Abstract
The occupational health and safety program is an integral component of a comprehensive animal care and use program. It is important to mitigate the risk of exposures of animal care and research personnel to allergens and physical, chemical, radiologic, and biologic hazards during the conduct of various tasks. This need is especially true in infectious disease and biocontainment research. One aspect of the program is the provision of personal protective equipment (PPE). Commercially available PPE should be carefully evaluated based on their material composition and performance according to manufacturer data. To help institutions and end users by providing them guidance on choosing appropriate PPE, we here discuss the regulatory framework, device standards, and materials engineering for various PPE, including gloves, shoe covers, head caps, gowns, aprons, masks, hearing and eye protection devices, and respirators. Ultimately, the choice of appropriate PPE is based on the risk assessment, which should include consideration for personnel comfort, correct device fitting, and the containment level for the hazard used.
Abbreviations: AAMI, Association for the Advancement of Medical Instrumentation; ANSI, American National Standards Institute; ASTM, American Society for Testing and Materials; NIOSH, National Institute of Occupational Safety and Health; NRL, natural rubber latex; PAPR, powered air-purifying respirator; PPE, personal protective equipment
The use of animals in research comes with the innate risk of accidental exposure of personnel to various hazards. Animals produce allergens from body secretions and products including dander, urine, and saliva. Chemicals like chlorine-based solutions and quaternary ammonium compounds are commonly used for environmental sanitation and disinfection. Others, like bromodeoxyuridine and tricaine methanesulfonate, and radioactive substances, such as bioimaging tracers, are used for animal experimentation. Biohazards include zoonotic agents (for example, Macacine herpesvirus 1 in macaques), infectious organisms used to model human disease, and more commonly, the use of recombinant and synthetic nucleic acid molecules and cells, organisms, and viruses containing such molecules. Finally, personnel exposure to high noise levels can occur during the care of certain animal species or when using noise-generating equipment, such as cage and rack washers.
A 3-fold management approach is needed, as enumerated in the Guide for the Care and Use of Animals to mitigate exposure risks.26 A robust occupational health and safety program can only be described in positive terms when associated with these components. First, engineering controls entail appropriate safety equipment provision and facility design and operation. Second, administrative controls need to be implemented to clearly describe processes and standard operating procedures. Finally, when exposure to hazards cannot be engineered completely out of normal operations and when safe work practices and other forms of administrative controls cannot provide sufficient additional protection, the use of personal protective equipment (PPE) provides a supplementary means of control and serves as the last line of defense for risk exposure. Education and training are embedded in these 3 components and will ensure full implementation of safety standards and practices and personnel compliance. In the current review, we aim to provide a reference for personnel on the selection and appropriate use of various PPE in full consideration of industry and regulatory standards. However, a thorough and comprehensive discussion of the standards is beyond the scope of this article, and we therefore direct readers to the standards for more information.
Regulatory Framework
The Occupational Safety and Health Act of 1970 (29 CFR 15) was promulgated to protect employees from hazards in the workplace.33 The Personal Protective Equipment standard (Subpart I 29 CFR 1910) requires PPE to be selected on the basis of the hazards present and that employers provide workers with appropriate PPE, such as those for the eyes, face, head, feet, and hands, which must be worn to reduce the potential for harm and injury.11 This PPE standard specifically puts primary responsibility on the employer, because the text outlines the process for the selection of appropriate PPE, training on its correct use, and its replacement and disposal.11 As a general rule, PPE must be provided, used, and maintained in reliable conditions whenever hazards in the workplace can cause injury or impairment from physical contact.11 The Occupational Health and Safety Administration (OSHA) requires that many categories of PPE meet or be equivalent to standards developed by the American National Standards Institute (ANSI).35 The National Institute of Occupational Safety and Health (NIOSH) is the responsible organization for testing and certification for respirators.16
OSHA's Bloodborne Pathogen Standard (29 CFR 1910.1030) requires employers to provide and ensure that employees use appropriate PPE such as, but not limited to, gloves, gowns, laboratory coats, face shields or masks, and eye protection when handling human blood or other potentially infectious materials.34 OSHA has indicated that “home laundering is unacceptable because the employer cannot ensure that proper handling or laundering procedures are being followed and because contamination could migrate to the homes of employees.”34 Employers are thus responsible for cleaning, laundering, and disposing of PPE.13
Other pertinent OSHA standards include 29 CFR 1910.134 (Respiratory Protection Standard)11 and 29 CFR 1910.95 (Occupational Noise Exposure Standard).10 The former was established to prevent potential occupational illnesses caused by exposure to airborne contaminants, including potentially infectious aerosols, whereas the latter was enacted to protect employees against the effects of high-intensity occupational noise.
Specific to animal research, an occupational health and safety program should be established based on the guidelines described in the Guide, which outlines that the program be consistent with federal, state, and local regulations.26 The Guide also encourages each institution to tailor needs, such as PPE, to its specific program.26 One of the foremost resources is Biosafety in Microbiologic and Biomedical Laboratories (5th edition) by the Centers for Disease Control and Prevention and NIH.18 This reference is considered to be the minimum standard of practice for all United States laboratories that handle infectious microorganisms and hazardous biologic materials. It provides information on good work practices, appropriate PPE, safety equipment, and laboratory facility design for each biosafety level. Table 3 of Section V (Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities) summarizes recommended practices, PPE, and primary and secondary barrier characteristics. Lastly, the NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (2016) describes the practices for constructing and handling recombinant and synthetic nucleic acid molecules, including those that are chemically or otherwise modified but can basepair with naturally occurring nucleic acid molecules, and cells, organisms, and viruses containing such molecules.29
Table 3.
Comparison of 3 types of gloves commonly used in health care and biomedical research settings
| Nature of material | Advantages | Disadvantages | Comments | |
| Latex | Natural rubber | Comfort and fit; dexterity (high level of touch sensitivity); elastic and strong; easy to put on; biodegradable; low cost | Can cause latex allergy;a poor for organic solvents; little chemical protection; difficult to detect puncture holes; frequently imported; may be poor quality | Petroleum-based hand lotions or creams may adversely affect the integrity of the gloves |
| Nitrile | Synthetic acrylonitrile butadiene rubber | Comfort and fit; dexterity (high level of touch sensitivity); superior puncture resistance; clear indication of tears and breaks; resists many chemicals; long shelf life | More expensive than latex and vinyl; stiffer than latex | Chemical accelerators and other additives commonly used in production may elicit allergy symptoms in sensitive persons. |
| Vinyl | Synthetic polyvinyl chloride | Less expensive; antistatic properties; easy to put on | Less durable; limited dexterity; looser fit; plasticizers can be stripped; frequently imported; may be poor quality; nonbiodegradable | Popular in industries (for example, food) where high levels of durability and protection are less of a priority |
Natural rubber latex gloves are mislabeled when packaging does not include the statement “Caution: This product contains natural rubber latex which may cause allergic reactions,” as required by 21 CFR 801.437 (User Labeling for Devices that Contain Natural Rubber).15
Risk Assessment
The first step in the selection of appropriate PPE is to conduct a risk assessment. In practical terms, risk assessment means reviewing the workplace to identify hazards or processes, evaluating the risk associated with those hazards, and determining the appropriate measures that should be in place to effectively eliminate or control the hazard. Personnel should be evaluated according to several factors including special medical conditions such as pregnancy, immune status, and ill health. For example, personnel with asthma likely may not be able to use N95 respirators because of their increased breathing resistance and should instead consider using a powered air-purifying respirator (PAPR). In addition, wearing cultural and religious clothing such as a headdress can provide a unique opportunity to assess potential accommodations for personnel protection. Furthermore, the animal species with which personnel would be working need to be considered as well. For example, working with NHP necessitates the use of additional PPE than what may be required during noninfectious research using mice. PPE must be chosen in light of the appropriate containment level as dictated by hazard identification. The nature of activities, especially the potential for aerosolization, is a significant consideration for risk assessment. Surgical procedures, especially those involving tissues that potentially can have high concentrations of an infectious agent, may pose a greater risk than routine husbandry procedures.
Issuing a form for personnel to complete can facilitate medical surveillance and risk assessment. Relevant information includes the person's medical status, the animals used, and any potential hazards exposure. In addition, risk assessment carefully evaluates the facility and its equipment and bridges the gap between engineering and administrative controls. PPE can be truly effective only when used correctly in the prescribed conditions (dependent on animal species, hazard, personnel considerations) and with sufficient and appropriate training. Full protective clothing can be worn for any procedure, but the clothing itself will not offer complete protection if it is not donned and doffed properly. Additional PPE should be considered when procedures are performed without engineering controls that minimize risk exposure. Respiratory protection, for example, may be needed in addition to a simple gown and gloves when changing rodent cages without the use of a cage-changing station. PPE is to be used only as a supplement to—and not as a replacement for—engineering standards and adequate administrative processes.24,37 Final determination of PPE requirements needs to be made collectively by a team which includes safety professionals, occupational health professionals, and veterinary and husbandry personnel and should include input from research personnel.
The risk assessment process is defined in various ways depending on the environment and associated hazards. However, a standard stepwise process in assessing and mitigating risks is generally accepted throughout industry covers most situations and serves as a building block for facilities to develop their own procedures. The process is broken down to first identifying the hazards within the workplace, systematically assessing those hazards to identify the probability of the hazard occurring and the severity of the hazard if it were to occur, identifying and applying controls to mitigate those risks to an acceptable tolerance level, and evaluating the success of the controls in actually mitigating the hazards. It is helpful to develop forms such as a risk assessment matrix and a risk assessment worksheet for establishing and reviewing the hazards as part of the process. A matrix is helpful in identifying the probability of the risk happening as compared with the severity if it does happen. Probability for the hazard to occur may be defined as unlikely, seldom, occasional, likely, and frequent. The severity of the hazard if it does occur may be defined as negligible, moderate, critical, or catastrophic. The actual risk level, determined by combining the severity and probability levels, may be defined as low risk, moderate risk, high risk, and extremely high risk.
The risk assessment worksheet should identify the main task, subtasks associated with the hazard, subhazards associated with the subtasks, the initial risk level as determined by the matrix, the residual risk level after controls are implemented, how the control will be implemented, who will supervise it, and whether the control was effective. For example, a main task is to draw blood on a nonsedated Old World NHP. A subtask may include drawing blood on a herpesvirus B-positive cynomolgus macaque. A subhazard bite risk exists to the person performing the procedure. When completing a risk assessment matrix, the probability of a monkey bite happening is determined as occasional, but the severity if it does happen is critical. The risk level determined by the matrix results in a high risk. A mitigating control includes the addition of para-aramid synthetic fiber reinforced outer gloves when performing the procedure. When completing the risk assessment worksheet, the initial level is high, but the residual level decreases the risk level to moderate when the use of reinforced outer gloves is added. Adding additional controls, such as animal handling training, may further decrease the risk level to low risk. The control is implemented by procuring cut-resistant gloves (for example, Kevlar) and training employees on the correct way to use them. The immediate supervisor observes correct use of the gloves and determines that the gloves are effective in decreasing the severity of bite wounds, if they were to occur.
It is important to understand that a strong hazard-analysis program is dependent on not just identifying and mitigating the risks but also on communicating to and training personnel of the hazards identified and the controls implemented. Training should be thorough and documented, and the effectiveness of controls in eliminating the hazards and controlling risks should be continually evaluated. Risk assessments should be reassessed regularly, to identify any new hazards that were not identified as part of the initial assessment and to document new risks that evolved during an activity. Risk assessments can be—and in many cases should be—intertwined with standard operating procedures and required as part of the periodic review of such procedures in place. In addition, a risk decision authority, who takes responsibility for the overall assessment and follow-up process in the facility, should be identified.
Terminology
Several terms need to be defined in describing the characteristics of various PPE. It is important to note that there is no industry consensus for using these terms. The FDA does not approve marketing PPE (especially surgical gowns or drapes) with labeling claims using the terminology of ‘fluid-resistant’ or ‘impermeable.’ Instead, the manufacturers must provide fabric or garment specifications associated with the standard test methods or standard classifications. For the purposes of the current review, ‘fluid-resistant’ applies to protective clothing that has been tested against water as the liquid challenge,30 whereas ‘impermeable’ is understood to mean that the material has demonstrated blockage of microorganisms in a recognized standard test method.30
PPE Components
Aprons, isolation gowns, coveralls, and sleeve protectors.
OSHA requires that aprons, isolation gowns, coveralls, and sleeve protectors meet or exceed standards developed by ANSI. The applicable standard developed by the Association for the Advancement of Medical Instrumentation (AAMI) and approved by ANSI for protective apparel is described in ANSI/AAMI PB70:2012—Liquid Barrier Performance and Classification of Protective Apparel and Drapes Intended for Use in Health Care Facilities (PB70).3 This second edition establishes a system of classification for protective apparel and drapes used in health care facilities on the basis of their liquid barrier performance. PB70 also specifies related labeling requirements and standardized test methods for determining compliance. Its scope covers all types of protective apparel that are labeled with liquid- barrier claims or liquid-borne microbial barrier claims (including single-use and multiple-use surgical gowns, decontamination garments, isolation gowns, aprons, sleeve protectors, laboratory attire, and other garments) and that are regulated by the FDA as medical devices under 21 CFR 878 (General and Plastic Surgery Devices).12 Items not covered by PB70 include protective apparel for the head, face and eyes, and feet, such as face shields, surgical caps, surgical masks, respirators, and shoe covers. Device standards, although primarily directed to the manufacturer, may also be of value to the device purchaser or user as a frame of reference for device evaluation.3
Protective apparel have a number of safety and performance characteristics that are based on PB70, including barrier effectiveness, abrasion resistance, strength, comfort, aesthetic acceptability, electrostatic properties, flammability, and strike-through (that is, the passage of a liquid that could contain microorganisms through a barrier product) investigation. The primary reason for the development of PB70 was the classification of barrier effectiveness on the basis of resistance to liquid and microbial penetration (Table 1). Briefly, the liquid challenge differs among the various barrier performance levels because the surface tension of water is much higher than that of blood, such that blood penetrates fabrics more readily than water does. Consequently, level 1, 2, and 3 test methods by the American Association of Textile Chemists and Colorists, which use water as a challenge agent, may not be representative for evaluating the barrier effectiveness of PPE and may overestimate the effectiveness of the PPE for bloodborne pathogens. Level 4 includes American Society for Testing and Materials (ASTM) F1670, which evaluates the resistance of surgical drape material to penetration by synthetic blood. Surgical and isolation gowns should be assessed in the viral penetration resistance test ASTM F1671, which measures the resistance of materials used in protective clothing to penetration by bloodborne pathogens by using a surrogate microbe under conditions of continuous liquid contact. Bacteriophage ΦX174 is specifically used because of its similar spherical morphology to HIV, hepatitis B virus, and hepatitis C virus, and at 27 nm in diameter, ΦX174 is similar in size to hepatitis C virus (diameter, 30 nm), the smallest bloodborne viral pathogen known.
Table 1.
Classification of barrier performance of surgical gowns, other protective apparel, surgical drapes, and drape accessories according to ANSI/AAMI PB70:2012 with examples of procedures from AAMI TIR11:2005
| Levela | Test | Liquid challenge | Result | Expected barrier effectiveness | Examples of procedures with anticipated exposure risksb |
| 1 | AATCC 42: impact penetrationc | Water | ≤4.5 g | Minimal water resistance (some resistance to water spray) | Simple excisional biopsies Excision of ‘lumps and bumps’ Ophthalmologic procedures Simple ear, nose, and throat procedures |
| 2 | AATCC 42: impact penetration | Water | ≤1.0 g | Low water resistance (resistant to water spray and some resistance to water penetration under constant contact with increasing pressure) | Tonsillectomies and adenoidectomies Endoscopic gastrointestinal procedures Simple orthopedic procedures with tourniquets |
| AATCC 127: hydrostatic pressured | Water | ≥20 cm | Open hernia repair Minimally invasive surgery Interventional radiology or catheter lab procedures | ||
| 3 | AATCC 42: impact penetration | Water | ≤1.0 g | Moderate water resistance (resistant to water spray and some resistance to water penetration under constant contact with increasing pressure) | Mastectomies Arthroscopic orthopedic procedures Endoscopic urological procedures (for example, transurethral prostate resections) |
| AATCC 127: hydrostatic pressure | Water | ≥50 cm | Open gastrointestinal and genitourinary procedures | ||
| 4 | ASTM F1670: synthetic blood penetration test (for surgical drapes)e | Surrogate blood | no penetration at 2 psi (13.8 kPa) | Blood and viral penetration resistance (2 psi) | Any procedure in which the surgeon's hands and arms are in a body cavity Orthopedic procedures without a tourniquet Open cardiovascular or thoracic procedures |
| ASTM F1671: viral penetration test (for surgical and isolation gowns)f | ΦX174 | no penetration at 2 psi (13.8 kPa) | Caesarean sections Trauma procedures |
Adapted with permission from AAMI.2
In order of increasing protection.
Examples are only general suggestions and should not be interpreted as absolutes or policy statements.
American Association of Textile Chemists and Colorists (AATCC) 42 determines the ability of a material to resist water penetration under spray impact.
AATCC 127 determines the ability of a material to resist water penetration under constant contact with increasing pressure.
American Society for Testing and Materials (ASTM) F1670, similar to ISO 16603, determines the ability of a material to resist the penetration of synthetic blood under constant contact.
ASTM F1671, similar to ISO 16604, determines the ability of a material to resist the penetration of a microorganism under constant contact. This is standard test for the barrier layer material and barrier layer seams used in the construction of garments, work gloves, face protection devices, footwear, and footwear covers (NFPA 1999).
The AAMI Protective Barriers Committee also developed the technical information report AAMI TIR11:2005—Selection and use of protective apparel and surgical drapes in health care facilities (TIR11)—to produce a reference that would enhance excellence in patient care practices involving protective apparel and drapes.2 This document was first issued in 1994 and addresses the selection and use of protective apparel and surgical drapes. TIR11 includes information on types of protective materials, safety and performance characteristics of protective materials, product evaluation and selection, levels of barrier performance, and care of protective apparel and drapes. A table in the report suggests barrier performance levels in accordance with PB70 for several patient-care procedures in light of anticipated exposure risks (Table 2).
Table 2.
Common materials in protective apparel
| Material | Characteristics | Uses | Product examplesa |
| Spunbonded polypropylene | Economical; maximal breathability; strong; lightweight; low linting; not liquid-resistant | Protects against dirt, grime, and some dry particulates in nonhazardous environments; ideal for less critical areas or pregowning entry rooms; food processing environments; general purposes | VWR Basic Protection SPP Lab Coats |
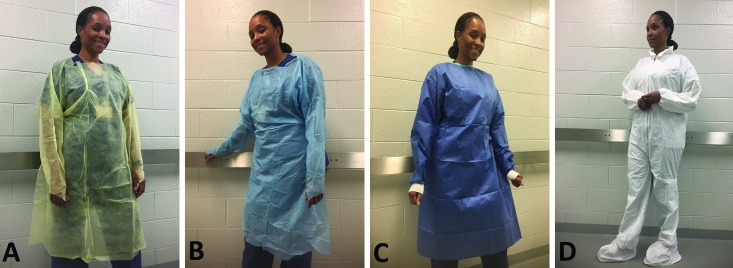
| Multiply polypropylene | Densely packed meltblown layers sandwiched between strong, spunbond outer layers; comes in multiple weights; low linting; may be liquid-resistant | General purposes or isolationb | Medline mediumweight and lightweight polypropylene gowns (Figure 2 A) |
| Low-density polyethylene | Low-cost, waterproof protection for light duty; convenient; flexible | General purposes, depending on the design | Medline Thumbs Up polyethylene gown (Figure 2 B) |
| Multilayered spunbonded–meltblown–spunbonded (SMS) fabric | High tensile strength; soft, comfortable, and breathable; low linting; resistant to tears and punctures | Light fluid and particulate barrier Isolationb | VWR Basic Protection SMS Gowns (Figure 2 C) Medline Eclipse Surgical Gowns |
| Flash-spun, high-density polyethylene | Lightweight; excellent abrasion resistance; expensive | Effective against hazardous dry particles and aerosols and nonhazardous light liquid splash; isolationb | DuPont Tyvek (Figure 2 D) |
| Monolithic filmc made from copolyesters | Good to very good breathability; no voids or holes in these types of films; high liquid repellency; excellent comfort when bonded to polyethylene terephthalate nonwovens or glued to polypropylene nonwovens | Isolationb | DuPont HyTrel DSM Arnitel |
| Microporous film, laminated | High strength; good dust and liquid repellent; great breathability | Isolationb | VWR Advanced Protection Coveralls Kimberly-Clark KleenGuard A40 Kappler ProVent 10000 |
| Spunbonded polypropylene with polyethylene coating | Comfort and flexibility during use; protects against fine sprays and particles; lightweight; low linting; hard-wearing | Isolationb | VWR BioClean-D, Clean-Tough Medline mediumweight and heavyweight coated isolation gowns |
Review of manufacturer information is needed. Final determination of the use of the PPE needs to be in consultation with the institutional occupational health and safety unit.
Isolation apparel needs to have at least level 1 barrier performance over its entire area.
Monolithic film is a polymer film, usually of urethane or copolyester material, which can pass water vapor but does not have physical voids or cells.
The barrier performance of protective apparel depends on the material composition and how the fabric was created. Polypropylene and polyethylene are their primary components (Table 2). Items made from spunbonded polypropylene are naturally low in lint and offer basic cover protection. In spunbonded fabric, the filaments have been extruded, drawn, and laid on a moving screen to form a web.4 Alternatively, protective apparel can be made of multiply polypropylene, in which the inner layers of meltblown polypropylene are sandwiched between outer layers of spunbond polypropylene. Meltblown fabric has polymer resins that are extruded and drawn molten with heated, high-velocity air to form fine filaments; the filaments are cooled and collected as a web onto a moving screen. The meltblown process is similar to the spunbond process, but meltblown fibers are much finer and thus generally measured in microns.4 In flash-spun fabric, such as Tyvek (a high-density polyethylene), the nondirectional fibers (plexifilaments) are first spun and then bonded together by using heat and pressure, without chemical binders.21 High-density polyethylene has little branching as compared with other types of polyethylene, giving it stronger intermolecular forces and tensile strength than low-density polyethylene. The difference in strength exceeds the difference in density, giving high-density polyethylene a high specific strength, making the fabric itself able to stand abrasion or being worn away. High-density polyethylene and monolithic films can easily be cut with scissors or a knife.
Fabric can be woven or nonwoven, with woven fabrics being stronger and higher in quality due to the layers created by weaving the threads over and under each another. Nonwoven materials are generally more affordable than woven fabrics. Seam production differs among protective apparel. Serged seams are produced when the threads are interlocked around the material edges for a strong stress-resistant seam. In ultrasonic welded seams, there are no thread or needle holes with this seam. The material is welded together, creating an excellent particle and fluid barrier.
The requirements for the design and construction of protective apparel reflect the anticipated location and degree of liquid contact during expected use. Critical zones of surgical and isolation gowns are identified as those where direct contact with blood, body fluids, or other potentially infectious materials is most likely to occur (Figure 1). Apparel that can be used for general purposes includes spunbond polypropylene gowns and over-the-head apron-style polyethylene gowns with waist ties and thumb-loop wrists (Figure 2 A and 2 B). Neither style meets the AAMI PB70 standard for isolation gowns, because barrier performance of at least level 1 is required for the entire gown (areas A, B, and C in Figure 1), including seams but excluding cuffs, hems, and bindings. Isolation gowns include those made from multilayered spunbonded-meltblown-spunbonded fabric (Figure 2 C), microporous laminate fabric and multiply polypropylene. Coveralls—sometimes generally referred to by the brand name ‘Tyvek suit’ (Figure 2 D)—are a type of isolation apparel commonly used at high biocontainment levels. Less commonly known materials that still meet AAMI level 4 criteria for barrier protection are monolilthic films. A 1-piece positive-pressure suit ventilated with a life-support system must be used to conduct all ABSL4 procedures.14 This suit, with a clear, flexible 360° hood, is supplied with fresh, filtered air through overhead tubing.
Figure 1.
Critical (gray) zones of an (A) isolation gown and (B) a surgical gown. The entire isolation gown (areas A, B, and C), including seams but excluding cuffs, hems, and bindings, is required to have a barrier performance of at least level 1. In contrast, only the entire front of the surgical gown (areas A, B, and C), and not the area that covers the back of the personnel (area D) is required to have a barrier performance of at least level 1. Note: the illustrations are not intended to reflect specific products or designs. A rendition of this figure is found in reference 3 (adapted with permission).
Figure 2.
Protective apparel. (A) Spunbond multiply polypropylene gown; (B) apron-style polyethylene gown. Because the polypropylene gown offers only basic protection and the polyethylene gown has an open back, neither meets the PB70 standard for isolation gowns. (C) Surgical gown made of multilayered (spunbonded-meltblown-spunbonded) fabric. (D) Flash-spun, high-density polyethylene (‘Tyvek’) suit.
Careful review of the manufacturer-provided information related to the barrier performance of each critical zone component is warranted when choosing protective apparel. Once the suitable type of apparel is identified, the appropriate sizes for personnel need to be determined. Personnel-use coveralls, especially those with attached boot covers and hood, should be one size larger than the person's body size, to allow for flexibility in movement. An undersized suit may compromise personal comfort and barrier integrity, especially if the fabric or seams and barrier layer is not sufficiently durable to withstand typical stresses applied during wear or use, such that garments might tear during kneeling, reaching, or bending. Conversely, oversized apparel may cause tripping accidents.
Sleeve protectors or covers—typically 16 to 18 in. in length, and tapered, with tunneled elastic at both ends—are used to cover the arm or garment's sleeve from the wrist and extending beyond the elbow area. Sleeve covers can be used alone, to protect the arms of personnel wearing scrubs and gloves. This PPE ensemble may be sufficient when working with animals not requiring containment housing and when using engineering standards, such as animal transfer stations and IVC. Sleeve protectors might also be used over a gown or coveralls for procedures with high splash potential (for example, necropsy or working with vomiting patients).
Gloves.
Gloves, the most commonly used PPE, primarily are used to prevent a person's exposure to the hazard and to reduce the risk of environmental and product contamination. ANSI does not have a standard for gloves. OSHA recommends basing glove selection on the tasks to be performed and the performance and construction characteristics of the glove material.35 Medical gloves are class I (general controls) reserved devices and are subject to general controls of the Federal Food, Drug, and Cosmetic Act.22
In 2008, the FDA issued the Medical Glove Guidance Manual to provide recommendations for premarket notification submissions and compliance with the quality system regulation for medical gloves.22 In this manual, patient examination gloves are classified into 5 subcategories—latex, vinyl (polyvinyl chloride), polymer (other than vinyl and including nitrile and polyurethane), finger cot, and specialty (includes chemotherapy).22 The manual also describes types of gloves (surgeon's, radiographic protection, and nonmedical, such as food and cleaning gloves) other than patient examination gloves. These include para-aramid synthetic fiber and leather gloves, which can be used on top of examination gloves and are cut-resistant, and cryogenic gloves, which are multilayered, insulated, and designed to prevent thermal injury. This section of the current review focuses on the 3 most commonly used types of patient-examination gloves (Table 3).
Manufacturers of gloves, especially surgeon's gloves, need to establish and maintain procedures to control the design of the device to ensure that specified design requirements are met.14 As described in the FDA manual,22 design specifications should include glove performance and efficacy; human factors such as fatigue and donning; glove length, cuff, size, and thickness; chemical safety, biocompatibility, environmental compatibility, and allergenicity (protein levels) of the glove material; pinhole acceptable quality level; and glove compatibility with blood, saline, and any intended chemical contact. The FDA also considers shelf life to be a significant factor in meeting user needs. Design validation, conducted under real or simulated conditions to determine whether the device meets user needs, assures that the donning ability, strength, thickness, feel, size, shape, texture, holding ability, tactile sensitivity, lack of fatigue, lack of irritation, color, and odor of the gloves are satisfactory to users.22 Medical-grade gloves have the label ‘Exam’ or ‘Medical Grade’ clearly marked on the packaging, meaning that they are FDA-approved for medical use. These gloves typically are not intended to be used as a chemical barrier.
Allergy to natural rubber latex (NRL) is one of the most- described occupational health and safety hypersensitivities. NRL is manufactured from a milky fluid derived mainly from the rubber tree, Hevea brasiliensi, and should not be confused with synthetic rubber, which is made from chemicals and found in products such as so-called ‘latex’ house paints.31 Allergic reactions to NRL products develop in persons who become allergic (or sensitized) to 1 or more of the 15 latex proteins known collectively as Hev b.17 There are 3 NRL reactions as described by the American Latex Allergy Association.1 The type I (immediate type) hypersensitivity is an IgE-mediated reaction to Hev b proteins; histamine is released, causing systemic symptoms. Type IV (delayed type) hypersensitivity is a T-cell–mediated response that typically occurs 48 to 96 h after exposure, typically in reaction to the processing chemicals used in NRL manufacturing. Generally localized to the area of contact, this reaction is also called allergic contact dermatitis, T-cell-mediated allergy, or chemical allergy. The third type of reaction to NRL is the nonallergic reaction, irritant contact dermatitis. Symptoms typically are dry, irritated, or fissured lesions. Preventive measures against NRL allergy include the use of low-protein, low-allergen, powder-free NRL gloves, a strategy that has proven to markedly reduce latex allergies in healthcare workers.8 In January 2017, FDA began to implement a ban on powdered medical gloves, based on its review of scientific literature and comments regarding the risks and benefits of such gloves.23 The ban specifically indicates that aerosolized glove powder on NRL gloves can carry proteins that might cause respiratory allergic reactions. All types of powdered gloves, including synthetic rubber, have been associated with other adverse events, including severe airway inflammation, wound inflammation, and postsurgical adhesions.23
Powder-free gloves, in which the inner coating is enhanced with a small amount of silicone or aloe, should be used to facilitate donning. Gloving creams, used to lubricate the user's hands, also are available and are classified as a class I device by the FDA. Oil-based creams should not be used, because they degrade the glove material (especially latex gloves).22 In addition to immune reactions to powder and latex proteins, sweat and moisture can have an irritant action, and the friction associated with wearing or removing gloves can contribute to dermatitis of the hands.9
Regular-length gloves are usually sufficient for general work in animal research. Using extended cuff gloves to cover the sleeves or cuffs of the gown or coveralls is advocated when there is a high risk of hazard exposure, such as when working in infectious disease research or working with animals like NHP that might carry debilitating zoonotic diseases. In addition, using para-aramid synthetic fiber and leather gloves over regular medical gloves should be considered strongly in special circumstances, such as when small NHP must be handled without chemical sedation or anesthesia.
There have been conflicting reports on the puncture resistance of various glove materials. In one study, nitrile and neoprene gloves showed 10-fold higher bacterial passage (Escherichia coli K12 [DSM 11250]) through a standardized puncture compared with latex gloves.5 In a study using an adapted version of ASTM F1342-91 (Standard Test Method for Protective Clothing Material Resistance to Puncture), nitrile gloves had significantly higher puncture resistance than latex gloves.36 An important consideration for needlestick injury prevention is the use of double gloving, which has proven to be more effective in reducing the number of glove perforations and blood stains on the skin27 and self-contamination38 than single gloving. The blood volume on a solid suture needle is reduced by as much as 95% when passing through 2 glove layers.7 In addition, in simulated needlestick injuries, significantly less fluid was transmitted through a double, thin glove layer compared with a single thick glove, and significantly more force was required to puncture the double layer compared with the single, albeit thicker, layer.20 A color-coded system for inner and outer gloves might be considered for safety and compliance, such that different glove colors could be used to differentiate sizing, prevent cross-contamination, or designate various types of glove material. The system might facilitate identifying a breach in a glove, for example, when the inner glove color is visible due to a pinhole or tear in the outer glove. Possible limitations of double gloving include compromised manual dexterity and tactile sensitivity.
It is important that personnel do not have the false security that wearing gloves is sufficient to prevent hazard exposure. Hand hygiene is necessary, because contamination can occur through small defects in gloves or during doffing. Variations in production processes can also significantly affect glove properties, such as abrasion resistance.39 Furthermore, alcohol-based disinfectants have been shown to permeate or degrade latex or synthetic gloves such that safety might be compromised.6
Head caps and boot or shoe covers.
The Occupational Safety and Health Standards indicate that surgical caps or hoods and shoe covers or boots shall be worn in instances when gross contamination can reasonably be anticipated (for example, autopsies, orthopedic surgery).13 Biosafety in Microbiologic and Biomedical Laboratories indicates that boots, shoe covers, or other protective footwear are to be used, where indicated, to conduct ABSL3 procedures.18 However, shoe covers do not improve bioexclusion and may actually compromise it, given the potential contamination of personnel from contact with shoe bottoms during donning;25 therefore this PPE may be unnecessary for ABSL1 procedures for rodents, especially in light of the common use of microisolation caging and ventilated rack housing.
One standard that applies to head caps and boot or shoe covers is the National Fire Protection Association's Standard on Protective Clothing and Ensembles for Emergency Medical Operations (NFPA 1999).28 The 5th edition (released in 2013) specifies minimal documentation, design, performance, testing, and certification requirements for new single-use and new multiple-use emergency medical operations protective clothing used by emergency medical responders prior to arrival at medical care facilities and by medical first receivers at medical care facilities during emergency medical operations.28 NFPA 1999 indicates that ASTM F1671 should be used for testing footwear materials and footwear cover materials.28 Commonly used shoe covers are made of polypropylene that is spunbonded, multiply, or coated (Table 2). Durability and antiskid properties are important for shoe covers (Figure 3). In addition, boot covers that extend to the knees and are made of durable and waterproof material such as flash-spun high-density polyethylene can be used and should be considered when performing procedures that may involve heavy floor soiling and splashes (for example, washing NHP cages or large animal pens), or higher biocontainment level. Boot and shoe covers made of flash-spun high-density polyethylene or coated polypropylene pass ASTM F1671; those made of low-density polyethylene, although with less traction because of their smooth bottoms, resist high levels of fluid.
Figure 3.
Shoe and boot covers made of (A) polypropylene with nonskid soles, (B) polyethylene, and (C) flash-spun high-density polyethylene.
Head caps are usually made of polypropylene and are either bouffant or surgeons’ caps. Although bouffant caps are made of a single material, the fabric for surgeons’ caps is divided into the side and crown material. The crown material is typically made of polypropylene, whereas the side materials can be scrim- reinforced material (a paper-like absorbent material), multilayer polypropylene, or spunlace. A surgeon's hood, with or without beard covers and typically made of polypropylene, can be used to provide complete head coverage.
Masks and respirators.
Respiratory protection is a significant component of the PPE ensemble in infectious disease research especially for hazards with a potential for aerosolization. As described earlier, personnel must wear appropriate respiratory protection18 or positive-pressure suits18 for ABSL3 and ABSL4, respectively. Respiratory protection should also be used for ABSL2 procedures as dictated by the risk assessment.18 These requirements are in accordance with the Occupational Health and Safety Standards indicating that masks in combination with eye protection devices, such as goggles and glasses with solid side shields, or chin-length face shields, shall be worn whenever splashes, spray, spatter, or droplets of blood or other potentially infectious materials may be generated and whenever eye, nose, or mouth contamination can be reasonably anticipated.13 This standard clearly defines the distinction between respiratory protection and mucous membrane protection.13
One of the primary functions of the National Personal Protective Technology Laboratory, which NIOSH created in 2001, is to carry out testing procedures and recommend respirators for approval. A respirator must be NIOSH-approved as in accordance with 42 CFR 84 (Approval of Respiratory Protective Devices) and meet the requirements of ASTM F2100 (Standard Specification for Performance of Materials Used in Medical Face Masks). The FDA also regulates surgical masks and surgical N95 respirators.28 As an example of labeling, when a respirator is cleared by the FDA as a surgical mask and certified by NIOSH as an N95 respirator mask, the FDA calls it a “surgical N95 respirator.”28
The Annex A Explanatory Material (NFPA 1999), although created as a reference (that is, not a part of the standard) for patient care providers, provides useful information on the use of surgical masks and respirators (Figure 4). Typical surgical masks are made of polypropylene and feature 3 pleats or folds to allow the user to expand the mask or of synthetic polyester and molded with an adjustable aluminum nosepiece and extend from the nose to under the chin of the wearer. Not covered by NIOSH, surgical masks are not designed or certified to prevent the inhalation of small airborne contaminants. Instead, they are worn to prevent patient exposure to the wearer's saliva and respiratory secretions and to protect the wearer against splashes of large droplets of potentially infected fluid, like blood. The other commonly used mask for patient care is N95 respirators, which entail medical clearance and fit testing to form a tight seal over the mouth and nose to ensure efficacy. N95 respirators filter out at least 95% of airborne particles during ‘worst-case’ testing using a ‘most-penetrating’ sized particle.28 Other kinds of respirators include those that filter out at least 99% and at least 99.97% (essentially 100%) of airborne particles, which respectively receive ratings of 99 or 100. In addition, disposable respirators are further rated for protection against oils, because some industrial oils can degrade filter performance: N respirators are not resistant to oil; R devices are somewhat resistant to oil; and P respirators are strongly resistant (that is, oil proof). Thus, there are 9 types of disposable respirators depending on the percentage filtration and oil resistance.
Figure 4.
Masks and respirators. (A) Three-pleated surgical mask. (B) Surgical molded mask. (C and D) N95 respirators with a metal band that seals the nose bridge area. Note: on a high nose arch or a thin nose, the metal band does not work well and may interefere with fit tests. (E) Flexible-fit design N95 respirator offers a pinch-free molded nose bridge for facial features that may not fit well with other models. (F) half-facepiece respirator with HEPA filter cartridge. (G) Full-facepiece respirator with HEPA filter cartridge. (H) Powered air-purifying respirator (PAPR) with HEPA filter cartridge within helmet.
Half- and full-facepiece elastomeric respirators are tight-fitting, air-purifying respirators with replaceable filters (for particulates) or cartridges or canisters (for gases and vapors), which are attached to a rubber or silicone facepiece that covers at least the nose and mouth. These devices need to be fit-tested and can be cleaned, decontaminated, and reused. One advantage of the full-facepiece respirator is the high level of protection it affords due to its sealing properties, particularly because it covers the user's eyes and face. Such respirators are generally used for specific volatile compounds that may be inhaled.
More commonly used in animal research than the N95 respirator is the loose-fitting PAPR, which is battery-operated and consists of a facepiece mask, helmet or hood, breathing tube, battery-operated blower, and HEPA filters. A PAPR may not necessarily be protective when the hazard is a volatile compound that can pass through a HEPA filter and instead is used when there is aerosol exposure risk with a biologic agent or a nonvolatile chemical particulate. A PAPR is a good option for personnel with facial hair or unusual facial features, which make respirator fitting difficult, or those with medical conditions like asthma. It is more comfortable to wear in biocontainment facilities because it provides a cooling effect in the hood and offers less breathing resistance than a standard tight-fitting respirator. PAPR provide a higher level of protection than most disposable respirators because they are considered to be as efficient as P100 respirators.29 A PAPR uses a blower to pass contaminated air through a HEPA filter, which removes the contaminant and supplies purified air to a facepiece. Some models use 2 shrouds, with one that needs to be tucked under the protective garment (typically coveralls). This inner shroud channels excess air into the garment and over the body for additional comfort. The reusable elements of PAPR should be cleaned and disinfected after use. The filters should be considered contaminated with infectious material and discarded safely when being replaced in accordance with manufacturer's recommendations.
Eye and face protection.
Eye and face protection is advised whenever the potential exists for exposure through splash, spray, or splatter of potentially infectious biologic materials to the eyes, nose, or mouth. For ABSL1 and ABSL2 procedures, eye and face protection should be used in rooms containing infected animals, as dictated by the risk assessment.18 Regular prescription glasses do not provide adequate eye protection; therefore safety glasses made of hardened glass or plastic should be considered minimal eye protection and worn to prevent injury from projectiles, minor splashes, or contact of contaminated hands with eyes.26 In addition, personnel who wear contact lenses are advised to wear eye protection.
Most safety glass lenses today are made of either polycarbonate (or varieties of this material) or the traditional hardened safety glass. However, polycarbonate lenses are typically more impact-resistant than glass lenses. Safety glasses must have side shields and should be chosen to conform to the wearers face, minimizing gaps around the glasses, through which materials could enter the unprotected eye. Safety goggles should be worn when there is a hazard from splashing, especially from corrosive chemicals that could be injurious to the eye, such as concentrated chlorine or phenolic disinfectants, or from flying objects or particles. All protective eye and face protection must comply with the ANSI Z87 (Standard for Occupational and Educational Eye and Face Protection).28
Both safety glasses and goggle lenses are susceptible to fogging as a result of increased body temperature during exertion and environmental factors such as heat and humidity. Lenses with antifog coating, which is applied to both the inside and outside of the lens, should be a consideration in selection of eyewear. Another option is a dual-pane lens, which has an air pocket between 2 layers of lens, thus helping to balance the temperature between the front of the eyewear and the back. Face shields, splash goggles worn with a mask, and masks with a built-in eye shield all offer greater protection to the face and neck area than safety glasses or goggles alone. To ensure full eye and face protection, best practice is to wear safety glasses or goggles in combination with a face shield, to prevent inadvertent airborne or splash that might be deflected under or around the face shield and thus injure the eye. It is important to remember that any device that is to be reused must be decontaminated appropriately.
Hearing protection.
The noise level in animal facility areas may reach potentially damaging levels, depending on the animal species being used (particularly pigs and dogs), the animal-related procedure, and the type of equipment being used (especially in cage-washing areas). The use of special equipment, such as ultrasound machines, that may produce sound inaudible to people can still result in hearing damage and may be covered through ANSI standards. In fact, when the frequency is below 20 kHz for ultrasonography, it is covered by the OSHA noise standard.32 Safety professionals can perform a noise exposure assessment to determine whether routine exposure is excessive or if previously monitored noise levels might have changed due to modifications to the process or in equipment. Wearing hearing protectors such as earmuffs or earplugs is required when exposure to high noise levels cannot be minimized through facility design, such as the use of quieter machinery or insulation or isolation of equipment, or through administrative controls, such as employee or task rotation to decrease exposure time. OSHA limits employee exposure to noise to 90 dBA averaged over an 8-h work shift.10 Where levels exceed 85 dBA, exposed employees need to participate in a hearing-conservation program that includes monitoring, audiometric testing, hearing protection, training, and record-keeping.10
The type of hearing protection must be selected carefully to ensure that it provides the right balance of comfort, noise attenuation, and ease of use and fit. No single type of hearing protection works for all personnel or situations. Some factors that should be considered when selecting protectors include individual comfort, size of ear canal, noise environments, work activities, and environmental conditions. The most common types of hearing protectors include earplugs and earmuffs. Foam earplugs provide sufficient noise reduction, are convenient, and are comfortable to use, but they can be difficult to fit correctly, especially for someone with a small ear canal. An alternative device is the molded or flanged plug; these come in a variety of sizes for individual fit and are easy to insert into the ear canal. Earmuffs that seal against the head and directly over the outer ear are designed with a foam or fluid material that is enclosed in an outer plastic envelope. Advantages include less attenuation variability among users and a consistent and reliable fit, and one size fits most people. However, earmuffs might be uncomfortable to wear in hot work areas and can restrict head motion.
Conclusion
There are many considerations in implementing institutional PPE requirements. With the aim of meeting compliance with regulatory agencies and adhering to best practice, PPE should primarily be selected based on risk assessment, level of containment involved, and its material composition, which dictates the level of barrier performance it provides. Additional consideration must be given to correct fit and wearer comfort.27 In addition, the manner in which the clothing is donned and doffed in sequence with other PPE is important because the ease or difficulty with which this process is achieved may affect the effectiveness of PPE and the potential for self-contamination during doffing. A ‘buddy system’ or the use of a step-by-step checklist might be considered for high-risk procedures. The requirements and all pertinent processes should be described in a standard operating procedure document that is used for training. Documentation of training for proficiency and competency in donning and doffing is necessary for work at high biocontainment levels. The training needs to include what PPE to use and its limitations; when and where to use it; how to correctly don, doff, adjust, and wear it; and its proper care, maintenance, and disposal.8 Periodic assessment of efficacy and applicability, together with review of standard operating procedures, is recommended. Although an integral component of the institutional occupational health and safety program, the use of PPE alone will not provide full protection against hazard exposure. Other practices such as good hygiene and laboratory techniques; the use of specialized instruments, supplies, and building infrastructure; and vaccinations, as appropriate, further mitigate risks.
Acknowledgments
We thank Ms LaJuanda Carter (Unit for Laboratory Animal Medicine, University of Michigan) for modeling the protective apparel (Figure 2) and Mr Josh Bennett (Department of Environment, Health, and Safety, University of Michigan) for modeling the masks and respirators (Figure 4).
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Army, Department of Defense, or the US government. The mention of trade names, commercial products, or organizations does not imply endorsement by the US government. The funding agency did not play a role in the study design, collection, analysis, or interpretation of data, in writing the report, or in the decision to submit the article for publication.
References
- 1.American Latex Allergy Association. [Internet]. 1996. Definition. [Cited 14 January 2017] Available at http://latexallergyresources.org/definition.
- 2.Association for the Advancement of Medical Instrumentation. 2005. TIR11:2005 Selection and use of protective apparel and surgical drapes in health care facilities. Arlington (VA): Association for the Advancement of Medical Instrumentation. [Google Scholar]
- 3.Association for the Advancement of Medical Instrumentation. 2012. ANSI/AAMI PB70:2012 Liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities. Arlington (VA): Association for the Advancement of Medical Instrumentation. [Google Scholar]
- 4.Association of the Nonwoven Fabrics Industry. [Internet]. 2012. Spunbond and melt–blown technology. [Cited 20 October 2016] Available at: http://www.inda.org/spunbond-melt-blown.html.
- 5.Bardorf MH, Jäger B, Boeckmans E, Kramer A, Assadian O. 2016. Influence of material properties on gloves’ bacterial barrier efficacy in the presence of microperforation. Am J Infect Control 44: 1645–1649. [DOI] [PubMed] [Google Scholar]
- 6.Baumann MA, Rath B, Fischer JH, Iffland R. 2000. The permeability of dental procedure and examination gloves by an alcohol-based disinfectant. Dent Mater 16:139–144. [DOI] [PubMed] [Google Scholar]
- 7.Bennett NT, Howard RJ. 1994. Quantity of blood inoculated in a needlestick injury from suture needles. J Am Coll Surg 178:107–110. [PubMed] [Google Scholar]
- 8.Blaabjerg MSB, Andersen KE, Bindslev-Jensen C, Mortz CG. 2015. Decrease in the rate of sensitization and clinical allergy to natural rubber latex. Contact Dermatitis 73:21–28. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. [Internet]. 2002. Morbidity and mortality weekly report: guideline for hand hygiene in healthcare settings. [Cited 20 October 2016] Available at: http://www.cdc.gov/mmwr/PDF/rr/rr5116.pdf.
- 10.Code of Federal Regulations (CFR). [Internet]. 1974. Title 29, part 1910. Occupational safety and health standards: subpart G. Occupational health and environmental control; standard number 1910.95, occupational noise exposure. Washington (DC): Office of the Federal Register; [Cited 20 October 2016]. Available at: https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=9735. [Google Scholar]
- 11.Code of Federal Regulations (CFR). [Internet]. 1974. Title 29, part 1910. Occupational safety and health standards: subpart I, personal protective equipment. Washington (DC): Office of the Federal Register; [Cited 20 October 2016]. Available at: https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=10118. [Google Scholar]
- 12.Code of Federal Regulations (CFR). [Internet]. 1988. Title 21 part 878. General and plastic surgery devices. Washington: Office of the Federal Register; [Cited 20 October 2016]. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=878. [Google Scholar]
- 13.Code of Federal Regulations (CFR). [Internet]. 1991. Title 29, part 1910. Occupational safety and health standard,; Subpart Z, toxic and hazardous substances; standard number 1910.1030, bloodborne pathogens. Washington (DC): Office of the Federal Register; [Cited 20 October 2016]. Available at: https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=10051. [Google Scholar]
- 14.Code of Federal Regulations (CFR). [Internet]. 1996. Title 21 part 820.30. Design controls. Washington (DC): Office of the Federal Register; [Cited 20 October 2016]. Available at: https://www.gpo.gov/fdsys/pkg/CFR-2012-title21-vol8/pdf/CFR-2012-title21-vol8-sec820-30.pdf. [Google Scholar]
- 15.Code of Federal Regulations (CFR). [Internet]. 1997. Title 21 part 801.437. User labeling for devices that contain natural rubber. Washington (DC): Office of the Federal Register; [Cited 20 October 2016]. Available at: https://www.gpo.gov/fdsys/pkg/CFR-2012- title21-vol8/pdf/CFR-2012-title21-vol8-sec801-437.pdf. [Google Scholar]
- 16.Code of Federal Regulations (CFR). [Internet]. 2004. Title 42, Part 84. Approval of respiratory protective devices. Washington (DC): Office of the Federal Register; [Cited 20 October 2016]. Available at: https://www.gpo.gov/fdsys/pkg/CFR-2012-title42-vol1/pdf/CFR-2012-title42-vol1-chapI-subchapG.pdf. [Google Scholar]
- 17.Crepy MN. 2016. Rubber: new allergens and preventive measures. Eur J Dermatol 26:523–530. [DOI] [PubMed] [Google Scholar]
- 18.Department of Health and Human Services. [Internet]. 2009. Biosafety in microbiological and biomedical laboratories, 5th ed. [Cited 20 October 2016] Available at: http://www.cdc.gov/biosafety/publications/bmbl5/BMBL.pdf. [Google Scholar]
- 19.Department of Energy. [Internet]. 2013. Safety glove selection guide. [Cited 23 April 2017] Available at: https://www1.aps.anl.gov/Safety-and-Training/Safety/Reference-Material/Safety-Glove-Selection-Guide.
- 20.Din SU, Tidley MG. 2013. Needlestick fluid transmission through surgical gloves of the same thickness. Occup Med (Lond) 64: 39–44. [DOI] [PubMed] [Google Scholar]
- 21.Dupont. [Internet]. 2002. Product handbook of Dupont Tyvek. [Cited 20 October 2016]. Available at: http://www.dupont.com/content/dam/dupont/products-and-services/fabrics-fibers- and-nonwovens/industrial-fabrics/documents/DPT_Tyvek_ Product_Handbook.pdf.
- 22.Food and Drug Administration. [Internet]. 2008. Guidance for industry and FDA staff: medical glove guidance manual. [Cited 20 October 2016] .Available at: http://www.fda.gov/downloads/ medicaldevices/deviceregulationandguidance/guidancedocuments/ucm428191.pdf.
- 23.Food and Drug Administration. [Internet]. 2017. Banned devices: powdered surgeon's gloves, powdered patient examination gloves, and absorbable powder for lubricating a surgeon's glove. [Cited 11 January 2017]. Available at: https://www.federalregister.gov/documents/2016/12/19/2016-30382/banned-devices-powdered-surgeons-gloves-powdered-patient-examination-gloves-and-absorbable-powder [PubMed]
- 24.Harrison DJ. 2001. Controlling exposure to laboratory animal allergens. ILAR J 42:17–36. [DOI] [PubMed] [Google Scholar]
- 25.Hickman-Davis JM, Nicolaus ML, Petty JM, Harrison DM, Bergdall VK. 2012. Effectiveness of shoe covers for bioexclusion within an animal facility. J Am Assoc Lab Anim Sci 51:181–188. [PMC free article] [PubMed] [Google Scholar]
- 26.Institute for Care and Use of Laboratory Animal Science. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academy Press. [Google Scholar]
- 27.Mischke C, Verbeek JH, Saarto A, Lavoie MC, Pahwa M, Ijaz S. 2014. Gloves, extra gloves, or special types of gloves for preventing percutaneous exposure injuries in healthcare personnel. Cochrane Database Syst Rev 3:CD009573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Fire Protection Association. 2013. NFPA 1999: Standard on protective clothing and ensembles for emergency medical operations. Quincy (MA): NFPA. [Google Scholar]
- 29.National Institutes of Health. [Internet] 2016. NIH guidelines for research involving recombinant or synthetic nucleic acid molecules. [Cited 16 January 2017] Available at: http://osp.od.nih.gov/sites/default/files/NIH_Guidelines.html.
- 30.National Institute for Occupational Safety and Health. [Internet] 2016. Considerations for selecting protective clothing used in healthcare for protection against microorganisms in blood and body fluids. [Cited 20 October 2016] Available at: https://www.cdc.gov/niosh/npptl/topics/protectiveclothing/.
- 31.National Institute for Occupational Safety and Health. [Internet] 1997. Preventing allergic reactions to natural rubber latex in the workplace. [Cited 14 January 2017] Available at: https://www.cdc.gov/niosh/docs/97-135/pdfs/97-135.pdf
- 32.National Research Council. 1997. Occupational health and safety in the care and use of research animals. Washington (DC): National Academy Press. [Google Scholar]
- 33.Occupational Health and Safety Administration. [Internet] 1970. Occupational Safety and Health Act of 1970. [Cited 14 January 2017] Available at: https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=OSHACT&p_id=2743.
- 34.Occupational Health and Safety Administration. [Internet] 2001. CPL 02-02-069: Enforcement procedures for the occupational exposure to bloodborne pathogens. [Cited 20 October 2016] Available at: https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=DIRECTIVES&p_id=2570.
- 35.Occupational Health and Safety Administration. [Internet] 2004. OSHA 3151-12R: personal protective equipment. [Cited 20 October 2016] Available at: https://www.osha.gov/Publications/osha3151.pdf.
- 36.Patel HB, Fleming GJ, Burke FJ. 2004. Puncture resistance and stiffness of nitrile and latex dental examination gloves. Br Dent J 196:695–700. [DOI] [PubMed] [Google Scholar]
- 37.Reeb-Whitaker CK, Harrison DJ, Jones RB, Kacergis JB, Myers DD, Paigen B. 1999. Control strategies for aeroallergens in an animal facility. J Allergy Clin Immunol 103:139–146. [DOI] [PubMed] [Google Scholar]
- 38.Verbeek JH, Ijaz S, Mischke C, Ruotsalainen JH, Mäkelä E, Neuvonen K, Edmond MB, Sauni R, Kilinc Balci FS, Mihalache RC. 2016. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst Rev 4:CD011621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh DL, Schwerin MR, Kisielewski RW, Kotz RM, Chaput MP, Varney GW, To TM. 2003. Abrasion resistance of medical glove materials. J Biomed Mater Res B Appl Biomater 68B:81 –87. [DOI] [PubMed] [Google Scholar]