Abstract
This paper explores how the regulatory approval process affects innovation incentives in medical technologies. Prior studies have found early mover regulatory advantages for drugs. I find the opposite for medical devices, where pioneer entrants spend 34 percent (7.2 months) longer than follow-on entrants in regulatory approval. Back-of-the- envelope calculations suggest that the cost of a delay of this length is upwards of 7 percent of the total cost of bringing a new high-risk device to market. Considering potential explanations, I find that approval times are largely unrelated to technological novelty, but are meaningfully reduced by the publication of objective regulatory guidelines. Finally, I consider how the regulatory process affects small firms’ market entry patterns and find that small firms are less likely to be pioneers in new device markets, a fact consistent with relatively higher costs of doing so for more financially constrained firms.
Keywords: Regulation, Innovation, FDA, Medical Devices
1 Introduction
How does entry regulation help or hinder pioneer innovators? On the one hand, first mover advantages in commercializing new technologies arise when firms can capture substantial market share, for example through exclusive patenting in settings with strong intellectual property protection. On the other hand, early innovators may pay large fixed costs in order to establish regulatory precedents in regulated industries and in doing so, allow subsequent entrants to “free ride” on the efforts and learnings of pioneers. Pioneer entrants’ ability to gain and sustain market share is shaped deeply by the context of market entry. Thus, the effect of novelty on early innovators’ market entry incentives is ambiguous.
In much of the health care sector as well as in ubiquitous industries such as transportation and energy, a regulator directly determines if and when a firm can enter a given market. Industry regulation, as such, is often associated with delayed or reduced firm entry: all else equal, increasing the (expected) time and/or costs that acrue between an innovation and its commercialization will reduce incentives to innovate. For example Budish et. al. (2015) find evidence of this phenomenon in cancer research and development (R&D). Reductions in firms’ innovation incentives will, in turn, have a downstream effect on whether they enter new markets. This paper explores one determinant of these incentives by considering the implications of being a first mover innovator in the context of U.S. medical technology markets.
In the United States, all medical products are regulated by a single agency, the U.S. Food and Drug Administration (FDA). The FDA regulates two trillion dollars worth of products every year, including 80 percent of the U.S. food supply, cosmetics, animal products, and all ethical drugs and medical devices (Babiarz and Pisano, 2008). The FDA also regulates emerging classes of medical products such as biologic drugs (therapeutic proteins often referred to simply as “biologics”), nanomedicines, tissue engineered products, and the use and applications of cellular and gene therapies.
Criticism of FDA regulation – and in particular, regulation’s potential effects on innovation – date back several decades. Peltzman’s (1974) essay on the regulation of pharmaceutical innovation is perhaps one of the best-known examples. Writing in response to the 1962 (“Kefauver-Harris”) amendments to the Food, Drug, and Cosmetic Act, which required new drugs to demonstrate not only safety but also efficacy before being brought to market, Peltzman argues that additional regulatory requirements increased the time and costs associated with bringing new products to market. Highlighting counterfactuals in which important drugs and vaccines would have been subject to post-1962 regulation and thus taken longer to reach patients, he estimates the additional mortality and morbidity cost that would have been associated with longer periods of regulatory approval for products including tuberculosis therapies, tranquilizers, and polio vaccines. Based on this exercise, Peltzman concludes that after 1962, reduced consumer spending on ineffective drugs was outweighed by the costs associated with both more expensive pharmaceuticals and “missed benefits (consumer surplus) from the reduced flow of new drugs” (Peltzman, 1974). However, this research has been heavily criticized: other researchers have pointed out that “the facts don’t fit the tidy conclusion” (Hilts, 2003). In reality, the number of drugs companies were producing actually began falling in the late 1950s – well before the Kefauver-Harris amendments – and the effects of the law likely wouldn’t have been felt as quickly as documented in Peltzman’s study (Carpenter, 2010; Hilts, 2003). Indeed, a more critical review of Pletzman (1974) suggests that declining drug innovation in the 1960s and beyond was not (just) a result of stricter regulation.1
Still, much remains to be understood with respect to how the anticipated length and stringency of regulatory review affect incentives for firms to bring new medical products to market. Unlike Peltzman, this study does not consider a major change to the regulation of new medical products as a source of identification. Rather, it focuses on understanding the innovation incentives inherent in the regulatory approval process for new medical devices, as it exists today. Specifically, this paper asks: what are the incentives for new product development created by the regulatory approval process currently in place? And under what circumstances do we expect firms to experience higher costs of market entry in the form of longer periods of regulatory approval?
Previous studies of medical innovation under FDA regulation have focused almost exclusively on the pharmaceutical drug industry (Goldman and Lakdawalla, 2012; Carpenter and Ting, 2007; Carpenter et. al., 2010), where early mover regulatory advantages have been documented. For example, Carpenter et. al. (2010) find that a one standard deviation increase in the log of order of entry (the order in which a product is approved within a given category) is associated with roughly 3.6-month increase in FDA approval time. Further, they find evidence that this pattern is most consistent with a model of political consumer co-optation by an “astute regulator.” In this model, the regulator appeals to the political demands of specific groups of consumers (e.g. HIV/AIDS patients and advocates), where the earliest products to enter the market result in the greatest marginal political satisfaction and are thus prioritized in the regulatory approval process. Relatedly, Dranove and Meltzer (1994) show that more important chemical drugs are developed and approved more rapidly. However, newer classes of medical products – in particular, medical devices – are characterized by a much larger degree of product heterogeneity and significant ex ante uncertainty about the nature of the regulatory process itself, changing the context and incentives for new product approval regulation.
At the same time, the regulation of new medical products has several unique features, including limited knowledge about product quality and relatedly, (potentially) endogenous submission. Carpenter and Ting (2007) describe these phenomena in the new drug context. They present a model in which the likelihood of type I error (approving unsafe products) is higher for new drugs submitted by firms with lower costs of experimentation – i.e. larger firms – and find evidence for this model in data from FDA drug approvals. Given this finding, it is especially important to control for firm regulatory experience in studies of new product approvals. Further, Carpenter and Ting’s (2007) model and the reality that type I errors occur serve as a reminder that new medical products have features of so-called “credence goods” – that is, their quality may not be fully knowable by regulators or innovator firms, even after some testing and experience. Thus, in the device setting, some amount of product quality uncertainty is likely to remain even following clinical trials.
I begin by contrasting detailed data on new medical device approvals to what is known about the approval dynamics of new drugs. Medical devices are an extremely heterogenous category of products including technologies as wide-ranging as pacemakers, coronary stents, and silicone breast implants. I find that, in contrast to the early entrant advantages observed in drug regulation, first entrants in medical device markets experience a strong disadvantage in the average length of their regulatory approval process. Using data spanning three decades of regulatory approvals (1977–2007), I show that pioneer entrants in new device categories spend 34 percent (7.2 months) longer in the approval process than the first (and subsequent) follow-on innovator(s) in that category. Back-of-the-envelope calculations suggest that the costs of a delay of this length are upward of 7 percent of the total R&D costs associated with bringing a new high-risk medical device to market.
I then ask how different components of regulatory uncertainty are related to approval times in the medical device setting. I first consider technological uncertainty – uncertainty on the part of the regulator that involves a lack of technological or scientific understanding of a specific type of product and its use in the human body. This definition of technological uncertainty is, by its nature, comprised of both uncertainty about how a product works as well as uncertainty about how the regulator will know that a new product works. In studying novel products, it is virtually impossible to disentangle these two types of regulatory uncertainty and as such, the analyses presented below capture both simultaneously. Technological uncertainty arises most clearly in the evaluation of novel medical devices, where the regulator needs to understand the scientific mechanisms through which a device works, but is unlikely to have a clear up-front understanding of the data required to be convinced of a product’s safety and effectiveness prior to the product’s appearance in the regulatory review queue.
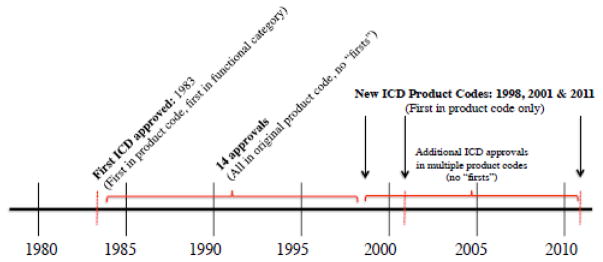
Consider, for example, the first time that the FDA was tasked with evaluating the safety and effectiveness of an implantable cardioverter defibrillator (ICD2) for use in patients. The first ICD was approved by the FDA in 1983 and at that time, the technological uncertainty faced by regulators was centered around understanding precisely how the device functioned as well as how it would interact with the heart and the surrounding tissues. Over the years, R&D on ICDs continued and to date, over two dozen later-generation ICDs have been approved by the FDA. Many of these ICDs were classified under the same product code as the originally approved device, but starting in 1998, some ICDs were given a new categorical designation (an FDA “product code”) due to modifications to the device. While these later ICDs were somewhat different than earlier models, it is also clearly the case that by the time they entered the regulatory approval process, the FDA had already established a good understanding of how ICDs work as well as an understanding of how the regulator can assess the safety and effectiveness of ICDs in clinical trials.
Importantly for this study, the assignment of FDA product codes occurs independently of the process of regulating new technologies and is discussed at length in section 5.2. Exploiting the fact that several products with known technological functions in the human body are assigned to a new product code at the start of the regulatory approval process, I am able to compare products that are both technologically and categorically novel to those that are only categorically novel. This exercise can be used to shed light on how much of the delay associated with pioneer entry can be explained by the introduction of truly novel technologies vs. the introduction of known technologies in newly designated regulatory categories.
I find that after controlling for the designation of being assigned to a new product code, knowing whether or not a device was technologically novel does not provide additional explanatory power in understanding regulatory approval times. This suggests that the regulator’s familiarity (or lack of familiarity) with the primary technology used in a new medical device is not the primary determinant of the length of the regulatory approval process. For example, the first ICDs regulated in later-established ICD product codes still experienced a regulatory delay associated with being “first-in-class,” despite the fact that the regulator already had significant experience with this type of device at the time they were considered for regulatory approval.
If technological novelty is not the primary driver of longer regulatory approval times for first mover innovators, than what else might be at play? The results suggest that there is something particular about the designation of being in a new product code that is of importance – that the categorical designation and administrative novelty associated with a new product code is itself predictive of longer regulatory approval times. With this in mind, I next consider the role of a different type of uncertainty: uncertainty about the content and format of information required for regulatory approval of a specific medical device. Content and format uncertainty occurs in the absence of clear guidelines for the protocol for evaluating a new product, leading to uncertainty as to how to present (on the part of the applicant firm) and assess (on the part of the regulator) the results of clinical studies and other (e.g. biocompatibility and engineering) tests. This type of uncertainty necessarily co-occurs with technological uncertainty for novel products, and without the establishment of clear evaluation standards, it may persist.
Content and format uncertainty is easiest to think about in a scenario in which a product and its functionality are known to the regulator, but evaluation criteria have not been formally articulated or informally established by precedent. This phenomenon can be observed in drug eluting stents3 (DESs), which were first submitted to the FDA for regulatory approval in 2002. It was not until 2008, however – after five different DESs had submitted applications for regulatory approval and four had been approved – that the FDA published a formal guidance document, detailing what testing and clinical criteria it would use to evaluate DESs moving forward.
I consider the release of FDA guidance on DESs and eight other unique medical devices. In each case, objective regulatory guidance was introduced for a group of already-established products (i.e. some number of approvals had already occurred). Conservative estimates indicate that on average, approval times for subsequent entrants fall by approximately 40 percent (6.1 months) after application content and evaluation procedures are made explicit through formal guidance. In contrast to technological uncertainty, uncertainty about content and format of new product applications appears to play a large role in explaining regulatory approval times for first movers, and overall.
These findings have implications for other emerging categories of medical technology such as tissue engineered products and cellular and gene therapies, as these are all contexts in which there is a large degree of uncertainty about the content and format of the new product regulatory approval process as well as how such products will be evaluated. This uncertainty is the result of both a short (or nonexistent) regulatory history for these types of products and dearth of formally or informally established regulatory criteria. Much like new medical devices, these products also “credence good” characteristics and will need to be evaluated as such by regulators and adopters. Further, in these new product categories, regulatory approval times for a given product are similarly likely to be substantially protracted until a time when clear(er) evaluation criteria are formalized and made available.
Finally, I consider how the implicit costs of early regulatory uncertainty for categorically novel medical devices may affect if and when firms enter into new markets. I evaluate the behavior of all cardiovascular device firms that brought new products to market over the three decades studied and find that small (more financially constrained) firms are less likely than large firms to enter new device markets as pioneers. On average, the fraction of small firms among pioneer entrants into new device markets is between 25 and 52 percent lower4 than among follow-on entrants. These findings are consistent with the prediction that small firms should be less willing to enter new markets when there is significant regulatory uncertainty and therefore higher associated costs of doing so.
In its regulatory decisions, the FDA is charged with ensuring the safety and effectiveness of new products. A long debate has engaged with the tradeoffs between regulatory speed and consumer safety. Former FDA Commissioner Margaret Hamburg and former Principal Deputy Commissioner Joshua Sharfstein have discussed the balance that the FDA must strike between risks to consumers and speed of regulation, arguing that “as a public health agency, the FDA should always ask whether delays in approval or safety problems can be prevented” (Hamburg and Sharfstein, 2009). This paper does not evaluate or weigh in on the balance between regulatory speed and consumer safety in current policies. Rather, it considers factors that may affect regulatory approval times given the regulatory system as experienced by medical device innovators in the United States today – and, as such, the length of development times experienced by firms. Thus, the analysis presented here concerns only the regulatory system currently in place, given a regulatory agency that works to protect both consumer safety and its own reputation. These dual goals are reflected in the conceptual framework presented below.
The rest of the paper proceeds as follows: the next section describes the markets for medical devices and the institutions that regulate their entry. Section 3 lays out a model of regulatory delay and subsequent firm choice given large anticipated costs for pioneer innovators. Section 4 describes the data on new product approvals used in the empirical analyses in Sections 5 and 6. Section 7 concludes.
2 Background: Markets and Regulatory Frameworks
2.1 Medical Products: Definitions and Markets
This paper considers a large category of medical products: medical devices. A medical device is defined5 by the FDA as an “instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article...intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease” and “which is not dependent upon being metabolized for the achievement of any of its principal intended purposes.” Examples of medical devices range from stethoscopes to breast implants, prosthetic limbs and pacemakers.
U.S. device markets are large: at an annual $140b, device spending makes up a meaningful share of the $2.7 trillion that is spent annually on health care in the United States.6,7. Moreover, devices and other emerging medical technologies make up a growing share of national health spending: spending on devices grew at an annual rate of approximately 6.0% over the five years leading up to 2012, versus 4.5% growth in overall health expenditures over the same period. In addition to representing a large medical product market in the United States, devices offer substantial research opportunities: detailed approval data are available across product classes and over the entire history of the FDA’s regulation of these products.
Other emerging categories of medical technology also comprise an increasing share of health spending. One prominent example is that of biologic drugs, a group of therapeutic proteins derived from living organisms, which are often the primary component of vaccines and cancer therapies. Because they are more complex than chemically-synthesized “small molecule” drugs and derived from living cells, biologic drugs are regulated separately from chemical drugs. Although biologics do not appear in the analysis below, they resemble devices in their heterogeneity and shorter regulatory history and are poised to increase in both economic importance8 and regulatory submissions over the coming years (Scott Morton et. al., 2016).9
Drugs are a relatively homogeneous category of products with a century-long history of regulation (see Appendix D for additional detail). By comparison, medical devices and other non-drug medical products are far more heterogeneous and have a much shorter history of FDA oversight. For these reasons, it is less feasible to define detailed regulatory procedures and standards for new device ex ante. Given the greater degree of resulting regulatory uncertainty facing innovators in the medical device industry, I explore what types of implicit costs and incentives have emerged from the regulatory system in place.
2.2 Medical Product Regulation in the United States: The FDA
In the United States, all medical technologies are regulated by a single agency, the U.S. Food and Drug Administration (FDA). The FDA is an agency of the Department of Health and Human Services and is responsible for the oversight of two trillion dollars worth of products every year, including all over-the-counter and prescription drugs and medical devices (Babiarz and Pisano, 2008; Hamburg and Sharfstien, 2009). The FDA also regulates all other existing and emerging classes of medical products. The precursor to the modern FDA was established through the Pure Food and Drug Act, which was signed by President Theodore Roosevelt in 1906. It was not until seven decades later, however, that the FDA’s regulatory scope grew to include medical devices, which came under FDA regulation in 1976.
The FDA is organized into centers, each of which is tasked with the oversight of a different type of product. The center most relevant to the analysis below is the Center for Devices and Radiological Health (CDRH), which regulates medical devices.10 Within the CDRH, the Office of Device Evaluation is responsible for the review and approval of medical devices. Other categories of products are also reviewed by specialty centers within the FDA.11
2.3 The FDA and the Regulation of Medical Devices
The foundation of the FDA’s modern-day statutory authority to regulate medical products is the Federal Food, Drug, and Cosmetic Act of 1938 (FDCA), which requires that new drugs be tested for safety and that those tests be submitted to the government for marking approval (Babiarz and Pisano, 2008; FDA, 2013), however the FDCA of 1938 did not impose any pre-approval requirements on medical devices, which instead were regulated at the state level at the discretion of each state’s legislature for several subsequent decades. Devices came into the spotlight and, in some cases, into the FDA’s jurisdiction following the United States v. Bacto-Unidisk Supreme Court case in 1969 (394 U.S. 784), in which the court ruled that the term “drug” should be considered more broadly, so as to include certain types of complex products that carried some of the same risks as drugs (Munsey, 1995). However, this new authority was only granted to the FDA on a case-by-case basis and only once devices were already in use. Combined with limited clarity about the scope of the FDA’s regulatory authority over medical devices, this meant that the FDA’s oversight of devices occurred primarily on an ad hoc basis following this ruling. It wasn’t until 1976, after a series of well-publicized medical device failures, that Congress passed the Medical Device Amendments Act (MDA) to the FDCA, which gave the FDA primary authority to regulate devices sold in the United States (Sall, 2008; Kramer et. al., 2012; Munsey 1995).
Devices are wide-ranging in their cost, invasiveness, function, and risk: they include products ranging from tongue depressors and stethoscopes (which the FDA classifies as “low-risk” devices) to hearing aids (“moderate-risk” devices) to pacemakers and prosthetic heart valves (“high-risk” devices). The MDA delineates these three risk classes and lays out the rules for regulating each class of products. This paper focuses only on approval regulation of “high-risk” (Class III) devices which are those that “support or sustain human life” and are of the highest risk (FDA, 2002).12 Unlike moderate and low risk devices, high-risk devices are subject to a rigorous regulatory process that resembles that used for new drugs (Zuckerman et. al. 2011; Goldman and Lakdawalla, 2012), requiring detailed product information and evidence of safety and effectiveness from clinical trials. While high risk devices represent only about one percent of the devices that the FDA regulates each year (Redberg and Dhruva, 2011), they represent an out-sized fraction of medical device spending: In 2008, spending on the six highest-cost implanted devices alone was about $13 billion (Meier, 2009), or approximately 10 percent of total U.S. medical device spending.
The regulatory approval process for high-risk devices is called “premarket approval” (PMA) and is necessary when a medical device developer wants to market a new high-risk device. Importantly, once the first device in a product code is approved through the PMA process, all subsequent devices in that product code must also be approved through the PMA process.13 In the data used here, the average approval time for a new high-risk device is 18.1 months, although the average for a device that is first within a product code is longer, at 22.5 months. Appendix Figures 1 and 2 provide additional information on the chronology and requirements of the PMA process and Appendix Figure 2 highlights similarities and differences between the requirements for the PMA and the New Drug Application (NDA).14
The PMA is a complex document filed by the manufacturer that contains information about the product and results of clinical trials. As is the case for drugs, Section 515 of the FDCA requires that a PMA provide scientific evidence of safety and effectiveness, typically in the form of data from a so-called “pivotal study.”15 However, as the next section explains, the types of trials that can constitute a pivotal study for a new high-risk medical device are highly heterogeneous and to a large extent, open to interpretation – an important difference between the regulatory approval processes for drugs and devices.
2.4 Drugs vs. Devices: Regulatory Differences
Importantly – and quite differently from drug trials – clinical trials for medical devices may take many different and often more flexible forms. In new drug studies, three phases of randomized controlled trials are the norm. In device trials, however, clinical evidence can come from a variety of sources: trials may take the form of well controlled investigations, partially controlled investigations, objective trials without matched controls, and other types of studies “from which it can fairly and responsibly be concluded by qualified experts that there is reasonable assurance of safety and effectiveness of a device under its conditions of use” (21 CFR 860.7(c)(2)).
This lack of ex ante specificity about the design and execution of clinical trials is largely the result of product and delivery-method heterogeneity across medical devices. Because of these sources of heterogeneity, regulators cannot articulate general rules or guidelines for medical device clinical trials and subsequent regulatory evaluation that are both sufficiently broad so as to be relevant for devices ranging from pacemakers to silicone breast implants, while still being sufficiently specific to guide the clinical trials and regulatory evaluation of all types of devices.
Moreover, while drugs are almost always delivered in one of just a few conventional ways (administered orally, injected intravenously or intramuscularly, inhaled, or administered topically), the delivery method and use of a new high-risk medical device is often a novel process with few (if any) related prior clinical trials to use as a precedent or guide. Thus, both the planning and execution of device trials are substantially more uncertain than those for new chemical drugs. Devices can be used, implanted, or otherwise administered in hundreds of ways. Furthermore, how a device is used or the method by which an implantable device is placed in the human body is often not only unique, but also critical to the success or failure of a trial (Sall, 2008). In summary, the large degree of heterogeneity across medical devices and in the processes required for their evaluation, combined with limited information about how clinical trials should proceed and be presented to regulators, results in a much greater degree of uncertainty about the content and format requirements for new device approvals (vs. new drug approvals).
Chatterji (2009) relays the anecdote of one pitfall of regulatory uncertainty: the company Acorn Cardiovascular “believed they were close to FDA approval in 2002 for their device that helps to shrink enlarged hearts, but the FDA instead recommended a much larger clinical trial that ended up taking three more years and costing the company $30 million.” While this represents an extreme example of delay due to regulatory uncertainty, it is true that there are typically at least two cycles of requests for additional information (by FDA) and responses (by the applicant firm) before a decision about a new PMA is made (Zenios et. al., 2010). This is because for many devices, the evaluation criteria that the FDA will use to assess a new product are not made explicit before the regulatory process begins. An important exception to this are cases where the FDA publishes regulatory guidance, a list of objective product evaluation criteria addressing application content and format that will be used to assess all devices of a certain type from the time of publication onward. The publication of such guidance and subsequent product approvals are considered in detail in Section 5.3 of this paper.
Appendix A presents additional case studies of firms’ experiences with regulatory uncertainty and delay. Case 1 in Appendix A presents a heart failure monitoring system that spent three years under consideration by the FDA. At the time of data collection for this paper, the device has already been through one large-scale controlled clinical trial and one follow-up study and at the time of writing, the device had been approved for marking in the United States. Case 2 presents an even later stage regulatory experience for a new high-risk device: following the completion of a randomized, double-blind, sham-controlled pivotal clinical trial that yielded statistically significant results supporting the device’s safety and efficacy, the FDA returned to the manufacturer with follow-up questions related to device testing and clinical data.
In sum, although device manufacturers need to present the FDA with evidence from clinical trials in order to bring a new product to market, the lack of specificity about what types of data to collect and present to regulators makes the regulatory process for devices far more uncertain than that of drugs. In the sections that follow, I explore how this uncertainty plays out in both new product approval times and firms’ strategies for entering new markets.
3 A Model of Approval Regulation and Firm Strategy
In many industries, government approval or licensing is a prerequisite for market entry. Examples include nearly all parts of the energy, health care and transportation industries. This paper considers the experiences of medical technology firms in their interactions with the FDA.
3.1 Framework and Regulator Decision-Making
The first empirical exercise builds on Carpenter et. al.’s (2010), model16 of the FDA drug approval process, in which a farsighted regulator discounts the future pipeline of product approvals and decides how rapidly to approve a new product in light of this discounting. In such a setting, the regulator gets greater utility from quickly approving an earlier entrant into a given market than a later entrant. Appendix B presents details of this model of approval by a farsighted regulator.
In the model, the regulator can also respond to political factors. This feature is consistent with existing evidence on the political economy of the FDA’s regulatory behavior. For example, studies show that the FDA responds to the demands of lobby groups representing (potential) drug consumers, such as cancer or AIDS organizations (Olson, 1995; Carpenter 2002; Carpenter et. al., 2010). Individual firms may also exert pressure on the FDA17, although research on pharmaceutical drug approvals has found limited evidence of firms’ influence on regulatory approval times (Carpenter et. al., 2010). In the model and analyses that follow, I account for firm and disease-specific factors that may influence the duration of the regulatory approval process. Acemoglu and Linn (2004) find that potential market size has a strong influence on the entry of non-generic drugs and new molecular entities and DuBois et. al. (2015), find similarly that global pharmaceutical innovation responds significantly to market size. Carpenter (2004) finds that firms submitting more new product applications may expect quicker and more likely approvals. Below, I deviate from Carpenter et. al. (2010) in defining a more general model of approval priorities for an uncertain regulator.
I begin with a simple, flexible empirical model of regulatory approval times that includes known covariates, such as those factors identified above. Both firms and the regulator observe the relationship between regulatory approval times and application characteristics. Approval time (T) of product p, in speciality area s, of entry order ϕ produced by firm f, in year t is then observed as:
| (1) |
where i denotes the application of subscripts p, s, ϕ, f, t and where Xs include:
Entry order (ϕ) within a product code (devices) or disease group (drugs), p.18
Fixed effects for the advisory panel (organized by medical specialty, s), product group (p), and applicant firm (f).
Year of review (t).
Applicant firm (f)’s cumulative regulatory experience at time, t.
Eligibility for expedited review – e.g. if a product addresses a rare/orphan/terminal disease or is the first to address a given condition – (p, ϕ).
If the regulator discounts the future pipeline of products and/or appeals to the political demands of specific groups of consumers (consumer co-optation), it will prefer to approve earlier products more quickly (see Appendix B). In this setting, ceteris paribus, review times increase in entry order as later products create less marginal value for consumers and the regulator: early entrants should benefit from a shorter regulatory process (with later products experiencing increasingly longer approval times) leading to “early mover advantages” in terms of approval speed in the regulatory approval process.
However, when there is regulatory uncertainty about how to evaluate a product, it will increase the time that a regulator spends on the approval decision in order to obtain an amount of product information that is sufficient to outweigh product risk (see Appendix B). Further, because the degree of regulatory uncertainty is likely to be inversely related to entry order (i.e. uncertainty is greater among the first products of a certain type to seek regulatory approval), greater regulatory uncertainty among early entrants would be expected to affect approval times in the opposite direction of the early mover regulatory advantages described above. Indeed, if the affect of regulatory uncertainty is large enough, it could lead to longer regulatory approval times for earlier entrants, even when the regulator has a preference for getting more novel products to market quickly, either as a result of political demands or future discounting.
To account for entry-order specific uncertainty, I modify Equation 1, relating review times to the set of determinants above as well as a term that captures the cost of regulatory uncertainty:
| (2) |
where
| (3) |
For simplicity, regulatory uncertainty, Uϕ, can be thought of as generating a fixed delay during the regulatory approval process, on average Dϕ, although a more specific framework would model Uϕ = g(ϕ)+ε, where g(ϕ) is decreasing in entry order (e.g. due to regulator learning). That is, among some set of the earliest entrants, there exists regulatory uncertainty such that a new product experiences approval times that are Dϕ longer, on average. As Dϕ becomes larger, expected approval times for products of entry order 1 through ϕ will increase commensurately. Thus, even when the regulator would otherwise approve early entrants more quickly – as is empirically true of the FDA’s approval of new drugs (Carpenter et. al., 2010) – a relatively large value of Dϕ implies that approval times for the earliest entrants could be longer than those of subsequent entrants. In the empirical section of this paper, I ask when there is evidence that Dϕ > 0 and for which values of ϕ this is the case. By knowing the values of ϕ (entry order), for which there are additional costs of regulatory approval, one can evaluate which set of entrants are disadvantaged in the form of extended approval times in the approval process.
In Section 5, I first focus on estimating the regulatory approval times associated with early entrants in device markets. I define first mover regulatory disadvantage as a setting in which first entrants experience longer regulatory approval periods than follow-on entrants. However, there are other additional and related costs likely to accrue to early innovators who experience longer approval times; these include shortened periods of market exclusivity and additional legal fees and are discussed in Section 5.
Additionally, the empirical section of the paper addresses the fact that Uϕ likely has several components. Any factor that increases Upϕ will, on average, also lead to approval times that are both longer and higher in variance. I consider two such factors specifically: technological uncertainty and uncertainty about application content and format. I am able to consider each of thse separately by taking advantage of two unique sources of variation in regulatory uncertainty in the new device approval data.
3.2 Firm Strategy
Finally, I present a testable hypothesis about firm strategy that emerges from the model described above. Both firms and the regulator observe to-date regulatory approvals, approval times, and the entry order of all prior products. Firms know that greater uncertainty increases time spent on regulatory approval and the variance thereof and decide which markets to enter, given anticipated costs and benefits. The first dimension on which a firm makes a decision is whether to enter a novel or existing market. All else equal, this decision will be influenced by the relative cost of novel vs. established product regulation.
Assume that each firm, f, has access to a certain amount of capital, Kf. Firms expect an uncertainty-driven delay of length Dϕ (as above) for innovating in a new market. For a firm, the implied cost of being a first mover is an increasing function of the length of the anticipated regulatory delay and a decreasing function of firm capital (as financially-constrained firms will have less capital allocated for R&D and/or higher costs of borrowing) such that Cf = c(Dϕ,Kf ). Now consider two firms: Firm A has access to a large amount of capital (e.g. Firm A is a large, publicly listed company), while Firm B is financially constrained (e.g. Firm B is small and/or has a finite amount of venture capital to deploy and therefore faces high costs of borrowing or additional fundraising) such that KA > KB. Then in a given product market at a given time, the relative cost of entry is greater for Firm B than for Firm A (i.e., CB > CA) because the expected value of Dϕ is the same for both firms.
Assume a distribution of the value to firms of pioneer entry into new markets, such that there is a range of potential profits, πϕ=1, that can be captured by the first entrant. Then each firm decides whether the expected marginal value of being the first entrant is greater than the marginal cost of being the first mover and enters when πϕ=1 > c(D,Kf ). Since the relative costs of new market entry are greater for Firm B than for Firm A, Firm B will be willing to enter fewer new markets than Firm A. More generally, small (financially-constrained) firms should be less inclined to enter new markets as pioneers when there are large costs (delays) associated with doing so. This leads to the following market entry hypothesis: In the presence of approval delays under regulatory uncertainty, small firms should be less likely to act as pioneer entrants than large firms.
4 Data
The first source of data I use is the FDA’s Premarket Approval (PMA) database. Later, I also use information from a detailed firm-level dataset, which was collected by hand from financial databases and firm websites and includes financial, ownership, and acquisition data for all cardiovascular device firms represented in the PMA data.
The data on high-risk device approvals come from the FDA’s PMA database,19 which includes an exhaustive record of all PMA approvals since the 1976 Medical Device Amendments to the Federal Food, Drug and Cosmetic Act. I include all submissions starting in calendar year 1977 – the first calendar after which the FDA first began regulating medical devices – and limit the data to include submissions through the year 2007.20 The medical device approval data summarized in Table 1 include 847 unique device approvals in 249 product codes. Product codes are specific definitions based on design and function that “delineate [a device’s] technology and indication.”21 Examples include drug-eluting stents or silicone breast implants. As an analog to the disease groups used to categorize drugs, device product codes are likely to be very good to excellent clinical substitutes for one another. A list of example device product code names as well as an example of a device product code definition from the FDA can be found in Appendix C. The PMA database also includes detailed information about the date of each application’s submission, date of FDA decision, the submitting firm’s identity, and an indicator for whether a product ever received “priority” or expedited review. According to the FDA, such a device “supports or sustains human life or is of substantial importance in preventing impairment of human health or presents a potential, unreasonable risk of illness or injury.”22
Table 1.
Summary Statistics
| Premarket Applications (PMAs) - Devices: N=847 Premarket Applications (Cardiovascular Devices): N=241 | ||||
|---|---|---|---|---|
|
| ||||
| Devices | CV Devices Only | |||
| Mean | S.D. | Mean | S.D. | |
| Approval Time (Months) | 18.12 | 15.84 | 17.31 | 12.96 |
| Approval Time (First Product) | 21.48 | 16.77 | 23.07 | 18.16 |
| Approval Time Ratio: First Device/Average Device | 1.18 | 1.06 | 1.33 | 1.40 |
| Entry Order | 6.37 | 8.79 | 5.06 | 4.32 |
| Priority Review | 0.10 | 0.30 | 0.10 | 0.29 |
| New Applications (Current) | 15.32 | 20.63 | 27.57 | 25.92 |
| Submission Year | 1994 | 8.46 | 1995 | 7.98 |
| Firm | 32 FEs | – | 15 FEs | – |
| Medical Device Product Code | 249 FEs | – | 55 FEs | – |
Summary statistics for the 847 medical devices used in the empirical analyses as well as separate descriptive statistics (right columns) for the subset of (241) cardiovascular devices alone. Approval Time measures months from PMA submission until FDA approval. Entry Order is based on the chronological ordering of PMA submissions. Priority Review is an indicator for whether a product was eligible for expedited FDA review. New Applications (Current) is a firm-specific, time-varying count of total successful new product applications that the applicant firm has completed at the time of a given submission. Submission year is the calendar year in which an application was sent to the FDA. Firm contains a set of dummy variables for each firm in the data set or a dummy indicator for being a “small” firm – i.e. one with fewer than five new applications over the entire period of observation.
For a subset of the empirical exercises that follow, I focus only on high-risk cardiovascular devices, which are those reviewed by the Circulatory System Devices Panel. Table 1 presents summary statistics for all devices as well as for the subset of cardiovascular devices only. Table 2 shows the distribution of medical device approvals by specialty: cardiovascular (circulatory system) devices make up by far the largest speciality area, comprising 241 out of the 847 applications in the data, or approximately 28.5 percent of the total device sample.
Table 2.
New Devices by Advisory Committee (Specialty)
| Advisory Committee | New Devices | Percent |
|---|---|---|
| Circulatory System | 241 | 28.45 |
| Opthalmic | 160 | 18.89 |
| Microbiology | 74 | 8.74 |
| General and Plastic Surgery | 60 | 7.08 |
| Gastroenterology-Urology | 53 | 6.26 |
| Orthopedic and Rehabilitation | 53 | 6.26 |
| Immunology | 38 | 4.49 |
| Obstetrics and Gynecology | 33 | 3.90 |
| Radiology | 28 | 3.31 |
| General Hospital and Personal use | 23 | 2.72 |
| Clinical Chemistry and Toxicology | 17 | 2.01 |
| Dental | 15 | 1.77 |
| Ear, nose and throat | 13 | 1.53 |
| Neurology | 13 | 1.53 |
| Anesthesiology | 12 | 1.42 |
| Physical Medicine | 8 | 0.94 |
| Hematology and pathology | 6 | 0.71 |
|
| ||
| (Total) | 847 | 100.00 |
This table shows the distribution of all 847 new devices analyzed in this study by FDA (specialty-specific) Advisory Committee.
Finally, for the set of firms that produce the high-risk cardiovascular devices in the PMA database, I collect detailed firm-level financial and ownership data. These include data on firm size (as measured by annual revenues), firm ownership (public vs. private), and whether and when a firm was acquired by another company – as well as the identity of that company and the year of acquisition, if relevant. Financial data were collected from Google Finance, NASDAQ, NYSE Euronext, and from firm websites.
Throughout the paper, I observe data on new product approvals, not on earlier innovation steps and other decisions prior to the regulatory approval process. This means that I do not observe those products that are abandoned before or during the Premarket Approval process, based on unpromising clinical results. In the drug setting, which shares many features of the device setting, Carpenter and Ting (2007) discuss circumstances under which regulatory submission may be endogenous.23 Yet conditional on looking at the PMA process, as I do here, selection bias at the stage of the approval decision is mitigated by the fact that the rate of non-approvals is negligible: a 2009 Government Accountability Office Report using FDA data found that “all PMA decisions during fiscal years 2003 through 2007 were approved or withdrawn. FDA did not deny approval of any PMA submissions during this period. According to FDA officials, when a PMA was seriously deficient, FDA issued a “not approvable” letter under 21 C.F.R. 814.44(f) and placed the submission on hold.” (GAO, 2009). A Freedom of Information Act request by the author (answered October 22, 2015) further revealed that the total count of all PMA applications denied by the FDA since 1980 yielded only two incidents of denial of approval (one in 1982 and another in 2013). As such, the approval time phenomena I observe and the effects that I calculate represent the effects of regulation on the regulated, and not the (deterrent) effect of regulation on those products that do not make it into (or through) the approval process.24
5 Empirical Estimation
I proceed with a series of estimates based on the framework above. I first establish patterns in device approval times following the model of regulatory approval times presented in Section 3. I then consider the specific case of medical device regulation to better understand regulatory experiences of novel and follow-on products. Finally, I test the hypothesis about firm market entry strategies in detailed firm-level data.
5.1 Approval Times and Entry Order
The first part of this analysis is grounded in the literature on the determinants of FDA approval times for new drugs, notably Carpenter et. al. (2010) and others. I account for potential political and institutional factors that may affect the approval process while estimating the relationship between product entry order and approval times for both drugs and devices. Carpenter et. al. (2010) define “entry order” as the order in which a drug within a given disease group submits an application for FDA approval. In exercises presented in Appendix D and Appendix Tables II and III, I replicate Carpenter et. al. (2010)’s findings for drugs and compare those results to the same specifications applied to medical devices. I extend this definition to its closest analog for medical devices: the order in which a medical device within a given product code submits an application for FDA approval.
To understand the entry order relationships further, the first column of Table 3 uses dummy variable indicators for a product being first, second, third, fourth, or greater than fifth in a product code (omitted category: entry order = 5), rather than a linear indicator of entry order. Column 2 estimates the same model as column 1, but uses months as the independent variable. Column 3 compares only the first entrant to the first unambiguous follow-on entrant – that is, the first PMA submitted in a product code vs. the first PMA submitted after the first PMA had been approved – and finds that relative to the first follow-on entrant, a pioneer entrant spends approximately 34 percent longer in the regulatory approval process. Column 4 converts this result into months, indicating that a pioneer spends an average of 7.2 months (approximately 219 days) longer in the regulatory approval process than the first unambiguous follow-on entrant into the same product code. The results from Table 325 also facilitate an approximation of the value of ϕ*: approval delays are only statistically significant for the first entrant into a product code, suggesting that the value of ϕ* is close to 1.
Table 3.
Quantifying Early Mover Disadvantage
| Outcome = Device Approval Time (Months) | ||||
|---|---|---|---|---|
|
| ||||
| (1) Ln Approval Time | (2) Approval Time (Months) | (3) Ln Approval Time | (4) Approval Time (Months) | |
| First in Product Code | 0.2157** (0.0890) | 5.7158*** (1.5015) | 0.3376*** (0.0914) | 7.1993*** (1.3238) |
| Second in Product Code | −0.0705 (0.0887) | 0.1781 (1.3966) | ||
| Third in Product Code | 0.1208 (0.1235) | 4.7995 (3.4273) | ||
| Fourth in Product Code | 0.0039 (0.0694) | 1.6371 (1.7781) | ||
| Greater than 5th in Product Code | −0.0536 (0.0732) | 0.9762 (1.0754) | ||
| Full Sample | X | X | ||
| Restricted Sample (1st + 1st Follow-on Only) | X | X | ||
| N | 847 | 847 | 342 | 342 |
| R2 | 0.0934 | 0.1073 | 0.1105 | 0.0986 |
p<0.05,
p<0.01,
p<0.001
Column 1 shows the relationship between the listed entry order dummies and the log of approval time. Column 2 converts these results into months. Column 3 considers only the difference in approval times between the first applicant (the pioneer) and the first unambiguous follow-on innovator in the same product code. Column 4 converts these results into months.
All models include firm and advisory committee fixed effects, a time trend (year), and are robust to the exclusion/inclusion of year fixed effects rather than a time trend. All models also include controls for whether a product was granted “priority” (expedited) review as well as a count of the applicant firm’s total approved applications at the time of submission. Standard errors are clustered at the product level.
Note: the large coefficient and standard error seen on the dummy variable for “Third in Product Code” above appear to be driven by a few outliers and can be roughly halved by windsorizing approval time at 45.6 months (the 95th percentile) without changing the magnitude or statistical significance of any of the coefficients on “First in Product Code” in the regressions presented in this table. For simplicity, this table presents regression results using the original, uncensored data.
With the brunt of the costs of delay borne by the first entrant, one might wonder about the financial implications of pioneer innovation. Consider the estimated value of D, the 7.2 month longer approval times estimated for pioneer entrants: how large is this? One benchmark is the length of delay relative to the length of the period of de facto market exclusivity that a pioneer can expect to have. In the full medical device sample, the first entrant into a product code has an average of 3.8 years as the sole product with regulatory approval (before the second product is approved for market entry – that is, the pioneer can expect an average of 3.8 years of de facto market exclusivity). For high-risk cardiovascular devices, this period is just 2.8 years. As a naïve benchmark comparison then, the additional time a pioneer medical device can expect to spend in regulation is between 15.8 and 21.4 percent of the total period of time it can expect to spend alone on the market. Of course this benchmark is problematic for many reasons. Above all these de facto periods of market exclusivity are endogenously determined and do not take into account any dynamic affects that may result from changing the speed of approval of the first product. Yet the calculation represents a useful basis for further consideration because it suggests that the type of time periods estimated in Table 3 are likely of an order of magnitude that is consequential for firms.
A more general way to think about the cost of delay is to calculate the implied opportunity cost of capital over that period of time. In medical product industries, the opportunity costs of capital are large. Assuming a discount rate such as that used for the biotechnology industry, one can calculate the opportunity cost of a 7.2 month delay. Makower et. al. (2010) survey roughly 20% of firms in the medical device industry and find that the average cost of bringing a high-risk medical device to market is about $94 million. Assuming a discount rate of 11.5% (DiMasi and Grabowski, 2007), the results suggest that the opportunity cost of the delay associated with being the first entrant in a product code is probably around $6.7 million, or more generally, upwards of 7 percent of the total cost of new device development.26
5.2 Sources of Uncertainty Part 1: Is Technological Novelty Associated with Longer Approval Times?
Given evidence of longer regulatory approval times for the first entrants in a medical device product code, I next turn to potential explanations. Regulatory delay has many possible components. One of the most obvious is technological uncertainty about the workings of a new product. Technological uncertainty broadly encompasses uncertainty on the part of the regulator due to a lack of scientific familiarity with or understanding of a specific type of product.
When a product is very novel – i.e. the regulator has never seen anything that performs its function before – technological uncertainty is high. An example can be seen in the historical approvals of implantable cardioverter defibrillators (ICDs) described in Section 1. However, after the technological uncertainty around a certain type of device has been largely resolved – for example through multiple successful, completed assessments and approvals of that type of product – one would expect to see a decrease in that component of approval delay that is associated with technological uncertainty.
I use the information embedded in FDA-defined, detailed device product names27 to assess product technological novelty in a subsample of high-risk cardiovascular devices. Device classification into product codes depends on the intended use and indications of a device28 and, importantly, occurs independently from the process of regulating new technologies. For example, cardiovascular devices are classified by category of use (diagnostic devices, monitoring devices, surgical devices, prosthetic devices, and therapeutic devices) as well as by specific purpose – e.g. among therapeutic devices, separate classifications exist for catheters used for percutaneous translumial coronary angioplasty (PTCA), ebolectomies and septostomies (Code of Federal Regulations, Title 21). The FDA explains how classification proceeds: “A device will be assigned an existing classification product code when it has the same intended use, indications for use, and relies on technology that does not raise new safety and effectiveness questions. However, if the proposed device differs significantly from the predicate device with respect to technology, intended use or indications for use or is found not substantially equivalent (NSE), a new product code should be assigned.”29 This analysis focuses on cardiovascular devices because this is by far the largest specialty area in the data, representing nearly 30% of all new device approvals and because this speciality includes the greatest number of unique product codes.
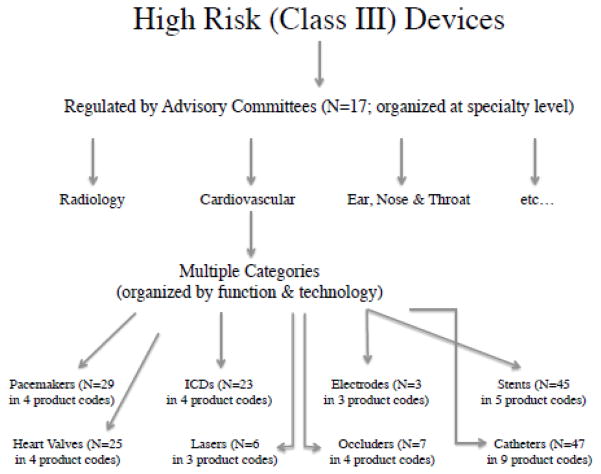
I identify eight “functional categories” of devices, each of which contains multiple unique device product codes, but all of which share a common function in the human body, making each category a natural setting for comparing highly related products. Examples of functional categories include stents, implantable cardioverter defibrillators (ICDs), and replacement heart valves. Each of these functional categories includes multiple products that have the same general function in the human body, but some variation in the materials from which they are made, their method of delivery, and/or the product design, resulting in designations of multiple product codes within each functional category. Figure 1 provides a guide to functional category construction for the subsample and Figure 2 gives a detailed timeline of ICD approvals within the ICD functional category. The eight functional categories analyzed and the number of products and product codes in each are listed in Table 4.
Figure 1.
Organization and Example Classification
Figure 2.
ICD Example - First in Product Code vs. First in Functional Category
Table 4.
Functional Category Composition (Cardiovascular Devices)
| Device Function (Category) | Number of Unique Product Codes | Number of Unique Devices (Total) |
|---|---|---|
| 1. Pacemaker | 4 | 29 |
| 2. Catheter | 9 | 47 |
| 3. ICD | 4 | 23 |
| 4. Electrodes | 3 | 3 |
| 5. Stents | 5 | 45 |
| 6. Valves | 4 | 25 |
| 7. Laser for Angioplasty | 3 | 6 |
| 8. Occluder | 4 | 7 |
This table presents the eight functional categories evaluated in Section 5.4. Each of the categories contains multiple unique product codes, making each a useful setting for comparing technologically similar products.
To evaluate the extent to which technological uncertainty affects approval times, I consider whether a prior approval within the a functional category is associated with reduced approval times for subsequent devices in that functional category. Devices in a functional category will, by definition, be highly similar to one another. Moreover, the prior approval of the first of a particular category of device (e.g. a stent) should lead to the regulator acquiring a technological understanding of that type of product, which in turn, will be relevant for subsequent approvals of products of that type. Thus I ask: when a device is first in its product code, but its primary technological function and components are already known to the regulator, are regulatory times shorter? In other words, controlling for the designation of being first within a product code, how much explanatory power (if any) can be gained from knowing whether a device was technologically novel? If technological novelty is a major driver of longer regulatory approval times for first entrants, the coefficient on a dummy variable indicator for being first in a functional category should be large and statistically significant.
I identify the earliest entrant in each functional category of products and then look for subsequent entrants into that category. These subsequent entrants were, by their timing, the clear beneficiaries of reduced technological uncertainty because the first product utilizing their primary technology had already been approved. (This is true regardless of entry order within the relevant device product code – which may or may not be different from entry order within the functional category).
Because this analysis is limited to a smaller sample of only cardiovascular devices, I first repeat the product-code-level analyses from Table 3 for the subsample of cardiovascular devices alone. The results of this analysis are presented in Panel A of Table 5 and yield coefficients of a very similar magnitude and statistical significance to those seen in Table 3: being first within a product code is associated with a regulatory approval process that is 6.8 months longer (similar to the coefficient of 7.2 calculated in the same regression model for the full data set). I next proceed with the analysis at the functional category level. I find little evidence of the importance of reduced technological uncertainty in explaining subsequent approval times (Panel B). The results suggest that on average, being first within a functional category is associated with a regulatory approval process that longer, but these results are not statistically significant at any conventional levels.30
Table 5.
Technological Novelty in Cardiovascular Devices
| (1) Ln Approval Time (Months) | (2) Approval Time | (3) Approval Time (Months) | |
|---|---|---|---|
| Panel A: Cardiovascular Subsample Only (by Prod. Code) | |||
|
| |||
| First in Product Code | 0.2334* (0.1292) | 5.1143** (2.4862) | 6.8224** (2.6205) |
| N | 183 | 183 | 163 |
| R2 | 0.5009 | 0.4372 | 0.4118 |
|
| |||
| Panel B: Devices in 8 Functional Categories | |||
|
| |||
| First in Category | 0.1624 (0.2767) | 2.7185 (5.3434) | 9.0857 (5.8699) |
| N | 183 | 183 | 179 |
| R2 | 0.4899 | 0.4206 | 0.4218 |
|
| |||
| Panel C: Controlling for Technological Uncertainty | |||
|
| |||
| First in Product Code | 0.2327* (0.1376) | 5.2872** (2.6466) | 7.1890** (2.8121) |
| First in Category | 0.0041 (0.2934) | −1.1056 (5.6446) | −2.1300 (5.7774) |
| N | 183 | 183 | 163 |
| R2 | 0.5009 | 0.4374 | 0.4125 |
p<0.05,
p<0.01,
p<0.001
This table looks at first entrants and their respective approval delays in a) product codes b) functional categories and c) both in the same model.
All models include firm and year fixed effects. Models also include controls for whether a product was granted “priority” (expedited) review and a count of the applicant firm’s approved applications at the time of submission.
Column 1 presents a log-linear model, while Column 2 translates the result into months. Column 3 restricts the sample to only the first entrant plus those subsequent entrants who submitted applications after the first entrant’s approval decision was finalized.
In Panel C, I ask how much – if any – of the delay observed for a new entrant in a product code is reduced when another device with its function has already completed the regulatory approval process. Specifically, because of the grouping of multiple products into functional categories, it is possible to control for the resolution of a large degree of technological uncertainty (at the functional category level) and then look at the residual relationship between product entry order and approval delay. The statistically and economically non-significant coefficients on “First in Category” suggest a very limited role for technological uncertainty itself in explaining regulatory delays. However, in this specification, being first within a product code is associated with a regulatory approval process that is 5.3 to 7.2 months longer and these coefficients are statistically significant at the 1 percent level. Thus, it seems that the delineation of a new product code itself, rather than the novelty of the technology involved in that product’s primary function is the strongest predictor of longer regulatory approval times.
5.3 Sources of Uncertainty Part 2: Regulatory Guidance to Reduce Uncertainty about Application Content and Format
This section considers cases in which uncertainty about new product application content and format is resolved through the publication of formal guidance documents. This type of uncertainty occurs in the absence of clear guidelines about the protocol for evaluating a new product, leading to uncertainty on the part of the regulator as to how to evaluate the results of clinical studies and other (e.g. biocompatibility and engineering) tests and uncertainty on the part of firms as to what information to submit to the regulator and in what format. A clear example of the resolution of this type of uncertainty can be seen in the publication of FDA guidance documents related to the regulation of drug eluting stents, which is described in Section 1.
The publication of formal FDA guidance about a specific product or class of products31 is the primary way in which protocols for evaluating a new medical device are formally established. In 1997, the FDA announced that it would formalize its Good Guidance Practices in order “to provide transparency and consistency in policy development” moving forward (FDA, 2007).32 Examples include documents that describe the “design, production,...manufacturing, and testing of regulated products; processing, content, and evaluation or approval of submissions; [and] inspection and enforcement policies.”
In recent years, the FDA has released several pieces of guidance related to medical devices, which are available from the Office of Device Evaluation (ODE). Of the 162 pieces of guidance released since the approval of GGPs, the vast majority deal with Class II (moderate-risk) devices and several others relate to general evaluation practices, rather than focusing on specific products. I consider the publication of four pieces of formal, product-specific guidance documents for high-risk cardiovascular devices. These pieces of guidance directly outline objective evaluation criteria relating to the PMA process for nine unique product codes of high-risk (Class III) cardiovascular devices. These guidance documents and the dates of their publication are listed in Table 6.
Table 6.
Case Studies, Publication of Objective Regulatory Guidance
| Product Type | Date Published | Product Code(s) Affected | Pre-Guidance Approval Time (Months) | Post-Guidance Approval Time (Months) | N (obs) |
|---|---|---|---|---|---|
| Drug-Eluting Stents | (3/1/2008) | 1 | 15.38 | 8.75 | 9 |
| Intravascular Stents | (4/18/2010) | 4 | 13.50 | 8.02 | 42 |
| Heart Valves | (1/20/2010) | 3 | 11.83 | 9.00 | 6 |
| Catheter Ablation Devices | (8/5/2008) | 1 | 14.29 (N=49) | 9.36 (N=15) | 7 (N=64) |
This table summarizes four recent cases in which objective regulatory guidelines were published by the FDA for major categories of cardiovascular devices. In each of the cases, regulatory delays fall substantially in the period after guidance is published. The data are raw and un-adjusted for potentially relevant covariates.
Unfortunately, we rarely see variation in this type of procedural uncertainty for first-in-class devices, due to the fact that such guidance documents are typically written with an existent set of products in mind. Nevertheless, it is illustrative to consider the impact of guidance publication on subsequent product review times in order to understand how the clarification of application content and format may impact the speed of regulatory review. In each of the cases outlined in Table 6, uncertainty around application content and format was largely resolved through the release of formal submission and evaluation guidelines for new product applications and in each of these cases, average approval times subsequently decreased. In the analysis that follows, I define post-guidance applications as those that were submitted one month or more after the publication of guidance for a given product or set of products. This ensures that all post-guidance applicants had the opportunity to incorporate information from the guidance into their application prior to submission.
Table 6 shows that (without any controls), following the publication of regulatory guidance, an average decrease in regulatory approval times of 2.8 to 6.6 months was observed in affected product codes. Table 7 includes statistically appropriate control variables and estimates the covariate-adjusted average decrease in approval time associated with the publication of guidance. All models in Table 7 include product code fixed effects and controls for whether a product was granted “priority” (expedited) review, year of submission, and a count of the applicant firms total approved PMAs at the time of submission. The first column of Table 7 presents a covariate-adjusted pre-post analysis of approval times with respect to the publication of regulatory guidance for all applications in affected categories. Column 2 excludes the first two entrants in each group so as not to bias the results by including applications in the pre-guidance average that are known to have longer approval times, as documented in Section 5.1.
Table 7.
Publication of Objective Regulatory Guidance Outcome = Approval Time
| (1) | (2) | (3) | (4) | |
|---|---|---|---|---|
| Post-Guidance | −10.0515** | −8.3711† | (4.7666) | (4.9293) |
| ATE (Post-Guideance) | −6.0696*** (0.8577) | |||
| ATT (Post-Guideance) | −8.5193*** (3.0972) | |||
| Controls | X | X | X | X |
| Excluding first 2 Entrants | X | |||
| Pre-Post Analysis | X | X | ||
| Matched Analysis | X | X | ||
| N | 64 | 51 | 192 | 192 |
| R2 | 0.3401 | 0.3944 |
p<0.10
p<0.05,
p<0.01,
p<0.001
This table shows the covariate-controlled results of a regression model of the relationship between new device approval times and the publication of regulatory guidance for the four cases presented in Table 8.
All models include product code fixed effects and controls for whether a product was granted “priority” (expedited) review, application year, and a count of the applicant firm’s approved applications at the time of submission. Columns 1 and 2 present results from a pre-post analysis of guidance publication. Column 1 shows results for all devices in affected categories. Column 2 excludes the first entrants so as not to bias the results by including a group that is known to have longer approval times in the pre-guidance average.
Columns 3 & 4 presents results from a “nearest neighbor” matching analysis in which each device in a “treated” product code is matched to two other “untreated” devices based on observables including entry order, submission year, submissions in the product code at the time of a given application, and average approval times in the product code. Both the average treatment effect (ATE) and average treatment effect on the treated (ATT) from this analysis are presented.
Although these results are consistent with the conclusion that uncertainty about application content and format is an important driver of first mover disadvantage in the medical device regulatory process, one should be concerned about likely endogeneity in the FDA’s decision to publish guidance for these particular devices. For example, it may be the case that more popular categories of medical devices were more likely to get regulatory guidance. To address potential selection, Column 3 presents results from a nearest neighbor matching analysis in which each device in a “treated” product code (i.e. one in which guidance was at some point published) is matched to two other “untreated” devices (other high-risk cardiovascular devices in product codes in which guidance was not published) based on ex ante observables about the applications and product codes including entry order, submission year, total PMA submissions in the product code at the time of a given application (device “popularity”), and average approval times in the product code. Both the average treatment effect (ATE) and average treatment effect on the treated (ATT) of the introduction of regulatory guidance are presented. Even the most conservative estimate, the ATE presented in Column 4, suggests that the resolution of procedural uncertainty through the publication of formal guidance is associated with a 6.1 month (approximately 185 day) reduction in regulatory approval times. In this subsample, that represents a 41 percent reduction in the average length of regulatory approval.
The results in this section complement prior findings on the procedurally-oriented determinants of entrepreneurial success in the device industry: Chatterji (2009) finds evidence that for venture capital funded companies, familiarity with protocols is more important than technical knowledge for predicting firm successes. These results in turn, suggest that uncertainty about the content and format of a new product application is more important than technological uncertainty about a product for predicting regulatory approval times.
5.4 Entrant Type and Strategy
The final empirical section of this paper considers the relationship between firm type and observed market entry patterns. The market entry hypothesis in Section 3.2, predicts that in the presence of delays under regulatory uncertainty, small (financially constrained) firms should be less likely to enter new device markets as pioneers. Looking within the ownership and financial data assembled for all cardiovascular drug and device firms in the data, I identify small firms. For this exercise, I define a small firm as one that a) is not publicly listed, b) does not have revenues of more than $500 million per year, and c) is not a fully or partially-owned subsidiary of a firm of type a or b above. This categorization identifies a set of small, privately held firms, none of which are subsidiaries of larger companies.
Using the criteria above, Table 8 considers how the proportion of small firms varies with the application of the above definition. The first definition (Definition 1) looks only at those firms that were defined as “small” at least one year before an application was submitted. The next definition (Definition 2) excludes those firms that were or became subsidiaries of large firms within a five year window of a given PMA submission. For example, Irvine biomedical’s percutaneous cardiac ablation catheter was submitted to the FDA for approval in 2004, acquired by St. Jude in the same year, and received approval in 2005. This product would count as belonging to a small firm under Definition 1, but not under Definition 2, which is more conservative. The third and most conservative definition (Definition 3) classifies small firms as those that never met criteria a, b, or c above – that is, they were never part of a large company.
Table 8.
Small Firms’ Market Entry Strategies
| (1) | (2) | (3) | |
|---|---|---|---|
| % Small Firms Among Pioneer Entrants | % Small Firms Among Follow-On Entrants | P[(1) = (2)]† | |
| Drug Firms (N=107) | |||
|
| |||
| Definition 1 | 54.5% | 46.9% | 0.6513 |
| Definition 2 | 45.4% | 35.4% | 0.5541 |
| Definition 3 | 36.4% | 11.5% | 0.1379 |
|
| |||
| Device Firms (N=275) | |||
|
| |||
| Definition 1 | 17.2% | 21.7% | 0.4430 |
| Definition 2 | 10.3% | 19.4% | 0.0657 |
| Definition 3 | 6.9% | 14.3% | 0.0751 |
P-values are from a 2-sided t-test with unequal variances.
A small firm is one that is not a) publicly listed, b) does not have revenues of more than $500 million per year, and c) is not a subsidiary of a firm of type a or b. Results not sensitive to using revenues of more than $1 billion as a cutoff.
Definition 1: only those firms that were defined as “small” at least one year before an application was submitted; Definition 2: excludes those firms that were or became subsidiaries of established firms within five years of a given PMA submission; Definition 3: firms that never met criteria a, b, or c above.
By these definitions, small device firms make up 6.9 to 17.2 percent of the sample among pioneer entrants but 14.3 to 21.7 percent of the sample among follow-on entrants. The difference between these samples is statistically significant at the 10% level for both Definitions 2 and 3 in two-sample t-tests of means with unequal variance. The difference between the two samples is not statistically significant based on Definition 1, likely a result of the small sample sizes used in this exercise. The results are consistent with the prediction that small firms will be less willing to take on the additional costs of entering new device markets, where the regulatory approval process is likely to be more costly.
6 Discussion and Conclusion
I have considered how regulatory uncertainty is related to approval times and therefore first mover advantages and disadvantages in the regulatory approval process for high-risk medical devices. The data on medical device approvals reveal large costs of early entry into new device markets: pioneer entrants in new device product codes spend 34 percent longer in the approval process than the first (and subsequent) follow-on innovator(s) in a product code. I estimate that the magnitude of the additional approval time pioneer innovators can expect to experience is approximately 7.2 months, a large delay relative to the 2.8 to 3.8 years of de facto market exclusivity that a pioneer innovator can expect and a potentially costly experience, given the high opportunity costs of capital in medical technology markets. Indeed, back-of-the-envelope calculations suggest that the cost of a delay of this length will be upwards of 7 percent of the total cost of bringing a high-risk medical device to market. Given these costs, it is not surprising that small firms are less likely to act as pioneer innovators in device markets, a result that is consistent with the prediction that firms with more capital should be better able and/or more willing to bear the additional regulatory costs associated with pioneer entry in the device setting.
I analyze regulatory approval times under two sources of uncertainty by looking at settings in which either technological uncertainty or uncertainty about application content and format are largely resolved. I find that large delays for the first entrant in a product code persist even when a great degree of technological uncertainty (around how a product works as well as how the regulator will know how a product works) has already been resolved. In contrast, I find that the resolution of uncertainty about application content and format through the publication of formal regulatory guidance to clarify product evaluation criteria is associated with substantially reduced approval times thereafter. These results complement other research into the importance of understanding regulatory protocols in the medical device industry; for example, Chatterji (2009) finds that regulatory and procedural knowledge is more important than technical knowledge for predicting firm success.
This paper contributes to a broad literature about the relationship between regulatory uncertainty and innovation incentives – in particular, with respect to medical devices and other emerging categories of medical products, where methods for effectively incentivizing innovation remain poorly understood (Xu et. al., 2013). Generally speaking, incentives for engaging in R&D activity are negatively influenced by increases in the costs and risks of developing new products (Grabowski et. al., 1976). This study is therefore related to research on how R&D incentives affect the pipeline of innovation. Budish et. al. (2015) find evidence that private firms’ incentives to innovate have meaningful impact on the level and composition of R&D investments. Moreover, they find that increases in R&D – in particular in cases where there may be underinvestment – have the potential to generate large improvements in patient health. This paper suggests that under regulatory uncertainty, the nature of the approval process for new medical products may create disincentives for pioneer entry by meaningfully increasing the length of the product development period for novel products. This, in turn, affects firms’ strategies for entering new markets and may encourage small firms to either a) avoid innovating in such markets or b) sell their intellectual property to larger firms before commercializing products in new markets, so as not to bear the regulatory and administrative costs of regulatory standardization and learning. Although it is beyond the scope of this paper, this phenomenon may also lead to the under-development of new high-risk medical devices as a result.
The results also suggest that under some circumstances, the regulation of medical technologies could be made more efficient through the earlier resolution of uncertainty about new product application content and format, where possible. This might be done, for example, through the earlier release of guidance documents about the content and format of new product applications and/or by establishing more formal channels through which firms can work with the FDA very early in the new product development process in order to help the FDA develop evaluation standards or formal guidelines for a new medical technology before a regulatory approval application is officially submitted.33 However, there may be limits to how early in a product’s life cycle guidance can be written. It simply may be the case that some regulatory experience with a product is a prerequisite for the composition of guidance. To the extent this is true, the scope for using guidance alone to accelerate regulatory approval of new products will be limited.
This study could be expanded in a number of ways. First, it would be interesting to know more about regulatory delays themselves: what happens over the period between PMA submission and the FDA’s ultimate approval decision? Relatedly, how much of an observed delay is due to the FDA requesting additional information from device companies and what types of information are requested? And finally, are certain types of information requests (e.g. additional product manufacturing specifications) faster to execute and/or evaluate than others (e.g. additional biocompatibility tests)? The data that I use in this study do not allow me to satisfactorily address these questions. In conversations and interviews with medical device companies, it has frequently been expressed that the FDA’s requesting of additional clinical or technical information is a major source of uncertainty for device firms entering a regulatory process in which the regulator’s expectations are unknown ex ante. Unfortunately, no existing data are able to shed light on the relative frequency or size of these types of delays. As such, this would be a very fruitful area for future data collection and aggregation – both within and beyond the context of medical device regulation.
The results do not address the onerous process of regulatory reform. While it seems likely that earlier clarity on regulatory procedure by the FDA could decrease some approval times, the process for implementing any large changes to formal regulatory policy is complex, time-consuming and institutionally entrenched. Former FDA Commissioner Margaret Hamburg has noted that “these challenges are not the FDA’s alone.” Indeed, she argues that in order “to truly leverage advances in science and technology, there must be a collaboration of all relevant stakeholders, including government, academia, and industry. The FDA must work with its partners to promote innovation and creativity at various points throughout the development process” (Hamburg, 2010).
New medical technologies are poised to continue to grow in importance over the coming years and a better understanding of the innovation incentives inherent in the regulatory approval system can help to shape future policies governing both new product development and new product regulation. This study suggests that mitigating application content and format uncertainty for medical device manufacturers has potential to accelerate the regulatory approval process on the intensive margin – that is, among the group of firms who enter a given market. Yet this study also finds that on the extensive margin, small firms are less likely to enter new device markets as pioneers. Future work should focus on understanding the extent to which this phenomenon is a reflection of efficient product licensing to larger firms that may, for example, be more capable of navigating the regulatory approval process vs. inefficient (welfare-reducing) innovation deterrents for small firms.
Highlights.
I explore how regulation affects innovation incentives in medical technology.
New devices spend 34 percent longer than follow-on entrants in regulatory approval.
Device approval times are not explained by a products technological novelty, but are meaningfully reduced by the publication of objective regulatory guidelines.
Small firms are less likely to enter new device markets than new drug markets.
Acknowledgments
I am especially grateful to Daniel Carpenter, Amitabh Chandra, David Cutler, and Scott Stern for detailed feedback. Leila Agha, Steve Cicala, Iain Cockburn, Innessa Colaiacovo, Mitsuru Igami, Larry Katz, Daniel Kramer, Bruce Sacerdote, Pian Shu, Jonathan Skinner, Bhaven Sampat, David Studdert, Heidi Williams, and many other colleagues and seminar participants provided helpful suggestions. I am also indebted to several medical device industry experts and FDA employees who provided guidance – in particular Richard Cohen, Elazer Edleman, Chip Hance, Clark Nardinelli, and Andreas Schick. Melissa Ouellet provided excellent research assistance. Funding from the National Institute on Aging, through Grant Number T32-AG000186 to the National Bureau of Economic Research, is gratefully acknowledged.
Appendix A: Firm Experiences in the PMA Process
Case 1: A Protracted Review Process for a New Device
The company CardioMEMS is the developer of the Champion Heart Failure Monitoring System device. This device is a permanently implantable pressure measurement system that sits in the pulmonary artery of heart failure patients and monitors pressure and heart rate, transmitting data wirelessly. It is intend to assist in the ambulatory management of heart failure and reduce associated hospital stays (Loh et. al., 2013).
The device was evaluated in the CHAMPION Trial in which 550 patients were randomized into treatment or control groups. In the treatment group, physicians were provided access to patients’ pulmonary artery pressure and all physicians were instructed in the adjustment of heart failure medications. According to CardioMEMS,
“The CHAMPION trial achieved all pre-specified primary efficacy and safety endpoints. Specifically, the rate of adjudicated hospitalizations for heart failure was significantly lower in the Treatment Arm (0.32) compared to the Control Arm (0.44) (28% reduction, p=0.0002), and the device exhibited an excellent safety and performance profile. All pre-specified secondary endpoints were also achieved.”
The results of this trial were submitted in the company’s Premarket Application to the FDA on December 14th, 2010. The reviewing panel raised concerns about potential bias in the efficacy analysis as well as concerns about the efficacy of the device in some subpopulations and the device was not approved following the first meeting at which it was considered in December, 2011 (Husten, 2013). CardioMEMS continued to pursue FDA approval, having completed an additional follow-up study, but at the next review in 2013, the reviewer reported that the FDA still found it “difficult to draw conclusions based on unrandomized and unblinded followup data of a segment of the original trial population” (Husten, 2013). On May 28, 2014 the FDA approved the CardioMEMs device and on May 30, just two days later, St. Jude reported that it closed its acquisition of the product, adding a payout of $375 to the $60 million (19% stake) it had already acquired in 201034.
Case 2: Requests for Additional Information Following PMA Submission and Completion of Pivotal Trials
EnteroMedics is a medical device company that develops neuroscience based technologies to treat obesity and metabolic disease. Its VBLOC therapy device is intended to help obese patients lose weight more comfortably by intermittently blocking the vagus nerve, which resides just above the intersection of the stomach and esophagus. This is accomplished by two small laparoscopically implanted electrodes that are put in contact with the vagus nerve.
EnteroMedics completed a randomized, double-blind, sham-controlled, multicenter pivotal clinical trial of the effectiveness of the VBLOC device in 239 patients at 10 sites (The control group received a non-functional device during the trial period). In February of 2013, EnteroMedics announced a statistically significant and clinically meaningful effect of the device on weight loss and “an excellent safety profile” of the device in trials. The results suggested excess weight loss of approximately 25 percent among treated patients, with over half of patients achieving at least 20 percent excess weight loss. Based on the results of the pivotal trial, a Premarket Application was submitted to the FDA.
In late September of 2013, EnteroMedics reported that it had “received a formal response...from the Food and Drug Administration (FDA) with regard to its Premarket Approval Application (PMA) for approval of the Maestro Rechargeable System as a treatment for obesity.” According to a press release by EnteroMedics, “the response contains follow-up questions related to the application, pertaining primarily to device testing and clinical data, including training programs for users and a post approval study.” (EnteroMedics, September 24, 2013) EnteroMedics said that it would respond to the FDA’s follow-up questions within the weeks immediately following the communication. The Premarket Application for the VBLOC device is still under review at the FDA and EnteroMedics hopes for an approval in 2014.
Case 3: Emerging Classes of Medical Technology and Procedural Uncertainty
There are several classes of emerging medical technologies that do not yet have formal regulatory approval pathways in place for entering U.S. Markets. Two examples (biosimilars and cellular and gene therapies) are presented below. These can be thought of as extreme cases of procedural uncertainty – that is, the complete absence of rules for regulating these new technologies has meant that they are not yet available to patients in the United States.
I. Biosimilars
Biologics are a group of large, complex and heterogeneous therapeutic proteins derived from living organisms, which are often the primary component of vaccines and cancer therapies. Because they are more complex and derived from living cells, biologic drugs are regulated separately from chemical drugs. Biosimilars or follow-on biopharmaceuticals differ from chemical drug generics in terms of their physical characteristics as well as in how they are regulated. Generic versions of chemically manufactured small molecule drugs are based on bioequivalence – that is, containing the same quantity of active substance(s) as the reference product. These generic drugs can be used in the same dose to treat the same disease with equal expected efficacy. Biosimilars, on the other hand, are much larger molecules and follow-on products are based on similarity to the reference product, such as biologically manufactured recombinant proteins (Manheim et. al. 2006; Rovira et. al., 2011).
At present, the FDA is finalizing its guidance covering the regulation of follow-on biological products. At the time of writing, the United States has only seen one biosimilar drug come to market. Europe, in contrast, has had biosimilars since 2006 following the establishment of a formal regulatory pathway for their approval (for additional detail on the early European experience with biosimilars, see Scott Morton et. al., 2016).
On February 9, 2012, the FDA issued three draft guidance documents on biosimilar product development and the FDA is currently accepting public comments these documents. There remains a fair amount of debate as to what FDA will require of biosimilars – in particular with respect to requirements to prove interchangeability (GaBI, 2012). In a February 2012 editorial, The Lancet urged the FDA “to integrate the data, experience, and lessons learned by the European Medicines Agency, which has approved a dozen biosimilars since 2006.” At present, ambiguity in the regulatory processes for approving biosimilars has meant that patients in the United States have much more limited access to these products than European patients.
II. Cellular and Gene Therapies
Several new cellular and gene therapies also provide examples of extreme procedural uncertainty. Cellular and gene therapies are regulated by the FDA’s Center for Biologics Evaluation and Research (CBER). Specific products and applications, in turn, are typically regulated following the publication of, and in accordance with, CBER guidance35 documents. As a corollary, the absence of CBER guidance on a specific therapeutic application typically means that it is unavailable to U.S. patients.
An example is that of retinal ganglion cell gene therapy for visual system repair. In this application, the cells in the retina are genetically modified using viral vectors in order to benefit patients with certain inherited degenerative conditions that compromise visual function (Hellström and Harvey, 2011). Several recent clinical trials have demonstrated that genetic modification can be of meaningful therapeutic benefit to patients and there is now evidence for the long-term expression of genes delivered through the vector, suggesting extended therapeutic effects of the therapy, following a single treatment/dose. However, clinical trials to-date have been heterogeneous in their use of viral vs. non-viral gene therapy vectors and even within viral vector therapies, multiple vectors have been studied in clinical trials (Hellström and Harvey, 2011). In the absence of FDA guidance on the regulation of such therapies and despite evidence of their effectiveness, no rental repair gene therapies are currently approved by the FDA for use outside of clinical trials.
Appendix B: Approval Regulation given a Farsighted Regulator
The first model tested in Section 5 is an extension of Carpenter et. al.’s (2010), model of the FDA drug approval process. In this model, drugs are indexed by i, diseases by j, and firms by k. I generalize this model to apply to multiple categories of medical technology products (e.g. drugs, devices, and others) and a common regulator, the FDA. New products can then be characterized by two parameters:
γij (where 0 <γij ≤ 1) is the curing probability of the product. Assume γij is fixed and known with certainty throughout agency’s decision problem.
-
μi is the danger of the drug or the expected number of people who will be harmed or killed by it over an interval of time, which can be normalized to 1 such that μi can be thought of as the rate of harming consumers. The greater is μi, the more its approval will harm the regulator’s reputation.
Note: for simplicity, it is helpful to assume that cov(μi, γij) = 0 – that is, danger and curing power are independently distributed.
The agency observes information (e.g. clinical trials) in which a product either harms or does not harm the consumer. Harm evolves according to a Wiener process Xit = X(t) a linear function of underlying danger (μ) plus a random component:
where μ and σ > 0 are constants and where z(t) is a standard normal variable with mean 0 and variance t. Then the agency applies Bayes’ Rule to the stochastic history of X(t) to learn about μ. In this model, assume that σ is the same across products, but that μ (normally distributed) differs across them and has a mean, m and variance s. Then, Carpenter et. al. (2010) note that for any product review of time t and accumulated harm X(t) = x, [x, t] constitutes a sufficient statistic for the agency’s problem.
Bayseian estimates of μ are then:
And
Where the posterior variance can be thought of as the FDA’s uncertainty about μ, the harm the product may induce (Carpenter et. al., 2010).
Approval Payoff
Scholars of the political economy of FDA drug approvals have found that the FDA may be more responsive to the demands of lobby groups representing (potential) drug consumers, such as cancer or AIDS organizations (Olson, 1995; Carpenter 2002; Carpenter et. al., 2010) and that individual firms may also exert pressure on the FDA. Once can think of a general model of payoff for the regulator as follows:
where:
γN is the curing probability, as noted above
NJ –1 is the number of products in the same product category that have already applied for FDA approval
ρK is the “political clout” of the submitting firm, K
θJ is a disease-specific factor that may represent the disease’s prevalence and/or the strength of its political lobby36
χ is a set of relevant specialty area and time-varying effects that may affect the payoff associated with product approval
Agency Decision-Making
As in Carpenter et. al. (2010), I can write an agency objective function, in which the Agency wants to maximize its approval payoff given information about μ̂:
where δ is the discount factor, T is approval time, μ* is the agency’s estimate of danger at the optimal stopping time for clinical trials and other data collection, ω represents an elementary event in probability space Ω and y is a variable of integration.
Early Entrant Advantages
In a model like the one used above, early entrant protection should be observed within a product category. All else equal, this is a result of a regulator making approval decisions in the present while expecting a discounted pipeline of future innovations. For example, given two products i = N and i = N + 1 with the same expected levels of danger (μN = μN+1) and curing probability, then the Nth product should have a shorter expected approval time. This result is, of course, in the absence of regulatory uncertainty, which is introduced and discussed in Section 3.
Appendix C: Product Code Examples
I. Examples of unique cardiovascular products
TITLE 21–FOOD AND DRUGS, CHAPTER I–FOOD AND DRUG ADMINISTRATION, DEPARTMENT OF HEALTH AND HUMAN SERVICES, SUBCHAPTER H–MEDICAL DEVICES
PART 870 CARDIOVASCULAR DEVICES
Subpart A–General Provisions
§870.1 - Scope.
§870.3 - Effective dates of requirement for premarket approval.
§870.9 - Limitations of exemptions from section 510(k) of the Federal Food, Drug, and Cosmetic Act (the act).
Subpart B–Cardiovascular Diagnostic Devices
§870.1025 - Arrhythmia detector and alarm (including ST-segment measurement and alarm).
§870.1100 - Blood pressure alarm.
§870.1110 - Blood pressure computer.
§870.1120 - Blood pressure cuff.
§870.1130 - Noninvasive blood pressure measurement system.
§870.1140 - Venous blood pressure manometer.
§870.1200 - Diagnostic intravascular catheter.
§870.1210 - Continuous flush catheter.
§870.1220 - Electrode recording catheter or electrode recording probe.
§870.1230 - Fiberoptic oximeter catheter.
§870.1240 - Flow-directed catheter.
§870.1250 - Percutaneous catheter.
§870.1270 - Intracavitary phonocatheter system.
§870.1280 - Steerable catheter.
§870.1290 - Steerable catheter control system.
§870.1300 - Catheter cannula.
§870.1310 - Vessel dilator for percutaneous catheterization.
§870.1330 - Catheter guide wire.
§870.1340 - Catheter introducer.
§870.1350 - Catheter balloon repair kit.
§870.1360 - Trace microsphere.
§870.1370 - Catheter tip occluder.
§870.1380 - Catheter stylet.
§870.1390 - Trocar.
§870.1425 - Programmable diagnostic computer.
§870.1435 - Single-function, preprogrammed diagnostic computer.
§870.1450 - Densitometer.
§870.1650 - Angiographic injector and syringe.
§870.1660 - Indicator injector.
§870.1670 - Syringe actuator for an injector.
§870.1750 - External programmable pacemaker pulse generator.
§870.1800 - Withdrawal-infusion pump.
§870.1875 - Stethoscope.
§870.1915 - Thermodilution probe.
Subpart C–Cardiovascular Monitoring Devices
§870.2050 - Biopotential amplifier and signal conditioner.
§870.2060 - Transducer signal amplifier and conditioner.
§870.2100 - Cardiovascular blood flowmeter.
§870.2120 - Extravascular blood flow probe.
§870.2300 - Cardiac monitor (including cardiotachometer and rate alarm).
§870.2310 - Apex cardiograph (vibrocardiograph).
§870.2320 - Ballistocardiograph.
§870.2330 - Echocardiograph.
§870.2340 - Electrocardiograph.
§870.2350 - Electrocardiograph lead switching adaptor.
§870.2360 - Electrocardiograph electrode.
§870.2370 - Electrocardiograph surface electrode tester.
§870.2390 - Phonocardiograph.
§870.2400 - Vectorcardiograph.
§870.2450 - Medical cathode-ray tube display.
§870.2600 - Signal isolation system.
§870.2620 - Line isolation monitor.
§870.2640 - Portable leakage current alarm.
§870.2675 - Oscillometer.
§870.2700 - Oximeter.
§870.2710 - Ear oximeter.
§870.2750 - Impedance phlebograph.
§870.2770 - Impedance plethysmograph.
§870.2780 - Hydraulic, pneumatic, or photoelectric plethysmographs.
§870.2800 - Medical magnetic tape recorder.
§870.2810 - Paper chart recorder.
§870.2840 - Apex cardiographic transducer.
§870.2850 - Extravascular blood pressure transducer.
§870.2855 - Implantable Intra-aneurysm Pressure Measurement System.
§870.2860 - Heart sound transducer.
§870.2870 - Catheter tip pressure transducer.
§870.2880 - Ultrasonic transducer.
§870.2890 - Vessel occlusion transducer.
§870.2900 - Patient transducer and electrode cable (including connector).
§870.2910 - Radiofrequency physiological signal transmitter and receiver.
§870.2920 - Telephone electrocardiograph transmitter and receiver.
Subpart D–Cardiovascular Prosthetic Devices
§870.3250 - Vascular clip.
§870.3260 - Vena cava clip.
§870.3300 - Vascular embolization device.
§870.3375 - Cardiovascular intravascular filter.
§870.3450 - Vascular graft prosthesis.
§870.3460 - Endovascular Suturing System.
§870.3470 - Intracardiac patch or pledget made of polypropylene, polyethylene terephthalate, or polytetrafluoroethylene.
§870.3535 - Intra-aortic balloon and control system.
§870.3545 - Ventricular bypass (assist) device.
§870.3600 - External pacemaker pulse generator.
§870.3610 - Implantable pacemaker pulse generator.
§870.3620 - Pacemaker lead adaptor.
§870.3630 - Pacemaker generator function analyzer.
§870.3640 - Indirect pacemaker generator function analyzer.
§870.3650 - Pacemaker polymeric mesh bag.
§870.3670 - Pacemaker charger.
§870.3680 - Cardiovascular permanent or temporary pacemaker electrode.
§870.3690 - Pacemaker test magnet.
§870.3700 - Pacemaker programmers.
§870.3710 - Pacemaker repair or replacement material.
§870.3720 - Pacemaker electrode function tester.
§870.3730 - Pacemaker service tools.
§870.3800 - Annuloplasty ring.
§870.3850 - Carotid sinus nerve stimulator.
§870.3925 - Replacement heart valve.
§870.3935 - Prosthetic heart valve holder.
§870.3945 - Prosthetic heart valve sizer.
Subpart E–Cardiovascular Surgical Devices
§870.4075 - Endomyocardial biopsy device.
§870.4200 - Cardiopulmonary bypass accessory equipment.
§870.4205 - Cardiopulmonary bypass bubble detector.
§870.4210 - Cardiopulmonary bypass vascular catheter, cannula, or tubing.
§870.4220 - Cardiopulmonary bypass heart-lung machine console.
§870.4230 - Cardiopulmonary bypass defoamer.
§870.4240 - Cardiopulmonary bypass heat exchanger.
§870.4250 - Cardiopulmonary bypass temperature controller.
§870.4260 - Cardiopulmonary bypass arterial line blood filter.
§870.4270 - Cardiopulmonary bypass cardiotomy suction line blood filter.
§870.4280 - Cardiopulmonary prebypass filter.
§870.4290 - Cardiopulmonary bypass adaptor, stopcock, manifold, or fitting.
§870.4300 - Cardiopulmonary bypass gas control unit.
§870.4310 - Cardiopulmonary bypass coronary pressure gauge.
§870.4320 - Cardiopulmonary bypass pulsatile flow generator.
§870.4330 - Cardiopulmonary bypass on-line blood gas monitor.
§870.4340 - Cardiopulmonary bypass level sensing monitor and/or control.
§870.4350 - Cardiopulmonary bypass oxygenator.
§870.4360 - Nonroller-type blood pump.
§870.4370 - Roller-type cardiopulmonary bypass blood pump.
§870.4380 - Cardiopulmonary bypass pump speed control.
§870.4390 - Cardiopulmonary bypass pump tubing.
§870.4400 - Cardiopulmonary bypass blood reservoir.
§870.4410 - Cardiopulmonary bypass in-line blood gas sensor.
§870.4420 - Cardiopulmonary bypass cardiotomy return sucker.
§870.4430 - Cardiopulmonary bypass intracardiac suction control.
§870.4450 - Vascular clamp.
§870.4475 - Surgical vessel dilator.
§870.4500 - Cardiovascular surgical instruments.
§870.4875 - Intraluminal artery stripper.
§870.4885 - External vein stripper.
Subpart F–Cardiovascular Therapeutic Devices
§870.5050 - Patient care suction apparatus.
§870.5100 - Percutaneous Transluminal Coronary Angioplasty (PTCA) Catheter.
§870.5150 - Embolectomy catheter.
§870.5175 - Septostomy catheter.
§870.5200 - External cardiac compressor.
§870.5225 - External counter-pulsating device.
§870.5300 - DC-defibrillator (including paddles).
§870.5310 - Automated external defibrillator system.
§870.5325 - Defibrillator tester.
§870.5550 - External transcutaneous cardiac pacemaker (noninvasive).
§870.5800 - Compressible limb sleeve.
§870.5900 - Thermal regulating system.
§870.5925 - Automatic rotating tourniquet.
Authority: 21 U.S.C. 351, 360, 360c, 360e, 360j, 371.
Source: 45 FR 7907–7971, Feb. 5, 1980, unless otherwise noted.
II. Detailed product code definition: implantable pacemaker pulse generator
PART 870 – CARDIOVASCULAR DEVICES
Subpart D–Cardiovascular Prosthetic Devices
Sec. 870.3610 Implantable pacemaker pulse generator. (a) Identification. An implantable pacemaker pulse generator is a device that has a power supply and electronic circuits that produce a periodic electrical pulse to stimulate the heart. This device is used as a substitute for the heart’s intrinsic pacing system to correct both intermittent and continuous cardiac rhythm disorders. This device may include triggered, inhibited, and asynchronous modes and is implanted in the human body.
(b) Classification. Class III (premarket approval).
(c) Date PMA or notice of completion of PDP is required. A PMA or notice of completion of a PDP is required to be filed with the Food and Drug Administration on or before September 20, 2012, for any implantable pacemaker pulse generator device that was in commercial distribution before May 28, 1976, or that has, on or before September 20, 2012, been found to be substantially equivalent to any implantable pacemaker pulse generator device that was in commercial distribution before May 28, 1976. Any other implantable pacemaker pulse generator device shall have an approved PMA or declared completed PDP in effect before being placed in commercial distribution.
[45 FR 7907, Feb. 5, 1980, as amended at 52 FR 17736, May 11, 1987; 77 FR 37576, June 22, 2012]
Appendix D: Additional Background and Analysis of Drug Approvals
Background
Chemical drugs are defined37 by the FDA as “articles intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease” and “articles (other than food) intended to affect the structure or any function of the body.” Examples of drugs include familiar ingestible or injectable products such as antibiotics and oral contraceptives. Drugs are regulated by the FDA’s Center for Drug Evaluation and Research (CDER). Within CDER, the Office of Drug Evaluation is responsible for the approval of new drugs.
The FDA and the Regulation of Drugs
The foundation of the FDA’s modern-day statutory authority to regulate medical products is the Federal Food, Drug, and Cosmetic Act of 1938 (FDCA), which requires that new drugs be tested for safety and that those tests be submitted to the government for marking approval (Babiarz and Pisano, 2008; FDA, 2013). The FDCA “endowed the FDA with sole authority to reject the ex ante marketability of any new pharmaceutical product” (Carpenter, 2010) and resulted in the establishment of the new drug application process (NDA), the “vehicle through which drug sponsors formally propose that the FDA approve a new pharmaceutical for sale and marketing in the U.S.”38 The goals of the NDA are to provide sufficient information on drug safety and effectiveness for proposed uses, to determine whether the contents of proposed labeling are appropriate, and to evaluate whether manufacturing methods used are adequate.
The NDA is organized into technical sections,39 which are evaluated by specialized review teams of experts (Monahan and Babiarz, 2008). The components of the NDA are specific and well-defined for all types of drugs. For example, for the information required about the drug’s manufacturing scheme, the applicant firm must describe the synthesis of the active ingredient, including details on all starting materials, solvents, reagents, intermediate substances and their compilations and analytical controls (Monahan and Babiarz, 2008).
The results of randomized, typically placebo-controlled clinical trials are also an important component of any NDA.40 During the FDA’s in-depth review of the NDA, the drug’s sponsor (the firm filing for regulatory approval) may also be required to submit additional information supporting the drug application (Babiarz and Pisano, 2008). In the data used in this appendix, the average approval time for a new drug is 23.5 months, although the average approval time for a drug that is the first to be approved in its “disease group” (a specific product category based on the function and target of a drug) is shorter, at 19.3 months. Figures 1 and 2 provide additional information on the chronology and requirements of the NDA process.
Drug Approval Data
In these exercises, I take advantage of an additional source of data, the FDA’s New Drug Approval (NDA) database. The NDA database includes a comprehensive list of all new drug approvals in the FDA’s regulatory history.41 For comparability in my empirical analyses and in order to focus on contemporaneous regulatory periods for both drugs and devices, I limit the years of drug application data used to only those applications that were submitted beginning in 1977 (the year after which the FDA first began regulating medical devices) and through 2007. While later data are available for some products, I deliberately limit the years considered in the approval data. This strategy avoids any bias that would be created by using a sample in which only the fastest approvals for more recent years would be observed.
I consider a final sample of 693 unique drug approvals that are indicated for 187 disease groups. “Disease groups” are specific product categories that are based on the function and target of a drug. Within a disease group, products are likely to be very good to excellent clinical substitutes for one another. For example, anti-inflammatory agents, contraceptives, and statins each represent specific disease groups. The data also include detailed information about the date of NDA submission, date of FDA decision, the submitting firm’s identity, and an indicator for whether a product received “priority” or expedited review (a drug could be eligible for expedited review because it is used for a rare or late-stage/terminal disease). I observe approval times as elapsed days or months from the date of the NDA submission to FDA decision.42 Summary statistics are presented in Appendix Table I.
Appendix Table I highlights several similarities and important differences between the drug and device approval data. While average approval times in the sample are longer for drugs (22.5 months) than devices (18.1 months), the average approval times for the first product in a given category are shorter for drugs (19.3 months) than for devices (21.5 months). Drugs tend to have more entry per product category (13.6 products on average) than devices (6.4 products on average) and drugs are also far more likely to be eligible for “priority” (expedited) review (44 percent of drugs vs. 10 percent of devices).
Additional Empirical Estimation
I replicate Carpenter et. al.’s (2010) results on the set of 693 new chemical drugs described above. Columns 1 and 3 of Appendix Table II show the results of both a parametric (lognormal) model and a semi-parametric (Cox proportional hazard) model. The log-normal model can be interpreted as the percentage change in approval time associated with a one unit increase in entry order. The Cox proportional hazard model (Cox, 1972) reports the effect of a unit increase in entry order with respect to the hazard rate of exiting the approval process. As previously documented in other studies, both models present evidence of a positive, statistically significant entry order gradient in approval times for new drugs that is persistent, robust to multiple statistical specifications, and tantamount to early mover advantage in the drug regulatory approval process.
On average, a one unit increase in entry order is associated with approximately a 2 percent increase in regulatory approval times for new drugs within a disease group (e.g. among statins, oral contraceptives, etc.), a magnitude that is highly comparable to that found in Carpenter et. al. (2010). The results are statistically significant at conventional levels and robust to the inclusion of firm and year fixed effects or a time trend, disease group fixed or random effects, and a time-varying indicator of a firm’s “expertise” in navigating the regulatory process (for which I proxy using a firm-specific, time-varying count of previously approved NDAs at the time of a given application).
I then conduct a parallel analysis for the approval times of new medical devices. In Columns 2 and 4 of Appendix Table II, I repeat both the parametric (log-normal) and semi-parametric (Cox proportional hazard) analyses on the dataset of 847 new medical devices. In the medical device sample, I document a statistically significant relationship, which is oppositely signed compared to that estimated for drugs: on average, a one unit increase in entry order is associated with approximately a 1 percent decrease in regulatory approval times for new medical devices. That is, the later a product enters a given market, the shorter the average time to regulatory approval. These medical device approval models also present results that are statistically significant at conventional levels and robust to the inclusion of firm and year fixed effects or a time trend, product code fixed or random effects, and a time-varying indicator of a firm’s “expertise” in navigating the regulatory process (again using as a proxy a firm-specific, time-varying count of previously approved products at the time of a given new application). F-tests comparing the drug versus device coefficients reject the equivalence of the relationships between entry order and approval times for these two categories of products at the 0.001 percent level in both sets of models.
Having found evidence of early mover regulatory advantages on average in drug approval times and early mover regulatory disadvantages on average for device approval times, I turn to understanding the drivers of these patterns in greater detail. If the relationship seen in device approvals is a result of first or early entrant regulatory disadvantage, there are three empirical facts that should be observable in the data. First, if observed patterns are being driven by the earliest entrants, one should expect to see stronger relationships in samples that include these entrants and should not expect to see the same patterns in samples that do not include early entrants. Second, it should be possible to identify those entrants for whom there are additional delays associated with entry order and third, to quantify their magnitude.
Appendix Table III tests the first case above. For reference, column 0 replicates the two sets of log-linear results in Appendix Table III: on average, approval times are decreasing in entry order for devices and increasing in entry order for drugs. Subsequent columns of Appendix Table IV then ask the question: “what is the relationship between entry order and approval times when considering only entrants beyond the Zth product?” While the positive entry order gradient documented in the regulatory approval of new drugs is stable over the product development lifecycle of a category of drugs, this is not the case for devices: the negative entry order gradient disappears as soon as the first entrants are excluded from the sample. The device results in Appendix Table IV thus suggest that delays accrue mostly to the first entrant in a device product code and that the inclusion of these early entrants drives overall averages in the data. With respect to Equation 3, this result is consistent with an empirical result of ϕ* < 2 for devices.
Footnotes
Moreover, it has been pointed out that a single important (“breakthrough”) drug might well provide more benefit than several new molecules, independent of the timing issues with Peltzman’s measurements (Carpenter, 2010).
An ICD is a small device that is surgically placed in the chest or abdomen, which is used to treat irregular heartbeats called arrhythmias. An ICD uses electrical pulses to help control life-threatening arrhythmias – in particular, those that can cause sudden cardiac arrest and subsequent death (http://www.nhlbi.nih.gov/health/health-topics/topics/icd)
Catheter-based procedures are frequently used to treat blockages in the arteries of the heart (coronary arteries). Often a stent is used to prevent restentosis (renarrowing) of the diseased artery. Stents are small metal tubes that are inserted and expanded into the artery wall and used to keep the previously narrowed artery segment open. Drug eluting stents (DESs) are medication-coated stents that reduce the chance of renarrowing of the blood vessel (Maisel and Lasky, 2007)
depending on the definition used; see Section 6 and Table 8 for detailed descriptions.
Source: National Health Expenditures, 2012
Drug spending is higher than device spending at roughly $320b annually
In 2010, seven of the top 20 drugs in the US were biologics (Lancet, 2012).
Another example of an emerging medical technology is that of nanomedicine – a term used to define the application of nanotechnology in medicine. Nanomedicine involves the use of particles in the size range of 100 nanometres (nm) or less and includes liposomes, polymer conjugates, protein/antibody conjugates, block polymer micelles, cross-linked (nano)gels, bioactive synthetic polymers/vesicles, nanoparticles and nano-sized drug crystals. Nanomedicines are mainly anticancer, anti-infective or immunomodulator drugs. The global nanomedicines market was valued at $72.8 billion in 2011 and is expected to reach $130.9 billion in 2016 (Generics and Biosimilars Initiative, 2013).
The CDRH also regulates radiation-emitting products such as X-ray and ultrasound machines.
For example, drugs are regulated by the FDA’s Center for Drug Evaluation and Research (CDER) and biologics and human cells, tissues, and cellular- and tissue-based products are reviewed by the FDA’s Center for Biologics Evaluation and Research (CBER).
The 510(k) process – the other primary regulatory pathway through which medical devices come to market – is intended for use with medium-risk devices only. The FDA notes that “a 510(k) is a premarket submission made to FDA to demonstrate that the device to be marketed is at least as safe and effective, that is, substantially equivalent, to a legally marketed device (21 CFR 807.92(a)(3)) that is not subject to PMA” (http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/PremarketSubmissions/PremarketNotification510k). The 510(k) process has been criticized for being used too freely and the Institute of Medicine has convened a committee to look at its use (Garber, 2010). This is an important area for further research, however this paper focuses only on those products that are explicitly designated for the PMA-track approval process.
The NDA process is described in Appendix D.
The clinical study report includes the study design and protocol, patient enrollment and exclusion data, primary and secondary endpoints of the study, data from all patients entered into the trial, and detailed statistical analysis of the results. Technical data on biocompatibility, stress and fatigue, shelf life, and other relevant non-clinical tests are also submitted (Zenios et. al., 2010)
This framework is also related to Carpentner (2004). In this model, “early entrants” into an exclusive market niche (disease) receive shorter expected approval times than later entrants, even when later entrants offer known quality improvements over earlier products.
Other work – e.g. Thomas (1990) has found that FDA regulations have heterogeneous effects on firm productivity by firm size.
These are described in detail in section 4; each represents a group of products that are good to excellent substitutes for one another.
The raw data are available at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm
For example: a device application that was submitted in 2007 and approved in 2010 would be included in the dataset. A device application submitted in 2011 and approved in 2012 would not because its submission occurred after the end of calendar year 2007.
FDA PMA Approvals overview: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/PMAApprovals/default.htm
In this model, under some circumstances, firms are more likely to submit when they expect to have a higher rate of type I error (i.e. having a bad product approved). Because the firms with higher acceptance rates are more experienced and face lower costs of experimentation, there may be selection into the decision of whether or not to apply for regulatory approval in the first place, given private information about product safety/effectiveness from early clinical trials.
While the fraction of PMAs that are rejected following the PMA process is negligible (zero in recent years), the fraction of devices that are granted investigational device exemptions and then never apply for approval through the PMA process is likely higher.
Similar results are suggested by Appendix Table III.
Yet another way to benchmark the costs of delay, yields a comparable result: according to the 2013 Annual Report from Medtronic, the world’s largest medical device company, 38 percent of 2013 revenues were from products introduced in the last three years (Medtronic, 2013). While it is only a rough estimation of foregone revenue, it is illustrative to think about what a 7.2-month regulatory delay means in this context: 7.2 months represents 20 percent of three years. If, on average, a medical device makes 38 percent of its total profits over its first three years on the market, then a 7.2 month delay in getting to market could translate into a decrease of approximately 8 percent of lifetime revenues per new device.
The FDA has 16 independent panels for device classification. These panels are found in 21 CFR 862–892. For each of the devices classified by the FDA the CFR gives a general description including the intended use, the class to which the device belongs (i.e., Class I, II, or III), and information about marketing requirements. (http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/ClassifyYourDevice)
Medical Device Classification Product Codes: Guidance for Industry and Food and Drug Administration Staff (April 11, 2013).
In models not presented, I also perform a “placebo test” in which I randomly assign each of the devices to one of eight arbitrary dummy categories and then run the same set of regressions. As would be expected, a prior approval of another randomly selected and unrelated cardiovascular device does not help in predicting approval times for subsequent cardiovascular devices.
The history of FDA guidance dates back to the 1970s, when the FDA began to issue “guidelines” for clinical trials, a regulatory norm (less stringent than formal rule-making) that would lead to an important role of “guidance documents” in communicating structures of clinical experiment and drug development to the pharmaceutical industry moving forward (Carpenter, 2010). Guidance documents continue to shape the FDA’s regulation of medical products to this day and their scope has expanded with that of the FDA to include medical devices and other products.
Guidance documents are issued by the FDA, however their standardization in the 21st century has been governed by a formal congressional regulation: on September 19th, 2000, Congress approved regulation (21 CFR 10.115), which outlined the FDA’s policies and procedures for developing, issuing, and using guidance documents. While the FDA had released various medical device guidance documents prior to 2000, they were not standardized and so their interpretability and significance were more limited.
Interviews with regulatory consultants revealed that this is a strategy that they often recommend.
All cellular and gene therapy guidance documents are available at http://www.fda.gov/BiologicsBloodVaccines/guidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/default.htm
Note that in contrast to Carpenter et. al. (2009), I am not interested in identifying the disease-specific effects per se. Rather, knowing that they may exist for some subset of illnesses, I control for them when estimating other model coefficients.
FD&C Act, sec. 201(g)(1)
Requirements are outlined in FDCA and Title 21 of the US Code of Federal Regulations part 314
Typically three phases of clinical trials are required in order for the FDA to be assured of a drug’s safety and effectiveness (although sometimes approval decisions are made early based on demonstrated need for a drug and very promising results in phase II trials). Phase I trials are typically very small (N=20 to 80) and are primarily for determining drug safety and establishing side effects. Assuming that Phase I trials don’t reveal unacceptable levels of harm, Phase II trials are conducted in a greater number of healthy subjects (as many as a few hundred, with the exception of drugs for diseases like cancer) and the focus is on establishing a product’s effectiveness. Phase III trials begin following evidence of effectiveness in Phase II and are usually very large studies (N= hundreds to 3000). Phase III studies are designed to have sufficient statistical power to confirm a product’s safety and effectiveness in different populations and different dosages (FDA, 2012; http://www.fda.gov/drugs/resourcesforyou/consumers/ucm143534.htm)
I am grateful to Daniel Carpenter for sharing the cleaned data from Carpenter, et. al. (2010) for this project. The raw data are available at http://www.fda.gov/Drugs/InformationOnDrugs/ucm135821.htm
One reader noted that it may be harder to recruit patients for clinical trials for non-first-in-class drugs, and that this could make the clinical trials last longer and extend commercialization lags for non-pioneers. While this may be true, it would represent an effect above and beyond what I observe in the FDA’s data on approval times, which measure time between submission and an approval decision and not pre-NDA-submission phenomena such as the duration of clinical trials.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acemoglu Daron, Linn Joshua. Market size in innovation: theory and evidence from the pharmaceutical industry. The Quarterly Journal of Economics. 2004;119(3):1049–1090. [Google Scholar]
- Babiarz Josephine C, Pisano Douglas J. Overview of FDA and drug development. In: Pisano Douglas J, Mantus David S., editors. FDA Regulatory Affairs: A Guide for Prescription Drugs, Medical Devices and Biologics. 2. Informa Healthcare; USA, New York: 2008. pp. 1–32. [Google Scholar]
- Bartel Ann P, Thomas Lacy Glenn. Predation through regulation: the wage and profit effects of the Occupational Safety and Health Administration and the Environmental Protection Agency. Journal of Law and Economics. 1987;30:239. [Google Scholar]
- Budish Eric, Roin Benjamin N, Williams Heidi. Do Firms Underinvest in Long-Term Research? Evidence from Cancer Clinical Trials. American Economic Review. 2015;105(7):2044–85. doi: 10.1257/aer.20131176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter Daniel P. Groups, the Media and Agency Waiting Costs: The Political Economy of FDA Drug Approval,” (2002) 46. American Journal of Political Science. 490505. [Google Scholar]
- Carpenter Daniel P. Protection without capture: Product approval by a politically responsive, learning regulator. American Political Science Review. 2004;98(4):613–31. [Google Scholar]
- Carpenter Daniel P. Reputation and Power: Organizational Image and Regulation at the FDA. Princeton: Princeton University Press; 2010. [Google Scholar]
- Carpenter Daniel P, Moffitt Susan I, Moore Colin D, Rynbrandt Ryan T, Ting Michael M, Yohai Ian, Zucker Evan James. Early entrant protection in approval regulation: Theory and evidence from FDA drug review. Journal of Law, Economics, and Organization. 2010;26(3):515–545. [Google Scholar]
- Carpenter D, Ting MM. Regulatory errors with endogenous agendas. American Journal of Political Science. 2007;51(4):835–852. [Google Scholar]
- Centers for Medicare & Medicaid Services. National Health Expenditures. Office of the Actuary, National Health Statistics Group; U.S. Department of Commerce, Bureau of Economic Analysis; and U.S. Bureau of the Census; 2012. [Google Scholar]
- Chatterji Aaron K. Spawned with a silver spoon? Entrepreneurial performance and innovation in the medical device industry. Strategic Management Journal. 2009;30(2):185–206. [Google Scholar]
- Dranove David, Meltzer David. Do important drugs reach the market sooner? The RAND Journal of Economics. 1994:402–423. [Google Scholar]
- Dubois Pierre, de Mouzon Olivier, Morton Fiona Scott, Seabright Paul. Market size and pharmaceutical innovation. The RAND Journal of Economics. 2015;46(4):844–871. [Google Scholar]
- Garber Alan M. Modernizing device regulation. New England Journal of Medicine. 2010;362(13):1161–1163. doi: 10.1056/NEJMp1000447. [DOI] [PubMed] [Google Scholar]
- Generics and Biosimilars Initiative (GaBI) The future of nanomedicines nanosimilars. 2013 Aug; Available at: http://www.gabionline.net.
- Goldman Dana, Lakdawalla Darius. Intellectual Property, Information Technology, Biomedical Research, and Marketing of Patented Products. In: Pauly Mark V, Mcguire Thomas G, Barros Pedro P., editors. Handbook of Health Economics. Vol. 2. New York: Elsevier; 2011. 825872. [Google Scholar]
- Grabowski Henry G, Vernon John M, Thomas Lacy Glenn. Estimating the effects of regulation on innovation: an international comparative analysis of the pharmaceutical industry. Journal of Law and Economics. 1978;21(1):133–163. doi: 10.1086/466914. [DOI] [PubMed] [Google Scholar]
- DiMasi JA, Grabowski HG. R&D Costs for New Biotech Compounds. Managerial and Decision Economics. 2007;28:469479. [Google Scholar]
- Government Accountability Office. Medical Devices: FDA Should Take Steps to Ensure that High-Risk Device Types are Approved through the Most Stringent Premarket Review Process. GAO-09-190. 2009 Jan; [Google Scholar]
- Guedj Ilan, Scharfstein David. NBER Working Paper 10933. 2004. Organizational Scope and Investment: Evidence from the Drug Development Strategies and Performance of Biopharmaceutical Firms. [Google Scholar]
- Hamburg Margaret A. Innovation, regulation, and the FDA. New England Journal of Medicine. 2010;363(23):2228–2232. doi: 10.1056/NEJMsa1007467. [DOI] [PubMed] [Google Scholar]
- Hamburg Margaret A, Sharfstein Joshua M. The FDA as a public health agency. New England Journal of Medicine. 2009;360(24):2493–2495. doi: 10.1056/NEJMp0903764. [DOI] [PubMed] [Google Scholar]
- Mats Hellström, Harvey Alan R. Retinal ganglion cell gene therapy and visual system repair. Current gene therapy. 2011;11(2):116–131. doi: 10.2174/156652311794940746. [DOI] [PubMed] [Google Scholar]
- Hilts PJ. Protecting America’s health: The FDA, business, and one hundred years of regulation. New York: Alfred A. Knopf; 2003. p. 23. [Google Scholar]
- Kaplan Aaron V, Baim Donald S, Smith John J, Feigal David A, Simons Michael, Jefferys David, Fogarty Thomas J, Kuntz Richard E, Leon Martin B. Medical Device Development From Prototype to Regulatory Approval. Circulation. 2004;109(25):3068–3072. doi: 10.1161/01.CIR.0000134695.65733.64. [DOI] [PubMed] [Google Scholar]
- Kramer Daniel B, Xu Shuai, Kesselheim Aaron S. Regulation of medical devices in the United States and European Union. New England journal of medicine. 2012;366(9):848–855. doi: 10.1056/NEJMhle1113918. [DOI] [PubMed] [Google Scholar]
- Lancet, The. Approval of biosimilars in the USA – dead ringers? The Lancet. 2012 Feb 25;379(9817):686. doi: 10.1016/S0140-6736(12)60290-6. [DOI] [PubMed] [Google Scholar]
- Lerner Josh, Wulf Julie. Innovation and incentives: Evidence from corporate R&D. The Review of Economics and Statistics. 2007;89(4):634–644. [Google Scholar]
- Maisel William H, Laskey Warren K. Drug-eluting stents. Circulation. 2007;115(17):e426–e427. doi: 10.1161/CIRCULATIONAHA.107.688176. [DOI] [PubMed] [Google Scholar]
- Maisel William H. Medical device regulation: an introduction for the practicing physician. Annals of Internal Medicine. 2004;140(4):296–302. doi: 10.7326/0003-4819-140-4-200402170-00012. [DOI] [PubMed] [Google Scholar]
- Meier Barry. Costs Surge for Medical Devices, but Benefits Are Opaque. The New York Times. 2009 Nov 5; [Google Scholar]
- Monahan Charles, Babiarz Josephine C. The New Drug Application. In: Pisano Douglas J, Mantus David S., editors. FDA Regulatory Affairs: A Guide for Prescription Drugs, Medical Devices and Biologics. 2. Informa Healthcare; USA, New York: 2008. pp. 69–108. [Google Scholar]
- Munsey Rodney R. Trends and events in FDA regulation of medical devices over the last fifty years. Food & Drug LJ. 1995;50:163. [PubMed] [Google Scholar]
- Olson Mary. Substitution in Regulatory Agencies: FDA Enforcement Alternatives,” 12. Journal of Law Economics and Organization. 1995:376407. [Google Scholar]
- Peltzman Sam. Regulation of pharmaceutical innovation: The 1962 amendments. Vol. 15. American Enterprise Institute for Public Policy Research; 1974. [Google Scholar]
- Redberg Rita F, Dhruva Sanket S. Medical Device Recalls: Get It Right the First Time: Comment on “Medical Device Recalls and the FDA Approval Process”. Archives of internal medicine. 2011;171(11):1011. doi: 10.1001/archinternmed.2011.27. [DOI] [PubMed] [Google Scholar]
- Sall Barry. FDA Medical Device Regulation. In: Pisano Douglas J, Mantus David S., editors. FDA Regulatory Affairs: A Guide for Prescription Drugs, Medical Devices and Biologics. 2. Informa Healthcare; USA, New York: 2008. pp. 69–108. [Google Scholar]
- Morton Scott, Fiona M, Stern Ariel Dora, Stern Scott. Harvard Business School Working Paper, No. 16–141. Jun, 2016. The Impact of the Entry of Biosimilars: Evidence from Europe. [Google Scholar]
- Thomas Lacy Glenn. Regulation and firm size: FDA impacts on innovation. The RAND Journal of Economics. 1990:497–517. [Google Scholar]
- Xu Shuai, Avorn Jerry, Kesselheim Aaron S. Origins of Medical Innovation The Case of Coronary Artery Stents. Circulation: Cardiovascular Quality and Outcomes. 2012;5(6):743–749. doi: 10.1161/CIRCOUTCOMES.112.967398. [DOI] [PubMed] [Google Scholar]
- Yang QZ, Sun JP, Zhu CF. Medical device innovation methods and case studies”. Vol. 7. SIMTech Technical Reports. 2006 [Google Scholar]
- Zenios Stefanos A, Makower Josh, Yock Paul G. Biodesign: the process of innovating medical technologies. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- Zuckerman Diana M, Brown Paul, Nissen Steven E. Medical device recalls and the FDA approval process. Archives of internal medicine. 2011;171(11):1006. doi: 10.1001/archinternmed.2011.30. [DOI] [PubMed] [Google Scholar]