Abstract
Background
Mycophenolic acid (MPA) is the active immunosuppressive substance in both mycophenolate mofetil and mycophenolate sodium, and it is widely used after organ transplantation. In women, taking MPA is teratogenic and may also influence spermatogenesis. There is a lack of knowledge regarding outcome of pregnancies fathered by men exposed to MPA.
Methods
We compared outcomes in pregnancies fathered by renal transplant men per whether they had been exposed to MPA or not at time of conception. A nationwide population-based retrospective cohort study was performed. Data from the Norwegian Renal Registry with all renal transplanted men alive between January 1, 1995 and December 31, 2015 were included, and relevant outcome data were extracted from the Medical Birth Registry of Norway.
Results
During the given time, 230 immunosuppressed renal transplanted men fathered 350 children (155 on MPA/195 not on MPA). There were no significant increased risks of malformation (3.9% vs. 2.6%, P = 0.49) in MPA exposed versus unexposed cohorts of children. The average dose (±SD) of mycophenolate was 1.42 ± 0.3 g/day and the individual median MPA trough concentration in the time period of anticipated conception and pregnancy was 2.8 ± 1.6 mg/L. Birth weight was similar in exposed and unexposed cohorts of children; 3381 ± 681 g vs. 3429 ± 714 g (P = 0.53).
Conclusions
Paternal exposure to MPA did not increase the risk of adverse birth outcomes in children fathered by male kidney transplanted patients. These results are reassuring and support the continuation of paternal MPA treatment before, during, and after conception.
Paternal exposure to mycophenolic acid (MPA) did not increase the risk of adverse birth outcomes in children fathered by male kidney transplanted patients and reassuring and support the continuation of paternal MPA treatment before, during and after conception.
End-stage renal disease patients commonly experience sexual disturbance and reduced fertility. Successful renal transplantation can restore these functions in both male and female patients.1,2 Women are, in general, recommended to refrain from taking any medication during pregnancy. Transplant recipients, regardless of sex, must however take lifelong immunosuppressive medication to preserve their transplant function. It has therefore been a focus on pregnancy safety and outcome for immunosuppressed women.3-5 Mycophenolic acid (MPA) is the active immunosuppressive substance in both mycophenolate mofetil (MMF) and mycophenolate sodium (MPS), and it reduces the incidence of acute rejection and benefits graft survival.6 From previous studies, it is well known that exposure to MPA in early pregnancy of female transplant recipients has been associated with increased rate of miscarriages and birth defects in the live born.7,8 Accordingly, common practice in female patients who wish to become pregnant is either to withdraw MPA or switch to azathioprine before conception. Recently, the manufacturers of MMF (CellCept; Roche, Basel) and MPS (Myfortic; Novartis, Basel) and the European Medicines Agency (EMA) recommended additional measurements to prevent MPA exposure in pregnancy.9 The warning states: “sexually active men taking mycophenolate are recommended to use condom for sex during treatment and for 90 days thereafter; partners of childbearing potential are also recommended to use highly effective contraception for the same period.”9 There is very limited published data on the effect of MPA on human sperm and the possible effects of children whose fathers used MPA at time of conception. Actually, the authors could only find one publication and the results published did not support the EMA recommendations.10 There is an increased risk of acute rejection and graft loss associated with withdrawal of MPA. The transplant community was therefore puzzled by the EMA warning.11,12 Was this EMA recommendation solely due to precautionary measures? Obviously, there was and is a need for more data. We therefore decided to perform a population-based nationwide retrospective study comparing outcomes in pregnancies fathered by renal transplant men either exposed to MPA or not at time of conception and/or pregnancy.
MATERIALS AND METHODS
A national population-based study was designed with focus on the time period from 1995 to 2015. Transplanted men alive between January 1, 1995 and December 31, 2015 were identified in the Norwegian Renal Registry (NRR). Data from the Medical Birth Registry of Norway (MBRN) was collected for the same time period. The registry was established in 1967 and was the first national medical birth registry in the world. It is based on compulsory notification of all live births and stillbirths from gestational week 16 initially (1967–2001) and since 2002 from week 12. To ensure medical notification of every newborn in Norway, all records in the MBRN are matched with files of the Central Person Registry.13 Linkage between the data from the NRR and MBRN were merged utilizing the unique 11-digit personal identification number given to every Norwegian citizen at birth.
Data on MPA exposure was extracted from NRR. Patients with registered use of MPA drugs the year of birth or the year before were defined as “MPA-exposed.” Trough concentrations of MPA for the same time period were searched for and obtained (when measured) from the hospital laboratory system for all patients in the MPA-exposed group. If no trough MPA concentration was measured, all patient charts were manually cross-checked for accuracy in reported MPA usage to NRR in all patients defined as MPA-exposed above.
Data extracted from the MBRN included demographics on mother and father, previous births and spontaneous abortions of the mother, health status of the mother before and during pregnancy, and pregnancy and birth outcomes including birth complications, pregnancy duration, birth weight, stillbirths, perinatal death, APGAR score, and malformations.
Immunosuppressive protocols used by the patients included in the study have varied depending primarily on time period of engraftment. All recipients have received steroids and a calcineurin inhibitor at time of engraftment. From 1995 to 2008, more or less all recipients received cyclosporine A. From January 1, 2009, recipients <50 years received tacrolimus, and from January 1, 2012, all recipients have received tacrolimus. Azathioprine was used from 1995 to December 31, 2000. After that, all recipients have received MPA. Induction with basiliximab was started in 2000. Some patients have been included in clinical trials with either switch to mTOR (everolimus/sirolimus), use of JAK 3-inhibitor, or belatacept. Data from these patients were not included in the final evaluation.
Statistical Analyses
Differences between the MPA exposed and MPA not exposed groups were statistically compared with logistic and linear regression methods adjusting for repeated fatherhood both within and between groups by clustered sandwich estimator for adjusting the standard errors. R was used for data management and statistical analyses.14,15
RESULTS
A total of 6032 renal transplant recipients were alive during this time, 3974 men/2058 women, and a total of 4000 had been exposed to MPA (2681 M/1319 F). During the time period, a total of 230 immunosuppressed renal transplanted males fathered 350 children. Moreover, 136 men fathered 1 child, 73 men fathered 2 children, and 21 fathered 3 or more children. At the estimated time of conception, there were 112 different male transplant recipients who fathered 155 children under MPA exposure and 133 male recipients who fathered 195 children without MPA exposure. Fifteen men fathered children both as exposed and unexposed to MPA. In the early phase of MPA use, trough measurements on stable maintenance patients were not always performed. We were, however, able to trace valid MPA trough concentration measurements for the actual time period for 117 of the 155 MPA exposed patients. The average dose (±SD) of mycophenolate was 1.42 ± 0.3 g/day and individual median MPA trough concentration in the time period of anticipated conception and pregnancy was 2.8 ± 1.6 mg/L. For the remaining 38 patients, we found verification in hospital journals of MPA use at time of conception. There were no clinical differences between the age of the fathers on MPA versus not on MPA (36.1 ± 5.6 vs. 35.7 ± 6.0 years, P = 0.59) or age of mothers to these children (31.9 ± 4.7 vs. 31.0 ± 4.8 years, P = 0.12). Mean gestational age was also not different between the groups, 38.8 ± 2.5 and 39.1 ± 2.7 weeks, respectively (P = 0.30). When comparing other available data from the MBRN, we could not detect any overall differences between outcomes in the two groups (Table 1).
TABLE 1.
Birth outcomes between exposed (MPA+) and not exposed (MPA−) groups
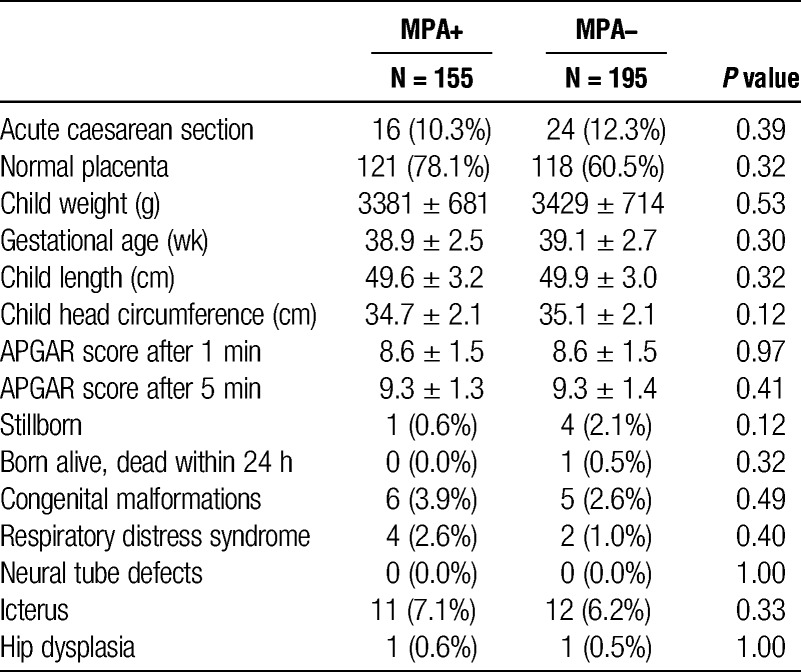
There was no difference in malformations in children fathered by MPA exposed versus nonexposed recipients. There were no significant differences with regards to mother’s health before or during respective pregnancy or medication used during pregnancy (data not shown). Preeclampsia occurred in 7 (4.5%) and 14 (7.3%) births fathered by exposed and nonexposed to MPA, respectively.
DISCUSSION
The results indicate no difference in congenital malformation or other unwanted outcomes when comparing pregnancies whether the father was exposed to or not exposed to MPA. This supports the previous findings published by Jones et al and questions the validity of the EMA recommendations.
After a successful organ transplantation, recipients are faced with the need of lifelong immunosuppressive medication to preserve their transplant function. Kidney transplantation by far outnumbers other organs. Because approximately 40% to 50% of men receiving a kidney transplant are younger than 50 years of age, the fertility and possibility of fatherhood of male renal transplant recipients is a concern. There have, however, been very few reports that have investigated the potential effect of immunosuppressants on the male fertility and on the offspring.10,16 Currently, the most common worldwide posttransplant immunosuppressive combination consists of glucocorticoids, tacrolimus, and MPA. Additional concomitant medications are often needed. Many drugs may affect the spermatogenesis. This makes it impossible to pinpoint one specific drug used by the father at time of conception as “the sinner” causing possible adverse birth outcomes. There have been some animal studies and some very few human studies addressing a possible difference in the calcineurin inhibitors cyclosporine A and tacrolimus on the spermatozoa.2,16,17 Results are conflicting. Recently, Nørgård et al published a large study focusing on birth outcome in children fathered by men treated with azathioprine due to inflammatory bowel disease,18 ie, not using other immunosuppressive drugs. They found no increased risk of adverse birth outcomes. There is no such study addressing monotherapy with MPA. For ethical reasons, randomized trials cannot be designed to evaluate the safety of drugs like MPA during conception. Therefore, we have to base clinical decisions on evidence from observational registry studies.
In this evaluation, we have not compared outcome of children conceived from immunosuppressed fathers with the outcome of the general population. This comparison was, however, recently performed by Morken et al,19 but from a different time interval and also including data from other organ transplant recipients. In their analysis, they compared data from 4614 pregnancies before and 474 after transplantation to the outcome in the general population and found an increased risk of preeclampsia. The risk of preeclampsia was increased (OR: 7.4, 95% CI: 1.1–51.4) after transplantation. They write that a “causal link between a specific immunosuppressive drug and preeclampsia is thus difficult to establish.” Importantly, no increased risk was found for congenital malformations or other outcomes when compared with pregnancies before transplantation or with the general population (2,511,506 births), ie, findings not supporting the EMA recommendations. Number of fathers exposed to MPA was not specified.
A strength of the current study is its design being a nationwide population-based cohort study. Oslo University Hospital is the only transplant center in Norway serving approximately 5 million inhabitants and has kept complete lifelong records of all transplanted individuals since 1969, including immunosuppressive drugs used. We do not have direct data on drug adherence but because MPA trough only is analyzed at the Oslo University Hospital laboratory, we could verify a valid trough value around time of conception for the majority of the male recipients. Additionally, we could evaluate mother’s health before or during respective pregnancy—finding no difference. A drawback is obviously the retrospective design of the study. It is well known that most miscarriages occur during the first trimester, and a caveat of our retrospective registration is that there may have been more miscarriages during the first trimester if the father was on immunosuppression. We are, however, not aware of any national registry capturing miscarriages this early.
In conclusion, the results indicate no difference in obstetrical and/or neonatal complications when the father is a transplanted man on MPA as compared to a transplanted man not on MPA. Obviously, even more data is needed but in the meantime we once again urge the EMA to reconsider their current recommendations regarding MPA.
ACKNOWLEDGMENTS
The Medical Birth Registry of Norway and the transplantation registry of Oslo University Hospital provided data and are acknowledged.
Footnotes
The authors declare no funding or conflicts of interest.
Karsten Midtvedt and Anders Åsberg both designed, collected data, wrote, and approved of the final manuscript. Anders Åsberg performed the statistical analysis. Stein Bergan collected the MPA exposure trough values and approved of the final manuscript. Anna V. Reisæter critically evaluated and approved of the final manuscript. Bjørn Egil Vikse critically evaluated and approved of the final manuscript.
REFERENCES
- 1.McKay DB, Josephson MA. Pregnancy in recipients of solid organs—effects on mother and child. N Engl J Med. 2006;354:1281–1293. [DOI] [PubMed] [Google Scholar]
- 2.Georgiou GK, Dounousi E, Harissis HV. Calcineurin inhibitors and male fertility after renal transplantation—a review. Andrologia. 2016;48:483–490. [DOI] [PubMed] [Google Scholar]
- 3.Rose C, Gill J, Zalunardo N, et al. Timing of pregnancy after kidney transplantation and risk of allograft failure. Am J Transplant. 2016;16:2360–2367. [DOI] [PubMed] [Google Scholar]
- 4.Coscia LA, Armenti DP, King RW, et al. Update on the teratogenicity of maternal mycophenolate mofetil. J Pediatr Genet. 2015;4:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl. 2010:65–85. [PubMed] [Google Scholar]
- 6.Wagner M, Earley AK, Webster AC, et al. Mycophenolic acid versus azathioprine as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev. 2015:Cd007746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deshpande NA, Coscia LA, Gomez-Lobo V, Moritz MJ, Armenti VT. Pregnancy after solid organ transplantation: a guide for obstetric management. Rev Obstet Gynecol. 2013;6(3–4):116–125. [PMC free article] [PubMed] [Google Scholar]
- 8.Kim M, Rostas S, Gabardi S. Mycophenolate fetal toxicity and risk evaluation and mitigation strategies. Am J Transplant. 2013;13:1383–1389. [DOI] [PubMed] [Google Scholar]
- 9.European Medicines Agency. EMA recommends additional measures to prevent use of mycophenolate in pregnancy. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2015/10/news_detail_002418.jsp&mid=WC0b01ac058004d5c1. Published October 23 2015. Accessed on September 24, 2016.
- 10.Jones A, Clary MJ, McDermott E, et al. Outcomes of pregnancies fathered by solid-organ transplant recipients exposed to mycophenolic acid products. Prog Transplant. 2013;23:153–157. [DOI] [PubMed] [Google Scholar]
- 11.Kuypers DR, Van Mieghem T, Meijers B, et al. Updated manufacturer and European Medicines Agency recommendations on the use of mycophenolate acid: balancing the risks for male allograft recipients. Transplantation. 2016;100:e50–e51. [DOI] [PubMed] [Google Scholar]
- 12.Midtvedt K, Åsberg A. Mycophenolate acid and balancing the risk for male allograft recipients. Transplantation. 2017;101:e39. [DOI] [PubMed] [Google Scholar]
- 13.Irgens LM. The medical birth registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand. 2000;79:435–439. [PubMed] [Google Scholar]
- 14.Achim Zeileis TH. Diagnostic checking in regression relationships. R News. 2002;2:7–10. [Google Scholar]
- 15.Graham N, Arai M, Hagströmer B. Multi-way standard error clustering. http://CRAN.R-project.org/package=multiwayvcov. Published May 5 2016.
- 16.Xu L, Han S, Liu Y, et al. The influence of immunosuppressants on the fertility of males who undergo renal transplantation and on the immune function of their offspring. Transpl Immunol. 2009;22:28–31. [DOI] [PubMed] [Google Scholar]
- 17.Miyata H, Satouh Y, Mashiko D, et al. Sperm calcineurin inhibition prevents mouse fertility with implications for male contraceptive. Science. 2015;350:442–445. [DOI] [PubMed] [Google Scholar]
- 18.Nørgård BM, Magnussen B, Larsen MD, et al. Reassuring results on birth outcomes in children fathered by men treated with azathioprine/6-mercaptopurine within 3 months before conception: a nationwide cohort study. Gut. 2016. [published online 2016]. doi: 10.1136/gutjnl-2016-312123. [DOI] [PubMed] [Google Scholar]
- 19.Morken NH, Diaz-Garcia C, Reisaeter AV, et al. Obstetric and neonatal outcome of pregnancies fathered by males on immunosuppression after solid organ transplantation. Am J Transplant. 2015;15:1666–1673. [DOI] [PubMed] [Google Scholar]


