Abstract
Study Objectives:
To estimate the association of restless legs syndrome (RLS) and its frequency with sleep-wake disturbances in pregnancy.
Methods:
A cohort of 1,563 women in their third trimester of pregnancy were recruited from prenatal clinics between March 2007 and December 2010. Demographic, pregnancy, and delivery data were extracted from medical records and sleep information was collected with questionnaires. To diagnose RLS, we used standardized criteria of RLS symptoms and frequency that were developed by the International Restless Legs Study Group. Logistic regression models were constructed to investigate the association of RLS and its frequency with sleep-wake disturbances (poor sleep quality, daytime sleepiness, poor daytime function) and delivery outcomes.
Results:
Overall 36% of the pregnant women had RLS, and half had moderate to severe symptoms. Compared to women without RLS, those with RLS were more likely to have poor sleep quality (odds ratio [OR] 2.2, 95% confidence interval [CI] 1.7–2.9), poor daytime function (OR 1.9, 95% CI 1.4–2.4), and excessive daytime sleepiness (OR 1.6, 95% CI 1.3–2.0). A dose-response relationship also was evident between RLS frequency and each of the sleep-wake disturbances. There was no evidence for any association between RLS and delivery outcomes.
Conclusions:
RLS is a significant contributor to poor sleep quality, daytime sleepiness, and poor daytime function, all common and often debilitating conditions in pregnancy. Obstetric health care providers should be aware of these associations and screen women for RLS.
Commentary:
A commentary on this article appears in this issue on page 857.
Citation:
Dunietz GL, Lisabeth LD, Shedden K, Shamim-Uzzaman A, Bullough AS, Chames MC, Bowden MF, O'Brien LM. Restless legs syndrome and sleep-wake disturbances in pregnancy. J Clin Sleep Med. 2017;13(7):863–870.
Keywords: delivery outcomes, excessive daytime sleepiness, poor daytime function, restless legs syndrome, sleep quality, sleep-wake disturbances, Willis-Ekbom disease
INTRODUCTION
Restless legs syndrome (RLS), also known as Willis-Ekbom disease, is a sensorimotor disorder composed of an urge to move, with or without associated discomfort that occurs with inactivity and improves with movement. It follows a circadian pattern, with a predilection for the evening and night.1 RLS is a common, underdiagnosed condition that affects up to 5% of the population worldwide,2–4 with up to 15% prevalence in Caucasians.5 The prevalence of RLS is positively correlated with age and body mass index (BMI) among both men and women.6,7 Although RLS has a genetic predisposition, it is also associated with other conditions such as end-stage renal disease, neuropathy, and pregnancy.8
RLS affects up to one-third of pregnant women9,10 peaks in the third trimester,9–11 and usually subsides after delivery.11 Multiparous women are affected up to three times more often than nulliparous women.10 The high prevalence of RLS during pregnancy has been attributed to hemodynamic and hormonal changes, iron and folate metabolism, and psychomotor behaviors.12 Although RLS is related to reduced quality of life and poor sleep in the general population13,14 data on RLS-associated maternal sleep-wake disturbances are lacking. Prior reports have linked sleep-wake disturbances to adverse pregnancy and delivery outcomes (eg, preterm delivery, prolonged labor, cesarean section deliveries, and postpartum depression).15–17 Therefore, the goal of this study was twofold: (1) to examine the frequency of RLS in a large, heterogeneous sample of pregnant women using standardized diagnostic criteria for RLS; and (2) to investigate the role of RLS in sleep-wake disturbances (ie, poor sleep quality, excessive daytime sleepiness, and poor daytime function) in addition to key delivery outcomes.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Restless legs syndrome (RLS) affects up to one-third of pregnant women, yet data on its associated maternal sleep-wake disturbances—which are predictors of adverse pregnancy outcomes—are scarce. This study examined the frequency of RLS and its association with sleep-wake disturbances in a large sample of pregnant women.
Study Impact: Results from this large cohort of pregnant women suggest that RLS is strongly associated with poor sleep quality, excessive daytime sleepiness, and poor daytime function, which are common symptoms frequently attributed to pregnancy. Screening and identification of RLS in pregnancy may alleviate the burden of symptoms in the majority of cases.
METHODS
Women in their third trimester of pregnancy were recruited from general and high-risk prenatal clinics at the University of Michigan between March 2007 and December 2010 to investigate the effect of sleep disturbance on adverse pregnancy outcomes. Informed consent was obtained from all study participants and the study was approved by the University of Michigan's Institutional Review Board.
All women reported whether or not they habitually snored, defined as “snoring at least 3 nights per week,” and completed several validated self-reported sleep questionnaires as detailed in the next paragraphs.
Questionnaires
Brief Restless Legs Scale
The Brief Restless Legs Scale18 is a 4-item questionnaire devised by the International Restless Legs Study Group (IRLSSG), a consensus workshop of international experts on RLS. This scale was used to diagnose and quantify the frequency of RLS. Four items were used as a minimum core for population-based epidemiologic studies. Participants were asked the following questions: (1) Do you have unpleasant sensations in your legs combined with the need to move your legs? (2) Do these feelings occur mainly or only at rest and do they improve with movement? (3) Are these feelings worse in the evening or night than in the morning? (4) How often do these feelings occur? A positive response to the first three questions indicated the presence of RLS. The fourth question, symptom frequency, provided an important indication of RLS frequency. Women were classified into 4 groups based on their reported frequency of RLS symptoms: < 1 time/mo, 1–4 times/mo, 2–3 times/wk, and ≥ 4 times/wk.
General Sleep Disturbance Scale
The General Sleep Disturbance Scale (GSDS)19 was used to determine poor sleep quality and poor daytime function. It is a 21-item instrument that assesses symptoms of sleep disturbance in the past week (ranging from 0 ‘‘not at all’’ to 7 ‘‘everyday’’). The GSDS contains several subscales, including a sleep quality subscale and a daytime function subscale, which were used in the current study. The sleep quality subscale includes 7 question items: difficulty getting to sleep, wake up during sleep period, wake up too early at the end of a sleep period, sleep poorly, satisfied with quality of sleep, get too little sleep, and fall asleep at an unscheduled time. Five question items constitute the daytime function subscale: feel sleepy during the day, struggle to stay awake during the day, feel irritable during the day, feel tired or fatigued during the day, and feel alert and energetic during the day. Subscale scores range between 0 and 7, with higher scores indicating greater frequency of sleep disturbance. A subscale score ≥ 3 indicates disturbed sleep on ≥ 3 nights during the past week. This threshold is used to distinguish good sleepers from poor sleepers and corresponds to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (1994) criteria for primary insomnia. Scores ≥ 3 on the respective subscales were used to distinguish those with poor sleep quality and those with poor daytime function. The GSDS has been used extensively in women20,21 and has been validated in pregnant women.21
Epworth Sleepiness Scale
The Epworth Sleepiness Scale22 was used to determine excessive daytime sleepiness. It is a validated, widely used, 1-page, 8-item questionnaire asking about the likelihood of dozing in variably sedentary situations such as driving a car or sitting and watching television. A score ≥ 10 on a scale that ranges from 0 to 24 suggests excessive sleepiness.
Demographic and Pregnancy Characteristics
Demographic and pregnancy characteristics were extracted from the medical records; these included maternal age, education, race, prepregnancy BMI, gestational weight gain, parity, smoking status, and key morbidities, such as hypertension and diabetes. Delivery outcomes were also collected, and included gestational age at delivery, cesarean section delivery, birth weight, and 1-minute and 5-minute Apgar scores.
Statistical Analysis
Descriptive statistics, chi-square test and linear regression, were used to compare maternal characteristics, pregnancy comorbidities, and sleep characteristics among women with or without reported RLS, and of RLS frequency by race. We used a Euler diagram to represent the prevalence of each sleep-related outcome and the relationships between them. Four separate multivariable logistic regression analyses were performed to investigate the association of RLS, modeled as the independent variable, and sleep-wake disturbances, defined as: (1) poor sleep quality; (2) poor daytime function; (3) excessive daytime sleepiness; and (4) the presence of at least one of these latter sleep-wake disturbances. Women with no reported sleep-wake disturbances served as the reference group. Next, we investigated whether RLS frequency was associated with each sleep-wake disturbance. Additional adjusted multivariable logistic regression models included race/ethnicity, hypertension, habitual snoring, parity, and prepregnancy BMI. We then tested for dose-response relationship of RLS frequency and each of the sleep-wake disturbances. Finally, we examined the association of RLS and key delivery and neonatal outcomes, including 1-minute and 5-minute Apgar score, cesarean section delivery, mean gestational age at delivery and mean birth weight centile. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, North Carolina, United States).
RESULTS
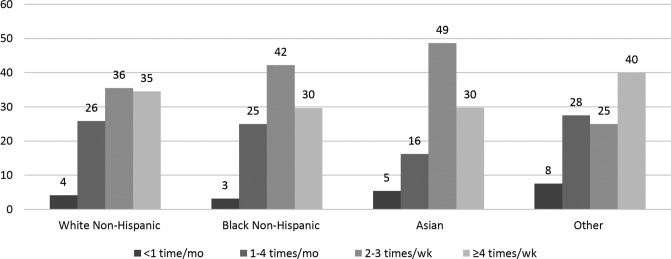
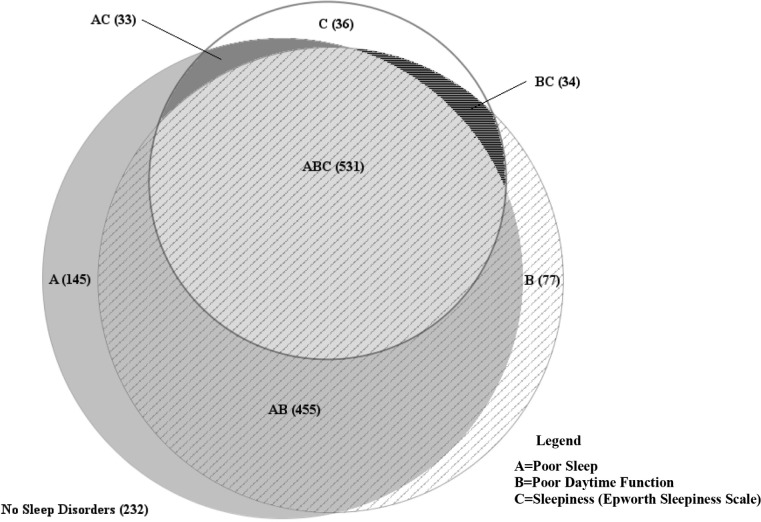
A total of 1,563 pregnant women in their third trimester were included in the sample, of whom 36% had RLS. Most of the pregnant women with RLS were white, parous women, with reported poor sleep quality and poor daytime function (Table 1). Prepregnancy BMI, habitual snoring and gestational comorbidities, hypertension, and diabetes were similar in women with and without RLS. One-fourth of the women with RLS reported the highest RLS frequency (ie, symptoms occurring ≥ 4 times/wk). For each race/ethnicity category, more than 65% of the pregnant women with RLS reported moderate to severe RLS; that is, RLS symptoms that occurred 2–3 times/wk or ≥ 4 times/wk (Figure 1). There was a substantial overlap of the three sleep-wake disturbances, such that a large proportion of women (34%) reported all three disturbances and only 14.8% women had none of the conditions (Figure 2).
Table 1.
Demographic, pregnancy and sleep characteristics of 1,563 pregnant women.

Figure 1. Distribution of RLS frequency by race among a cohort of 1,563 pregnant women.
Other = Hispanic, American-Indian, Native American, or multiracial. RLS = restless legs syndrome.
Figure 2. Frequencies of sleep-wake disturbances in a cohort of 1,563 pregnant women.
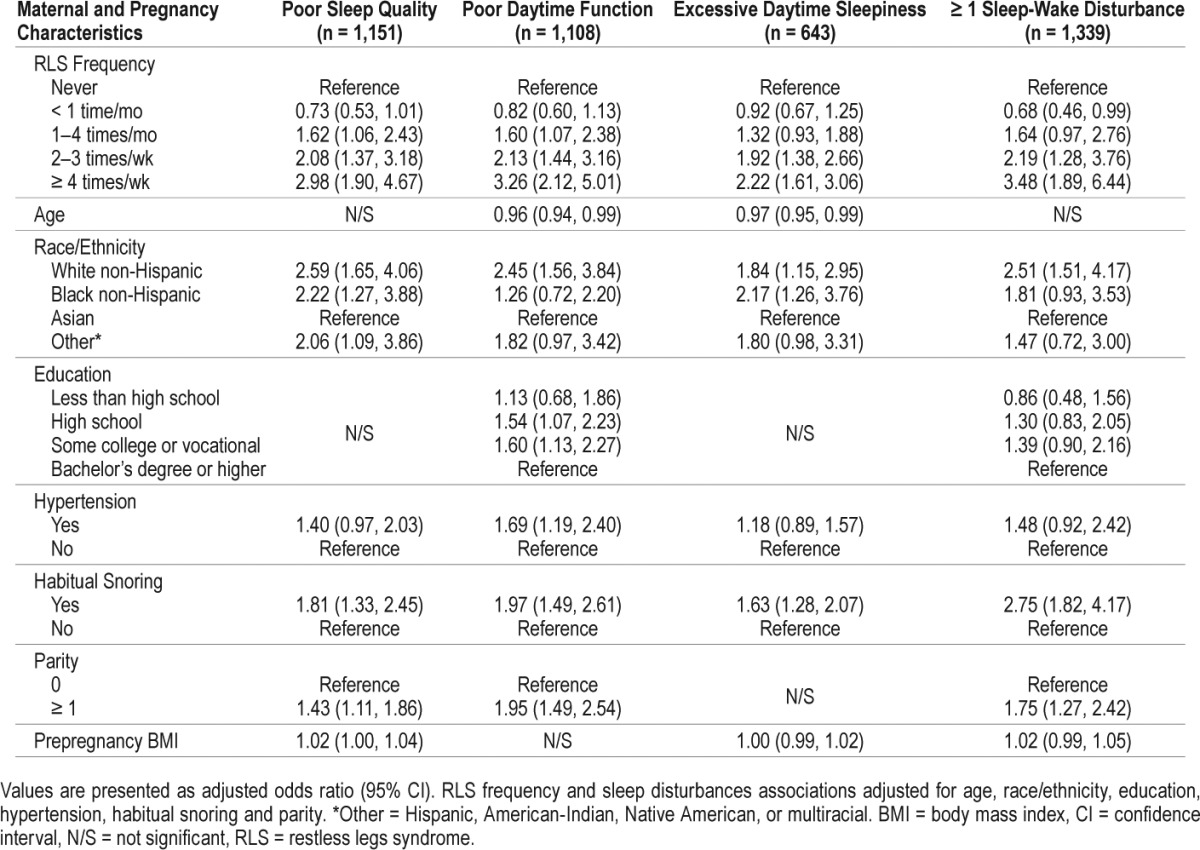
There was a significant association between RLS and poor sleep quality, poor daytime function, and excessive daytime sleepiness. Compared with pregnant women without RLS, those with RLS were more than twice as likely to have poor sleep quality, when controlled for race/ethnicity, hypertension, habitual snoring, parity, and prepregnancy BMI. Significantly increased odds for poor daytime function, excessive daytime sleepiness, or at least one of the three sleep-wake disturbances were also observed among women with RLS compared to those with no reported RLS. Habitual snoring, race/ethnicity, and parity were independently and significantly associated with each of the sleep-wake disturbances (Table 2).
Table 2.
Associations of RLS, poor sleep quality, poor daytime function and excessive daytime sleepiness in a cohort of 1,563 pregnant women.
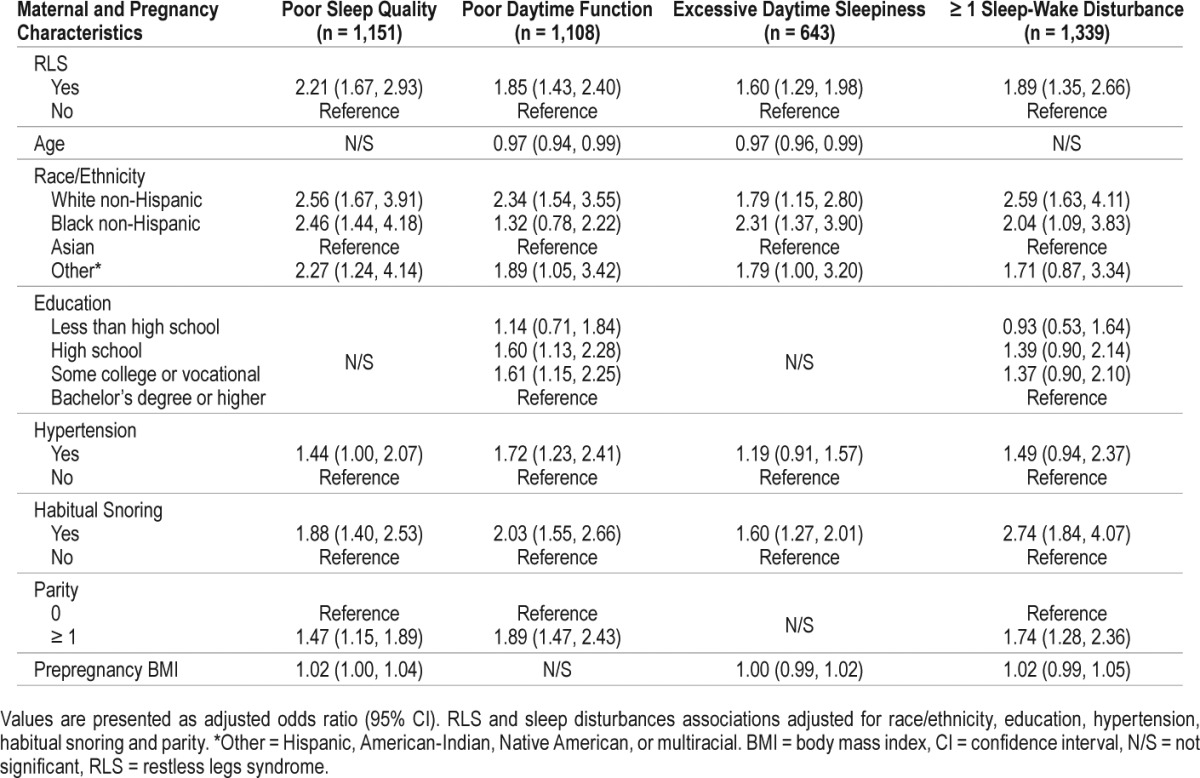
The associations of RLS frequency and sleep-wake disturbances represented dose-response relationships, that is, as RLS frequency increased, so did the odds for sleep-wake disturbances. For example, after controlling for pregnancy and maternal characteristics, compared to pregnant women without RLS, women who reported RLS 1–4 times/mo, 2–3 times/wk, or nearly every day, had increased odds of 1.62 (95% confidence interval [CI] 1.06–2.43), 2.08 (95% CI 1.37–3.18) and 2.98 (95% CI 1.90–4.67) for poor sleep quality. Similar dose-response effects were seen for poor daytime function and excessive daytime sleepiness (Table 3). This increase in odds ratios of each sleep-wake disturbance along RLS frequency was statistically significant.
Table 3.
Associations of RLS frequency, poor sleep quality, poor daytime function and excessive daytime sleepiness in a cohort of 1,563 pregnant women.
There were no associations between RLS status and several key delivery outcomes, such as cesarean section delivery (39% versus 35%, P = .2), mean birth weight centiles (51st versus 50th, P = .4), mean gestational age at birth (38.9 weeks versus 39.0 weeks, P = .4) and 1-minute (7.6 versus 7.5, P = .4) or 5-minute mean Apgar scores (8.7 versus 8.7, P = .3). These results remained nonsignificant among all RLS frequency groups.
DISCUSSION
In a large cohort we have demonstrated that 36% of third-trimester pregnant women have RLS as diagnosed by standard IRLSSG criteria and that approximately half of these women have moderate to severe RLS symptoms. Of note, significant dose-response relationships were found between RLS frequency and poor sleep quality, poor daytime function, and excessive daytime sleepiness, all common complaints in pregnancy that are typically attributed to normal physiological changes during gestation. We found no evidence for associations between RLS or its frequency and delivery outcomes.
Compared with studies that used the same diagnostic tool, our estimated RLS prevalence was similar to reported prevalence among European populations9,11,23–28 but higher than Asian populations29–32; this is consistent with the lower prevalence of RLS generally found in Asian populations compared to Caucasian populations. In a cohort of 300 pregnant European women, 60% reported symptoms either daily or 16–25 times/mo.9 In contrast, our findings are more modest, with severe RLS (≥ 4 times/wk) occurring in one-fourth of pregnant women. The wide range of RLS prevalence and frequency may be attributed to differences among study populations—particularly with regard to genetic background, distinct gestational age at enrollment, and small sample sizes.
Several studies have found associations between RLS and sleep-wake disturbances during pregnancy but few have investigated frequency of RLS. In one Swedish study, 30% of pregnant women with RLS reported impaired sleep quality and more than 40% experienced daytime sleepiness or fatigue.33 In another Swedish population, RLS was linked to daytime fatigue and excessive daytime sleepiness in the third trimester.28 Among a Chinese cohort, RLS was associated with a high frequency of poor sleep (82%) and excessive daytime sleepiness (40%).32 Other Asian studies have found an association between RLS and excessive daytime sleepiness,30,31,34 one of which reported a dose-response relationship with RLS frequency (1–6 times/wk and ≥ 7 times/wk).31 However, not all studies have found an association of RLS and daytime sleepiness.35
The association of RLS and pregnancy disorders such as gestational hypertension, preeclampsia, and gestational diabetes is controversial. In this study we showed similar frequencies of hypertension and diabetes in both the RLS and control groups. Null associations between RLS and hypertension/diabetes have been reported previously.29,33 In a large sample of almost 4,000 pregnant Chinese women, those with RLS had a higher frequency of pregnancy-induced hypertension, preeclampsia, and cardiovascular disease,31,32 but similar frequency of diabetes.31 RLS has also been associated with preeclampsia.36 In a group of American women, a history of pregnancy-induced hypertension had a positive association with RLS (odds ratio [OR] 1.9, 95% CI 1.1–3.6) that increased with RLS frequency (OR 3.8, 95% CI 1.9–7.6).37 However, these studies are association studies and none investigated the temporal link with hypertension or diabetes. Nonetheless, in nonpregnant adults, metabolic and autonomic factors have been suggested to play an important role in the development of RLS,38–40 which supports a possible etiologic relationship.
Data on the associations of RLS and key delivery outcomes are sparse and, to our knowledge, we report the first study to utilize the IRLSSG scale in pregnant women in the United States. Similar to the current study, several previous—and notably smaller—studies from other countries that have included birth outcomes such as cesarean section delivery, preterm birth, gestational age, Apgar score, and birth weight, have also failed to find associations with RLS.9,41–43 However, in a study of 1,000 American women in the immediate postpartum period using a surrogate RLS question about jerky leg movements during pregnancy,44 having such movements “always” was associated with an increased incidence of preterm birth and a lower birth weight. In addition, in a New Zealand cohort of over 600 women between 35–37 weeks gestation, pregnancy-onset leg twitching was associated with a threefold higher odds of fetal distress, defined as a diagnostic code in the medical record related to maternal hypoxia, fetal heart rate concerns, or evidence of fetal stress.45 Whether the leg movements in the latter studies truly reflect RLS, or are manifestations of other sleep disorders, is unclear. As far as we are aware, no current study using standard RLS criteria has found positive associations between RLS and delivery outcomes.
A major strength of this study is that it is the largest study, thus far, to investigate RLS in a United States population of pregnant women utilizing standard diagnostic criteria. The detailed information collected on RLS and other sleep problems allowed a comprehensive investigation of the presence and frequency of RLS and its association with sleep-wake disturbances and delivery outcomes in a heterogeneous sample of pregnant women. However, this study has several limitations. Of the three validated questionnaires used, only the GSDS has been validated in pregnancy, but all questionnaires are used in clinical obstetric care. Although key morbidities such as BMI and diabetes were adjusted for, other disorders that could mimic symptoms, such as peripheral vascular disease and neuropathy not related to diabetes, were not excluded. However, these conditions are rare in pregnant population, and were reported by one woman with diabetes in this cohort, thus unlikely to confound our results.
In addition, we did not collect data on family history of RLS and RLS was only measured once in the third trimester such that development across pregnancy could not be determined. Nonetheless, RLS in pregnancy is often a transient condition that typically resolves at delivery11 and has a low prevalence, estimated at 6% among North American women,46 suggesting that most of our cohort was composed of incident cases.
In light of the high prevalence of severe RLS during pregnancy and its association with common sleep-wake disturbances often characteristic of pregnancy, obstetric health care providers should be aware of RLS and ideally screen for its presence. Given the burden of RLS on nocturnal and daytime complaints, symptom management is important in the setting of pregnancy. Nonpharmacological treatment is preferable during pregnancy for mild to moderate symptoms. Recommended approaches typically include moderate exercise, elastic stockings, or pneumatic compression devices, and avoidance of caffeinated and alcoholic beverages. Pharmacological treatment approaches during pregnancy are discouraged due to lack of data regarding safety and efficacy. However, there may be benefit in treating women with persistent, severe RLS symptoms with oral iron supplementation; women could benefit from ferritin is < 75 mcg/L and are likely to benefit from ferritin is < 30 mcg/L.47 If symptoms persist, and the maternal benefit outweighs any risks to the fetus, other treatment options may include dopaminergic drugs, benzodiazepines, opioids, or anti-epileptic agents.12,48 A recent consensus guideline provides further recommendations for the treatment of RLS in pregnancy.47
In summary, RLS is common in pregnant women and our findings demonstrate a significant role for RLS in poor sleep quality, excessive daytime sleepiness, and poor daytime function, which are common symptoms frequently attributed to pregnancy. Obstetric health care providers should be aware that RLS may underlie these often dismissed complaints.
DISCLOSURE STATEMENT
This work was performed at the University of Michigan's Medical School. Financial disclosure: GLD, LDL, KS, QAS, ASB, MCC, MFB and LMO have no financial relationships relevant to this study to disclose. Funding source: This work was partially supported by a T32 Grant from the National Institute of Neurological Disorders and Stroke (NIH/NINDS T32 NS007222) and by the Gene and Tubie Gilmore Fund for Sleep Research, the University of Michigan Institute for Clinical and Health Research (MICHR) grant UL1TR000433, MICHR seed pilot grant F021024, and the National Heart, Lung, and Blood Institute (R21 HL089918). During the course of this study Dr. O'Brien was also supported by a career grant from the National Heart, Lung, and Blood Institute (K23 HL095739) and in part by R21 HL087819. Conflicts of interest: None of the authors has a financial conflict of interest with the work presented.
ACKNOWLEDGMENTS
The authors are grateful to the women who participated in this study. We thank Ms. Carol Shannon, Associate Librarian, for her assistance.
ABBREVIATIONS
- BMI
body mass index
- CI
confidence interval
- GSDS
General Sleep Disturbance Scale
- IRLSSG
International Restless Legs Study Group
- OR
odds ratio
- RLS
restless legs syndrome
REFERENCES
- 1.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 2.Allen RP, Bharmal M, Calloway M. Prevalence and disease burden of primary restless legs syndrome: results of a general population survey in the United States. Mov Disord. 2011;26(1):114–120. doi: 10.1002/mds.23430. [DOI] [PubMed] [Google Scholar]
- 3.Hening WA. Restless legs syndrome: the most common and least diagnosed sleep disorder. Sleep Med. 2004;5(5):429–430. doi: 10.1016/j.sleep.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Ohayon MM, O'Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16(4):283–295. doi: 10.1016/j.smrv.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavigne G, Montplaisir J. Restless legs syndrome and sleep bruxism: prevalence and association among Canadians. Sleep. 1994;17(8):739–743. [PubMed] [Google Scholar]
- 6.Bjorvatn B, Leissner L, Ulfberg J, et al. Prevalence, severity and risk factors of restless legs syndrome in the general adult population in two Scandinavian countries. Sleep Med. 2005;6(4):307–312. doi: 10.1016/j.sleep.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Phillips B, Young T, Finn L, Asher K, Hening WA, Purvis C. Epidemiology of restless legs symptoms in adults. Arch Intern Med. 2000;160(14):2137–2141. doi: 10.1001/archinte.160.14.2137. [DOI] [PubMed] [Google Scholar]
- 8.Manconi M, Govoni V, De Vito A, et al. Pregnancy as a risk factor for restless legs syndrome. Sleep Med. 2004;5(3):305–308. doi: 10.1016/j.sleep.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Minár M, Habánová H, Rusňák I, Planck K, Valkovič P. Prevalence and impact of restless legs syndrome in pregnancy. Neuro Endocrinol Lett. 2013;34(5):366–371. [PubMed] [Google Scholar]
- 10.Berger K, Luedemann J, Trenkwalder C, John U, Kessler C. Sex and the risk of restless legs syndrome in the general population. Arch Intern Med. 2004;164(2):196–202. doi: 10.1001/archinte.164.2.196. [DOI] [PubMed] [Google Scholar]
- 11.Manconi M, Govoni V, De Vito A, et al. Restless legs syndrome and pregnancy. Neurology. 2004;63(6):1065–1069. doi: 10.1212/01.wnl.0000138427.83574.a6. [DOI] [PubMed] [Google Scholar]
- 12.Gupta R, Dhyani M, Kendzerska T, et al. Restless legs syndrome and pregnancy: prevalence, possible pathophysiological mechanisms and treatment. Acta Neurol Scand. 2016;133(5):320–329. doi: 10.1111/ane.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abetz L, Allen R, Follet A, et al. Evaluating the quality of life of patients with restless legs syndrome. Clin Ther. 2004;26(6):925–935. doi: 10.1016/s0149-2918(04)90136-1. [DOI] [PubMed] [Google Scholar]
- 14.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165(11):1286–1292. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 15.Lee KA, Gay CL. Sleep in late pregnancy predicts length of labor and type of delivery. Am J Obstet Gynecol. 2004;191(6):2041–2046. doi: 10.1016/j.ajog.2004.05.086. [DOI] [PubMed] [Google Scholar]
- 16.Dolatian M, Mehraban Z, Sadeghniat K. The effect of impaired sleep on preterm labour. West Indian Med J. 2014;63(1):62. doi: 10.7727/wimj.2012.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang JJ, Pien GW, Duntley SP, Macones GA. Sleep deprivation during pregnancy and maternal and fetal outcomes: is there a relationship? Sleep Med Rev. 2010;14(2):107–114. doi: 10.1016/j.smrv.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology: a report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 19.Lee KA. Self-reported sleep disturbances in employed women. Sleep. 1992;15(6):493–498. doi: 10.1093/sleep/15.6.493. [DOI] [PubMed] [Google Scholar]
- 20.Gay CL, Lee KA, Lee S-Y. Sleep patterns and fatigue in new mothers and fathers. Biol Res Nursing. 2004;5(4):311–318. doi: 10.1177/1099800403262142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee KA, DeJoseph JF. Sleep disturbances, vitality, and fatigue among a select group of employed childbearing women. Birth. 1992;19(4):208–213. doi: 10.1111/j.1523-536x.1992.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 22.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea: the Epworth Sleepiness Scale. Chest. 1993;103(1):30–36. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 23.Neau J-P, Marion P, Mathis S, et al. Restless legs syndrome and pregnancy: follow-up of pregnant women before and after delivery. Eur Neurol. 2010;64(6):361–366. doi: 10.1159/000322124. [DOI] [PubMed] [Google Scholar]
- 24.Uglane MT, Westad S, Backe B. Restless legs syndrome in pregnancy is a frequent disorder with a good prognosis. Acta Obstet Gynecol Scand. 2011;90(9):1046–1048. doi: 10.1111/j.1600-0412.2011.01157.x. [DOI] [PubMed] [Google Scholar]
- 25.Sarberg M, Bladh M, Svanborg E, Josefsson A. Postpartum depressive symptoms and its association to daytime sleepiness and restless legs during pregnancy. BMC Pregnancy Childbirth. 2016;16(1):137. doi: 10.1186/s12884-016-0917-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neau J-P, Porcheron A, Mathis S, et al. Restless legs syndrome and pregnancy: a questionnaire study in the Poitiers District, France. Eur Neurol. 2010;64(5):268–274. doi: 10.1159/000321413. [DOI] [PubMed] [Google Scholar]
- 27.Tunç T, Karadağ YS, Doğulu F, İnan LE. Predisposing factors of restless legs syndrome in pregnancy. Mov Disord. 2007;22(5):627–631. doi: 10.1002/mds.21291. [DOI] [PubMed] [Google Scholar]
- 28.Sarberg M, Josefsson A, Wiréhn AB, Svanborg E. Restless legs syndrome during and after pregnancy and its relation to snoring. Acta Obstet Gynecol Scand. 2012;91(7):850–855. doi: 10.1111/j.1600-0412.2012.01404.x. [DOI] [PubMed] [Google Scholar]
- 29.Chen PH, Liou KC, Chen CP, Cheng SJ. Risk factors and prevalence rate of restless legs syndrome among pregnant women in Taiwan. Sleep Med. 2012;13(9):1153–1157. doi: 10.1016/j.sleep.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Harano S, Ohida T, Kaneita Y, et al. Prevalence of restless legs syndrome with pregnancy and the relationship with sleep disorders in the Japanese large population. Sleep Biol Rhythms. 2008;6(2):102–109. [Google Scholar]
- 31.Liu G, Li L, Zhang J, et al. Restless legs syndrome and pregnancy or delivery complications in China: a representative survey. Sleep Med. 2016;17:158–162. doi: 10.1016/j.sleep.2015.02.541. [DOI] [PubMed] [Google Scholar]
- 32.Ma S, Shang X, Guo Y, Liu G, Yang J, Xue R. Restless legs syndrome and hypertension in Chinese pregnant women. Neurol Sci. 2015;36(6):877–881. doi: 10.1007/s10072-015-2094-4. [DOI] [PubMed] [Google Scholar]
- 33.Wesström J, Nilsson S, Sundström-Poromaa I, Ulfberg J. Restless legs syndrome among women: prevalence, co-morbidity and possible relationship to menopause. Climacteric. 2008;11(5):422–428. doi: 10.1080/13697130802359683. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki K, Ohida T, Sone T, et al. The prevalence of restless legs syndrome among pregnant women in Japan and the relationship between restless legs syndrome and sleep problems. Sleep. 2003;26(6):673–677. doi: 10.1093/sleep/26.6.673. [DOI] [PubMed] [Google Scholar]
- 35.Ismailogullari S, Ozturk A, Mazicioglu MM, Serin S, Gultekin M, Aksu M. Restless legs syndrome and pregnancy in Kayseri, Turkey: a hospital based survey. Sleep Biol Rhythms. 2010;8(2):137–143. [Google Scholar]
- 36.Ramirez J, Cabrera S, Hidalgo H, et al. Is preeclampsia associated with restless legs syndrome? Sleep Med. 2013;14(9):894–896. doi: 10.1016/j.sleep.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Innes KE, Kandati S, Flack KL, Agarwal P, Selfe TK. The relationship of restless legs syndrome to history of pregnancy-induced hypertension. J Women's Health (Larchmt) 2016;25(4):397–408. doi: 10.1089/jwh.2015.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Innes KE, Selfe TK, Agarwal P. Restless legs syndrome and conditions associated with metabolic dysregulation, sympathoadrenal dysfunction, and cardiovascular disease risk: a systematic review. Sleep Med Rev. 2012;16(4):309–339. doi: 10.1016/j.smrv.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Szentkirályi A, Völzke H, Hoffmann W, Happe S, Berger K. A time sequence analysis of the relationship between cardiovascular risk factors, vascular diseases and restless legs syndrome in the general population. J Sleep Res. 2013;22(4):434–442. doi: 10.1111/jsr.12040. [DOI] [PubMed] [Google Scholar]
- 40.Szentkirályi A, Völzke H, Hoffmann W, Trenkwalder C, Berger K. Multimorbidity and the risk of restless legs syndrome in 2 prospective cohort studies. Neurology. 2014;82(22):2026–2033. doi: 10.1212/WNL.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 41.Meharaban Z, Yahya S, Sadegniiat K. Restless legs syndrome during pregnancy and preterm birth in women referred to health centers of Ardabil. Iranian Red Crescent Med J. 2015;17(12):e24438. doi: 10.5812/ircmj.24438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma S, Nehra A, Sinha S, et al. Sleep disorders in pregnancy and their association with pregnancy outcomes: a prospective observational study. Sleep Breath. 2016;20(1):87–93. doi: 10.1007/s11325-015-1188-9. [DOI] [PubMed] [Google Scholar]
- 43.Vahdat M, Sariri E, Miri S, et al. Prevalence and associated features of restless legs syndrome in a population of Iranian women during pregnancy. Int J Gyanecol Obstet. 2013;123(1):46–49. doi: 10.1016/j.ijgo.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 44.Oyieng'o DO, Kirwa K, Tong I, Martin S, Rojas-Suarez JA, Bourjeily G. Restless legs symptoms and pregnancy and neonatal outcomes. Clin Ther. 2016;38(2):256–264. doi: 10.1016/j.clinthera.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howe LD, Signal TL, Paine S-J, et al. Self-reported sleep in late pregnancy in relation to birth size and fetal distress: the E Moe, Māmā prospective cohort study. BMJ Open. 2015;5(10):e008910. doi: 10.1136/bmjopen-2015-008910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohayon MM, O'Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16(4):283–295. doi: 10.1016/j.smrv.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Picchietti DL, Hensley JG, Bainbridge JL, et al. Consensus clinical practice guidelines for the diagnosis and treatment of restless legs syndrome/ Willis-Ekbom disease during pregnancy and lactation. Sleep Med Rev. 2015;22:64–77. doi: 10.1016/j.smrv.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 48.Srivanitchapoom P, Pandey S, Hallett M. Restless legs syndrome and pregnancy: a review. Parkinsonism Relat Disord. 2014;20(7):716–722. doi: 10.1016/j.parkreldis.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]