Abstract
Immunosuppression can allow organisms that are not usually pathogenetic to cause disease; under such circumstances, Aspergillus species frequently form large masses of fungal elements. We describe the case of a 12-year-old girl with hematologic remission of leukemia. She had a left ventricular pedunculated mass that was detected by echocardiographic study; at surgery, the presence of Aspergillus terreus was confirmed.
Key words: Aspergilloma; aspergillosis; Aspergillus/pathogenicity; Aspergillus terreus; child; echocardiography, transthoracic; endocarditis/complications; female; heart ventricle/pathology; human; immunocompromised host; leukemia; opportunistic infections/etiology
Cardiac fungal infections, although rare, are being seen more often because of the rise in placement of central venous lines and indwelling catheters, implantation of prosthetic heart valves, and the use of anti-neoplastic agents and antibiotics, among other reasons.1 Such infections are most often found in cases of myocarditis, endocarditis, and intracardiac fungal mass.1 Aspergillus endocarditis typically occurs in severely immunosuppressed patients and is generally considered fatal because it is so difficult to diagnose and treat.2,3 Fungal mural endocarditis is rarely diagnosed antemortem, and then only when a cardiac mass is involved. In such cases, 2-dimensional echocardiography is valuable for early diagnosis. Because a pedunculated mass in the left ventricle presents a clinical problem and raises the possibility of thromboembolism with potentially catastrophic consequences, prompt surgical intervention is necessary.
We describe a rare case of a pedunculated left ventricular mass that was found by use of transthoracic echocardiography (TTE). The mass was confirmed to be a fungal vegetation at surgery.
Case Report
A 12-year-old girl was diagnosed at our institution with acute lymphoblastic leukemia in September 2002. Polychemotherapy was initiated, and complete hematologic remission was achieved in November 2002. The next month, however, the patient was hospitalized due to persistent fever, nausea, vomiting, and abdominal pain.
Laboratory investigations disclosed anemia (hemoglobin, 7 g/dL), thrombocytopenia, and leukopenia. A bone marrow biopsy revealed no sign of leukemic infiltration. The patient did not have pulmonary or urinary tract infection. Hepatomegaly was found. Results of all blood cultures were negative.
Auscultation revealed a pericardial friction rub; therefore, TTE was performed. The echocardiogram revealed a pedunculated mobile mass in the apex of the left ventricle (Fig. 1A). Embolic infarctions in the spleen, liver, and kidneys were detected by computed tomographic (CT) scanning.
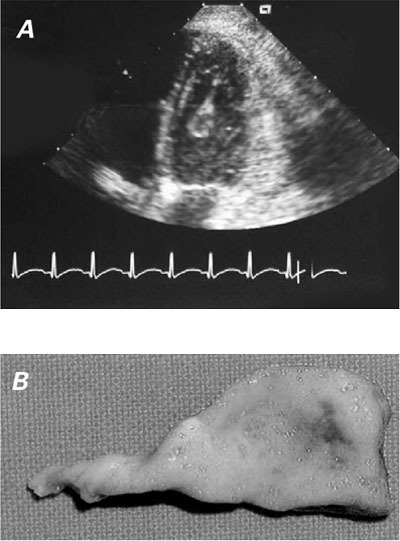
Fig. 1 A) Transthoracic echocardiogram shows a pedunculated mass in the left ventricle. B) Left ventricular fungal vegetation.
Treatment with vancomycin (60 mg/kg), gentamycin (5 mg/kg), and amphotericin B (1 mg/kg) was started 1 day after admission. Because of the risk of embolization due to the mobile nature of the mass, the patient was taken to surgery the next day.
The chest was opened by means of a median sternotomy. The pericardium did not appear to be affected by the mass. After cardiopulmonary bypass was established, the aorta was cross-clamped, and anterograde cold blood cardioplegic solution was administered. A left ventriculotomy was performed, and a large pedunculated mass (3.6 cm in length × 1.2 cm maximum width) was seen attached to the apex and was excised (Fig. 1B). There were small yellow nodules on the left ventricular wall; therefore, a small myectomy was performed. The mitral valve was normal. The patient was weaned successfully from cardiopulmonary bypass.
The patient's recovery was uneventful, with no sign of recurrence of the aspergillus endocarditis. On the 28th postoperative day, however, she was walking and experienced severe headache and dizziness; after that, she went into a coma. Computed tomographic scans showed a cerebral hemorrhage that involved the right parietal lobe. The patient died 1 day later; autopsy was refused by her parents.
Cultures of the mass and nodules of the left ventricle wall, collected during surgery, grew Aspergillus terreus. Pathologic study of the left ventricular tissue samples, also obtained during surgery, showed dichotomously branched septate hyphae of aspergillus within the tissue (Fig. 2).
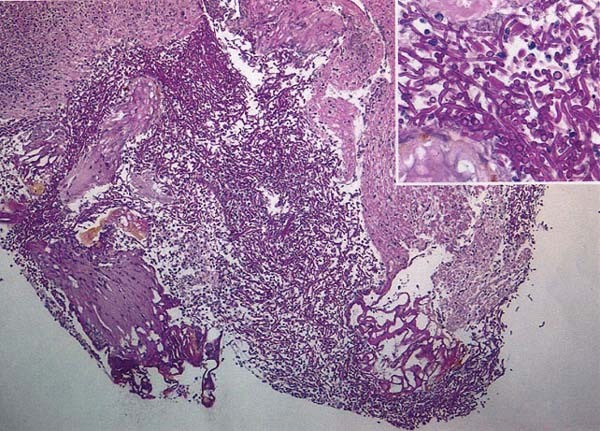
Fig. 2 Pathologic examination of the fungal mass shows septated, dichotomous hyphae.
Discussion
Aspergillus endocarditis is an opportunistic infection that usually ends in the death of the affected patient because of the difficulty of diagnosis and treatment.3 The prognosis of aspergillus endocarditis appears to be equally grim whether it occurs on a native or prosthetic valve, and at least 80% of cases of aspergillus endocarditis occur on the prosthetic valves.4 The remainder are associated with disseminated aspergillus in immunosuppressed patients in whom both endocarditis and myocardial abscesses develop.5
Immunosuppression can allow organisms that are not usually pathogenic to cause disease. Aspergillus species frequently form large fungal masses, which can then embolize. Two-dimensional echocardiography is a reliable diagnostic tool to identify a mass in the heart. In our patient, TTE showed a pedunculated mass in the left ventricle and, because she was imunosuppressed, we suspected the diagnosis of apergillus endocarditis. Once the diagnosis of an intracavitary mass was established, we initiated prompt surgical intervention.
Walsh and Hutchins5 found a 40% prevalence of aspergillus mural endocarditis in patients who had aspergillosis with cardiac involvement. The first occurrence of endocardial vegetations can occur as a subendocardial focus,5 as in our patient, in whom small yellow nodules were found on the left ventricular wall and aspergillus growth was demonstrated.
The aspergilloma, a noninvasive form of aspergillosis,6 can develop in a healthy host. In such cases, the organism colonizes a cavity that has been created by some form of destructive process that has previously involved the heart, such as leukemia. Cardiac leukemic infiltrates are found in most postmortem studies of patients with acute leukemia; therefore, infective endocarditis, commonly fungal, can complicate cases of acute leukemia.7,8
We conclude that, given the progressive increase in the number of immunosuppressed patients, as well as the increasing prevalence of cardiac fungal infections, clinicians should be aware of all possible presentations of invasive or noninvasive aspergillosis.
Footnotes
Address for reprints: José Rubio Alvarez, MD, Framan – Bugallido, 15866 La Coru-a, Spain
E-mail: framan1@hotmail.com
References
- 1.Alam M, Higgins R, Alam Z, Janakiraman N, Gorman M. Aspergillus fungal mass detected by transesophageal echocardiography. J Am Soc Echocardiogr 1998;11(1):83–5. [DOI] [PubMed]
- 2.Shett G, Casati B, Willinger B, Weinlander G, Binder T, Grabenwoger F, et al. Endocarditis and aortal embolization caused by Aspergillus terreus in a patient with acute lymphoblastic leukemia in remission: diagnosis by peripheral-blood culture. J Clin Microbiol 1998;36(11):3347–51. [DOI] [PMC free article] [PubMed]
- 3.Navabi MA, Ajami H, Amirghofran A, Peyravian F. Aspergillus endocarditis: rare but serious Aspergillus ball obstructing the pulmonary artery. Eur J Cardiothorac Surg 1998;14:530–2. [DOI] [PubMed]
- 4.Kammer RB, Utz JP. Aspergillus species endocarditis. The new face of a not so rare disease. Am J Med 1974;56:506–21. [DOI] [PubMed]
- 5.Walsh TJ, Hutchins GM. Aspergillus mural endocarditis. Am J Clin Pathol 1979;71(6):640–4. [DOI] [PubMed]
- 6.Khan ZU, Kortom M, Marouf R, Chandy R, Rinaldi MG, Sutton DA. Bilateral pulmonary Aspergilloma caused by an atypical isolate of Aspergillus terreus. J Clin Microbiol 2000;38(5):2010–4. [DOI] [PMC free article] [PubMed]
- 7.Hunkeler N, Canter CE. Antemorten diagnosis of gross cardiac metastasis in childhood leukemia: Echocardiographic demonstration. Pediatr Cardiol 1990;11(4):225–6. [DOI] [PubMed]
- 8.Perry DJ, McCormick D, Veasey S, Cohen IS. Right heart obstruction due to intracavitary prolymphocytic leukemia. Am J Med 1986;81(1):131–4. [DOI] [PubMed]


