Abstract
Viral vector research presents unique occupational health and safety challenges to institutions due to the rapid development of both in vivo and in vitro gene-editing technologies. Risks to human and animal health make it incumbent on institutions to appropriately evaluate viral vector usage in research on the basis of available information and governmental regulations and guidelines. Here we review the factors related to risk assessment regarding viral vector usage in animals and the relevant regulatory documents associated with this research, and we highlight the most commonly used viral vectors in research today. This review is particularly focused on the background, use in research and associated health and environmental risks related to adenoviral, adeno-associated viral, lentiviral, and herpesviral vectors.
Abbreviations: AAV, adeno-associated viral vector; ABSL, animal biosafety level; BMBL, Biosafety in Microbiologic and Biomedical Laboratories; RG, risk group
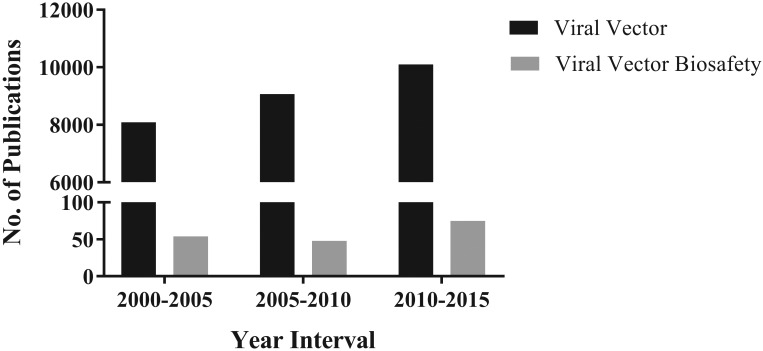
The use of viral vectors in research is increasing, both in animal research and human gene therapy trials. As of June 2012, a total of 1843 gene therapy trials have been undertaken in 31 countries, most of which have used transduction (Figure 1) as a gene-delivery tool.22 A PubMed search with keywords viral vector showed a consistent increase in publications in this arena over the last 15 y (Figure 2). However, research in viral vector biosafety in the laboratory animal setting has not risen in parallel, which is reflected in the relatively few publications during the same period.
Figure 1.
Common viral vector terminology.
Figure 2.
PubMed searches for the terms ‘viral vector’ compared with ‘viral vector biosafety’ for the indicated time intervals returned results indicating a steadily increasing and disproportionately higher number of publications focused on viral vector research as compared with publications focused specifically on viral vector biosafety. Searches performed on 24 September 2016.
In assessing risk regarding viral vector use in animal research, one must consider the hazards inherent to the viral vector itself, the animal model, the inserted gene construct, and the proposed research manipulations. A great deal is known about the original virus from which the vectors are genetically engineered, including replicative ability, oncogenic potential, environmental stability, tissue and cellular tropism (amphotropic compared with ecotropic; Figure 1), and pathogenic characteristics—all of which helps to guide risk assessment.13,16-18,20,31 In addition, information regarding shedding duration, excretion routes, biodistribution, and other components vital to appropriate risk assessment of vectors is available from basic research studies and human gene therapy trials.44,47 However, informative data from these clinical trials does not always translate to animal models, thus leaving a huge gap in the knowledge base needed to make accurate risk assessments. Genetic manipulations to increase safety such as pseudotyping (Figure 1) and the creation of conditionally replicating viral vectors must also be considered. The use of humanized mice has been proposed as a final step in preclinical viral vector risk assessment, but this condition does not address the inherent risks involved in animal research.3 Hazards implicit in working with animals such as bites and scratches can only be mitigated so much by engineering controls and personal protective equipment. In addition, the expansive use of sometimes uncharacterized transgenic animals makes it impossible to adequately anticipate all potential risk scenarios. Often the greatest unknown is the effect of the transgene itself within the specific animal model. Institutions have more control over administrative practices meant to mitigate risks specific to the experimental protocol, including assurance of proper training and proficiency and occupational health assessment of research personnel.
The hazards associated with viral vectors make it incumbent on institutions to conduct risk assessment to make informed decisions regarding policies and guidelines. Even with the large number of prior viral vector gene therapy clinical trials performed with no significant ill effects in participants, sometimes life-threatening effects have been observed in a small number of participants and thus highlight potential repercussions from inappropriate handling and usage.43,49 This assessment is challenging for animal care and use programs, given the level of expertise in virology, human and animal physiology, and other disciplines needed to appropriately evaluate literature. In this review, we present current regulations, literature, and industry practices for some of the most widely used viral vector agents (adenoviruses, lentiviruses, herpesviruses, and adeno-associated viruses [AAV]). We evaluate general characteristics, known hazards, and risk reduction practices in light of recent advances in molecular biology and viral engineering, in the hope of aiding institutions in their individual evaluations.
Current Regulations
Institutions receiving federal funding are subject to the NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules, impl mented by the Recombinant DNA Advisory Committee.2 These guidelines stipulate protocol review by the Institutional Biosafety Committee and outline required containment and safety procedures. Most viral vectors are classified by the NIH as belonging to either Risk Group (RG) 1 (not associated with disease in healthy human adults) or RG2 (associated with human disease which is rarely serious and for which preventive or therapeutic interventions are often available). Biosafety level (BL) containment requirements are stipulated for each risk group (BL1 or BL2).39 In addition to RG1 agents, viral vectors containing less than 2/3 of a eukaryotic viral genome may be handled under BL1 conditions. However, most viral vectors used in animal research are either RG2 agents or do not meet this size requirement and therefore require BL2 containment and procedures during preparation, manipulation, and injection. These precautions include restricted access, an appropriate laboratory set-up and signage, staff training, sharps safety, decontamination of waste prior to disposal, and personal protective equipment to prevent skin and mucous membrane exposure.7
For animal experiments involving most viral vectors used in research, the NIH Guidelines recommend BL2N (BL2–Animal; Figure 1) containment practices. Because of the operational challenges of continuous BL2N containment, further clarification was released for lentiviral vectors, recommending BL2N containment housing for 3 to 7 d.52 As discussed earlier, viral–host interactions cannot always be predicted when viral vectors are used in transgenic animals, and accordingly, the NIH Guidelines stress that “serious consideration should be given to increasing the containment conditions.”39 Biosafety in Microbiologic and Biomedical Laboratories (BMBL, 5th edition) can also be used as a guidance document for working with poorly characterized viral vectors.12 Many institutions do not distinguish between animal biosafety level (ABSL) 1 through 4 (as described in the BMBL) and BLN1 through 4, choosing instead to follow specifications provided in the BMBL, which also covers work with other infectious agents. As such, we reference ABSL containment levels throughout this document for clarity.
Adenoviral vectors
Replication-competent and -deficient adenoviruses are classified as RG2 agents. Adenoviruses are nonenveloped double-stranded DNA viruses (Baltimore classification group 1) that have wide species and cellular tropism (amphotropic). Without alterations, adenoviruses do not integrate efficiently into the genome and therefore expression is generally transient. In adenoviral vectors, most of which are derived from type 5 adenoviruses,28 the key replication elements are deleted. New ‘gutless’ systems consist of only the adenoviral packaging system and contain no adenoviral genomic material, thus increasing biosafety in terms of immunogenicity and cytotoxicity.13 Conditionally replicating adenoviruses, which replicate selectively in tumor cells, are available.13
Given the ubiquity of adenoviruses in the environment, most people have previously been exposed to adenoviruses although immunity to the virus is generally strain-specific. Adenoviruses most commonly cause mild respiratory disease or conjunctivitis in healthy adults but can cause more serious illness, especially in immunocompromised persons. These viruses are transmitted by direct contact, ingestion, aerosol, and percutaneous exposure.12 Even in experimental animal models, establishing vertical transmission or germline transduction of adenoviruses is very difficult.24 Long-term shedding can occur through respiratory secretions and feces from asymptomatic carriers. However, humans who received an adenoviral vector through intravenous administration had minimal shedding in biologic fluids after 48 h, due to sequestration by the liver.28 Hamsters and cotton rats (Sigmodon hispidus) are the only 2 common laboratory species in which unmanipulated human adenoviruses can replicate.13
Although natural disease is generally mild, adenoviruses are notoriously immunogenic. Adenoviral vectors are preferred for vaccination vectors, given their ready uptake by antigen presenting cells, particularly Kupffer cells.10 Several episodes of inflammatory responses to adenovirus vectors in clinical trials have been documented, including one death after direct injection of an adenoviral vector into the hepatic artery.33 Some first-generation adenoviral vectors, which retain a great proportion of the genome, have initiated dose-dependent apoptosis of various target cells in vitro and in multiple mouse models in vivo, indicating a potential for direct cytotoxicity.51,56 The widespread distribution and tropism of adenoviruses supports the potential for intracellular recombination or complementation (Figure 1) of the vector with previously acquired wild-type adenovirus in either packaging cell lines or in vivo, thus making the adenoviral vector replication-competent. This risk can be reduced considerably by using cell lines with decreased or no homology between vector and helper sequences.21 Another risk-reduction strategy is regular quality-control checks of viral vector stocks for low levels of contamination with replication-competent virus. No standards for this kind of monitoring are available currently, and threshold levels are determined arbitrarily by investigators.44
Adeno-associated Viral Vectors
Adeno-associated viral vectors (AAV), the most common parvoviral vectors, are generally classified under RG1. Exceptions include synthetic or recombinant AAV constructs produced in the presence of a helper virus and those that contain a potentially harmful transgene (Appendix B1 of the NIH Guidelines).39 Parvoviruses are nonenveloped single-stranded DNA viruses (Baltimore classification II). Although parvoviruses generally have a narrow host range, they are of interest in viral vector research because of their ability to infect both dividing and nondividing cells.15 However, they do not integrate efficiently into the host genome, and as such their expression is generally transient. AAV vectors get their name from their requirement for a helper plasmid or virus for propagation. Effective helper viruses include adenovirus, herpes simplex virus, vaccinia virus, and human papilloma virus. AAV cellular tropism is strain-dependent.18 AAV can infect humans and other primate species but are not known to cause disease. Approximately 80% of humans are seropositive to AAV strains.5
AAV vectors have a favorable safety record in clinical trials and preclinical animal studies.18,35 In one clinical trial, recombinant AAV vectors administered beneath the retina resulted in no detectable systemic vector dissemination and no indication of any mounted immune response to the vector.22 Most risks associated with AAV viral vectors are theoretical in nature. For example, although wild-type AAV inserts at a known locus in the human genome with no ill effects, recombinant AAV vectors can integrate randomly or at other points with potential prooncogenic results.26,40 In some animal models, the integration of recombinant AAV has been associated with an increased incidence of tumor formation, but this association has not been noted to occur in humans.45 Although ineffective in replication as an isolated agent, AAV have the potential for replication competence in the presence of a helper plasmid or virus, and stock solutions should be tested for the presence of other agents. However, autonomous replication has occurred under stressful genotoxic conditions (UV light exposure, hydroxyurea incubation) in some cell lines1,55 and differentiating keratinocytes.34 Finally, as hydrophilic nonenveloped viruses, parvoviruses are known for their environmental stability.
Herpesviral Vectors
Herpesviruses are double-stranded enveloped DNA viruses (Baltimore classification 1) with relatively large genomes. Herpesviruses are ubiquitous in the animal kingdom but species-specific in their host tropism and are classified into 1 of 3 subfamilies (α, β, and γ). Herpes simplex virus types 1 and 2 (HSV1 and HSV2) are αherpesviruses, which are epitheliotropic cytolytic viruses that can form lifelong infections by establishing latency in neural tissue with intermittent recrudescence in response to stress and other factors.29 Because they are human pathogens, herpesviral vectors are classified under RG2. In all HSV vectors, genes that encode for accessory or essential proteins have been manipulated, such that their products minimally to completely inhibit viral replication and lytic ability, respectively.29 Viral stocks are then produced by using cells lines that provide these protein products through complementation.
Compared with the other viruses described in this review, HSV vectors are used only rarely in clinical trials, but their neural tropism and ability to establish latency allowing a sustained transgene expression make it an attractive target for gene therapy for peripheral neuropathies.7,23 Genetic manipulation has led to the creation of 3 types of HSV vectors: amplicon, replication-defective, and replication-competent. Created in the late 1980s, amplicon vectors contain the origin of replication and packaging genes but lack lytic function. Amplicons require transduction with a helper virus for competent infection and are used extensively in preclinical research due to their ability to accept very large inserts (100 kb).48 Improvements were made to avoid stock contamination with helper viruses by further deletion of packaging genes. Replication-defective HSV vectors have come a long way since their inception in the early 1990s, with the deletion of essential replicative genes. One of the largest roadblocks to the use of replication-deficient HSV vectors is direct cytotoxicity, presumptively due to replication-independent mechanisms of cytoskeletal disruption.4,27 In 2015, the development of a third-generation HSV vector capable of long-term gene expression without cytotoxicity or interference with cell division represents one of the final breakthroughs needed to make HSV a widely useful gene-delivery technology.36 Finally, replication-competent HSV vectors are in the advanced stage of development for oncolytic cancer therapy.
Although molecular alterations to the viral genome have dramatically reduced virus-associated risks, HSV remains a known human pathogen with inflammatory and immunomodulatory effects. Although the seroprevalence of both HSV1 and HSV2 is high in the United States (approximately 53% and 16%, respectively),6 few solid data that support in vivo recombination or complementation are available. Primary infection with HSV1 can be mild or inapparent and usually occurs in early childhood. In active infections, lesions are generally limited to the mucocutaneous junctions, genitalia (HSV2), or epidermis. However, fatal meningoencephalitis can occur, especially in immunocompromised persons.7,31 HSV are transmitted by direct contact and mucous membrane exposure with body excretions and respiratory droplets.2 Apparently asymptomatic carriers can shed sporadically. In rats infected with wild-type HSV, subsequent intracranial injection of HSV vectors did not lead to viral reactivation of the vector.54 However, multiple strains of HSV were isolated from the neuronal tissue of a single patient, thus perhaps supporting intratypic recombination rather than successive infection.33 As an enveloped virus, HSV is inherently unstable in the environment. Although susceptible to many common disinfectants (bleach, Lysol [Reckitt Benckiser, Parsippany, NJ], Alcide [Ecolab, St Paul, MN]), HSV2 is more thermolabile than HSV1, and increased disinfectant concentrations or prolonged contact times may be required for inactivation, depending on the subtype.14
Lentiviral Vectors
Lentiviruses are a type of retrovirus (Baltimore classification VII) and consist of a single-stranded positive-sense RNA sequence that is transcribed into a DNA and integrated into the host genome, causing persistent infection. Lentiviruses have several unique characteristics that may make them preferred over vectors derived from other genera of retroviruses, such as gammaretroviruses, including integration into nondividing cells and the ability to transduce stem cells more efficiently, such that lower infection doses can be used.38 Most lentiviral vector systems are based on altered HIV types 1 and 2 and contain deletions or alterations of some or all pathogenic (vpr, vpx, and nef) and replication-dependent (gag–pol and env) genetic elements, depending on which lentiviral vector generation is used.16 Unmanipulated HIV1 and HIV2 are in RG3, but manipulated lentiviruses used as viral vectors (that is, altered HIV1 and HIV2) are in RG 2.39
Wildtype HIV1 and HIV2 infect CD4+ T helper cells, causing an immunosuppressed state and eventual progression to AIDS, which is characterized by the development of opportunistic infections.16 In comparing the 2 subtypes, HIV2 has a lower infectivity rate, longer asymptomatic period, and slower disease progression than HIV1. Although HIV1 infections are the most common in the United States, HIV2 is an important cause of disease in other regions. Coinfection with HIV1 and HIV2 is infrequent compared with the well-recognized intercladal and intracladal HIV1 coinfections.9 HIV transmission occurs through mucous membrane exposure, direct contact with bodily fluids including sexual transmission, percutaneous exposure, and vertical transmission.16 Antiviral therapy has been established to be effective in preventing HIV infection if used appropriately in the context of postexposure prophylaxis.
Lentiviral vectors are generally considered to be relatively safe, given their extensive genetic alterations in recent years through which components necessary for virus production have been split across multiple plasmids, some of which are integrated into the viral vector, and others that are expressed only within the packaging system.16 Each split of the lentiviral genome makes propagation outside of the cell line packaging system more difficult through mechanisms of complementation or recombination. Second-generation lentiviral vectors use 3 plasmids, third-generation vectors use 4 plasmids, and fourth-generation vectors use 5 plasmids.19,25,46,50 Other alterations to increase safety include the production of self-inactivating vectors (Figure 1) and improved packaging systems that use less HIV genomic material.19,37
Clinical trials in which lentiviral vectors were used to treat adrenoleukodystrophy, a fatal demyelinating disease, yielded no adverse effects.22 However, the use of lentiviral vectors in research is still associated with potential risks, and the long-term safety of these clinical interventions is still being evaluated. An initial in vitro study showed no propagation of replication-deficient lentiviral vectors in cell culture after 3 washes with PBS.1 However a later study found that cells incubated with lentiviral vectors can expose secondary target cells to infection despite repeated washings (that is, 2 washes) and in the absence cell-to-cell contact both in vitro and in vivo.41 Although these findings conflict, they might indicate a potential route of transmission to nontarget tissues through spill-over shedding of viral particles into the cellular supernatant.41 Although lentiviral vectors are less associated with insertional mutagenesis (Figure 1) than other retroviruses, these vectors still provide evidence regarding off-target effects. For example, one participant in a clinical trial to treat β-thalassemia by cells transduced by using self-inactivating lentivirus demonstrated the emergence of a partially dominant cell clone of myeloid progenitor origin after injection with transduced hematopoietic stem cells. This can either represent a stochastic event in the context of a low initial number of transduced cells or the precursor to a potentially malignant progression which is always a concern for lentiviral integration events.11 Long-term follow-up of this patient is ongoing at this time.11 Nevertheless, lentiviruses are not stable in the environment and are susceptible to most disinfectants, making decontamination straightforward.
Discussion
In 2015, a survey to determine common containment housing and practices among institutions involved in viral vector use in animal research was conducted by using an online questionnaire (Research Suite, Qualtrics, Provo, UT) sent through the CompMed listserve (AALAS, Memphis, TN). Responses were received from a total of 44 institutions, of which 41 (93%) were academic institutions and nonprofit organizations. Table 1 presents information gathered from the participants, some of whom returned responses only to select questions of the survey. In summary, most institutions house animals that received lentiviral and adenoviral vectors under ABSL2 conditions for a few days after administration or for the duration of the experiment. However, institutions may choose to downgrade containment from ABSL2 to ABSL1 for animal housing, provided that ABLS2 practices are followed during vector administration or that specific genetic alterations have been made to the viral vector itself (such as pseudotyping or replication incompetence).
Table 1.
Summary of CompMed survey responses
| Lentiviral vectors | Adenoviral vectors | |
| ABSL1 | 0 (0%) | 1 (3%) |
| Animals receive washed, transduced cells | ||
| Evidence of replication incompetence | ||
| ABSL1 with special procedures | 3 (7%) | 2 (6%) |
| Use of biosafety cabinet for vector preparation and administration to animals | ||
| All wastes are considered biohazardous | ||
| ABSL2 for 1 to 7 d after administration | 29 (66%) | 21 (60%) |
| Generally at least 72 h | ||
| ABSL2 for duration of experiment | 9 (20%) | 10 (29%) |
| Only for replication-competent vectors | ||
| Other measures | 3 (7%) | 1 (3%) |
| Total | 44 (100%) | 35 (100%) |
Containment housing strategies are indicated.
When performing a risk assessment to determine containment levels, consider the host species and its immune status, relevant viral vector modifications, inoculation strategy (titer, route of delivery, and tissue), and gene insert. Intravenous administration of adenoviral, AAV, and lentiviral vectors into either immunocompetent and immunocompromised (NOD-SCID) mice does not increase the risk of viral vector shedding when E1a/b-, E3-deleted type 5 adenoviruses, rAAV2/2, or third-generation lentiviral vectors are used.44 Once delivered, the replication-deficient vectors express proteins in target tissues but show no evidence of propagation. However, some data suggest that intravenous delivery can lead to leaking at the point of injection. Both lentiviral and adenoviral vectors can be found on tail swabs for as long as 72 h after injection, although this positivity was localized, and no vector was recovered from the bedding.44 To further minimize the risk of contamination of the environment, the injection site should be wiped with a disinfectant, given that viral vectors are rapidly cleared (that is, within 24 h) from the blood.44 Vectors persist on caging or in bedding for much less time than do wildtype virus.53 Furthermore, intracranial delivery methods are designed as closed injection systems that use small volumes such that the potential of superficial contamination is minimal when properly performed.
Because of the potential for the presence of viral vectors in the environment after inoculation, sanitize caging and equipment between uses to prevent transmission by fomites. When placed on untreated plastic, recombinant AAV and adenoviral vectors were recoverable by cell culture for 3 and 14 d respectively.44 In an evaluation of several disinfectants for in vitro efficacy against viral vectors (lentiviral, adenoviral, and AAV), only Virkon S (Dupont, Wilmington, DE) demonstrated robust surface disinfection and minimal aversion, making it preferable for use on surfaces that rodents contact.8 For many studies, sanitation through a cage wash system is appropriate because temperatures achieved by most industrial cage washers (74 °C for 6 min) are sufficient to eliminate the risk of adenoviral transmission.32,42 Similar precautions likely will be sufficient for lentiviruses and herpesviruses as well, given their relative instability in the environment.
For standard replication-deficient adenoviral, third-generation herpesviral vectors, and third- and fourth-generation lentiviral vectors, we recommend an initial 72-h period of ABSL2 containment followed by reclassification to ABSL1 housing after a complete cage change. Following the recommendations of the NIH Guidelines together with the demonstrated safety of AAV vectors, ABSL1 housing is sufficient provided that the encoded transgene is deemed safe and appropriate precautions are taken during vector preparation and administration.
References
- 1.Bagutti C, Schmidlin M, Mueller M, Brodmann P. 2012. Washout kinetics of viral vectors from cultured mammalian cells. Appl Biosaf 17:188–197. [Google Scholar]
- 2.Baldo A, van den Akker E, Bergmans HE, Lim F, Pauwels K. 2014. General considerations on the biosafety of virus-derived vectors used in gene therapy and vaccination. Curr Gene Ther 13:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer G, Dao MA, Case SS, Meyerrose T, Wirthlin L, Zhou P, Wang X, Herrbrich P, Arevalo J, Csik S, Skelton DC, Walker J, Pepper K, Kohn DB, Nolta JA. 2008. In vivo biosafety model to assess the risk of adverse events from retroviral and lentiviral vectors. Mol Ther 16:1308–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berto E, Bozac A, Marconi P. 2005. Development and application of replication-incompetent HSV1 based vectors. Gene Ther 12 Suppl 1:S98–S102. [DOI] [PubMed] [Google Scholar]
- 5.Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, Masurier C. 2010. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther 21:704–712. [DOI] [PubMed] [Google Scholar]
- 6.Bradley H, Markowitz LE, Gibson T, McQuillan GM. 2013. Seroprevalence of herpes simplex virus types 1 and 2—United States, 1999 to 2010. J Infect Dis 209:325–333. [DOI] [PubMed] [Google Scholar]
- 7.Braun A. 2006. Biosafety in handling gene transfer vectors. Chapter 12. 1–12. Current protocols on CD [electronic resource]. Unit 12 11. [DOI] [PubMed] [Google Scholar]
- 8.Campagna MV, Faure-Kumar E, Treger JA, Cushman JD, Grogan TR, Kasahara N, Lawson GW. 2016. Factors in the selection of surface disinfectants for use in a laboratory animal setting. J Am Assoc Lab Anim Sci 55:175–188. [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell-Yesufu OT, Gandhi RT. 2011. Update on human immunodeficiency virus HIV2 infection. Clin Infect Dis 52:780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, Tang A, Chen M, Huang L, Harris V, Freed DC, Wilson KA, Dubey S, Zhu DM, Nawrocki D, Mach H, Troutman R, Isopi L, Williams D, Hurni W, Xu Z, Smith JG, Wang S, Liu X, Guan L, Long R, Trigona W, Heidecker GJ, Perry HC, Persaud N, Toner TJ, Su Q, Liang X, Youil R, Chastain M, Bett AJ, Volkin DB, Emini EA, Shiver JW. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol 77:6305–6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, Down J, Denaro M, Brady T, Westerman K, Cavallesco R, Gillet-Legrand B, Caccavelli L, Sgarra R, Maouche-Chretien L, Bernaudin F, Girot R, Dorazio R, Mulder GJ, Polack A, Bank A, Soulier J, Larghero J, Kabbara N, Dalle B, Gourmel B, Socie G, Chretien S, Cartier N, Aubourg P, Fischer A, Cornetta K, Galacteros F, Beuzard Y, Gluckman E, Bushman F, Hacein-Bey-Abina S, Leboulch P. 2010. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature 467:318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention, Public Health Service, National Institutes of Health, 2009. Biosafety in microbiological and biomedical laboratories, 5th ed. Washington (DC): Human Health Services. [Google Scholar]
- 13.Chuah MK, Collen D, VandenDriessche T. 2003. Biosafety of adenoviral vectors. Curr Gene Ther 3:527–543. [DOI] [PubMed] [Google Scholar]
- 14.Croughan WS, Behbehani AM. 1988. Comparative study of inactivation of herpes simplex virus types 1 and 2 by commonly used antiseptic agents. J Clin Microbiol 26:213–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daya S, Berns KI. 2008. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev 21:583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debyser Z. 2003. Biosafety of lentiviral vectors. Curr Gene Ther 3:517–525. [DOI] [PubMed] [Google Scholar]
- 17.Deichmann A, Schmidt M. 2014. Biosafety considerations using γ-retroviral vectors in gene therapy. Curr Gene Ther 13:469–477. [DOI] [PubMed] [Google Scholar]
- 18.Dismuke DJ, Tenenbaum L, Samulski RJ. 2014. Biosafety of recombinant adeno-associated virus vectors. Curr Gene Ther 13: 434–452. [DOI] [PubMed] [Google Scholar]
- 19.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. 1998. A 3rd generation lentivirus vector with a conditional packaging system. J Virol 72:8463–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dupont F. 2003. Risk assessment of the use of autonomous parvovirus-based vectors. Curr Gene Ther 3:567–582. [DOI] [PubMed] [Google Scholar]
- 21.Fallaux FJ, Bout A, van der Velde I, van den Wollenberg DJ, Hehir KM, Keegan J, Auger C, Cramer SJ, van Ormondt H, van der Eb AJ, Valerio D, Hoeben RC. 1998. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum Gene Ther 9:1909–1917. [DOI] [PubMed] [Google Scholar]
- 22.Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. 2013. Gene therapy clinical trials worldwide to 2012—an update. J Gene Med 15:65–77. [DOI] [PubMed] [Google Scholar]
- 23.Glorioso JC. 2014. Herpes simplex viral vectors: late bloomers with big potential. Hum Gene Ther 25:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon JW. 2001. Direct exposure of mouse ovaries and oocytes to high doses of an adenovirus gene therapy vector fails to lead to germ cell transduction. Mol Ther 3:557–564. [DOI] [PubMed] [Google Scholar]
- 25.Hanawa H, Persons DA, Nienhuis AW. 2005. Mobilization and mechanism of transcription of integrated self-inactivating lentiviral vectors. J Virol 79:8410–8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janovitz T, Klein IA, Oliveira T, Mukherjee P, Nussenzweig MC, Sadelain M, Falck-Pedersen E. 2013. High-throughput sequencing reveals principles of adeno-associated virus serotype 2 integration. J Virol 87:8559–8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson PA, Miyanohara A, Levine F, Cahill T, Friedmann T. 1992. Cytotoxicity of a replication-defective mutant of herpes simplex virus type 1. J Virol 66:2952–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khare R, Chen CY, Weaver EA, Barry MA. 2011. Advances and future challenges in adenoviral vector pharmacology and targeting. Curr Gene Ther 11:241–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lachmann R. 2004. Herpes simplex virus-based vectors. Int J Exp Pathol 85:177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis ME, Leung WC, Jeffrey VM, Warren KG. 1984. Detection of multiple strains of latent herpes simplex virus type 1 within individual human hosts. J Virol 52:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim F, Khalique H, Ventosa M, Baldo A. 2014. Biosafety of gene therapy vectors derived from herpes simplex virus type 1. Curr Gene Ther 13:478–491. [DOI] [PubMed] [Google Scholar]
- 32.Maheshwari G, Jannat R, McCormick L, Hsu D. 2004. Thermal inactivation of adenovirus type 5. J Virol Methods 118:141–146. [DOI] [PubMed] [Google Scholar]
- 33.Marshall E. 1999. Gene therapy death prompts review of adenovirus vector. Science 286:2244–2245. [DOI] [PubMed] [Google Scholar]
- 34.Meyers C, Mane M, Kokorina N, Alam S, Hermonat PL. 2000. Ubiquitous human adeno-associated virus type 2 autonomously replicates in differentiating keratinocytes of a normal skin model. Virology 272:338–346. [DOI] [PubMed] [Google Scholar]
- 35.Mingozzi F, High KA. 2011. Immune responses to AAV in clinical trials. Curr Gene Ther 11:321–330. [DOI] [PubMed] [Google Scholar]
- 36.Miyagawa Y, Marino P, Verlengia G, Uchida H, Goins WF, Yokota S, Geller DA, Yoshida O, Mester J, Cohen JB, Glorioso JC. 2015. Herpes simplex viral-vector design for efficient transduction of nonneuronal cells without cytotoxicity. Proc Natl Acad Sci USA 112:E1632–E1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM. 1998. Development of a self-inactivating lentivirus vector. J Virol 72:8150–8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mostoslavsky G, Kotton DN, Fabian AJ, Gray JT, Lee JS, Mulligan RC. 2005. Efficiency of transduction of highly purified murine hematopoietic stem cells by lentiviral and oncoretroviral vectors under conditions of minimal in vitro manipulation. Mol Ther 11:932–940. [DOI] [PubMed] [Google Scholar]
- 39.NIH Office of Science Policy. 2016. NIH guidelines for research involving recombinant or synthetic nucleic acid molecules. [Cited 16 February 2017]. Available at: http://osp.od.nih.gov/sites/default/files/NIH_Guidelines.html.
- 40.Nowrouzi A, Penaud-Budloo M, Kaeppel C, Appelt U, Le Guiner C, Moullier P, von Kalle C, Snyder RO, Schmidt M. 2012. Integration frequency and intermolecular recombination of rAAV vectors in non-human primate skeletal muscle and liver. Mol Ther 20:1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan YW, Scarlett JM, Luoh TT, Kurre P. 2006. Prolonged adherence of human immunodeficiency virus-derived vector particles to hematopoietic target cells leads to secondary transduction in vitro and in vivo. J Virol 81:639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porter JD, Lyons RM. 2002. Virucidal effects of rodent cage-cleaning practices on the viability of adenovirus vectors. Contemp Top Lab Anim Sci 41:43–46. [PubMed] [Google Scholar]
- 43.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, Wilson JM, Batshaw ML. 2003. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab 80:148–158. [DOI] [PubMed] [Google Scholar]
- 44.Reuter JD, Fang X, Ly CS, Suter KK, Gibbs D. 2012. Assessment of hazard risk associated with the intravenous use of viral vectors in rodents. Comp Med 62:361–370. [PMC free article] [PubMed] [Google Scholar]
- 45.Rosas LE, Grieves JL, Zaraspe K, La Perle KM, Fu H, McCarty DM. 2012. Patterns of scAAV vector insertion associated with oncogenic events in a mouse model for genotoxicity. Mol Ther 20:2098–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schambach A, Zychlinski D, Ehrnstroem B, Baum C. 2013. Biosafety features of lentiviral vectors. Hum Gene Ther 24:132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schenk-Braat EA, van Mierlo MM, Wagemaker G, Bangma CH, Kaptein LC. 2007. An inventory of shedding data from clinical gene therapy trials. J Gene Med 9:910–921. [DOI] [PubMed] [Google Scholar]
- 48.Spaete RR, Frenkel N. 1985. The herpes simplex virus amplicon: analyses of cis-acting replication functions. Proc Natl Acad Sci USA 82:694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stein S, Ott MG, Schultze-Strasser S, Jauch A, Burwinkel B, Kinner A, Schmidt M, Kramer A, Schwable J, Glimm H, Koehl U, Preiss C, Ball C, Martin H, Gohring G, Schwarzwaelder K, Hofmann WK, Karakaya K, Tchatchou S, Yang R, Reinecke P, Kuhlcke K, Schlegelberger B, Thrasher AJ, Hoelzer D, Seger R, von Kalle C, Grez M. 2010. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat Med 16:198–204. [DOI] [PubMed] [Google Scholar]
- 50.Takara Bio. [Internet]. 2015. Fourth generation lentiviral packaging systems. [Cited 23 November 2016]. Available at: http://www.clontech.com/US/Products/Viral_Transduction/Lentiviral_Packaging/Lentiviral_Packaging_Overview.
- 51.Teodoro JG, Shore GC, Branton PE. 1995. Adenovirus E1A proteins induce apoptosis by both p53-dependent and p53-independent mechanisms. Oncogene 11:467–474. [PubMed] [Google Scholar]
- 52.US Department of Health and Human Services. [Internet]. 2006. Biosafety considerations for research with lentiviral vectors. [Cited 12 March 2016].Available at: http://osp.od.nih.gov/office-biotechnology-activities/event/2006-03-15-140000/biosafety-considerations-research-lentiviral-vectors.
- 53.Valtierra HN. 2008. Stability of viral pathogens in the laboratory environment. Appl Biosaf 13:21. [Google Scholar]
- 54.Wang Q, Guo J, Jia W. 1997. Intracerebral recombinant HSV1 vector does not reactivate latent HSV1. Gene Ther 4:1300–1304. [DOI] [PubMed] [Google Scholar]
- 55.Yalkinoglu AO, Heilbronn R, Burkle A, Schlehofer JR, zur Hausen H. 1988. DNA amplification of adeno-associated virus as a response to cellular genotoxic stress. Cancer Res 48:3123–3129. [PubMed] [Google Scholar]
- 56.Zou A, Atencio I, Huang WM, Horn M, Ramachandra M. 2004. Overexpression of adenovirus E3-11.6K protein induces cell killing by both caspase-dependent and caspase-independent mechanisms. Virology 326:240–249. [DOI] [PubMed] [Google Scholar]