Abstract
Background and Purpose—
Ultrasound markers of carotid atherosclerosis may be related to cognitive status. We hypothesized that individuals with greater carotid intima–media thickness (cIMT) and carotid plaque burden would exhibit worse cognition.
Methods—
One thousand one hundred sixty-six stroke-free participants from the NOMAS (Northern Manhattan Study) underwent carotid ultrasound and neuropsychological examination. Among them, 826 underwent a second neuropsychological examination an average of 5 years later. cIMT and plaque were assessed by a standardized B-mode ultrasound imaging and reading protocol. We used multivariable linear regression to examine cIMT, carotid plaque presence, and carotid plaque area as correlates of domain-specific neuropsychological Z scores cross-sectionally and over time. We also investigated possible effect modification by APOE ε4 allele, age, and race/ethnicity.
Results—
Participants had a mean (SD) age of 70 (9) years and were 60% women, 66% Hispanic, 15% white, and 18% black. Those with greater cIMT exhibited worse episodic memory after adjustment for demographics and vascular risk factors (β=−0.60; P=0.04). APOE ε4 carriers with greater cIMT exhibited worse episodic memory (β=−1.31; P=0.04), semantic memory (β=−1.45; P=0.01), and processing speed (β=−1.21; P=0.03). Participants with greater cIMT at baseline did not exhibit significantly greater cognitive decline after adjustment. APOE ε4noncarriers with greater cIMT exhibited greater declines in executive function (β=−0.98; P=0.06). Carotid plaque burden was not significantly associated with cognition at baseline or over time.
Conclusions—
Subclinical carotid atherosclerosis was associated with worse cognition among those at higher risk for Alzheimer disease. Interventions targeting early stages of atherosclerosis may modify cognitive aging.
Keywords: alleles, Alzheimer disease, atherosclerosis, carotid intima–media thickness, cognitive, dysfunction
Ultrasound markers of carotid atherosclerosis are associated with various vascular risk factors and incident cardiovascular disease.1,2 Two distinct measures of carotid atherosclerosis are carotid plaque burden, quantified by plaque presence or area, and carotid intima–media thickness (cIMT). Plaque represents significant atherosclerotic disease in the vessel lumen, whereas cIMT reflects thickening of the intimal and medial layers of the vessel wall. Although cIMT may better represent subclinical vascular disease amendable to preventative measures, current evidence suggests that plaque burden may be a stronger predictor of cardiovascular disease than cIMT.3 Although cardiovascular disease has been previously associated with cognitive impairment, less is known about the ability of these carotid ultrasound markers to predict cognitive impairment.3,4
Dementia and age-related cognitive decline represent leading threats to public health.5 Evidence suggests that cIMT and carotid plaque may be associated with cognitive impairment and dementia.6–9 These data suggest that early intervention of carotid atherosclerosis may prevent cognitive impairment.9,10 However, more research is needed in racially and ethnically diverse populations, which have a disproportionate cardiovascular disease burden compared with whites.11 Further, results from previous studies have been inconsistent, especially on which cognitive domains are most affected.
We hypothesized that greater carotid plaque burden and cIMT would be associated with domain-specific cognitive performance and decline in the racially and ethnically diverse NOMAS (Northern Manhattan Study). Carotid atherosclerosis represents a potentially valuable target for early intervention and risk stratification for those at risk for cognitive decline.
Methods
Study Population
The study sample includes a subset of participants from the NOMAS, as previously described.12 The NOMAS is an ongoing longitudinal cohort study of stroke in a multiethnic urban population. From 1993 to 2001, participants were identified by random digit dialing with the following eligibility criteria: (1) had never been diagnosed with a stroke, (2) were >40 years of age, and (3) resided in Northern Manhattan for ≥3 months, in a household with a telephone. The telephone response rate was 91% (9% refused to be screened), and 75% of these participants were enrolled with an in-person baseline interview and assessment by trained bilingual research assistants (enrollment n=3497). Beginning in 2001, we recruited a subgroup of 1290 participants during telephone follow-up who remained clinically stroke-free for MRI and neuropsychological assessments, after providing written informed consent. The following eligibility criteria applied: (1) age >55 and (2) no contraindications to MRI. Of the 1290 in this NOMAS subsample, 1166 had carotid ultrasound imaging. The current study included the NOMAS participants with both MRI/neuropsychological assessments and carotid ultrasound imaging (n=1166). Of this sample of 1166 participants, 836 people had available neuropsychological data at time 2 (average of 5 years after the initial neuropsychological assessment). The NOMAS was approved by the institutional review boards at the University of Miami and Columbia University, and all participants provided written informed consent.
Enrollment Evaluation
The enrollment evaluation has been previously described.12 Race/ethnicity was self-reported through questions modeled after the US Census and conforming to standard definitions outlined by Directive 15. Standardized questions were adapted from the Behavioral Risk Factor Surveillance System by the CDC on vascular risk factors. Smoking was categorized as current (within the past year), former, or never smoker of cigarettes, cigars, or pipes. Moderate alcohol use was defined as current drinking of >1 drink per month and ≤2 drinks per day. Moderate-to-heavy physical activity level was defined as engaging in one or more of selected rigorous physical activities in a typical 14-day period, as described previously.12 Blood pressure was obtained from the right brachial artery after a 10-minute rest in a seated position (Dinamap Pro100; Critikon, Inc), measured twice, before and after each examination, and averaged. Hypertension was defined as a blood pressure ≥140/90 mm Hg, the subject’s self-report of hypertension, or antihypertensive medication use. Diabetes mellitus was defined by the subject’s self-report of such a history, use of insulin or oral anti-diabetic medication, or fasting glucose ≥126 mg/dL. Hypercholesterolemia was defined as having a total cholesterol level of >200 mg/dL, cholesterol-lowering medication use, or self-reported history of hypercholesterolemia. Body mass index was calculated as kilograms per meter squared.
DNA samples were extracted from peripheral blood white cells using HhaI digestion and amplified by polymerase chain reaction as previously described.13 APOE ε4 carriers were identified as individuals with a genotype of ApoE4/4, ApoE4/3, and ApoE4/2.
Carotid Ultrasound
Carotid IMT and plaque were measured as part of the NOMAS starting in 2000 using high-resolution B-mode ultrasound imaging (GE LogIQ 700; 9- to 13- MHz linear-array transducer) by trained and certified sonographers, as previously described.14,15 Plaque was defined as a focal wall thickening or protrusion in the lumen >50% of the surrounding thickness. The automated computerized edge tracking software program M’Ath (M’Ath, Inc, Paris, France) was used to measure cIMT (mm) and quantify carotid plaque area (mm2).16 Total carotid plaque area was defined as the sum of all plaque areas measured in any of the carotid artery segments within an individual. M’Ath uses an automated plaque edge detection algorithm to detect boundaries of plaque from multiple images, such that the total plaque area is averaged across multiangle plaque images. cIMT was measured in areas without plaque and was calculated as a composite average measure of the IMT measured in the near and far walls of the common carotid artery, bifurcation, and internal carotid artery, bilaterally. cIMT was examined continuously as a mean of the maximum measurements of the 12 carotid sites.
Neuropsychological Examination
A neuropsychological examination (NPE) was conducted in English or Spanish in a quiet room by trained bilingual research assistants as described previously.17 Tests were grouped into 4 cognitive domains based on an exploratory factor analysis and a review of the literature. Z scores for each domain were calculated by averaging Z-transformed neuropsychological test scores. Episodic memory was assessed with 3 subscores on a 12-word, 5-trial list-learning task: list-learning total score, delayed recall score, and delayed recognition score.18 Executive function was assessed with 2 subscores: the difference in time to complete the color trails test form 1 and form 219 and the sum of the odd-man-out subtests 2 and 4.20 Processing speed was assessed with the Grooved Pegboard task in the nondominant hand,21 the color trails test form 1,19 and the visual–motor integration test.22 Semantic memory was assessed with the picture naming (modified Boston naming) test,23 category fluency (animal naming),24 and phonemic fluency test (C, F, and L in English speakers and F, A, and S in Spanish speakers).24 A mini–mental state examination was also performed.25 An average of 5 years after the initial assessment, participants were invited to participate in a second NPE.
For the change from initial to second NPE, we subtracted scores of second NPE from the first NPE and regressed the result on the corresponding initial test score adjusting for age, education, and time interval between the 2 assessments using linear regressions and used the standardized residuals from the regression models as the standardized change NPE test scores. The domain-specific individual standardized residuals were then averaged to compute the values for the change in neuropsychological domain scores.
At the time of the first NPE, brain MR imaging was performed on a 1.5T MRI system (Philips Medical Systems, Best, the Netherlands) at the Hatch Research Center. MRI scans were processed to estimate white matter hyperintensity and cerebral and intracranial volumes and identify subclinical brain infarcts, as described previously.26,27
Statistical Analysis
The primary exposures of interest were (1) cIMT, (2) carotid plaque presence, and (3) total carotid plaque area. As a secondary exposure, we also examined total carotid plaque area in quartiles. These exposures were examined in relation to the 4 cognitive domains at initial assessment and to the change in cognitive domains over time in a series of multivariable linear regression models. Model 1 was adjusted for age at NPE, years of education, time from baseline to carotid ultrasound, and time from carotid ultrasound to NPE. Model 2 was further adjusted for sex, race/ethnicity, Medicaid/no insurance status, physical activity, alcohol use, smoking, body mass index, diabetes mellitus, hypercholesterolemia, and hypertension. Model 3 further adjusted for brain MRI markers, including white matter hyperintensity, brain volume, and presence of subclinical brain infarcts, allowing us to examine the association of interest independently from subclinical brain pathology. Analyses of cognitive decline in cognition over time did not include age and education as covariates because they were included in the regression models created to derive the Z scores. We examined the potential effect modification by APOE ε4 carrier status by including interaction terms between APOE genotype and the carotid atherosclerosis phenotypes for all of the cognitive domain outcomes, controlling for the variables in model 2 and APOE genotype. We also examined the potential effect modification by age and race/ethnicity. When the interaction term P was <0.10, we performed stratified analyses.
A set of sensitivity analyses were conducted in participants free of cognitive impairment at enrollment, defined as a mini–mental state examination score of ≥17 among those with <8 years of education, and a mini–mental state examination score of ≥24 among those with ≥8 years of education, consistent with previous studies.28 These sensitivity analyses excluded 154 participants (95 with repeated NPEs). All analyses were performed with SAS 9.3 (SAS, Cary, NC).
Results
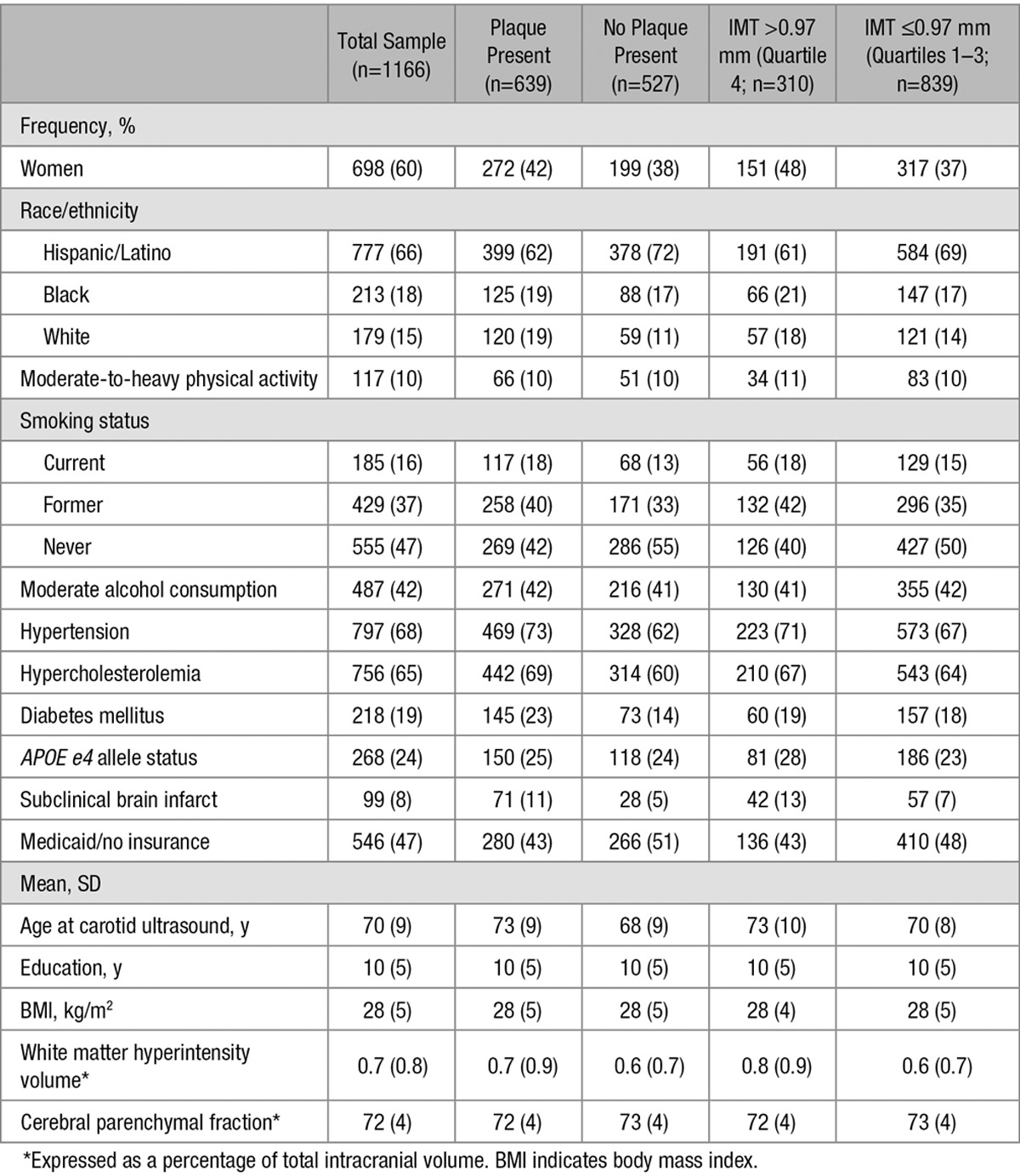
A total of 1166 NOMAS participants had both carotid ultrasound data and neuropsychological assessment (n=836 with follow-up NPE). Availability of follow-up neuropsychological assessment was related neither to cIMT nor APOE4 carrier status (data not shown). The characteristics of the study population are stratified by plaque presence and the top quartile of cIMT (0.97 mm; Table 1). The mean (SD) age at initial NPE was 70 (9) years. At initial NPE, the sample had 698 (60%) women, 777 (66%) Hispanics, 179 (15%) non-Hispanic whites, and 213 (18%) non-Hispanic blacks. Plaque was present in 639 people, which was 55% of the study sample. Mean cIMT was 0.93±0.09 mm. Participants with carotid plaques were more likely to be older, non-Hispanic blacks and whites, current and former smokers, hypertensive, hypercholesterolemic, and diabetics as compared with those without plaque. Participants with carotid plaques also had greater white matter hyperintensity, but less cerebral volume than those without plaque (Table 1). Participants with cIMT in the top quartile were more likely to have an APOE ε4 allele, subclinical brain infarct, and greater white matter hyperintensity and age than those who had cIMT less than the top quartile (Table 1).
Table 1.
Sample Characteristics
Carotid IMT was inversely associated with episodic memory at initial NPE after adjustment for demographics and vascular factors (Table 2; β=−0.604; P=0.042). This association attenuated and did not reach statistical significance after adjusting for brain MRI markers (β=−0.576; P=0.052). Overall, the inverse associations between cIMT and initial assessment of semantic memory, executive function, and processing speed did not reach statistical significance. The APOE ε4 allele was an effect modifier of the association between cIMT and cognition (P for interaction <0.10 for episodic memory, semantic memory, and processing speed). Stratified analyses showed that cIMT was inversely associated with episodic memory (β=−1.313; P=0.035), semantic memory (β=−1.451; P=0.008), and processing speed (β=−1.211; P=0.025) at initial assessment only among APOE ε4 carriers (24% of individuals). Carotid plaque presence and area were not significantly associated with any of the cognitive domains at the initial assessment, nor was there significant effect modification by APOE ε4 allele status (Table 2). When examined in quartiles, plaque area was also not significantly associated with baseline cognitive assessment (data not shown). There was no effect modification by age or race/ethnicity for the association of cIMT, carotid plaque presence, or area with any cognitive domain (data not shown). The results were similar when we restricted the analysis to those cognitively unimpaired at enrollment (data not shown).
Table 2.
Association of Carotid Atherosclerosis Markers and Baseline Cognitive Performance (n=1166)
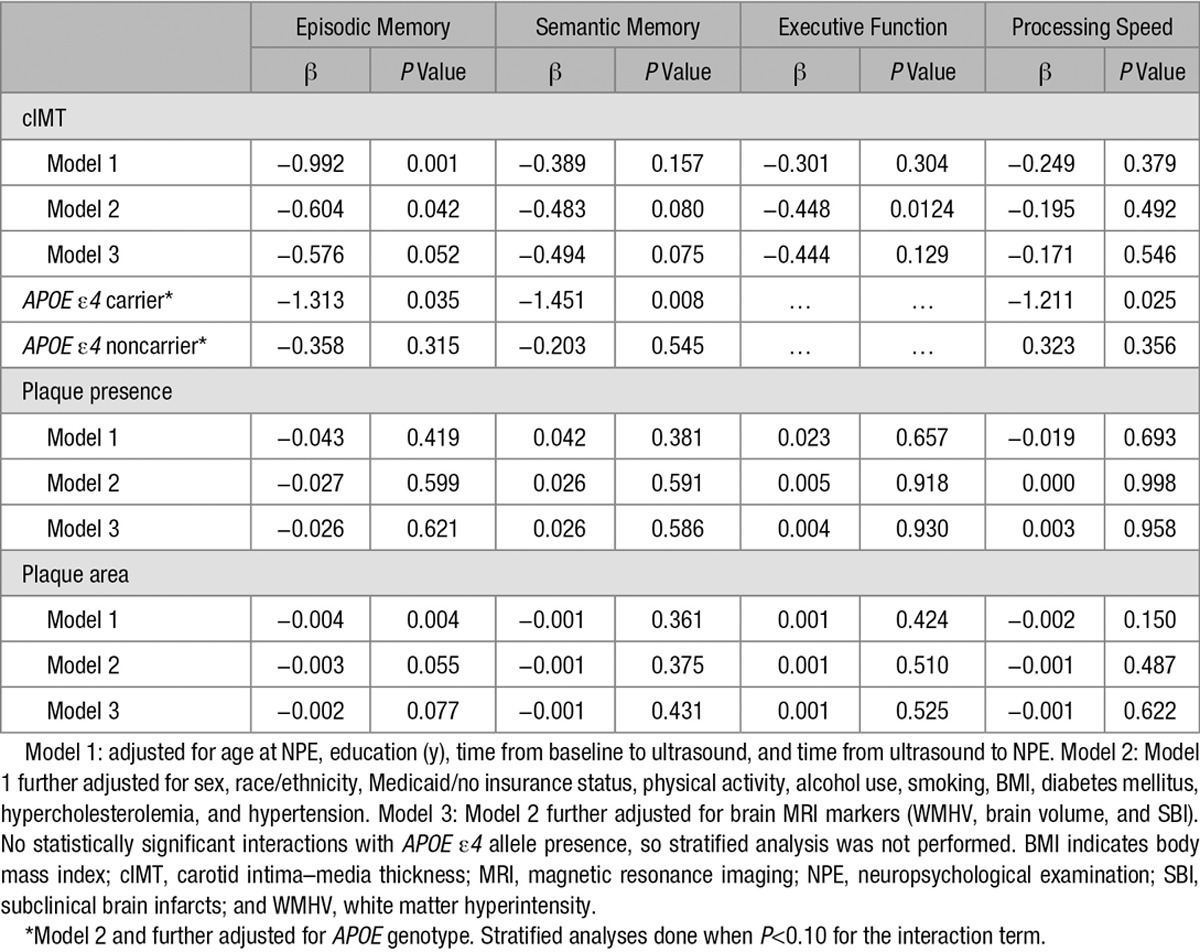
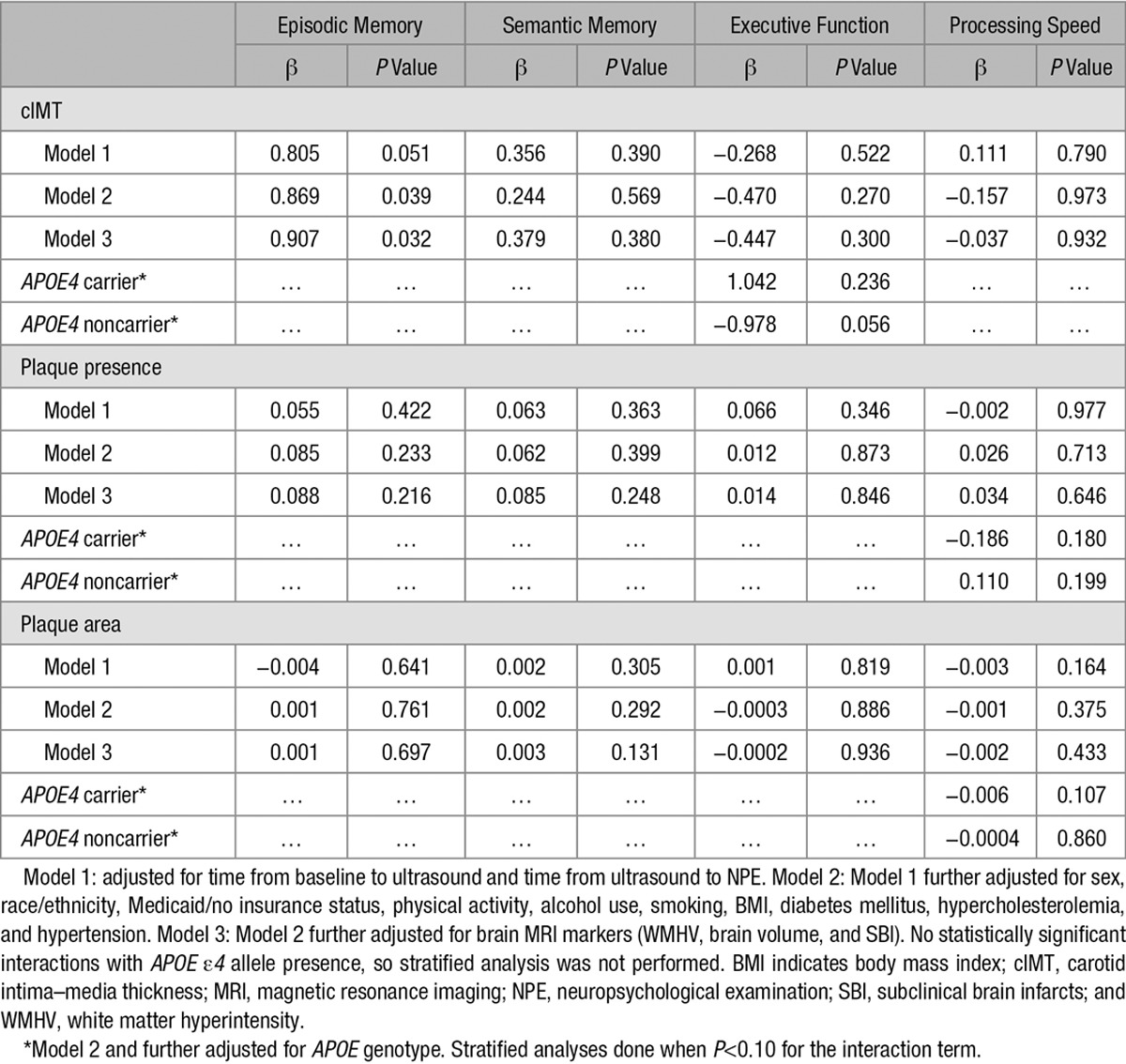
Table 3 shows the relationship between carotid atherosclerosis markers and change in cognitive performance over time (mean time between the initial and repeated NPE was 5 years). cIMT was positively associated with change in episodic memory, suggesting less decline in episodic memory with increased cIMT at baseline (model 3; β=0.907; P=0.032). APOE ε4 was a significant effect modifier of the association between cIMT and executive function performance (P=0.04). Only APOE ε4 noncarriers with greater cIMT had more decline in executive function, although this did not reach statistical significance (β=−0.978; P=0.056). Plaque presence and area were not associated with change in any of the cognitive domains (Table 3). There was no effect modification by age or race/ethnicity for any of the exposures (data not shown). There were no significant associations between quartiles of plaque area and cognitive decline (data not shown). The results were similar when we restricted the analysis to those cognitively unimpaired at enrollment (data not shown).
Table 3.
Association of Carotid Atherosclerosis Markers and Change in Cognitive Performance (n=836)
Discussion
In this longitudinal, racially and ethnically diverse, population-based study, subclinical carotid atherosclerosis was associated with worse episodic memory. APOE ε4 carriers with greater cIMT exhibited worse cognition across most domains, suggesting that subclinical vascular disease may exacerbate cognitive dysfunction in those who are at higher risk for Alzheimer disease. In contrast, carotid plaque presence and burden were not significant predictors of cognitive performance. None of the carotid atherosclerosis markers were significantly associated with cognitive decline after repeated NPE an average of 5 years apart, and greater cIMT was associated with less decline in episodic memory, contradicting our initial hypothesis. This may be explained by competing mortality or a healthy cohort effect in our study.
Several large cross-sectional studies have associated greater cIMT with worse cognition, but these studies examined general, and not domain-specific, cognition.29,30 Studies in middle-aged and nondemented older adults that examine domain-specific cognition show that elevated cIMT is associated with decline in executive function and processing speed, but not verbal memory.10,31 We found similar results in our longitudinal analysis for APOE ε4 noncarriers, consistent with the pathogenesis of vascular cognitive impairment.
Our cross-sectional results also demonstrate that episodic memory is the primary domain affected by subclinical carotid atherosclerosis, especially in APOE ε4 carriers, which is consistent with results from a large Brazilian cohort.32 Greater cIMT could result in increased arterial stiffness, causing impaired episodic memory because of chronic cerebral hypoperfusion. An animal model of carotid artery stenosis has demonstrated hippocampal atrophy and impaired performance in working memory tasks resulting from prolonged bilateral carotid artery stenosis.33 Our domain score for episodic memory is heavily weighted toward free recall, which also involves prefrontal demands similar to executive control. Therefore, although episodic memory was preferentially affected in our study, this finding may still represent the effect of large vessel disease on prefrontal processes that underlie executive function. In this context, our findings are consistent with previous studies. Additionally, we found an association between APOE ε4 allele presence and greater cIMT in the NOMAS (article in preparation), supporting the APOE ε4 allele as a possible mediator between cIMT and cognition. These data suggest that individuals in our cohort at risk for Alzheimer disease because of their APOE allele status may be more vulnerable to the deleterious effects of large vessel disease on cognitive function related to prefrontal processes.
Other studies have found significant associations between plaque presence, number, and total plaque area and cognition,34,35 but we did not. This may be because of acoustic shadow in the assessment of carotid ultrasounds affecting the accuracy of our plaque measurements. Also, those with carotid plaques may have received primary prevention treatment that attenuated the natural history of the disease and subsequently the effect of atherosclerosis on cognition. Plaque may not be an effective marker of cognitive performance in our cohort.
There were no statistically significant findings in our longitudinal analysis, except for episodic memory, which paradoxically exhibited less decline on average with greater cIMT. This may be explained by the healthy cohort effect because participants who were available for a second NPE are likely healthier than those who did not complete the second NPE. Competing risk of mortality is also a concern in this older cohort. Because our cohort was older at the initial NPE, pathology affecting cognition may have already progressed substantially; so a change in cognition may not be readily detected. Evidence suggests that midlife risk factor burden is more important for predicting cognitive performance than late-life risk factors.36 Therefore, our longitudinal analysis may not be sensitive to changes in cognition in this elderly cohort and should be interpreted with caution.
Strengths of the current study include its population-based design in a mostly Hispanic/Latino community, multiple time points of domain-specific cognitive assessments, and various measures of subclinical carotid atherosclerosis. The limitations of this study are important to note. The current study was conducted within a subcohort of NOMAS participants, and the findings may not generalize to the full study cohort, especially given the survival bias and competing risk of mortality that is common in longitudinal cohort study designs. Although our ultrasound protocol specifies the use of multiple angles of insonation to improve the accuracy of plaque area measurements, acoustic shadowing may still decrease the accuracy of these measurements. Longitudinal cognitive data were missing for 340 participants in this study, but the missingness of follow-up NPE was unrelated to cIMT and unrelated to APOE ε4 allele status, limiting the likelihood of selection bias for the analysis of cognitive decline.
Summary
Increased cIMT was associated with cognitive performance but not with cognitive decline. Further studies should explore the mediating effects of the APOE ε4 allele on vascular wall changes and cognition.
Sources of Funding
This study was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke (R01 NS 29993) and the Evelyn F. McKnight Brain Institute.
Disclosures
Dr Elkind receives compensation for providing consultative services for BioTelemetry/Cardionet, Bristol–Myers Squibb-Pfizer Partnership, Boehringer Ingelheim, Daiichi-Sankyo, Janssen Pharmaceuticals, and Sanofi-Regeneron Partnership; receives research support from diaDexus, Inc, Bristol–Myers Squibb/Sanofi Pharmaceuticals Partnership, and the National Institutes of Health/National Institute of Neurological Disorders and Stroke; has given expert legal opinions on behalf of Organon (NuvaRing and stroke litigation) and Hi-Tech; and serves on the National, Founders Affiliate, and New York City Chapter Boards of the American Heart Association/American Stroke Association. He receives royalties from UpToDate for chapters related to Stroke.
Dr Sacco receives federal grant support (R01 NS 29993), private foundation support (American Heart Association Bugher Center), and pharma research support (Boehringer Ingelheim) and Dr Wright receives royalties for 2 chapters on Vascular Dementia from UpToDate. The other authors report no conflicts.
Footnotes
H. Gardener and M.R. Caunca contributed equally.
Guest Editor for this article was Georgios Tsivgoulis, MD.
Presented in part at the International Stroke Conference, Los Angeles, CA, February 17–19, 2016, and the 141st American Neurological Association Annual Meeting, Baltimore, MD, October 16–18, 2016.
References
- 1.Rundek T, Arif H, Boden-Albala B, Elkind MS, Paik MC, Sacco RL. Carotid plaque, a subclinical precursor of vascular events: the Northern Manhattan Study. Neurology. 2008;70:1200–1207. doi: 10.1212/01.wnl.0000303969.63165.34. doi: 10.1212/01.wnl.0000303969.63165.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Oord SC, Sijbrands EJ, ten Kate GL, van Klaveren D, van Domburg RT, van der Steen AF, et al. Carotid intima-media thickness for cardiovascular risk assessment: systematic review and meta-analysis. Atherosclerosis. 2013;228:1–11. doi: 10.1016/j.atherosclerosis.2013.01.025. doi: 10.1016/j.atherosclerosis.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 3.Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7:1025–1038. doi: 10.1016/j.jcmg.2013.11.014. doi: 10.1016/j.jcmg.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Gardener H, Wright CB, Rundek T, Sacco RL. Brain health and shared risk factors for dementia and stroke. Nat Rev Neurol. 2015;11:651–657. doi: 10.1038/nrneurol.2015.195. doi: 10.1038/nrneurol.2015.195. [DOI] [PubMed] [Google Scholar]
- 5.Alzheimer’s Association. 2014 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2014;10:e47–e92.. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Moon JH, Lim S, Han JW, Kim KM, Choi SH, Park KS, et al. Carotid intima-media thickness is associated with the progression of cognitive impairment in older adults. Stroke. 2015;46:1024–1030. doi: 10.1161/STROKEAHA.114.008170. doi: 10.1161/STROKEAHA.114.008170. [DOI] [PubMed] [Google Scholar]
- 7.Wendell CR, Waldstein SR, Ferrucci L, O’Brien RJ, Strait JB, Zonderman AB. Carotid atherosclerosis and prospective risk of dementia. Stroke. 2012;43:3319–3324. doi: 10.1161/STROKEAHA.112.672527. doi: 10.1161/STROKEAHA.112.672527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buratti L, Balestrini S, Altamura C, Viticchi G, Falsetti L, Luzzi S, et al. Markers for the risk of progression from mild cognitive impairment to Alzheimer’s disease. J Alzheimers Dis. 2015;45:883–890. doi: 10.3233/JAD-143135. doi: 10.3233/JAD-143135. [DOI] [PubMed] [Google Scholar]
- 9.Zhong W, Cruickshanks KJ, Huang GH, Klein BE, Klein R, Nieto FJ, et al. Carotid atherosclerosis and cognitive function in midlife: the Beaver Dam Offspring Study. Atherosclerosis. 2011;219:330–333. doi: 10.1016/j.atherosclerosis.2011.07.013. doi: 10.1016/j.atherosclerosis.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al Hazzouri AZ, Vittinghoff E, Sidney S, Reis JP, Jacobs DR, Jr, Yaffe K. Intima-media thickness and cognitive function in stroke-free middle-aged adults: findings from the coronary artery risk development in young adults study. Stroke. 2015;46:2190–2196. doi: 10.1161/STROKEAHA.115.008994. doi: 10.1161/STROKEAHA.115.008994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–360.. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 12.Sacco RL, Gan R, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, et al. Leisure-time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. Stroke. 1998;29:380–387. doi: 10.1161/01.str.29.2.380. [DOI] [PubMed] [Google Scholar]
- 13.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 14.Rundek T, Elkind MS, Pittman J, Boden-Albala B, Martin S, Humphries SE, et al. Carotid intima-media thickness is associated with allelic variants of stromelysin-1, interleukin-6, and hepatic lipase genes: the Northern Manhattan Prospective Cohort Study. Stroke. 2002;33:1420–1423. doi: 10.1161/01.STR.0000015558.63492.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rundek T, Hundle R, Ratchford E, Ramas R, Sciacca R, Di Tullio MR, et al. Endothelial dysfunction is associated with carotid plaque: A cross-sectional study from the population based northern manhattan study. BMC Cardiovasc Disord. 2006;6:35. doi: 10.1186/1471-2261-6-35. doi: 10.1186/1471-2261-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo F, Gardener H, Dong C, Cabral D, Della-Morte D, Blanton SH, et al. Traditional cardiovascular risk factors explain the minority of the variability in carotid plaque. Stroke. 2012;43:1755–1760. doi: 10.1161/STROKEAHA.112.651059. doi: 10.1161/STROKEAHA.112.651059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siedlecki KL, Stern Y, Reuben A, Sacco RL, Elkind MS, Wright CB. Construct validity of cognitive reserve in a multiethnic cohort: the Northern Manhattan Study. J Int Neuropsychol Soc. 2009;15:558–569. doi: 10.1017/S1355617709090857. doi: 10.1017/S1355617709090857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siedlecki KL, Rundek T, Elkind MS, Sacco RL, Stern Y, Wright CB. Using contextual analyses to examine the meaning of neuropsychological variables across samples of english-speaking and spanish-speaking older adults. J Int Neuropsychol Soc. 2012;18:223–233. doi: 10.1017/S135561771100155X. doi: 10.1017/S135561771100155X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Elia LF, Satz P, Uchiyama CL, White T. Color Trails Test Professional Manual. Odessa, FL: Psychological Assessment Resources; 1996. [Google Scholar]
- 20.Flowers KA, Robertson C. The effect of Parkinson’s disease on the ability to maintain a mental set. J Neurol Neurosurg Psychiatry. 1985;48:517–529. doi: 10.1136/jnnp.48.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews CG, Klove H. Instruction Manual for the Adult Neuropsychology Test Battery. Madison, WI: University of Madison Medical School; 1964. [Google Scholar]
- 22.Beery KE. Developmental Test of Visual-Motor Integration (VMI) Manual. Chicago, IL: Follett; 1967. [Google Scholar]
- 23.Flanagan JL, Jackson ST. Test-retest reliability of three aphasia tests: performance of non-brain-damaged older adults. J Commun Disord. 1997;30:33–42. doi: 10.1016/s0021-9924(96)00039-1. quiz 42. [DOI] [PubMed] [Google Scholar]
- 24.Harrison JE, Buxton P, Husain M, Wise R. Short test of semantic and phonological fluency: normal performance, validity and test-retest reliability. Br J Clin Psychol. 2000;39(pt 2):181–191. doi: 10.1348/014466500163202. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Prabhakaran S, Wright CB, Yoshita M, Delapaz R, Brown T, DeCarli C, et al. Prevalence and determinants of subclinical brain infarction: the Northern Manhattan Study. Neurology. 2008;70:425–430. doi: 10.1212/01.wnl.0000277521.66947.e5. doi: 10.1212/01.wnl.0000277521.66947.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong C, Nabizadeh N, Caunca M, Cheung YK, Rundek T, Elkind MS, et al. Cognitive correlates of white matter lesion load and brain atrophy: the Northern Manhattan Study. Neurology. 2015;85:441–449. doi: 10.1212/WNL.0000000000001716. doi: 10.1212/WNL.0000000000001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardener H, Wright CB, Dong C, Cheung K, DeRosa J, Nannery M, et al. Ideal cardiovascular health and cognitive aging in the Northern Manhattan Study. J Am Heart Assoc. 2016;5:e002731. doi: 10.1161/JAHA.115.002731. doi: 10.1161/JAHA.115.002731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yue W, Wang A, Liang H, Hu F, Zhang Y, Deng M, et al. Association between carotid intima-media thickness and cognitive impairment in a chinese stroke population: a cross-sectional study. Sci Rep. 2016;6:19556. doi: 10.1038/srep19556. doi: 10.1038/srep19556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Z, Zhao Y, Wang X, Zhang H, Cui Y, Diao Y, et al. Low carotid artery wall shear stress is independently associated with brain white-matter hyperintensities and cognitive impairment in older patients. Atherosclerosis. 2016;247:78–86. doi: 10.1016/j.atherosclerosis.2016.02.003. doi: 10.1016/j.atherosclerosis.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Frazier DT, Seider T, Bettcher BM, Mack WJ, Jastrzab L, Chao L, et al. The role of carotid intima-media thickness in predicting longitudinal cognitive function in an older adult cohort. Cerebrovasc Dis. 2014;38:441–447. doi: 10.1159/000366469. doi: 10.1159/000366469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suemoto CK, Santos IS, Bittencourt MS, Pereira AC, Goulart AC, Rundek T, et al. Subclinical carotid artery atherosclerosis and performance on cognitive tests in middle-aged adults: baseline results from the ELSA-Brasil. Atherosclerosis. 2015;243:510–515. doi: 10.1016/j.atherosclerosis.2015.10.008. doi: 10.1016/j.atherosclerosis.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Nishio K, Ihara M, Yamasaki N, Kalaria RN, Maki T, Fujita Y, et al. A mouse model characterizing features of vascular dementia with hippocampal atrophy. Stroke. 2010;41:1278–1284. doi: 10.1161/STROKEAHA.110.581686. doi: 10.1161/STROKEAHA.110.581686. [DOI] [PubMed] [Google Scholar]
- 34.Arntzen KA, Schirmer H, Johnsen SH, Wilsgaard T, Mathiesen EB. Carotid atherosclerosis predicts lower cognitive test results: a 7-year follow-up study of 4,371 stroke-free subjects - the Tromsø Study. Cerebrovasc Dis. 2012;33:159–165. doi: 10.1159/000334182. doi: 10.1159/000334182. [DOI] [PubMed] [Google Scholar]
- 35.Arntzen KA, Schirmer H, Johnsen SH, Wilsgaard T, Mathiesen EB. Carotid artery plaque progression and cognitive decline: the Tromsø Study 1994-2008. Eur J Neurol. 2012;19:1318–1324. doi: 10.1111/j.1468-1331.2012.03728.x. doi: 10.1111/j.1468-1331.2012.03728.x. [DOI] [PubMed] [Google Scholar]
- 36.Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer R, et al. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014;129:1560–1567. doi: 10.1161/CIRCULATIONAHA.113.004798. doi: 10.1161/CIRCULATIONAHA.113.004798. [DOI] [PMC free article] [PubMed] [Google Scholar]


