B, D).
Additionally, we are not able at this point to determine whether our results stem from glial or neuronal Cb2 induction, though Cb2 receptors do exist on neurons (Van Sickle et al., 2005; Atwood and Mackie, 2010; Li and Kim, 2015; Ronan et al., 2016; Stempel et al., 2016). In the hippocampus particularly, Li and Kim, as well as Stempel et al., report that most of the gene expression for Cb2 receptors is neuronal, with expression in excitatory and inhibitory neurons in CA1, CA3 and DG (Li and Kim, 2015; Stempel et al., 2016). Although other reports suggest the opposite (Franklin and Stella, 2003; Carrier et al., 2004; Nunez et al., 2004; Maresz et al., 2005; Palazuelos et al., 2009; Bu et al., 2016), they suggest that Cb2 gene expression is rarely found in microglia, at least in hippocampus. Additionally, only Cb2 receptors in hippocampus were presumably responsive to anxiety plus fear conditioning (or exercise); Cb1 gene expression in vDG was not induced by anxiety and fear conditioning, and showed minor downregulation with compounding effects of anxiety and exercise. Similarly, chronic unpredictable stress has been demonstrated to downregulate Cb1 protein expression in the hippocampus of male, but not female, rats (Reich et al., 2009). By comparison, Cb2 receptor gene expression in dDG was upregulated by the combination of anxiety and exercise. It isn’t clear what mechanism produces the combinatorial effects of fear conditioning and anxiety, or exercise and anxiety, to stimulate greater Cb2 gene expression.
There has been significant research suggesting divergent functional modulation of specific behavioral outcomes from the dorsal and ventral poles of the hippocampus; with the ventral hippocampus proposed to be primarily responsible for anxiety and emotional responsiveness (Fanselow and Dong, 2010; Schoenfeld et al., 2013). As such, we hypothesized that endocannabinoid receptor gene expression associated with anxious states would be enriched at the ventral pole. In a recently published census of neuronal gene expression in the hippocampus, a broad array of genes (not including cannabinoid receptors) were explicitly segregated between the dorsal and ventral pole; however, many genes were enriched between the two, and some genes showed heightened expression at both poles (Cembrowski et al., 2016). Given the roles of ventral and dorsal areas in anxiety and learning, perhaps it is not surprising that elevated Cb2 expression following conditioning and social defeat was found at both poles.
CONCLUSIONS
Our data demonstrate for the first time that Cb2 receptor gene expression may be highly inducible in the brain, as well as in the periphery. While anxiety is not a sufficient stimulus on its own to upregulate hippocampal gene expression of Cb2 receptors, when paired with fear conditioning gene expression for these receptors may be potently and rapidly upregulated as much as four-fold in both dorsal and ventral hippocampi. Upregulation occurred not only at both poles of the hippocampus, but also in regions containing granule and pyramidal cells (Li and Kim, 2015), suggesting that integrative events regulated by the hippocampus, such as learning (Li and Kim, 2016a) and anxiety, may be regulated comprehensively. Up- and downregulation of Cb2 gene expression in the dCA1 were significantly correlated with anxious and anxiolytic behaviors, respectively.
Highlights.
Rapid induction of Cb2 receptor gene expression occurs in hippocampus
Social defeat + fear conditioning induces Cb2 gene expression in hippocampal subregions
Enhanced Cb2 receptor gene expression occurs in dorsal and ventral hippocampus
Stress-Alternatives Model Escape phenotype diminishes Cb2 gene expression in vCA1
Exercise also influences expression of Cb2 receptor mRNA
Acknowledgments
We thank Fadi Haroun for assistance with video and data analysis. We thank Joseph Madison for assistance with genome database analysis. Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R15MH104485, and by NIH grant and P20 RR15567, a CBBRe Research Enhancement Pilot grant, by an anonymous donor to the Summers’ lab via the USD Foundation, and by funding from the BCAAP SD 2010 Research Center and the Great Plains Medical Research Foundation (to PJR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguado T, Monory K, Palazuelos J, Stella N, Cravatt B, Lutz B, Marsicano G, Kokaia Z, Guzman M, Galve-Roperh I. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005;19:1704–1706. doi: 10.1096/fj.05-3995fje. [DOI] [PubMed] [Google Scholar]
- Aguado T, Palazuelos J, Monory K, Stella N, Cravatt B, Lutz B, Marsicano G, Kokaia Z, Guzman M, Galve-Roperh I. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J Neurosci. 2006;26:1551–1561. doi: 10.1523/JNEUROSCI.3101-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. The hippocampus and Pavlovian fear conditioning: reply to Bast et al. Hippocampus. 2002;12:561–565. doi: 10.1002/hipo.10071. [DOI] [PubMed] [Google Scholar]
- Arendt DH, Ronan PJ, Oliver KD, Callahan LB, Summers TR, Summers CH. Depressive behavior and activation of the orexin/hypocretin system. Behav Neurosci. 2013;127:86–94. doi: 10.1037/a0031442. [DOI] [PubMed] [Google Scholar]
- Arendt DH, Smith JP, Bastida CC, Prasad MS, Oliver KD, Eyster KM, Summers TR, Delville Y, Summers CH. Contrasting hippocampal and amygdalar expression of genes related to neural plasticity during escape from social aggression. Physiol Behav. 2012;107:670–679. doi: 10.1016/j.physbeh.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160:467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bouchard J, Truong J, Bouchard K, Dunkelberger D, Desrayaud S, Moussaoui S, Tabrizi SJ, Stella N, Muchowski PJ. Cannabinoid receptor 2 signaling in peripheral immune cells modulates disease onset and severity in mouse models of Huntington’s disease. J Neurosci. 2012;32:18259–18268. doi: 10.1523/JNEUROSCI.4008-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachman RA, Lehmann ML, Maric D, Herkenham M. Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. J Neurosci. 2015;35:1530–1538. doi: 10.1523/JNEUROSCI.2278-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR. The functional neuroanatomy of brain cannabinoid receptors. Neurobiol Dis. 1998;5:417–431. doi: 10.1006/nbdi.1998.0229. [DOI] [PubMed] [Google Scholar]
- Brusco A, Tagliaferro P, Saez T, Onaivi ES. Postsynaptic localization of CB2 cannabinoid receptors in the rat hippocampus. Synapse. 2008;62:944–949. doi: 10.1002/syn.20569. [DOI] [PubMed] [Google Scholar]
- Bu W, Ren H, Deng Y, Del Mar N, Guley NM, Moore BM, Honig MG, Reiner A. Mild Traumatic Brain Injury Produces Neuron Loss That Can Be Rescued by Modulating Microglial Activation Using a CB2 Receptor Inverse Agonist. Front Neurosci. 2016;10:449. doi: 10.3389/fnins.2016.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets-Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita A. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry. 2011;70:479–486. doi: 10.1016/j.biopsych.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Raborn ES, Griffin L, Dennis J, Marciano-Cabral F. CB2 receptors in the brain: role in central immune function. Br J Pharmacol. 2008;153:240–251. doi: 10.1038/sj.bjp.0707584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolongo P, Morena M, Scaccianoce S, Trezza V, Chiarotti F, Schelling G, Cuomo V, Roozendaal B. Novelty-induced emotional arousal modulates cannabinoid effects on recognition memory and adrenocortical activity. Neuropsychopharmacology. 2013;38:1276–1286. doi: 10.1038/npp.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RE, Summers CH. Learning strategies during fear conditioning. Neurobiol Learn Mem. 2009;91:415–423. doi: 10.1016/j.nlm.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier EJ, Kearn CS, Barkmeier AJ, Breese NM, Yang W, Nithipatikom K, Pfister SL, Campbell WB, Hillard CJ. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol Pharmacol. 2004;65:999–1007. doi: 10.1124/mol.65.4.999. [DOI] [PubMed] [Google Scholar]
- Cembrowski MS, Bachman JL, Wang L, Sugino K, Shields BC, Spruston N. Spatial gene-expression gradients underlie prominent heterogeneity of CA1 pyramidal neurons. Neuron. 2016 doi: 10.1016/j.neuron.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Concannon RM, Okine BN, Finn DP, Dowd E. Upregulation of the cannabinoid CB2 receptor in environmental and viral inflammation-driven rat models of Parkinson’s disease. Exp Neurol. 2016;283:204–212. doi: 10.1016/j.expneurol.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Dine J, Ionescu IA, Avrabos C, Yen YC, Holsboer F, Landgraf R, Schmidt U, Eder M. Intranasally applied neuropeptide S shifts a high-anxiety electrophysiological endophenotype in the ventral hippocampus towards a “normal”-anxiety one. PLoS One. 2015;10:e0120272. doi: 10.1371/journal.pone.0120272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Fan X, Li Y, Guo J, Xia D, Ding L, Zheng Q, Wang W, Xue F, Chen R, Liu S, Hu L, Gong Y. Blunted inflammation mediated by NF-kappaB activation in hippocampus alleviates chronic normobaric hypoxia-induced anxiety-like behavior in rats. Brain Res Bull. 2016;122:54–61. doi: 10.1016/j.brainresbull.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. doi: 10.1186/1471-2288-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandreau EI, Ressler KJ, Owens MJ, Nemeroff CB. Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology. 2012;37:27–38. doi: 10.1016/j.psyneuen.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin A, Stella N. Arachidonylcyclopropylamide increases microglial cell migration through cannabinoid CB2 and abnormal-cannabidiol-sensitive receptors. Eur J Pharmacol. 2003;474:195–198. doi: 10.1016/s0014-2999(03)02074-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Garcia-Bueno B, Zoppi S, Leza JC, Manzanares J. Chronic blockade of cannabinoid CB2 receptors induces anxiolytic-like actions associated with alterations in GABA(A) receptors. Br J Pharmacol. 2012;165:951–964. doi: 10.1111/j.1476-5381.2011.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Manzanares J. Overexpression of CB2 cannabinoid receptors decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. J Psychopharmacol. 2011;25:111–120. doi: 10.1177/0269881110379507. [DOI] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Ortega-Alvaro A, Busquets-Garcia A, Perez-Ortiz JM, Caltana L, Ricatti MJ, Brusco A, Maldonado R, Manzanares J. Synaptic plasticity alterations associated with memory impairment induced by deletion of CB2 cannabinoid receptors. Neuropharmacology. 2013;73:388–396. doi: 10.1016/j.neuropharm.2013.05.034. [DOI] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Perez-Ortiz JM, Gutierrez-Adan A, Manzanares J. Depression-resistant endophenotype in mice overexpressing cannabinoid CB(2) receptors. Br J Pharmacol. 2010;160:1773–1784. doi: 10.1111/j.1476-5381.2010.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Williams CM, Whalley BJ, Stephens GJ. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacology & Therapeutics. 2012;133:79–97. doi: 10.1016/j.pharmthera.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Hill MN, Patel S. Translational evidence for the involvement of the endocannabinoid system in stress-related psychiatric illnesses. Biol Mood Anxiety Disord. 2013;3:19. doi: 10.1186/2045-5380-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ. Stress regulates endocannabinoid-CB1 receptor signaling. Semin Immunol. 2014;26:380–388. doi: 10.1016/j.smim.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47(Suppl 1):345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Jennions MD, Moller AP. A survey of the statistical power of research in behavioral ecology and animal behavior. Behavioral Ecology. 2003;14:438–445. [Google Scholar]
- Julien B, Grenard P, Teixeira-Clerc F, Van Nhieu JT, Li L, Karsak M, Zimmer A, Mallat A, Lotersztajn S. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology. 2005;128:742–755. doi: 10.1053/j.gastro.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Kandel ER. From metapsychology to molecular biology: explorations into the nature of anxiety. Am J Psychiatry. 1983;140:1277–1293. doi: 10.1176/ajp.140.10.1277. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Ruscio AM, Shear K, Wittchen HU. Epidemiology of anxiety disorders. Curr Top Behav Neurosci. 2010;2:21–35. [PubMed] [Google Scholar]
- Kim J, Li Y. Chronic activation of CB2 cannabinoid receptors in the hippocampus increases excitatory synaptic transmission. J Physiol. 2015;593:871–886. doi: 10.1113/jphysiol.2014.286633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Cagniard B, Yamazaki M, Nakayama J, Sakimura K, Kirino Y, Kano M. Task-specific enhancement of hippocampus-dependent learning in mice deficient in monoacylglycerol lipase, the major hydrolyzing enzyme of the endocannabinoid 2-arachidonoylglycerol. Front Behav Neurosci. 2015;9:134. doi: 10.3389/fnbeh.2015.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Cooper HA, Maric D, Herkenham M. Social defeat induces depressive-like states and microglial activation without involvement of peripheral macrophages. J Neuroinflammation. 2016;13:224. doi: 10.1186/s12974-016-0672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MH, Suchland KL, Ingram SL. Compensatory activation of cannabinoid CB2 receptor inhibition of GABA release in the rostral ventromedial medulla in inflammatory pain. J Neurosci. 2017;37:626–636. doi: 10.1523/JNEUROSCI.1310-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kim J. Neuronal expression of CB2 cannabinoid receptor mRNAs in the mouse hippocampus. Neuroscience. 2015;311:253–267. doi: 10.1016/j.neuroscience.2015.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kim J. CB2 Cannabinoid Receptor Knockout in Mice Impairs Contextual Long-Term Memory and Enhances Spatial Working Memory. Neural Plast. 2016a;2016:9817089. doi: 10.1155/2016/9817089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kim J. Deletion of CB2 cannabinoid receptors reduces synaptic transmission and long-term potentiation in the mouse hippocampus. Hippocampus. 2016b;26:275–281. doi: 10.1002/hipo.22558. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005;95:437–445. doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- McHugh SB, Deacon RM, Rawlins JN, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci. 2004;118:63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- McKim DB, Niraula A, Tarr AJ, Wohleb ES, Sheridan JF, Godbout JP. Neuroinflammatory Dynamics Underlie Memory Impairments after Repeated Social Defeat. J Neurosci. 2016;36:2590–2604. doi: 10.1523/JNEUROSCI.2394-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecha M, Feliu A, Carrillo-Salinas FJ, Rueda-Zubiaurre A, Ortega-Gutierrez S, de Sola RG, Guaza C. Endocannabinoids drive the acquisition of an alternative phenotype in microglia. Brain Behav Immun. 2015;49:233–245. doi: 10.1016/j.bbi.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Moran MD. Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos. 2003;100:403–405. [Google Scholar]
- Morena M, Campolongo P. The endocannabinoid system: an emotional buffer in the modulation of memory function. Neurobiol Learn Mem. 2014;112:30–43. doi: 10.1016/j.nlm.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Morena M, Patel S, Bains JS, Hill MN. Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology. 2016;41:80–102. doi: 10.1038/npp.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behavioral Ecology. 2004;15:1044–1045. [Google Scholar]
- Nasehi M, Hajikhani M, Ebrahimi-Ghiri M, Zarrindast MR. Interaction between NMDA and CB2 function in the dorsal hippocampus on memory consolidation impairment: an isobologram analysis. Psychopharmacology (Berl) 2017;234:507–514. doi: 10.1007/s00213-016-4481-9. [DOI] [PubMed] [Google Scholar]
- Navarrete F, Rodriguez-Arias M, Martin-Garcia E, Navarro D, Garcia-Gutierrez MS, Aguilar MA, Aracil-Fernandez A, Berbel P, Minarro J, Maldonado R, Manzanares J. Role of CB2 cannabinoid receptors in the rewarding, reinforcing, and physical effects of nicotine. Neuropsychopharmacology. 2013;38:2515–2524. doi: 10.1038/npp.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G, Morales P, Rodriguez-Cueto C, Fernandez-Ruiz J, Jagerovic N, Franco R. Targeting Cannabinoid CB2 Receptors in the Central Nervous System. Medicinal Chemistry Approaches with Focus on Neurodegenerative Disorders. Front Neurosci. 2016;10:406. doi: 10.3389/fnins.2016.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez E, Benito C, Pazos MR, Barbachano A, Fajardo O, Gonzalez S, Tolon RM, Romero J. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an immunohistochemical study. Synapse. 2004;53:208–213. doi: 10.1002/syn.20050. [DOI] [PubMed] [Google Scholar]
- O’Loughlin EK, Pakan JM, McDermott KW, Yilmazer-Hanke D. Expression of neuropeptide Y1 receptors in the amygdala and hippocampus and anxiety-like behavior associated with Ammon’s horn sclerosis following intrahippocampal kainate injection in C57BL/6J mice. Epilepsy Behav. 2014;37:175–183. doi: 10.1016/j.yebeh.2014.06.033. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, Brusco A, Akinshola BE, Hope B, Lujilde J, Inada T, Iwasaki S, Macharia D, Teasenfitz L, Arinami T, Uhl GR. Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: from mice to human subjects. PLoS One. 2008a;3:e1640. doi: 10.1371/journal.pone.0001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, Brusco A, Akinshola BE, Liu QR, Chirwa SS, Hope B, Lujilde J, Inada T, Iwasaki S, Macharia D, Teasenfitz L, Arinami T, Uhl GR. Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Ann N Y Acad Sci. 2008b;1139:434–449. doi: 10.1196/annals.1432.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE, Liu QR, Hope B, Iwasaki S, Arinami T, Teasenfitz L, Uhl GR. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Ortega-Alvaro A, Aracil-Fernandez A, Garcia-Gutierrez MS, Navarrete F, Manzanares J. Deletion of CB2 cannabinoid receptor induces schizophrenia-related behaviors in mice. Neuropsychopharmacology. 2011;36:1489–1504. doi: 10.1038/npp.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Alvaro A, Ternianov A, Aracil-Fernandez A, Navarrete F, Garcia-Gutierrez MS, Manzanares J. Role of cannabinoid CB2 receptor in the reinforcing actions of ethanol. Addict Biol. 2015;20:43–55. doi: 10.1111/adb.12076. [DOI] [PubMed] [Google Scholar]
- Palazuelos J, Aguado T, Pazos MR, Julien B, Carrasco C, Resel E, Sagredo O, Benito C, Romero J, Azcoitia I, Fernandez-Ruiz J, Guzman M, Galve-Roperh I. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. Brain. 2009;132:3152–3164. doi: 10.1093/brain/awp239. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci. 2015;16:579–594. doi: 10.1038/nrn4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince MA, Achua JK, Smith JP, Robertson JM, Ronan PJ, Summers TR, Summers CH. Decision-making during social interaction in mice is not driven by aggression, but is related to ventral dentate gyrus expression of orexin receptors. Society for Neuroscience Abstracts. 2015;45:419. [Google Scholar]
- Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res. 2009;203:264–269. doi: 10.1016/j.bbr.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JM, Prince MA, Achua JK, Carpenter RE, Arendt DH, Smith JP, Summers TL, Summers TR, Blanchard DC, Summers CH. Nuance and behavioral cogency: How the Visible Burrow System inspired the Stress-Alternatives Model and conceptualization of the continuum of anxiety. Physiol Behav. 2015;146:86–97. doi: 10.1016/j.physbeh.2015.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronan PJ, Wongngamnit N, Beresford TP. Molecular Mechanisms of Cannabis Signaling in the Brain. Prog Mol Biol Transl Sci. 2016;137:123–147. doi: 10.1016/bs.pmbts.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- Schoenfeld TJ, Rada P, Pieruzzini PR, Hsueh B, Gould E. Physical exercise prevents stress-induced activation of granule neurons and enhances local inhibitory mechanisms in the dentate gyrus. J Neurosci. 2013;33:7770–7777. doi: 10.1523/JNEUROSCI.5352-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JP, Achua JK, Summers TR, Ronan PJ, Summers CH. Neuropeptide S and BDNF gene expression in the amygdala are influenced by social decision-making under stress. Frontiers in Behavioral Neuroscience. 2014;121:1–13. doi: 10.3389/fnbeh.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JP, Prince MA, Achua JK, Robertson JM, Anderson RT, Ronan PJ, Summers CH. Intensity of anxiety is modified via complex integrative stress circuitries. Psychoneuroendocrinology. 2016;63:351–361. doi: 10.1016/j.psyneuen.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stempel AV, Stumpf A, Zhang HY, Ozdogan T, Pannasch U, Theis AK, Otte DM, Wojtalla A, Racz I, Ponomarenko A, Xi ZX, Zimmer A, Schmitz D. Cannabinoid type 2 receptors mediate a cell type-specific plasticity in the hippocampus. Neuron. 2016;90:795–809. doi: 10.1016/j.neuron.2016.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers TR, Achua JK, Smith JP, Prince MA, Robertson JM, Summers CH. Exercise modifies orexin receptor, BDNF, and TrKB receptor gene expression during social stress. Ventral versus dorsal hippocampal regions. 2015;45:519. [Google Scholar]
- Toth I, Neumann ID. Animal models of social avoidance and social fear. Cell Tissue Res. 2013;354:107–118. doi: 10.1007/s00441-013-1636-4. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J Neurosci. 2014;34:2583–2591. doi: 10.1523/JNEUROSCI.3723-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AS, Klap R, Sherbourne CD, Wells KB. The quality of care for depressive and anxiety disorders in the United States. Arch Gen Psychiatry. 2001;58:55–61. doi: 10.1001/archpsyc.58.1.55. [DOI] [PubMed] [Google Scholar]
- Young LJ. The neurobiology of social recognition, approach, and avoidance. Biol Psychiatry. 2002;51:18–26. doi: 10.1016/s0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]
- Zhu PJ. Endocannabinoid signaling and synaptic plasticity in the brain. Crit Rev Neurobiol. 2006;18:113–124. doi: 10.1615/critrevneurobiol.v18.i1-2.120. [DOI] [PubMed] [Google Scholar]