Abstract
Background—
The objective of this study is to test the effect of permanent right internal mammary artery device closure on coronary collateral function and myocardial ischemia.
Methods and Results—
This was a prospective, open-label clinical trial in 50 patients with coronary artery disease. The primary study end point was coronary collateral flow index as obtained during a 1-minute proximal right coronary artery (RCA) and left coronary artery balloon occlusion at baseline before and at follow-up examination 6 weeks after distal right internal mammary artery device closure. Collateral flow index is the ratio between simultaneously recorded mean coronary occlusive pressure divided by mean aortic pressure, both subtracted by central venous pressure. Secondary study end points were fractional flow reserve during vessel patency, the quantitative intracoronary ECG ST-segment elevation, and angina pectoris during the same 1-minute coronary occlusion. Collateral flow index in the untreated RCA and left coronary artery changed from 0.071±0.082 at baseline to 0.132±0.117 (P<0.0001) at follow-up examination and from 0.106±0.092 to 0.081±0.079 (P=0.29), respectively. RCA fractional flow reserve increased significantly (P=0.0029) from baseline to follow-up examination, despite deferral of coronary intervention in all patients. There was a decrease in intracoronary ECG ST-elevation during RCA occlusion from baseline to follow-up examination (P=0.0015); it did not change in the left coronary artery. Angina pectoris during RCA occlusion tended to occur in fewer patients at follow-up versus baseline examination (P=0.06).
Conclusions—
Permanent right internal mammary artery device closure seems to augment extracardiac ipsilateral coronary supply to the effect of reducing ischemia in the dependent myocardial region.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02475408.
Keywords: arteriogenesis, collateral circulation, coronary circulation, internal mammary artery, myocardial ischemia
WHAT IS KNOWN
A recent investigation in patients with stable coronary artery disease has shown that temporary balloon occlusion of the internal mammary artery induces a functional, ischemia-reducing extracardiac coronary artery supply via ipsilateral natural internal mammary artery bypasses.
WHAT THE STUDY ADDS
The new finding of the present study is that over the course of 6 weeks, permanent right internal mammary artery occlusion by a vascular closure device augments extracardiac right coronary artery but not left coronary artery supply and reduces myocardial ischemia during a 1-minute ostial right coronary artery balloon occlusion.
Permanent internal mammary artery device closure deserves further evaluation as a potential treatment option for alleviation of myocardial ischemia in patients with chronic coronary artery disease not amenable to conventional revascularization or refractory to medical therapy.
The prevalence of patients with stable coronary artery disease (CAD) undergoing indicated but incomplete revascularization is close to 30%, and all-cause mortality among these patients has been described elevated in comparison to those with complete revascularization (3-year cumulative mortality rate of 14.8% versus 6.6%).1 Patients with incomplete revascularization and refractory angina pectoris require novel therapeutic strategies aimed at improving survival by reducing myocardial ischemia because in stable CAD, outcome after medical therapy irrespective of percutaneous coronary intervention (PCI) is dependent on the amount of myocardial ischemia.2 In the event of coronary occlusion, ischemia is influenced by its duration, the size of the ischemic territory, the lack of collateral supply, the absence of ischemic preconditioning prior to occlusion, and myocardial oxygen consumption at the time of occlusion.3 The clinical benefit on survival of a functional intercoronary collateral circulation in chronic stable CAD has been documented.4,5 Additionally, extracardiac coronary collateral supply via the internal mammary artery (IMA) has been described.6–8 Seven case reports have documented the existence of naturally occurring angiographic anastomoses between one of the IMAs and the human coronary circulation.9–15 Blair et al measured increased coronary artery blood flow after experimental IMA ligation. More recently, a study in 8 dogs undergoing coronary artery constriction and IMA ligation has failed to reveal myocardial microsphere perfusion via IMA in the single animal, which survived the study protocol.17 In patients with CAD, our group has provided evidence for a functional, ischemia-reducing extracardiac coronary artery collateral supply during temporary ipsilateral IMA balloon occlusion.18
See Editorial by Kern and Seto
Thus, the present conceptual study tested the hypotheses that after 6 weeks of follow-up, permanent right IMA (RIMA) device closure results in augmented collateral function of the right coronary artery (RCA), but not the left coronary artery (LCA), and that this is reflected by respective changes in myocardial ischemia.
Methods
Study Design and Patients
This was a prospective, open-label, longitudinal interventional clinical trial in 50 patients undergoing diagnostic coronary angiography in the context of chest pain. The primary study end point was quantitative coronary collateral function (collateral flow index, CFI; see below for calculation) as obtained during a 1-minute proximal coronary artery balloon occlusion at baseline before and at follow-up examination 6 weeks after RIMA device occlusion (Figure 1). Secondary study end points were fractional flow reserve during vessel patency (FFR), the quantitatively determined intracoronary (IC) ECG ST-segment elevation, and angina pectoris during the same 1-minute coronary occlusion. Study end points were obtained in the RCA and LCA. Eligibility criteria for study inclusion were age >18 years, angina pectoris under conventional medical therapy, written informed consent to participate in the study, and 1- to 3-vessel chronic stable CAD. Exclusion criteria were acute coronary syndrome, previous myocardial infarction in the vascular region undergoing CFI measurement, and severe hepatic or renal failure (creatinine clearance <15 mL/min per 1.73 m2).
Figure 1.
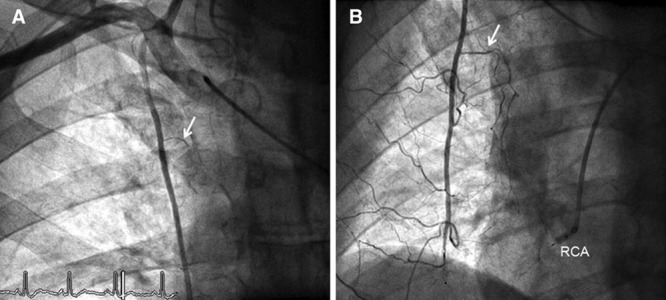
A, Anteroposterior angiogram of the truncus brachiocephalicus (site of the catheter tip) depicting the right internal mammary artery (RIMA) with its pericardiophrenic branch (arrow) taken at the baseline examination. B, RIMA angiography of the same patient as in A taken during follow-up examination. Simultaneous occlusion of the distal RIMA (by vascular occlusion device) and the ostial right coronary artery (RCA, by angioplasty balloon). The pericardiophrenic branch (arrow) and the other RIMA branches are larger than those at baseline (A).
The study was approved by the ethics committee of the Kanton of Bern, Switzerland, and all patients gave written informed consent to participate (in accordance with the author guidelines of this journal).
Cardiac Catheterization and Coronary Angiography
Patients underwent left heart catheterization and coronary angiography for diagnostic purposes from the right femoral artery approach via a 6F introducer sheath. Biplane left ventriculography was performed followed by coronary angiography. Coronary artery stenoses were assessed quantitatively as percent diameter reduction using the guiding catheter for calibration. Aortic pressure (Pao) was acquired via a 6F guiding catheter. Central venous pressure (CVP) was measured by a 5F pigtail catheter as right atrial pressure via the right femoral vein.
Study End Points
Primary Study End Point
Coronary occlusive collateral flow relative to normal antegrade flow through the nonoccluded coronary artery (CFI) was determined using coronary pressure measurements. A 0.014-inch pressure monitoring angioplasty guidewire (Pressure Wire; St Jude Medical, Eschborn, Germany) was set at zero, calibrated, advanced through the guiding catheter, and positioned in the distal part of the vessel of interest (identical position at baseline and follow-up examination). CFI was determined by simultaneous measurement of mean aortic pressure (Pao, mm Hg), the mean distal coronary artery pressure during balloon occlusion (Poccl, mm Hg), and the mean CVP (mm Hg; Figure 2) as measured during the last 30 seconds of the 1-minute coronary balloon occlusions. CFI was calculated as (Poccl−CVP) divided by (Pao−CVP).19 The accuracy of pressure-derived CFI measurements in comparison to ECG signs of myocardial ischemia during occlusion and to absolute myocardial perfusion measurements has been documented previously.5,19–21
Figure 2.
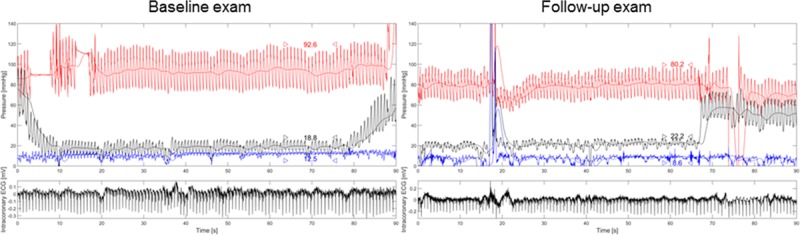
Left, Simultaneous recording of phasic and mean aortic pressure (Pao, red curve), ostial right coronary artery (RCA) occlusive pressure (Poccl, black curve), central venous pressure (CVP, blue curve), and intracoronary ECG (bottom, black curve) as obtained during baseline examination. Coronary balloon occlusion starts at ≈2 seconds on the time scale (horizontal axis) and lasts until 79 seconds (up-slope of Poccl). Pao, Poccl, and CVP are taken as temporal mean values between the arrowheads: collateral flow index, CFI=(Poccl−CVP)/(Pao−CVP)=(18.8−12.5)/(92.6−12.5)=0.079. Right, Simultaneous recording of phasic and mean Pao, ostial RCA Poccl, CVP, and intracoronary ECG during follow-up examination in the same patient as in the left panel. CFI=0.190.
Secondary Study End Points
Coronary pressure–derived FFR measurements were obtained. Signs of myocardial ischemia were assessed simultaneously to CFI measurement as quantitatively determined IC ECG ST-segment elevation in millivolt (Figure 2); the IC ECG lead was obtained from the angioplasty guidewire via a cross-clamp to a precordial lead.22 Myocardial ischemia during the 1-minute coronary occlusions was also characterized by the presence or absence of angina pectoris.
Study Protocol
Before the diagnostic examination, 2 puffs of oral isosorbidedinitrate were given. After diagnostic coronary angiography and at the start of the invasive baseline and follow-up study procedure, all patients received 5000 U of heparin intravenously. End point measurements were first obtained in the LCA, followed by the RCA. FFR was performed prior to CFI and in the same vessels as those undergoing CFI measurement. FFR was determined with the pressure guidewire positioned distally in the nonoccluded main vessel of interest using intravenous adenosine at 140 μg/min per kg for hyperemia induction: FFR=distal coronary pressure divided by Pao. The LCA (left anterior descending artery, LAD, and left circumflex coronary artery) undergoing CFI measurements was chosen on the basis of the presence of a stenotic lesion requiring PCI or on the basis of ease of access in case of a nonstenotic vessel. For CFI, an adequately sized angioplasty balloon catheter was positioned in the ostial part of the vessel while the pressure guidewire remained distally. Before imminent coronary balloon occlusion, the patient was asked not to talk and to breath normally for the following 1.5 minutes (prevention of CVP variability). Coronary balloon inflation occurred at a pressure of 1 to 2 atmospheres. Complete coronary occlusion was ascertained by angiography. During the 1-minute occlusion, simultaneous Poccl, Pao, and CVP were recorded for the calculation of CFI (Figure 2). During the entire procedure, the IC ECG obtained from the guidewire was recorded. Immediately after CFI measurement, the patient was asked about the occurrence of angina pectoris during coronary artery balloon occlusion. At an FFR>0.75, PCI of the LCA was deferred to the end of the invasive follow-up examination (ie, after all end point measurements), which took place at 6 weeks after the baseline examination; at an FFR≤0.75, PCI of the LCA occurred at baseline before end point measurements in the RCA. End point measurements in the RCA were repeated as just described.
At the end of the baseline examination, permanent RIMA device occlusion was performed using a 4F IMA catheter and a Radiofocus 0.032-inch, 260-cm stiff guidewire (Terumo, Eschborn, Germany), the latter of which was placed with its tip below the diaphragm. The IMA catheter was then exchanged for a 4F multipurpose catheter, which was engaged in the IMA until its tip reached the level of the right atrium (anteroposterior projection). Subsequently, an Amplatzer vascular plug 4 (St Jude Medical, Eschborn, Germany) was inserted via the multipurpose catheter into the RIMA at the level of the right atrium (Figure 1). Invasive follow-up examinations at 6 weeks after RIMA device occlusion consisted of identical measurements as described earlier.
Statistical Analysis
Sample size calculation was based on values for the primary end point CFI found in the previous work by Stoller et al,18 which had means±standard deviations of 0.116±0.079 and 0.091±0.067 with and without RIMA balloon occlusion, respectively, measured cross-sectionally. Using a 1-sided paired Student’s t test for intraindividual changes and a correlation of 0.65 between the matched data pairs (baseline and 6-week follow-up RCA CFI), an alpha level of 0.05, and a power of 0.80, the calculated sample size was 40 using the GPower program. For the purpose of data presentation, 2 study groups were established based on the absence or presence of percent diameter RCA stenosis ≤ or >50% as determined quantitatively. Intraindividual comparison of CFI, FFR, IC ECG ST-segment elevation, and heart rate obtained at baseline versus follow-up examination was performed by a paired Student’s t test. Between-group comparison of continuous demographic, clinical, angiographic, hemodynamic variables, CFI, FFR, and IC ECG data was performed by unpaired Student’s t test. A χ2 test was used for comparison of categorical variables among the study groups. Statistical significance was defined at a P level <0.05. Continuous variables are given as mean and standard deviation.
Results
Thirty-seven patients belonged to the group with an RCA lesion ≤50% in diameter, and 13 patients were in the group with at least 1 stenosis >50% (Table 1). PCI of the RCA was postponed to after follow-up end point measurements or not performed (n=40) in all 50 patients; in case of the LCA, PCI was deferred in 33 patients, and it was performed at baseline in 11 patients. In 6 patients, no LCA end point measurement at baseline and follow-up was performed, that is, it occurred only in the RCA.
Table 1.
Patient Characteristics and Clinical Data at Baseline
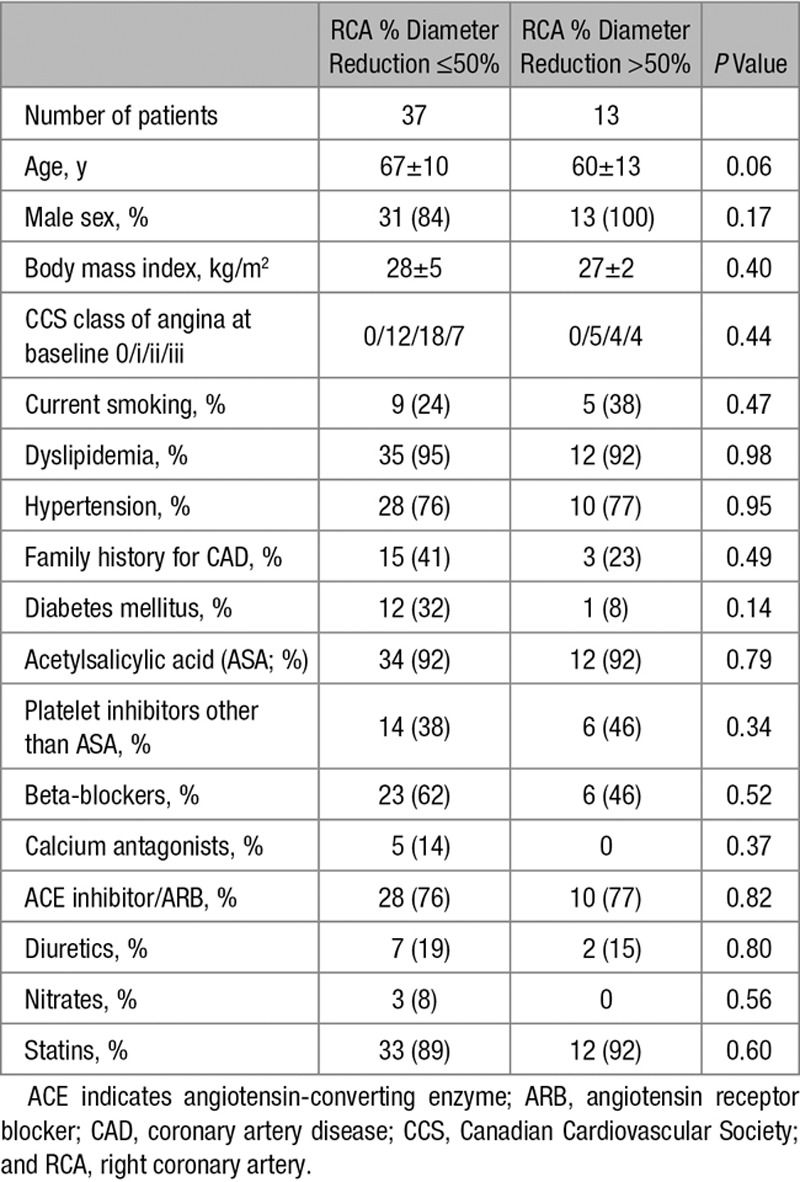
Patient Characteristics and Clinical Data at Baseline
There were no statistically significant differences between the groups regarding age, sex, body mass index, Canadian Cardiovascular Society class of angina pectoris, cardiovascular risk factor prevalence, and cardiovascular medication (Table 1).
Hemodynamic and Coronary Structural and Functional Data at Baseline
There were no statistical differences between the groups in heart rate, left ventricular ejection fraction and end-diastolic pressure, and mean arterial venous pressure and CVP (Table 2). The number of coronary arteries with stenotic lesions >50% was similar between the groups. Study end points were determined in all patients in the RCA and with similar frequency among the groups in the LAD or left circumflex coronary artery (in 44 LCA vessels at baseline). RCA percent diameter stenosis and FFR differed according to the group definition (Table 2). FFR of the LCA was significantly higher in the group with no or irrelevant RCA stenosis than in the group with significant RCA stenosis.
Table 2.
Hemodynamic Data at Baseline and Coronary Structural and Functional Data
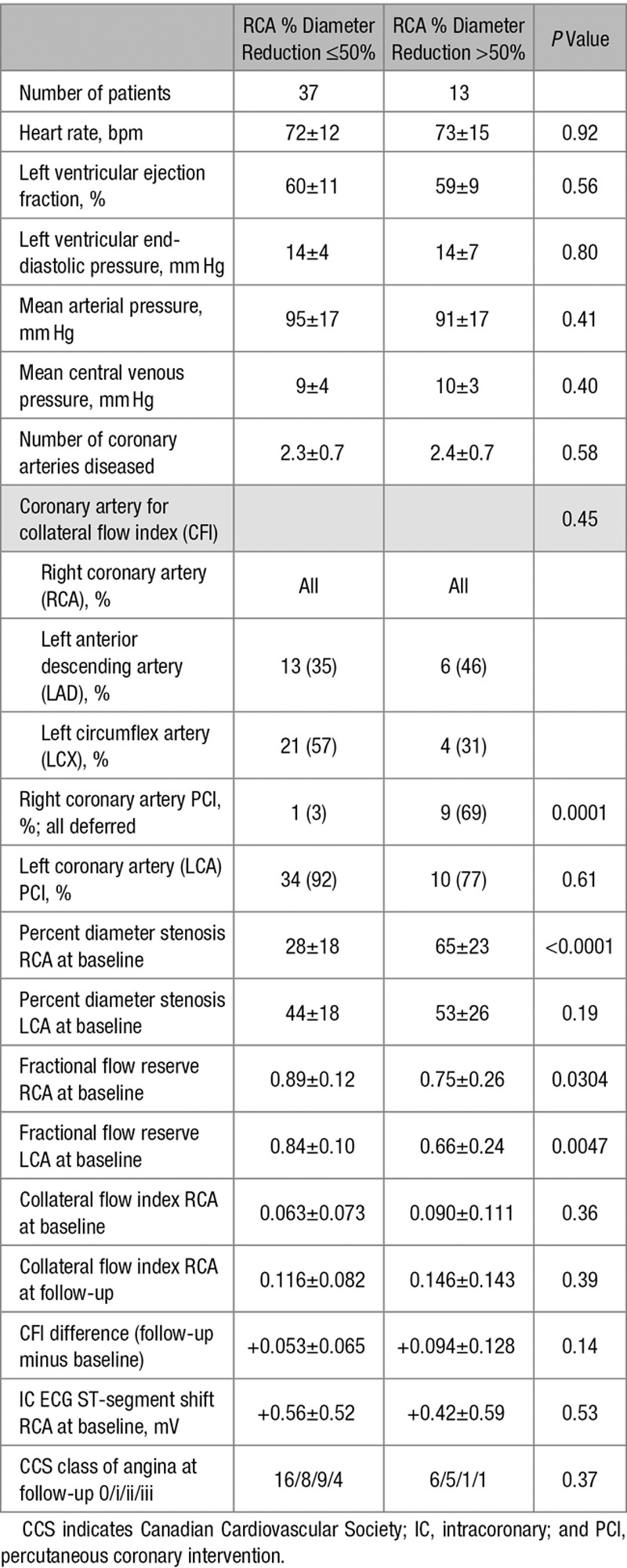
Changes of Coronary Function and Myocardial Ischemia During Follow-Up
Primary Study End Point
Overall, CFI in the RCA changed from 0.071±0.082 at baseline to 0.132±0.117 (P<0.0001) at follow-up examination (Figure 3 and Table 2). CFI in the untreated LCA changed from 0.106±0.092 to 0.081±0.079 (P=0.29). Absolute CFI change in the RCA over the 6-week course of follow-up was +0.067±0.094, and it was −0.012±0.112 in the LCA (P<0.0001; Figure 3). There was a trend to higher CFI increase in the group of patients with relevant RCA stenosis (Table 2).
Figure 3.
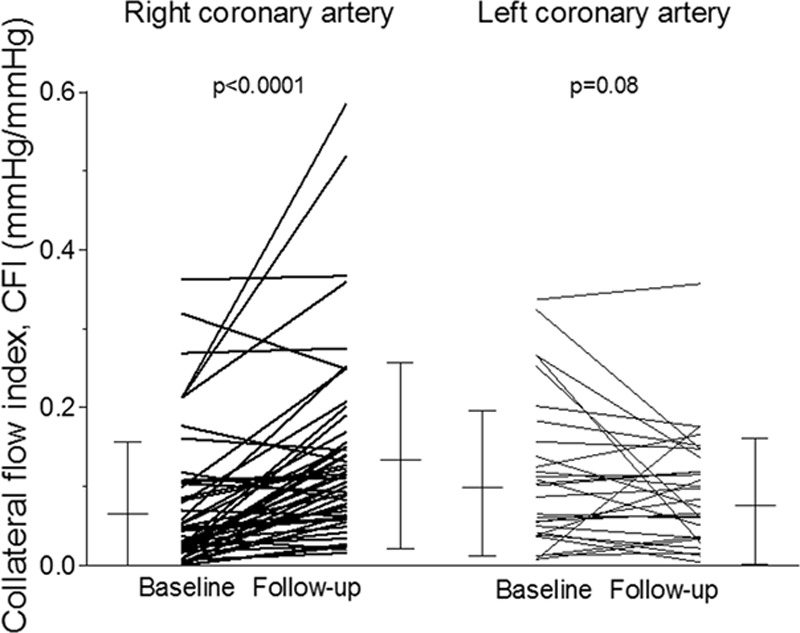
Individual values of collateral flow index (CFI, vertical axis) as obtained at baseline and follow-up examination in the right and left coronary artery. Error bars indicate mean values and standard deviation.
Secondary Study End Points
FFR in the RCA increased significantly from baseline to follow-up examination (Figure 4), and it remained unchanged in the LCA (PCI deferred until after FFR measurement in all vessels). There was a decrease in IC ECG ST-elevation during RCA occlusion from baseline to follow-up examination (Figure 4); IC ECG ST-elevation did not change significantly in the LCA. There was a trend to fewer events of angina pectoris during RCA occlusion at follow-up versus baseline examination and in comparison to LCA occlusion irrespective of the examination (Figure 5).
Figure 4.
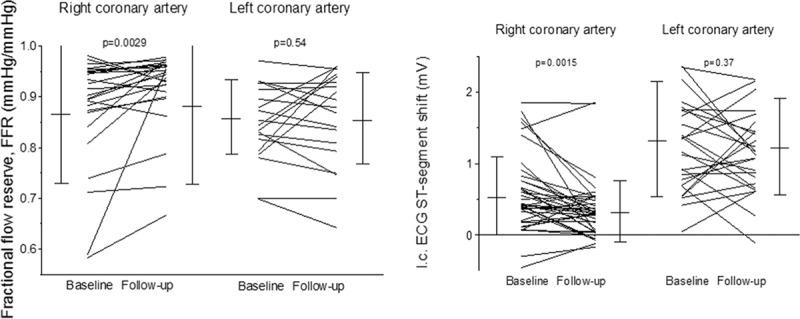
Left, Individual values of fractional flow reserve (FFR, vertical axis) as obtained at baseline and follow-up examination in the right and left coronary artery. Error bars indicate mean values and standard deviation. Right, Individual values of intracoronary ECG ST-segment shift (i.c. ECG ST-segment shift, vertical axis; ST-elevation as positive values) as obtained at baseline and follow-up examination in the right and left coronary artery. Error bars indicate mean values and standard deviation.
Figure 5.
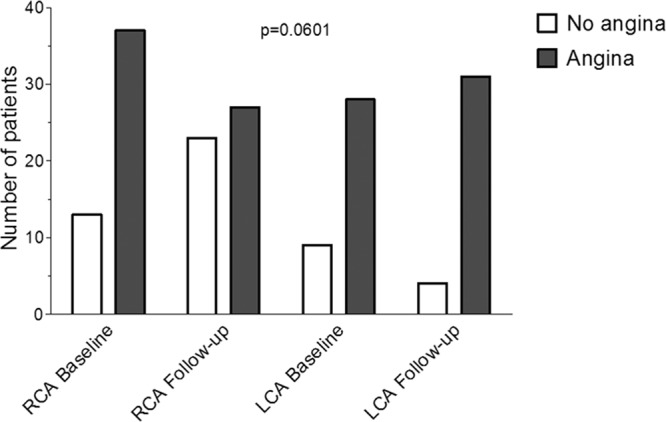
Number of patients with and without angina pectoris (vertical axis) during the 1-minute proximal right coronary artery (RCA) and left coronary artery (LCA) balloon occlusions at baseline and follow-up examination, which were performed for simultaneous pressure and intracoronary ECG measurements (see also Figure 2).
Canadian Cardiovascular Society class of angina pectoris class 0 at follow-up was observed more frequently than at baseline examination (Tables 1 and 2).
Procedural Feasibility of RIMA Closure
RIMA device closure was successful in all patients. In 12 patients, the right femoral artery access had to be switched to right radial artery or right brachial artery (n=2) access to achieve procedural success. Except for 2 cases of proximal RIMA dissection, no procedure-related complications occurred. The dissections were left untreated. During the 6 weeks of follow-up, all patients remained asymptomatic with regard to the RIMA device closure. Angiographic control of the RIMA at follow-up revealed incomplete occlusion at the site of the device in 11 of 50 cases.
Discussion
The present conceptual clinical trial found that permanent RIMA device closure results in augmented right (but not left) coronary collateral function (CFI), which was reflected by improved coronary hemodynamics as obtained during vessel patency (FFR) and by reduced signs of myocardial ischemia during vessel occlusion.
Treatment Options for Refractory Angina Pectoris
Refractory angina pectoris is defined as a chronic condition (>3 months) characterized by the presence of angina caused by coronary insufficiency in the presence of CAD, which cannot be controlled by a combination of medical therapy, angioplasty, and coronary bypass surgery.23 There are numerous treatment options aiming at alleviating myocardial ischemia using angiogenic therapeutic approaches (by the so-called myocardial laser revascularization,24 external shock wave therapy,25 and myocardial stem cell application26), diastolic coronary pressure augmentation by external counterpulsation,27 coronary arteriogenesis with induction of large collateral artery remodeling,28 coronary sinus venous backpressure augmentation,29,30 and ischemic preconditioning.31,32 The latter represents the default therapy applied by any primary or secondary prevention program because physical exercise with myocardial ischemia is its cornerstone. The only potentially useful treatment option exerting a permanent and not just temporary effect is coronary sinus pressure augmentation using a venous caliber reducer stent.33 All the other modalities require repetitive applications for a lasting effect, the fact of which substantially reduces the tolerability of a procedure, especially if it is as strenuous and time-consuming as, for example, external counterpulsation with its daily 1-hour sessions.
In comparison, the anatomic connections between the IMAs and the coronary circulation represent the basis for a sustainably improved extracardiac myocardial supply source.8 Essentially, this applies also to the extracardiac source of collateral connections between the bronchial artery and the RCA, respectively, the left circumflex coronary artery.34 However, these circulatory anastomoses are even less explored than the IMA coronary artery network.
Extracardiac Coronary Artery Supply via the IMAs
Anatomically, the IMAs on each side and the coronary circulation are connected via the second IMA branch, that is, the pericardiophrenic or pericardiacophrenic artery, which takes off proximally at the second intercostal space and supplies the pericardium.8 During IMA angiography, the pericardiophrenic branch is recognizable as the only IMA branch moving with the heart beat. At the follow-up examination of our study, enlargement of this IMA branch could be observed often (see Figure 1). The pericardiophrenic artery with its pericardial branches finds access to atrial branches of the epicardial coronary circulation via the sites of pericardial reflections located at the entry into the pericardium of the caval veins.35 This well-documented anatomic knowledge6–8,35,36 led to the first surgical attempt of myocardial revascularization. More than 20 years before the first clinical coronary artery bypass grafting in 1960,37 the ligation of the IMAs aiming at redirecting blood flow to the pericardiophrenic branch was introduced as a treatment concept for angina pectoris by Fieschi.38 In the course of this procedure, transthoracic surgical access to the IMAs was gained under local anesthesia by a small incision between the second and third rib, and they were ligated via this entry site. The primary end point of the first clinical IMA ligation trials was angina pectoris and, inconsistently, ECG signs of myocardial ischemia. Battezzati et39 reported the results of their uncontrolled trial in 304 CAD patients, with only half of them having angina pectoris: the symptoms of nearly all of them had improved sustainably. In a further uncontrolled trial among 50 CAD patients (2/3 with angina pectoris), Kitchell et al40 reported similarly favorable results with symptomatic relief in 68% of the patients.
Surgical bilateral IMA ligation has not gained historical stature because of its success, but because of its failure. The latter was—apparently—exposed by the then introduced new research design tool of a sham control group. The small, but sham-controlled negative trials by Cobb et al41 and by Dimond et al42 (totaling 35 patients) coined the term surgery as placebo.43,44 The fraction of trial participants (based on single digit numbers) experiencing relief of angina pectoris during long-term follow-up was similar between the really and sham-operated group.41,42 To infer IMA ligation inefficacious on the basis of those trials was unwarranted, but desirable for the inauguration of an essential scientific element into clinical research; this must be the current conclusion because of their low power in connection with a soft primary clinical end point (chest pain).
Furthermore, a recent clinical investigation in 120 CAD patients undergoing temporary distal IMA and simultaneous ostial coronary artery balloon occlusion has found an ischemia-reducing, enhanced extracardiac coronary artery supply via ipsilateral (but not contralateral) natural IMA bypasses.18 Specifically, the CFI difference as obtained with versus without IMA occlusion was highest and most consistently positive during left IMA with LAD occlusion and during RIMA with RCA occlusion: +0.033±0.04 and +0.025±0.03.18
Permanent IMA Closure
Permanent distal IMA device occlusion is the logical consequence of the above observations collected during temporary IMA balloon occlusion. The concept had to be tested in the setting of the less frequently grafted RIMA in order not to waste an arterial graft for future use. At our institution, RIMA coronary artery bypass grafts account for 5% to 8% of all coronary grafts. In addition, the distal occlusion site at the height of the right atrium in most cases still allows the RIMA to be used as a graft artery because its length may be sufficient to be anastomized to the RCA. The site of RIMA device closure in the present study was identical to temporary balloon occlusion in the investigation by Stoller et al.18 This location was chosen because it ought to be safely distal of and certainly not on the bifurcation of the pericardiophrenic branch. In all of the 4 cases, in whom the pericardiophrenic branch was inadvertently occluded by an IMA dissection or the device itself, no CFI increase was observed at follow-up examination. Because the IMA connects directly to the ipsilateral external iliac artery, the CFI of this vessel during ostial balloon occlusion amounts to ≈0.75 to 0.8, and thus, an effect, though a lower one, on coronary CFI could also be expected in case of ostial as opposed to distal IMA device occlusion. Certainly, this functional connection of the IMA between the upper and lower extremities is primarily responsible for the safety of the procedure in our study population; the risk of tissue necrosis in the RIMA-dependent territory is most likely low, which can also be expected, because no IMA side branches are affected by IMA device closure as opposed to IMA bypass grafting.
The amount of CFI increase in response to permanent RIMA occlusion observed in this study exceeds the above described +0.025 during temporary occlusion by a factor of 2.7 (+0.067±0.094). In (an informal) comparison to other, clinically tested coronary arteriogenic treatment options, such as granulocyte–macrophage colony-stimulating factor,45 granulocyte colony–stimulating factor,46 ivabradine,47 a 3-month physical exercise training,48 and 90 hours of external counterpulsation,27 permanent right IMA occlusion reached the largest CFI increase. The intraindividual comparison of CFI change between the right and left coronary territory in response to RIMA closure showed no effect on LCA CFI, the fact of which is in agreement with the ipsilateral anatomic connection between the IMA and the coronary circulation. The plausibility of the primary end point results (CFI) is enhanced by their coherence with the study’s secondary end points. The effect of RIMA device closure on CFI of the RCA is reflected by a decrease in functional stenosis severity, that is, an increased FFR in the absence of direct RCA intervention; RCA PCI was performed only after follow-up end point measurements. Finally, IC ECG signs of myocardial ischemia during the 1-minute ostial RCA occlusion were quantitatively diminished at follow-up examination, and there was a trend toward fewer events of angina pectoris during the same procedure.
Study Limitations
This conceptual and feasibility study of permanent RIMA closure was not a controlled trial, with randomized allocation to device closure or no closure. Thus, the results of this study should be confirmed in a larger, randomized controlled trial, possibly with a sham-control design and the presently used surrogate, as well as clinical end-points. In the above context, the important goal of technical feasibility and initial safety of the procedure could be determined more swiftly than by selecting a controlled study design. IMA device closure may, theoretically, carry a risk for ischemia in the dependent territories. None of our patients reported symptoms associable with IMA closure during the 6 weeks of follow-up or later. In comparison to the surgical use of an IMA for bypass grafting, IMA device occlusion does not affect IMA side branches by ligation. The RIMA was not entirely occluded at follow-up in 22% of the patients. Because this was a situation more likely favoring the null hypothesis than that of CFI augmentation, it rather supports than weakens the study findings. LCA PCI was not deferred in all, but only in 33 patients. In theory, LCA revascularization on its own could have augmented RCA CFI in this minority of patients. A subanalysis of the population with total PCI deferral (n=33) revealed effects of RIMA closure, consistent with those of the entire study cohort.
Acknowledgments
We thank Raphael Grossenbacher, RN, Hélène Steck, RN, Christine Tschannen, RN, and Reto Kurmann, MD, for their valuable work in patient recruitment and expert technical assistance during data acquisition.
Sources of Funding
This study was supported by a grant from the Swiss National Science Foundation for research (grant No 3200B_163256/1 to C. Seiler).
Disclosures
None.
References
- 1.Williams B, Menon M, Satran D, Hayward D, Hodges JS, Burke MN, Johnson RK, Poulose AK, Traverse JH, Henry TD. Patients with coronary artery disease not amenable to traditional revascularization: prevalence and 3-year mortality. Catheter Cardiovasc Interv. 2010;75:886–891. doi: 10.1002/ccd.22431. doi: 10.1002/ccd.22431. [DOI] [PubMed] [Google Scholar]
- 2.Hachamovitch R, Rozanski A, Shaw LJ, Stone GW, Thomson LE, Friedman JD, Hayes SW, Cohen I, Germano G, Berman DS. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J. 2011;32:1012–1024. doi: 10.1093/eurheartj/ehq500. doi: 10.1093/eurheartj/ehq500. [DOI] [PubMed] [Google Scholar]
- 3.Reimer KA, Ideker RE, Jennings RB. Effect of coronary occlusion site on ischaemic bed size and collateral blood flow in dogs. Cardiovasc Res. 1981;15:668–674. doi: 10.1093/cvr/15.11.668. [DOI] [PubMed] [Google Scholar]
- 4.Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: a meta-analysis. Eur Heart J. 2012;33:614–621. doi: 10.1093/eurheartj/ehr308. doi: 10.1093/eurheartj/ehr308. [DOI] [PubMed] [Google Scholar]
- 5.Seiler C, Engler R, Berner L, Stoller M, Meier P, Steck H, Traupe T. Prognostic relevance of coronary collateral function: confounded or causal relationship? Heart. 2013;99:1408–1414. doi: 10.1136/heartjnl-2013-304369. doi: 10.1136/heartjnl-2013-304369. [DOI] [PubMed] [Google Scholar]
- 6.Hudson CL, Moritz AR, Wearn JT. The extracardiac anastomoses of the coronary arteries. J Exp Med. 1932;56:919–925. doi: 10.1084/jem.56.6.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moberg A. Anastomoses between extracardiac vessels and coronary arteries. II. Via internal mammary arteries. Post-mortem angiographic study. Acta Radiol Diagn (Stockh) 1967;6:263–272. doi: 10.1177/028418516700600306. [DOI] [PubMed] [Google Scholar]
- 8.Loukas M, Hanna M, Chen J, Tubbs RS, Anderson RH. Extracardiac coronary arterial anastomoses. Clin Anat. 2011;24:137–142. doi: 10.1002/ca.21088. doi: 10.1002/ca.21088. [DOI] [PubMed] [Google Scholar]
- 9.Singh RN, Varat MA. Acquired internal mammary-to-coronary artery communication. Cathet Cardiovasc Diagn. 1982;8:281–285. doi: 10.1002/ccd.1810080312. [DOI] [PubMed] [Google Scholar]
- 10.Kajinami K, Takekoshi N, Yoshio H. Internal mammary-to-coronary artery communication in a patient with occluded right coronary artery. Am Heart J. 1993;125(5 pt 1):1428–1430. doi: 10.1016/0002-8703(93)91017-9. [DOI] [PubMed] [Google Scholar]
- 11.Knight C, Webster G, Mulcahy D. Collateral growth between left internal mammary and left anterior descending coronary arteries following coronary artery bypass surgery. Int J Cardiol. 1994;43:107–109. doi: 10.1016/0167-5273(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 12.Salachas A, Antonellis I, Margaris N, Ifantis G, Moustakas I, Tsoukas A, Tavernarakis A. Communication between a nongrafted left internal mammary artery and left anterior descending coronary artery following saphenous vein bypass grafting. Cathet Cardiovasc Diagn. 1997;40:170–172. doi: 10.1002/(sici)1097-0304(199702)40:2<170::aid-ccd11>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto T, Kasagami Y, Ohba T, Saeki K, Kanemura M, Munakata K, Takano T. Left internal mammary-to-left circumflex coronary artery collateral pathway in a patient with occluded left circumflex artery. Jpn Circ J. 2001;65:753–754. doi: 10.1253/jcj.65.753. [DOI] [PubMed] [Google Scholar]
- 14.Aras D, Topaloglu S, Cagli K, Ergun K, Ozeke O, Korkmaz S. A rare form of communication between the left internal thoracic artery and the left anterior descending artery. J Invasive Cardiol. 2006;18:E209–E210. [PubMed] [Google Scholar]
- 15.Numata S, Yamazaki S, Tsutsumi Y, Ohashi H. Natural right internal mammary artery to left circumflex artery bypass. Circulation. 2014;130:e76–e78. doi: 10.1161/CIRCULATIONAHA.114.011126. doi: 10.1161/CIRCULATIONAHA.114.011126. [DOI] [PubMed] [Google Scholar]
- 16.Blair CR, Roth RF, Zintel HA. Measurement of coronary artery blood-flow following experimental ligation of the internal mammary artery. Ann Surg. 1960;152:325–329. doi: 10.1097/00000658-196008000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picichè M, Fadel E, Kingma JG, Jr, Dagenais F, Robillard J, Simard D, Voisine P. Blood flow to the heart from noncoronary arteries: an intriguing but challenging research field. Cardiovasc Revasc Med. 2012;13:25–29. doi: 10.1016/j.carrev.2011.07.001. doi: 10.1016/j.carrev.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Stoller M, de Marchi SF, Seiler C. Function of natural internal mammary-to-coronary artery bypasses and its effect on myocardial ischemia. Circulation. 2014;129:2645–2652. doi: 10.1161/CIRCULATIONAHA.114.008898. doi: 10.1161/CIRCULATIONAHA.114.008898. [DOI] [PubMed] [Google Scholar]
- 19.Seiler C, Fleisch M, Garachemani A, Meier B. Coronary collateral quantitation in patients with coronary artery disease using intravascular flow velocity or pressure measurements. J Am Coll Cardiol. 1998;32:1272–1279. doi: 10.1016/s0735-1097(98)00384-2. [DOI] [PubMed] [Google Scholar]
- 20.Matsuo H, Watanabe S, Kadosaki T, Yamaki T, Tanaka S, Miyata S, Segawa T, Matsuno Y, Tomita M, Fujiwara H. Validation of collateral fractional flow reserve by myocardial perfusion imaging. Circulation. 2002;105:1060–1065. doi: 10.1161/hc0902.104719. [DOI] [PubMed] [Google Scholar]
- 21.Vogel R, Zbinden R, Indermühle A, Windecker S, Meier B, Seiler C. Collateral-flow measurements in humans by myocardial contrast echocardiography: validation of coronary pressure-derived collateral-flow assessment. Eur Heart J. 2006;27:157–165. doi: 10.1093/eurheartj/ehi585. doi: 10.1093/eurheartj/ehi585. [DOI] [PubMed] [Google Scholar]
- 22.Friedman PL, Shook TL, Kirshenbaum JM, Selwyn AP, Ganz P. Value of the intracoronary electrocardiogram to monitor myocardial ischemia during percutaneous transluminal coronary angioplasty. Circulation. 1986;74:330–339. doi: 10.1161/01.cir.74.2.330. [DOI] [PubMed] [Google Scholar]
- 23.Mannheimer C, Camici P, Chester MR, Collins A, DeJongste M, Eliasson T, Follath F, Hellemans I, Herlitz J, Lüscher T, Pasic M, Thelle D. The problem of chronic refractory angina; report from the ESC Joint Study Group on the Treatment of Refractory Angina. Eur Heart J. 2002;23:355–370. doi: 10.1053/euhj.2001.2706. doi: 10.1053/euhj.2001.2706. [DOI] [PubMed] [Google Scholar]
- 24.Briones E, Lacalle JR, Marin-Leon I, Rueda JR. Transmyocardial laser revascularization versus medical therapy for refractory angina. Cochrane Database Syst Rev. 2015;2:CD003712. doi: 10.1002/14651858.CD003712.pub3. doi: 10.1002/14651858.CD003712.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cassar A, Prasad M, Rodriguez-Porcel M, Reeder GS, Karia D, DeMaria AN, Lerman A. Safety and efficacy of extracorporeal shock wave myocardial revascularization therapy for refractory angina pectoris. Mayo Clin Proc. 2014;89:346–354. doi: 10.1016/j.mayocp.2013.11.017. doi: 10.1016/j.mayocp.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Povsic TJ, Henry TD, Traverse JH, Fortuin FD, Schaer GL, Kereiakes DJ, Schatz RA, Zeiher AM, White CJ, Stewart DJ, Jolicoeur EM, Bass T, Henderson DA, Dignacco P, Gu Z, Al-Khalidi HR, Junge C, Nada A, Hunt AS, Losordo DW RENEW Investigators. The RENEW Trial: Efficacy and Safety of Intramyocardial Autologous CD34(+) Cell Administration in Patients With Refractory Angina. JACC Cardiovasc Interv. 2016;9:1576–1585. doi: 10.1016/j.jcin.2016.05.003. doi: 10.1016/j.jcin.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Gloekler S, Meier P, de Marchi SF, Rutz T, Traupe T, Rimoldi SF, Wustmann K, Steck H, Cook S, Vogel R, Togni M, Seiler C. Coronary collateral growth by external counterpulsation: a randomised controlled trial. Heart. 2010;96:202–207. doi: 10.1136/hrt.2009.184507. doi: 10.1136/hrt.2009.184507. [DOI] [PubMed] [Google Scholar]
- 28.Seiler C, Stoller M, Pitt B, Meier P. The human coronary collateral circulation: development and clinical importance. Eur Heart J. 2013;34:2674–2682. doi: 10.1093/eurheartj/eht195. doi: 10.1093/eurheartj/eht195. [DOI] [PubMed] [Google Scholar]
- 29.Banai S, Ben Muvhar S, Parikh KH, Medina A, Sievert H, Seth A, Tsehori J, Paz Y, Sheinfeld A, Keren G. Coronary sinus reducer stent for the treatment of chronic refractory angina pectoris: a prospective, open-label, multicenter, safety feasibility first-in-man study. J Am Coll Cardiol. 2007;49:1783–1789. doi: 10.1016/j.jacc.2007.01.061. doi: 10.1016/j.jacc.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 30.Stoller M, Traupe T, Khattab AA, de Marchi SF, Steck H, Seiler C. Effects of coronary sinus occlusion on myocardial ischaemia in humans: role of coronary collateral function. Heart. 2013;99:548–555. doi: 10.1136/heartjnl-2012-303305. doi: 10.1136/heartjnl-2012-303305. [DOI] [PubMed] [Google Scholar]
- 31.Billinger M, Fleisch M, Eberli FR, Garachemani A, Meier B, Seiler C. Is the development of myocardial tolerance to repeated ischemia in humans due to preconditioning or to collateral recruitment? J Am Coll Cardiol. 1999;33:1027–1035. doi: 10.1016/s0735-1097(98)00674-3. [DOI] [PubMed] [Google Scholar]
- 32.Williams RP, Manou-Stathopoulou V, Redwood SR, Marber MS. ‘Warm-up Angina’: harnessing the benefits of exercise and myocardial ischaemia. Heart. 2014;100:106–114. doi: 10.1136/heartjnl-2013-304187. doi: 10.1136/heartjnl-2013-304187. [DOI] [PubMed] [Google Scholar]
- 33.Verheye S, Jolicœur EM, Behan MW, Pettersson T, Sainsbury P, Hill J, Vrolix M, Agostoni P, Engstrom T, Labinaz M, de Silva R, Schwartz M, Meyten N, Uren NG, Doucet S, Tanguay JF, Lindsay S, Henry TD, White CJ, Edelman ER, Banai S. Efficacy of a device to narrow the coronary sinus in refractory angina. N Engl J Med. 2015;372:519–527. doi: 10.1056/NEJMoa1402556. doi: 10.1056/NEJMoa1402556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otsuka Y, Imoto H, Kono M, Koyama T, Nakamura K, Kodama S, Noguchi H, Saito T. Chronic total coronary occlusion with bronchocoronary collateral circulation failed to visualize by conventional angiography. JACC Cardiovasc Interv. 2014;7:e197–e199. doi: 10.1016/j.jcin.2014.07.016. doi: 10.1016/j.jcin.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 35.Moberg A. Anastomoses between extracardiac vessels and coronary arteries. Acta Med Scand Suppl. 1968;485:5–26. [PubMed] [Google Scholar]
- 36.Battezzati M, Tagliaferro A, de Marchi G. [Ligation of the two internal mammary arteries in vascular disorders of the myocardium; preventive note concerning the first experimental and clinical findings]. Minerva Med. 1955;46:1178–1188. [PubMed] [Google Scholar]
- 37.Haller JD, Olearchyk AS. Cardiology’s 10 greatest discoveries. Tex Heart Inst J. 2002;29:342–344. [PMC free article] [PubMed] [Google Scholar]
- 38.Fieschi D. Criteri anatomo-fisiologici per intervento chirurgico lieve in malati di infarto e cuore di angina. Arch Ital Chir. 1942;63:305–310. [Google Scholar]
- 39.Battezzati M, Tagliaferro A, Cattaneo AD. Clinical evaluation of bilateral internal mammary artery ligation as treatment coronary heart disease. Am J Cardiol. 1959;4:180–183. doi: 10.1016/0002-9149(59)90245-0. [DOI] [PubMed] [Google Scholar]
- 40.Kitchell JR, Glover RP, Kyle RH. Bilateral internal mammary artery ligation for angina pectoris; preliminary clinical considerations. Am J Cardiol. 1958;1:46–50. doi: 10.1016/0002-9149(58)90074-2. [DOI] [PubMed] [Google Scholar]
- 41.Cobb LA, Thomas GI, Dillard DH, Merendino KA, Bruce RA. An evaluation of internal-mammary-artery ligation by a double-blind technic. N Engl J Med. 1959;260:1115–1118. doi: 10.1056/NEJM195905282602204. doi: 10.1056/NEJM195905282602204. [DOI] [PubMed] [Google Scholar]
- 42.Dimond EG, Kittle CF, Crockett JE. Comparison of internal mammary artery ligation and sham operation for angina pectoris. Am J Cardiol. 1960;5:483–486. doi: 10.1016/0002-9149(60)90105-3. [DOI] [PubMed] [Google Scholar]
- 43.Beecher HK. Surgery as placebo. A quantitative study of bias. JAMA. 1961;176:1102–1107. doi: 10.1001/jama.1961.63040260007008. [DOI] [PubMed] [Google Scholar]
- 44.Miller FG. The enduring legacy of sham-controlled trials of internal mammary artery ligation. Prog Cardiovasc Dis. 2012;55:246–250. doi: 10.1016/j.pcad.2012.09.002. doi: 10.1016/j.pcad.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Zbinden S, Zbinden R, Meier P, Windecker S, Seiler C. Safety and efficacy of subcutaneous-only granulocyte-macrophage colony-stimulating factor for collateral growth promotion in patients with coronary artery disease. J Am Coll Cardiol. 2005;46:1636–1642. doi: 10.1016/j.jacc.2005.01.068. doi: 10.1016/j.jacc.2005.01.068. [DOI] [PubMed] [Google Scholar]
- 46.Meier P, Gloekler S, de Marchi SF, Indermuehle A, Rutz T, Traupe T, Steck H, Vogel R, Seiler C. Myocardial salvage through coronary collateral growth by granulocyte colony-stimulating factor in chronic coronary artery disease: a controlled randomized trial. Circulation. 2009;120:1355–1363. doi: 10.1161/CIRCULATIONAHA.109.866269. doi: 10.1161/CIRCULATIONAHA.109.866269. [DOI] [PubMed] [Google Scholar]
- 47.Gloekler S, Traupe T, Stoller M, Schild D, Steck H, Khattab A, Vogel R, Seiler C. The effect of heart rate reduction by ivabradine on collateral function in patients with chronic stable coronary artery disease. Heart. 2014;100:160–166. doi: 10.1136/heartjnl-2013-304880. doi: 10.1136/heartjnl-2013-304880. [DOI] [PubMed] [Google Scholar]
- 48.Zbinden R, Zbinden S, Meier P, Hutter D, Billinger M, Wahl A, Schmid JP, Windecker S, Meier B, Seiler C. Coronary collateral flow in response to endurance exercise training. Eur J Cardiovasc Prev Rehabil. 2007;14:250–257. doi: 10.1097/HJR.0b013e3280565dee. doi: 10.1097/HJR.0b013e3280565dee. [DOI] [PubMed] [Google Scholar]


