Abstract
Purpose of review
A collaboration of comparative effectiveness research trials of pulse oximeter saturation (SpO2) targeting in extremely low gestational age neonates (ELGANs) have begun to report their aggregate results. We will examine the results of those trials, collectively referred to as the Neonatal Oxygenation Prospective Meta-analysis, or NeOProM. We will also discuss the uncertainties that remain and the clinical challenges that lie ahead.
Recent findings
The primary outcome from NeOProM was a composite of death or disability at 18–24 months corrected age. Earlier this year, the last of these reports was published. Although there were no differences in the primary outcome overall, analyses of secondary outcomes and data subsets following a pulse oximeter revision show significant treatment differences between targeting a lower compared to a higher SpO2.
Summary
NeOProM represents the largest collaborative clinical research study of SpO2 targets in ELGANs. While aggregate results give us some insight into the feasibility and efficacy of SpO2 targeting in this population, many questions remain. A patient-level analysis, tracking individual outcomes based on actual SpO2 experienced, may shed some light on these questions. However, finding a single optimal SpO2 range seems unlikely.
Keywords: Pulse oximetry, oxygen saturation, ELGAN, NeOProM, comparative effectiveness research
Introduction
For the past sixty years clinicians and researchers have been concerned about the safe use of supplemental oxygen in preterm infants, arguably the single most common therapeutic intervention in the neonatal intensive care unit (NICU). Like warmth, water, and food, oxygen is vital to sustaining life, but in excess it can be harmful. Retinopathy of prematurity (ROP) first brought our attention to the harms of excess oxygen in the 1950’s, but soon after, reports of increased rates of disability or death following oxygen restriction suggested that absolute oxygen restriction might also be harmful. [1] What emerged was a need to better understand the risks at both ends of the oxygenation spectrum, in order to find the optimal balance of competing adverse outcomes.
NeOProM: Study Designs
In 2003, research clinicians and clinical trials experts from several countries conceived a plan to harmonize several planned comparative effectiveness research trials (CERTs) on SpO2 targeting. [2] By designing CERTs with similar populations, methods and endpoints, the goal would be a prospective individual patient meta-analysis of the data from all trials. Five trials were designed under three study groups; the Benefits of Oxygen Saturation Targeting (BOOST-II) trials, which included Australia, New Zealand (NZ), and the United Kingdom (UK); the Canadian Oxygen Trial (COT); and the Surfactant, Positive Pressure and Pulse Oximetry Randomized Trial (SUPPORT) in the United States.
All five trials randomly assigned infants less than 28 weeks gestation to one of two SpO2 target ranges: a lower saturation group (85–89%) and a higher saturation group (91–95%). [3–5] Blinding was maintained by using oximeters with software modification to read a 3% offset (either lower or higher) when in the SpO2 range of 88–92%; clinicians were then instructed to target this range for all study infants. Alarm limits (as distinct from target ranges) were pre-specified (COT), recommended (BOOST-II), or merely suggested (SUPPORT); for BOOST-II, the recommended alarm limits differed among the three trials. Targeting was initiated after initial stabilization but before 24 hours of age; in one trial (SUPPORT), targeting was initiated before 2 hours of age. In all trials targeting continued until 36 weeks postmenstrual age or when the infant was in ambient air.
The primary outcome for each of the trials was a composite of death or disability by 18–24 months corrected age; SUPPORT also had a primary short-term composite outcome of severe ROP or death before hospital discharge. [5] The Bayley Scales of Infant Development (BSID) were used to assess neurodevelopmental outcomes but the application of this tool differed among studies. Some of the centers in BOOST-NZ used the BSID-II while all other centers used the BSID-III. [6, 7] Since the BSID-III was known to underestimate disability compared to the BSID-II, [8] the NZ investigators used different cutoffs to define disability: 70 (−2SD) for the BSID-II and 85(−1SD) for the BSID-III. Although the other four trials all used the BSID-III, SUPPORT used a cutoff of 70 to define disability, [5] while COT and the other two BOOST trials (Australia and UK) used 85, similar to BOOST-NZ. Secondary outcomes included intraventricular hemorrhage, necrotizing enterocolitis (NEC), bronchopulmonary dyplasia, and ROP; death was a pre-specified secondary outcome only in the BOOST-II studies, but specific criteria (e.g., timing, cause) differed among the three BOOST study centers. [4]
NeOProM: Revised Pulse Oximeter Algorithms
In early 2009, the BOOST-II investigators in the UK found an unexpectedly low frequency of SpO2 readings in the range 87–90% among study subjects. [9] The oximeter manufacturer determined that this was due to a discontinuity in the calibration; they supplied revised software, but not before more than half of the BOOST-II study subjects, including all of the NZ subjects, had completed the targeting phase of the study. This change also occurred about midway in COT, but not in SUPPORT, as that trial had already been completed. Although not experienced by the COT investigators, the BOOST-II investigators reported improved SpO2 targeting; [10] this led them to specify a comparative analysis plan for subjects enrolled before and after pulse oximeter software revision.
NeOProM Results: No Differences in Primary Outcomes
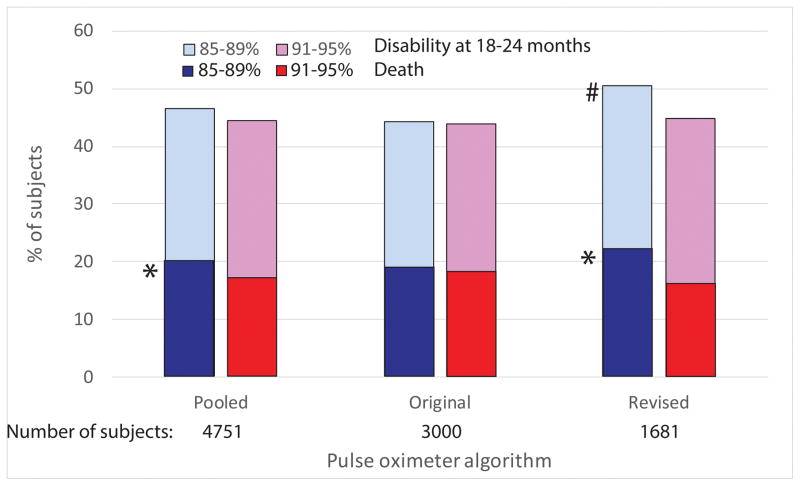
The first study to report a primary outcome was SUPPORT, and they found no difference between groups in the composite outcome of severe ROP or death before hospital discharge. [5] For the NeOProM primary outcome of death or disability by 2 years corrected age, neither SUPPORT nor COT found a difference between the two SpO2 target groups. [3, 11] The BOOST-II investigators reported a significant difference between groups in the combined outcome of death or disability, but only when they excluded the NZ cohort; [7] when all three BOOST-II trials are analyzed together no difference in the primary outcome was found. [12] The BOOST-II investigators reported an unadjusted aggregate meta-analysis of all five trials showing an overall difference between SpO2 groups in the primary outcome of death or disability. [13] However, this did not take into account the different BSID cutoffs between SUPPORT and the other trials in defining disability; in separate meta-analyses using a uniform BSID cutoff and with additional data provided by the SUPPORT investigators, no difference in the primary outcome was found (Figure 1). [14, 15]
Figure 1.
Rates of death and disability at 18–24 months from pooled (original and revised pulse oximeter algorithms), original pulse oximeter algorithm only and revised algorithm only. Data from BOOST-II (UK, Australia and NZ), COT and SUPPORT are combined. *mortality, p < 0.05 compared to 91–95% SpO2 target group; # combined outcome of death and disability, p < 0.05 compared to 91–95% SpO2 target group. Pooled data includes 70 additional infants from the COT trial who were monitored with both the original and revised algorithms; these are not included in the subgroup analyses.
NeOProM Results: Secondary Outcomes Raise Concerns
At first blush, the careful planning and hard work of the NeOProM study teams have left us with a definitive conclusion, that targeting a SpO2 of 91–95% versus 85–89% does not affect either the composite outcome of severe ROP or death, or the composite outcome of death or disability by 2 years corrected age. However, published analyses of secondary outcomes have cast doubt on the relative safety of a lower versus a higher SpO2 target range. Concerns were raised when SUPPORT investigators noted significant but opposing trends in the two elements composing the primary outcome; death before discharge was more likely in the lower SpO2 group, while severe ROP requiring treatment was more likely in the higher SpO2 group. While the difference in ROP might have been anticipated, the increase in mortality was not, but nevertheless suggested that oxygen targeting could represent a balance between competing adverse clinical outcomes.
The difference in mortality between study groups has prompted many NICUs to raise their SpO2 target ranges for ELGANs, but some have advised caution in adopting this approach. [16] In a meta-analysis of outcomes before and after study oximeter software revision, differences in mortality were not seen among more than 3000 infants monitored with the original software. [14, 15] Some have attributed the differences seen following the revised algorithm to better separation between study groups; however, post hoc analyses show no improvement in study group separation following software revision, [17] and no enhancement of treatment effect (mortality differences) among NICUs that achieved better separation. [18] An additional confounder in interpreting the mortality results is that two of the BOOST-II trials were stopped early based on an interim analysis of mortality, [10] thus biasing the results towards this outcome; this may explain why these two trials show the largest differences in death between study groups in the NeOProM collaborative.
An explanation for the increased mortality in the lower SpO2 group remains unclear. In all NeOProM trials, infants in the lower saturation group spent relatively more time with SpO2 < 85%, as might be expected; however, a systematic review could not demonstrate a relationship between time spent <85% and mortality. [15] Also, when comparing the original to the revised oximeter data, a greater mortality difference was seen although the amount of time infants spent <85% did not change. [4] Considering that the revised algorithm only affected readings between 87–91%, there may be modifying factors that place a subset of ELGANs at increased risk of mortality, even when subjected to only mild hypoxemia (SpO2 85–89%); for example, post hoc analyses of SUPPORT found that SGA but not AGA infants in the lower SpO2 group were at increased risk of mortality. [19, 20] This concept needs to be further explored in the complete NeOProM dataset.
If there were no concerns for adopting the higher SpO2 target range studied in the NeOProM trials, confirming a true mortality difference becomes less important. However, as might be expected based on past experience, and reaffirmed by a recent NeOProM meta-analysis, [21] the risk of severe ROP increases when a SpO2 target range of 91–95% is imposed; indeed, a recent observational study from a NICU in Australia found that their rate of severe ROP more than doubled after they changed their SpO2 target range from 88–92% to 91–95%. [22]
Limitations of Pulse Oximetry
Pulse oximetry gives a rough estimate of SaO2 and hence, arterial blood oxygen content. The oximeter used in the NeOProM trials had a reported accuracy of ± 3% (1SD); [9, 23] this means that 5% of subjects would have an actual SaO2 that is more than 6 points higher or lower than the reported SpO2. While this degree of inaccuracy doesn’t preclude its clinical usefulness for individual patients, it significantly limits the ability of pulse oximetry to discriminate between patients, or in the case of NeOProM, study group subjects.
More importantly, peripheral SaO2, which pulse oximetry is designed to estimate, tells us nothing about what is going on at the tissue level in terms of perfusion and oxygen transfer. It is therefore unclear how SpO2 targeting can be optimized to improve overall clinical outcomes. This weakness has been underscored in recent clinical studies in extremely preterm infants where near infrared spectroscopy (NIRS) was used as a measure of cerebral tissue oxygen saturation (SctO2), in conjunction with pulse oximetry. In studies of spontaneous desaturation episodes, SctO2 was almost always maintained during peripheral desaturation, even when deep (SpO2<70) or prolonged (>180 sec); [24, 25] in another study, investigators found that hyperoxia assessed by SctO2 but not SpO2 correlated with development of severe ROP. [26] While a useful adjunct for the management of infants who may require cardiorespiratory support, the utility of pulse oximetry in preventing oxygen-related tissue injury should be seriously questioned. Like the blind men trying to appraise the elephant, pulse oximetry gives us only one aspect of a complex situation, and taken alone can lead us to false conclusions.
Optimal SpO2 as a “Moving Target”
The optimal SpO2 for preterm infants has been described as a “moving target”, primarily because of the uncertainty that exists regarding the most appropriate range. [27] We would extend this argument, noting that it is physiologically implausible for a single SpO2 target range to be “optimal” for all preterm infants, or even for a single preterm infant across the duration of a NICU stay. Newborn infants undergo dramatic developmental changes during the first weeks of life; some of these changes, like the transition from fetal to adult hemoglobin (which is often accelerated in the NICU setting by frequent transfusions) significantly alter the physiology of tissue transfer oxygen for any given SpO2. [28] Coming from a moderately hypoxic intrauterine environment, the newborn infant is likely to have residual tolerance to hypoxemia, at least during the early postnatal period. [29] Perhaps most importantly, the adverse outcomes we are trying to prevent (e.g., ROP and NEC) have different postnatal vulnerability periods; since the “optimal” SpO2 target is a balance between competing outcomes, differences in pathophysiologic timing for different adverse outcomes suggest that the optimal SpO2 will also depend on postnatal and developmental age.
Conclusions
So how should be apply this information to clinical practice? We know that higher SpO2, especially during the first postnatal weeks, increases the risk of severe ROP; epidemiologic and biologic evidence to support this link is quite strong. The NeOProM trials further support that link but also suggest that lower SpO2 increases the risk of NEC and death. In trying to reconcile these competing clinical outcomes we note that the published results from the NeOProM collaboration seem to leave us with more questions than answers. Can we reconcile the differences between individual trials? How reliable are outcome differences in light of the tremendous overlap in SpO2 distributions between the two study groups? Why didn’t better separation between study groups lead to larger treatment effects? What is the effect on a secondary outcome measurement when trials are stopped early for evidence of benefit in that secondary outcome? Are there patient characteristics that affect the risk of either a lower or higher SpO2 target? Why was there such a dramatic change in treatment effect in some centers after using oximeters with the revised software? Is there biologic plausibility for an increased risk of adverse outcomes for modest (87–90%) but not moderate (<85%) hypoxemia? Can targeting SpO2 optimize clinical outcomes?
We must be careful when interpreting the results from NeOProM. While the collective results from these trials support using a SpO2 target range of 91–95% compared to 85–89%, they give us no guidance about any other target range. In particular, given the high degree of overlap between study groups, as well as the significant deviation beyond the specified target ranges, it is possible that a wider target range that partially (or even totally) encompasses both study group ranges may be associated with similar outcomes. For example, while it seems prudent to avoid a target range of 85–89%, that does not mean that a target range of 86–94% would not be preferable to 91–95%. It is interesting to note that the European Consensus Guidelines chose a target SpO2 range of 90–94% for preterm infants with RDS after evaluating the NeOProM results; they also noted this was a weak recommendation based on the quality of evidence. [30] It may also make physiologic sense to titrate SpO2 target ranges based on individual patient characteristics, such as gestational age, postmenstrual age, and transfusion status; some have embraced this concept of a “moving target” for SpO2. [31]
The NeOProM trials also give us insight into narrower target ranges, not just different target ranges. These trials suggest that narrowing the target range increases the time spent outside that range; infants in NeOProM were only within target about 50% of the time. This tendency to stray outside narrowly confined target ranges may explain the increased risk of severe ROP in the higher SpO2 target group (which spent relatively more time above 95%) and the increased mortality in the lower SpO2 target group (which spent relatively more time below 85%). If a wider SpO2 target range, say 87–95%, were allowed, it is likely that infants would spend less time either below 85% or above 95%, compared to infants in the lower and higher target groups of the NeOProM, respectively.
A detailed individual patient data analysis of the NeOProM trials, wherein individual infant outcomes are correlated with their actual SpO2 frequency distributions, regardless of target group assignment, is sorely needed as it could shed light on many of the questions raised above. This was the goal of NeOProM and we eagerly await these analyses. However, we remain skeptical that a single, optimal SpO2 target for the extremely low gestational age infant will be clinically useful. Rather, efforts should be directed at minimizing supplemental oxygen exposure, reducing wide fluctuations in oxygenation, and eliminating alarm fatigue – factors known to be associated with poorer outcomes. Advances in avoiding oxygen-related organ injury, either from too little or too much, will come from reliable methods to assess tissue oxygenation in the clinical setting and a better understanding of the pathophysiology of oxygen-related organ injury in this highly vulnerable population.
Key Points.
SpO2 is a poor estimator of tissue oxygenation.
Targeting SpO2 is a balance of competing adverse outcomes.
Carefully conducted CERTs suggest that targeting an upper SpO2 limit <90% may be harmful.
Individual (patient-level) data analyses from NeOProM are necessary to fully examine the relationship between SpO2 and clinical outcomes.
It is unlikely that a single SpO2 target range for the ELGAN will be clinically useful.
Acknowledgments
Funding
Dr. Lakshminrusimha was supported by RO1 HD072929.
Footnotes
Conflicts of Interest
Dr. Cummings is a paid consultant for ONY, Inc (study chair) and Glaxo-Smith-Kline (data monitor). He is also a co-investigator for a clinical trial sponsored by Windtree Therapeutics but receives no direct compensation for that activity.
Dr. Lakshminrusimha has no conflicts of interest.
Financial support and sponsorship
N/A
Contributor Information
James J. Cummings, Professor and Vice Chair of Pediatrics, The Children’s Hospital at Albany Medical Center, Department of Pediatrics, 43 New Scotland Avenue, Mailstop 88, Albany, NY 12208, Phone: (518) 262-5421.
Satyan Lakshminrusimha, Professor and Vice Chair of Pediatrics, Women and Children’s Hospital of Buffalo, Department of Pediatrics, 219 Bryant Street, Buffalo, NY 14222, Phone: (716) 878-7673.
References
- 1**.Cummings JJ, Polin RA AAP Committee On Fetus and Newborn. Oxygen targeting in extremely low birth weight infants. Pediatrics. 2016;138(2):e20161576. doi: 10.1542/peds.2016-1576. This Clinical Report from the AAP discusses the limitations of pulse oximetry for assessing oxygenation and summarizes CERTs of SpO2 targeting trials. It addresses implications for clinical practice, noting that the ideal SpO2 for the preterm infant is unknown and likley to be dynamic as well as a compromise among negative outcomes. [DOI] [PubMed] [Google Scholar]
- 2.Cole C, Wright K, Tarnow-Mordi W, et al. Resolving our uncertainty about oxygen therapy. Pediatrics. 2003;112:1415–1419. doi: 10.1542/peds.112.6.1415. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt B, Whyte RK, Asztalos EV, et al. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA. 2013;309(20):2111–2120. doi: 10.1001/jama.2013.5555. [DOI] [PubMed] [Google Scholar]
- 4.Stenson BJ, Tarnow-Mordi WO, Darlow BA, et al. Oxygen saturation and outcomes in preterm infants. N Engl J Med. 2013;368(22):2094–2104. doi: 10.1056/NEJMoa1302298. [DOI] [PubMed] [Google Scholar]
- 5.SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362(21):1959–1969. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.The BOOST-II Australia and United Kingdom Collaborative Groups. Outcomes of two trials of oxygen-saturation targets in preterm infants. N Engl J Med. 2016;374(8):749–760. doi: 10.1056/NEJMoa1514212. This is the last primary outcome report from the NeOProM trials, summarizing the results of two of the three BOOST-II trials. (The primary outcome for BOOST-NZ had already been reported.) There was no difference in the composite outcome of death or disability at 2 years of age in each of two trials. However, in a post hoc, unadjusted analysis combining the two trials the authors report an increased incidence of death or disability in the lower-target group compared to the higher-target group, driven primarily by significant differences seen after the pulse oximeter algorithm was revised. [DOI] [PubMed] [Google Scholar]
- 7.Darlow BA, Marschner SL, Donoghoe M, et al. Randomized controlled trial of oxygen saturation targets in very preterm infants: two year outcomes. J Pediatr. 2014;165(1):30–35. e32. doi: 10.1016/j.jpeds.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Anderson PJ, De Luca CR, Hutchinson E, et al. Underestimation of developmental delay by the new Bayley-III scale. Arch Pediatr Adolesc Med. 2010;164(4):352–356. doi: 10.1001/archpediatrics.2010.20. [DOI] [PubMed] [Google Scholar]
- 9.Johnston ED, Boyle B, Juszczak E, et al. Oxygen targeting in preterm infants using the Masimo SET Radical pulse oximeter. Arch Dis Child Fetal Neonatal Ed. 2011;96(6):F429–433. doi: 10.1136/adc.2010.206011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stenson B, Brocklehurst P, Tarnow-Mordi W. Increased 36-week survival with high oxygen saturation target in extremely preterm infants. N Engl J Med. 2011;364(17):1680–1682. doi: 10.1056/NEJMc1101319. [DOI] [PubMed] [Google Scholar]
- 11.Vaucher YE, Peralta-Carcelen M, Finer NN, et al. Neurodevelopmental outcomes in the early CPAP and pulse oximetry trial. N Engl J Med. 2012;367(26):2495–2504. doi: 10.1056/NEJMoa1208506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cummings JJ, Lakshminrusimha S, Polin RA. Oxygen-saturation targets in preterm infants (letter) N Engl J Med. 2016;375(2):186–187. doi: 10.1056/NEJMc1604023. [DOI] [PubMed] [Google Scholar]
- 13*.Tarnow-Mordi W, Stenson B, Kirby A. Oxygen-saturation targets in preterm infants (reply) N Engl J Med. 2016;375(2):187–188. doi: 10.1056/NEJMc1604023. In a reply to a letter to the editor, the BOOST-II UK and Australia group provided an unadjusted meta-analysis of aggregate data on death or major disability from NeOProM trials using definitions of composite outcome as reported in the individual trials. The authors reported that this combined outcome was more common in the lower SpO2 target group. However, one of the trials (SUPPORT) used a BSID-III cutoff score of < 70 to define major disability, whereas the other trials used a BSID-III cutoff score of < 85. [DOI] [PubMed] [Google Scholar]
- 14.Davis P. Oxygen saturation targeting in preterm infants. Cochrane Database Syst Rev. 2016 in press. [Google Scholar]
- 15**.Manja V, Saugstad OD, Lakshminrusimha S. Oxygen saturation targets in preterm infants: Death and disability at 18–24 mo: A systematic review. Pediatrics. 2016 doi: 10.1542/peds.2016-1609. in press. This systematic review used previously unpublished data from SUPPORT in order to redefine disability based on a BSID-III cutoff score of < 85, thereby aligning disability definitions among all five NeOProM trials. A pooled analysis of these trials showed no difference in the primary, composite outcome of death or disability at 18–24 months. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sola A, Golombek SG, Montes Bueno MT, et al. Safe oxygen saturation targeting and monitoring in preterm infants: can we avoid hypoxia and hyperoxia? Acta Paediatr. 2014;103:1009–1018. doi: 10.1111/apa.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whyte RK, Nelson H, Roberts RS, Schmidt B. Oximeter software revision and infant saturations in the Benefits of Oxygen Saturation Targeting (BOOST) Trials. J Pediatr. 2016 doi: 10.1016/j.jpeds.2016.11.076. in press. [DOI] [PubMed] [Google Scholar]
- 18*.Schmidt B, Whyte RK, Shah PS, et al. Effects of targeting higher or lower oxygen saturations in centers with more versus less separation between median saturations. J Pediatr. 2016;178:288–291. e282. doi: 10.1016/j.jpeds.2016.08.002. The COT investigators conducted a subgroup analysis to compare outcomes of preterm infants in centers with more versus less separation between median SpO2 for the two study groups. Centers with more separation did not observe larger treatment effects favoring the higher SpO2 group; in fact, centers with more separation actually reported lower rates of death, disability, or the composite outcome in the lower SpO2 target range group than in the higher SpO2 target range group. These post hoc analyses support the COT authors’ previous conclusion that targeting an SpO2 range of 85–89% vs 91–95% does not affect important clinical outcomes like death or disability. [DOI] [PubMed] [Google Scholar]
- 19.Walsh MC, DiFiore JM, Martin RJ, et al. Association of oxygen target and growth status with increased mortality in small for gestational age infants: Further analysis of the Surfactant, Positive pressure, and Pulse Oximetry Randomized Trial. JAMA Pediatr. 2016;170(3):292–294. doi: 10.1001/jamapediatrics.2015.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Fiore JM, Martin RJ, Li H, et al. Patterns of oxygenation and mortality in the Surfactant, Positive pressure and Oxygen Trial (SUPPORT) cohort. J Pediatr. 2016 doi: 10.1016/j.jpeds.2017.01.057. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Askie L, Darlow B, Finer N, et al. Oxygen saturation targeting in extremely preterm infants: the prospectively planned, NeOProM individual participant data meta-analysis (abstract) European Journal of Pediatrics. 2016 in press. [Google Scholar]
- 22*.Manley BJ, Kuschel CA, Elder JE, et al. Higher rates of retinopathy of prematurity after increasing oxygen saturation targets for very preterm infants: Experience in a single center. J Pediatr. 2016;168:242–244. doi: 10.1016/j.jpeds.2015.10.005. In this single center, observational study, the authors found that a change from an SpO2 target range of 88–92% to 91–95% was associated with an increased rate and severity of ROP. For infants less than 28 weeks gestation at birth, the rate of Stage II or worse ROP increased from 16 to 35%. Although an increase in ROP was not unexpected, it occurred despite a relatively modest change in SpO2 targets compared to the NeOProM trials. [DOI] [PubMed] [Google Scholar]
- 23.Masimo Corporation. Radical-7 Signal Extraction Pulse Co-Oximeter with Rainbow Technology Operator’s Manual. 2010. [Google Scholar]
- 24.Schmid M, Hopfner R, Lenhof S, et al. Cerebral oxygenation during intermittent hypoxemia and bradycardia in preterm infants. Neonatology. 2015;107(2):137–146. doi: 10.1159/000368294. [DOI] [PubMed] [Google Scholar]
- 25.Waitz M, Schmid MB, Fuchs H, et al. Effects of automated adjustment of the inspired oxygen on fluctuations of arterial and regional cerebral tissue oxygenation in preterm infants with frequent desaturations. J Pediatr. 2015;166(2):240–244. e241. doi: 10.1016/j.jpeds.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Vesoulis ZA, Lust CE, Liao SM, et al. Early hyperoxia burden detected by cerebral near-infrared spectroscopy is superior to pulse oximetry for prediction of severe retinopathy of prematurity. J Perinatol. 2016;36(11):966–971. doi: 10.1038/jp.2016.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Askie LM. Optimal oxygen saturations in preterm infants: a moving target. Curr Opin Pediatr. 2013;25(2):188–192. doi: 10.1097/MOP.0b013e32835e2c00. [DOI] [PubMed] [Google Scholar]
- 28.Evans RG. Oxygen regulation in biological systems. Am J Physiol Regul Integr Comp Physiol. 2016;310(8):R673–678. doi: 10.1152/ajpregu.00004.2016. [DOI] [PubMed] [Google Scholar]
- 29.Martin DS, Khosravi M, Grocott MP, Mythen MG. Concepts in hypoxia reborn. Critical Care Med. 2010;14:315–321. doi: 10.1186/cc9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30**.Sweet DG, Carnielli V, Greisen G, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome - 2016 Update. Neonatology. 2017;111:107–125. doi: 10.1159/000448985. In the latest, revised version of these consensus guidelines, an SpO2 target of 90–94% was recommended in preterm babies receiving oxygen. After considering all the data available from the NeOProM trials, the quality of evidence was rated as ‘moderate’ and the strength of recommendation was assessed as ‘weak’. [DOI] [PubMed] [Google Scholar]
- 31.Cayabyab R, Arora V, Wertheimer F, et al. Graded oxygen saturation targets and retinopathy of prematurity in extremely preterm infants. Pediatr Res. 2016;80(3):401–406. doi: 10.1038/pr.2016.98. [DOI] [PubMed] [Google Scholar]