Abstract
Study Objectives:
To assess the benefit and tolerance of autotitrating positive airway pressure (APAP) versus continuous positive airway pressure (CPAP) in subjects who experience aerophagia.
Methods:
This is the report of a prospective, two-week, double-blinded, randomized crossover trial set in an Australian clinical sleep laboratory in a tertiary hospital. Fifty-six subjects who reported symptoms of aerophagia that they attributed to CPAP were recruited. Full face masks were used by 39 of the 56 subjects recruited. Subjects were randomly and blindly allocated to either CPAP at their treatment recommended pressure or APAP 6–20 cm H2O, in random order. Subjects spent two weeks on each therapy mode. Therapy usage hours, 95th centile pressure, maximum pressure, 95th centile leak, and residual apnea-hypopnea index (AHI) were reported at the end of each two-week treatment period. Functional Outcome of Sleepiness Questionnaire, Epworth Sleepiness Scale, and visual analog scale to measure symptoms of aerophagia were also completed at the end of each 2-week treatment arm.
Results:
The median pressure (P < .001) and 95th centile pressure (P < .001) were reduced with APAP but no differences in compliance (P = .120) and residual AHI were observed. APAP reduced the symptoms of bloating (P = .011), worst episode of bloating (P = .040), flatulence (P = .010), and belching (P = .001) compared to CPAP. There were no differences in Epworth Sleepiness Scale or Functional Outcome of Sleepiness Questionnaire outcomes between CPAP and APAP.
Conclusions:
APAP therapy reduces the symptoms of aerophagia while not affecting compliance when compared with CPAP therapy.
Clinical Trial Registration:
Australian and New Zealand Clinical Trials Registry at https://www.anzctr.org.au, trial number ACTRN12611001250921.
Commentary:
A commentary on this article appears in this issue on page 859.
Citation:
Shirlaw T, Hanssen K, Duce B, Hukins C. A randomized crossover trial comparing autotitrating and continuous positive airway pressure in subjects with symptoms of aerophagia: effects on compliance and subjective symptoms. J Clin Sleep Med. 2017;13(7):881–888.
Keywords: aerophagia, autotitrating positive airway pressure (APAP), continuous positive airway pressure (CPAP), CPAP side effects, sleep apnea therapy
INTRODUCTION
Obstructive sleep apnea (OSA) is a prevalent disorder that is associated with daytime sleepiness,1 reduced quality of life,2 increased risk of motor vehicle accidents,3 and cardiovascular disease.4 The treatment of OSA is predominantly through continuous positive airway pressure (CPAP), which delivers pressurized air to splint the upper airway open and increase end-expiratory lung volume.5 CPAP has been demonstrated to reduce the apnea-hypopnea index (AHI), improve objective and subjective sleepiness, and improve quality of life.6
Despite the obvious efficacy of CPAP, uptake and compliance with this therapy is still an ongoing problem. Reports of long-term CPAP compliance vary between 40% to 80%,7,8 although some of this variation can be attributed to different definitions of compliance. This has led many clinicians to examine the side effects associated with CPAP as a contributor to poor compliance rates. Very early on, it was recognized that a significant proportion of patients experienced side effects with CPAP.9 These side effects experienced by patients include claustrophobia, nasal dryness, pressure intolerance, and interface leak (with sleep fragmentation arising from this phenomenon).10 Another reported side effect of CPAP therapy is aerophagia or bloating.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Aerophagia is a known side effect of continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea. Currently there is no evidence for the use of autotitrating positive airway pressure (APAP) in reducing the symptoms of aerophagia in affected patients.
Study Impact: This study showed that using APAP reduces the symptoms of aerophagia but does not increase compliance with therapy. APAP is a viable alternative to CPAP in patients experiencing aerophagia.
Aerophagia appears to occur when some of the pressurized air is swallowed and collects in the gastrointestinal system. This air causes abdominal discomfort and reports of excessive belching and/or flatulence. In some cases, the aerophagia can be severe enough for the patient to discontinue CPAP therapy. Aerophagia in CPAP patients has a prevalence of 16%,9 and has been reported in infants as well as adults.11 It may also be associated with gastroesophageal reflux disease.12 Current clinical management of aerophagia in CPAP therapy is largely anecdotal and includes empiric reductions in therapeutic pressure or the use of autotitrating positive airway pressure (APAP) therapy. Even if reduction of therapeutic pressure is successful in relieving the symptoms of aerophagia in some patients, these pressure reductions can also leave the patient under-treated with recurrence of OSA symptoms. Clinicians have therefore utilized APAP in order to effectively treat the OSA while lowering the mean pressure across the night.13 There is, however, no published evidence to demonstrate that APAP is effective in adequately treating the OSA while reducing the symptoms of aerophagia.
In this study, we examine the effectiveness of APAP in CPAP patients who report aerophagia symptoms. Measures to determine the effectiveness of APAP in this cohort included objective therapy compliance, aerophagia symptoms, and sleep-related quality of life.
METHODS
Study Design and Sample Size Determination
This was a double-blind, randomized, crossover study of APAP versus CPAP (Autoset Spirit S8, ResMed Ltd., Sydney, Australia) with no washout period. The study took place at the Sleep Disorders Centre, an Australian accredited clinical sleep laboratory in a tertiary hospital. Data collection occurred at baseline, and after two weeks of each trial arm. Our primary outcome measure in this study was compliance with positive airway pressure (PAP) therapy. We determined that a 30-minute difference in average nightly therapy was clinically significant. Based on average CPAP compliance recorded within the previous 12 months, a priori power calculations indicated that a sample size of 56 subjects was required to detect a 30-minute difference in nightly objective usage with a power of 0.8 (α = 0.05). Secondary outcomes measures were residual AHI, device pressure and leak, Epworth Sleepiness Scale (ESS), Functional Outcomes of Sleep Questionnaire (FOSQ), and an in-house visual analog scale (VAS) of aerophagia symptoms questionnaire. The aerophagia symptoms questionnaire was developed with input from the Princess Alexandra Hospital Gastroenterology Department and is available from the corresponding author.
Subject Selection
Subjects were recruited between 2009 and 2013. Subjects who reported symptoms of aerophagia attributed to CPAP use (during medical review) were invited to participate. Aerophagia was confirmed by responding positively to the following question: Do you experience any bloating, excessive flatulence, excessive belching or abdominal pain or discomfort, due to CPAP use? Subjects were excluded if they were younger than 18 years, had an intellectual impairment, had comorbid central sleep apnea (central apnea index ≥ 5 events/h), regularly used sedatives or narcotics, had preexisting lung or psychiatric disease, or were unable to attend the protocol appointments. The study protocol was approved by the Institutional Human Research Ethics Committee (HREC/09/QPAH/088) and written consent was obtained from all subjects. The study is registered in the Australian and New Zealand Clinical Trials Registry at https://www.anzctr.org.au, trial number ACTRN12611001250921.
Study Protocol
All outcomes were assessed on three occasions, at baseline prior to randomization and then at the end of each of the two-week treatment arms. Subjects were randomized by a clinical staff member not involved in data collection for this study (KH) using the technique of shuffled sealed envelopes containing equal numbers of each treatment arm. An Autoset Spirit S8 was then programmed for subject use and used for both treatment arms. The APAP device was programmed by clinical staff not involved in the patient recruitment and data collection (KH, BD). The device display screen was then covered to discourage discovery of therapeutic mode. The clinical staff member (TS) who collected study data was also blinded to the therapeutic mode.
APAP therapy was delivered using a minimum pressure of 6 cm H2O and a maximum pressure of 20 cm H2O according to our standard laboratory protocol. CPAP therapy was delivered according to their sleep physician-recommended setting that was determined during a Type 1 CPAP titration study. Subjects were asked to use the same interface throughout the study, and remain either on or off humidification use for the duration of the study. Subjects were provided with a diary and asked to make a daily record of the incidence of belching and flatulence as well as consumption of aerated drinks, the time between eating and retiring to bed and time of sleep onset. The subjects were also asked to measure their abdominal girth upon waking and to rate their abdominal discomfort on waking between 0 (none) and 10 (severe). Education about completing the diary was provided both verbally and in written form.
After the first two weeks of either fixed pressure CPAP at their recommended setting or autotitrating PAP with a window of 6 to 20 cm H2O, subjects were reassessed in clinic. At this visit, PAP usage (hours per night used for all nights in the trial arm, % of nights used and % of nights used > 4 hours), aerophagia symptoms (using VAS), weight, ESS, FOSQ and clinical global index of change were recorded and the 2-week diary was retrieved. The APAP device data was downloaded using ResScan software (version 4.3, ResMed Ltd., Sydney, Australia) and then the device was reprogrammed to the alternate therapy. Another diary was provided. Subjects continued on the alternate therapy for two weeks and were then reassessed in clinic. PAP usage (hours per night used for all nights in the trial arm, % of nights used and % of nights used > 4 hours), aerophagia symptoms (using VAS), weight, ESS, FOSQ, and clinical global index of change were again recorded and the two-week diary was retrieved. At this final visit, subjects were asked which therapy they preferred, and the main reason for this. Subjects were then given the opportunity to have the order of therapy type revealed and to discuss their ongoing management with a nonblinded researcher (KH, BD).
Statistical Analysis
All statistical analyses were undertaken on an intention-to-treat basis. Subgroup analysis was also conducted to examine the effects of subjects using a full face mask compared to subjects using a nasal mask. Statistical analyses were performed using GraphPad Prism 7.01 (GraphPad Software, La Jolla, California, United States). Tests of period and carryover effects were calculated according to Senn.14 The normality of group data collected was determined by the D'Agostino-Pearson omnibus K2 test.15 Data are presented as mean ± standard deviation or median (interquartile range) where appropriate. Differences between baseline, CPAP, and APAP arms were compared using the Friedman test with Dunn posttest (non-parametric). Differences between the CPAP and APAP arms were compared using the Wilcoxon matched-pairs signed-rank test (nonparametric). The effect size of changes between the two arms was calculated according to Nakagawa and Cuthill (nonparametric).16 Pearson r was selected to determine effect size with the cutoffs of r = 0.10 small effect, r = 0.30 medium and r = 0.50 large effect.17 A value of P < .05 was considered statistically significant.
RESULTS
Subject Flow
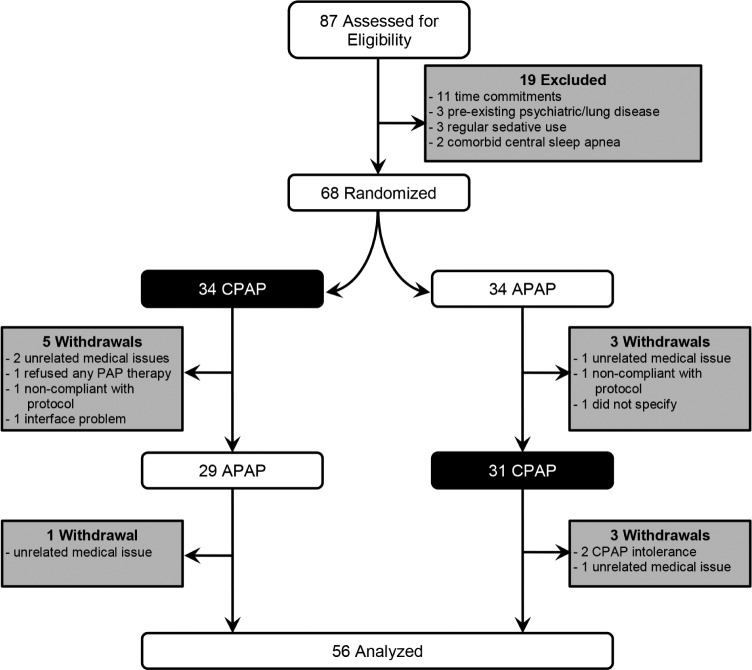
A total of 87 subjects were assessed for eligibility. The study flow chart is shown in Figure 1. Sixty-eight subjects who volunteered aerophagia symptoms were randomized for the trial. Thirty-four subjects were randomly selected to receive CPAP and the other 34 to receive APAP during the first arm of the trial. Five subjects withdrew from CPAP in the first arm for the reasons of: hospitalization for unrelated medical issues (two subjects), did not wish to continue with any PAP therapy (one subject), did not wish to complete any questionnaires or diary (one subject), and could not replace broken mask interface (one subject). Three subjects withdrew from APAP in the first arm for the reasons of: cracked ribs after a fall (one patient), did not wish to attend clinic (one patient), and did not wish to specify (one subject). This left 29 subjects to continue on to APAP in the second arm of their trial and 31 subjects to continue on to CPAP in the second arm of their trial. One subject withdrew from the APAP arm for the reason of hospitalization for an unrelated medical issue. Three subjects withdrew from the CPAP arm for the reasons of: intolerance of CPAP (two subjects) and on the advice of their general physician due to worsening cardiac history (one subject).
Figure 1. Trial flow chart.
APAP = autotitrating positive airway pressure, CPAP = continuous positive airway pressure, PAP = positive airway pressure.
Subject Characteristics
The baseline demographic and clinical characteristics of the group are shown in Table 1. The recruited subjects were of older age, obese, and predominantly male. Comorbidities such as hypertension, depression, gastroesophageal reflux disease, and hyperlipidemia were common in this group. Subjects were diagnosed with severe OSA. Obstructive respiratory events were spread evenly throughout non-rapid eye movement and rapid eye movement sleep. There was a supine predominance in the occurrence of these obstructive respiratory events. During their CPAP titration study, subjects were manually titrated to a little over 14 cm H2O. Subjects were predominantly using a full face mask and had been on therapy for almost one year. None of the included patients had a history of previous uvulopalatopharyngoplasty.
Table 1.
Patient characteristics of the study cohort.

Therapy Outcomes
Statistical analysis of our primary outcome data (average compliance between each trial arm) demonstrated no period effect (P = .62), carryover effect P = .63) or interaction be -tween treatment and period (P = .57). Objective compliance during the CPAP and APAP trial arms are shown in Figure 2 and Table 2. Both the CPAP and APAP arms demonstrated increased objective compliance compared to baseline CPAP therapy (P = .010 and P < .001 for CPAP and APAP therapy arms, respectively). There was no statistically significant difference between CPAP arm and APAP arm compliance (P = .12) during the trial. The device-calculated AHI was within clinically acceptable limits for both trial arms. The median pressure and the 95th centile pressure were reduced in the APAP trial arm (P < .001 for both median pressure and 95th centile pressures). Similarly, the median pressure and the 95th centile leak were also reduced in the APAP trial arm (P < .001 for both median leak and 95th centile leak). At the end of the trial 35 subjects preferred APAP, 11 subjects preferred CPAP, and 10 subjects had no specific preference between the therapy modes.
Figure 2. Compliance with CPAP and APAP in all subjects after two weeks of treatment.
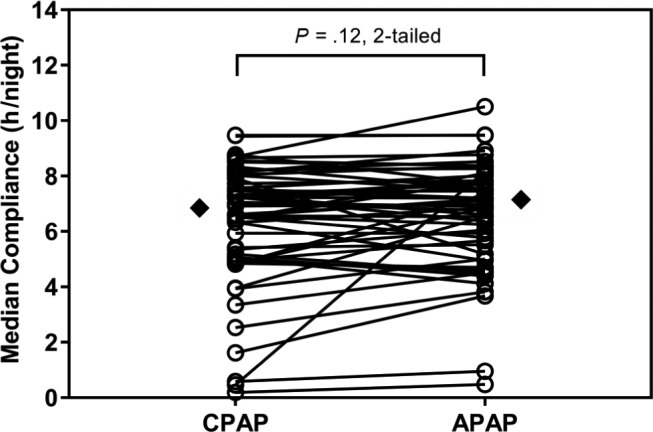
The diamonds mark the median value for each treatment arm. APAP = autotitrating positive airway pressure, CPAP = continuous positive airway pressure.
Table 2.
Comparison of CPAP and APAP outcome measures.

Symptoms of Aerophagia
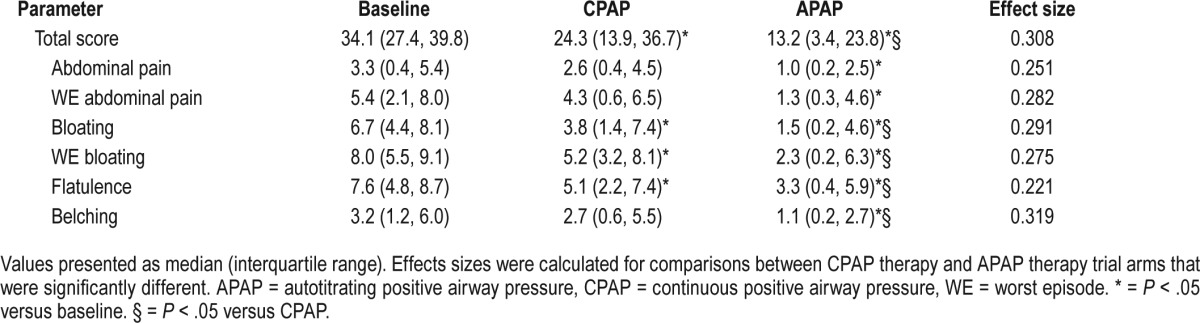
The effect of CPAP and APAP therapy on aerophagia symptoms in this group is shown in Table 3. Both CPAP and APAP arms described less total aerophagia symptoms compared to baseline (P = .021 and P < .001 for CPAP and APAP, respectively). APAP therapy was associated with a moderate reduction in total aerophagia symptoms compared with CPAP therapy (P = .003; r = 0.308). The individual symptoms of bloating (P = .010), worst episode of bloating (P = .006), and flatulence (P < .001) were significantly less with CPAP therapy compared with baseline symptoms. The individual symptoms of abdominal pain (P = .010), worst episode of abdominal pain (P = .003), and belching (P < .001) were also significantly less with APAP therapy in addition to the individual symptoms of bloating (P < .001), worst episode of bloating (P < .001), and flatulence (P < .001) when compared with baseline symptoms. APAP therapy was associated with moderate reductions in bloating (P = .011; r = 0.291), worst episode of bloating (P = .040; r = 0.275), flatulence (P = .010; r = 0.221), and belching (P = .001; r = 0.319) compared with CPAP therapy. There were no differences in morning abdominal girth (CPAP: 117 ± 14 cm versus APAP: 116 ± 14 cm, P = .780), subject-reported average daily consumption of aerated drinks (P = .914), and interval between consumption of last meal and bedtime (P = .439) between each arm.
Table 3.
Comparison of aerophagia-related symptoms during two weeks of CPAP and APAP treatment.
Sleep-Related Outcomes
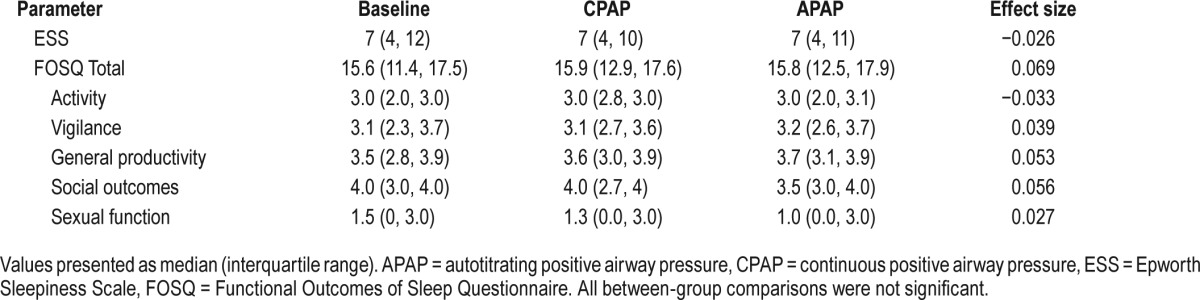
The effect of CPAP and APAP therapy on subjective sleepiness and sleep-related quality of life is shown in Table 4. At baseline, subjects ESS was within the normal range (< 10) and remained the same after the CPAP and APAP trial arms (P < .066). Subjects also described their quality of life as not being affected by their disorder or side effects. Both subjective sleepiness and their quality of life remained the same after the CPAP and APAP trial arms.
Table 4.
Comparison of subjective sleep and sleep-related outcomes scores during two weeks of CPAP and APAP treatment.
Nasal Mask Versus Full Face Mask Subjects
The subjects using a nasal mask were similar to subjects using a full face mask in nearly all aspects. No statistical differences were observed at baseline in body mass index (34 kg/m2 versus 33 kg/m2 for nasal mask vs full face mask, respectively, P = .121), diagnostic AHI (40.9 events/h versus 31.3 events/h for nasal mask versus full face mask, respectively, P = .400) or titrated pressure (12.0 cm H2O versus 14.5 cm H2O for nasal mask versus full face mask, respectively, P = .126). Compliance with therapy between the two mask types was also similar throughout the study (baseline: 6.0 h/night versus 5.5 h/night, P = .369; CPAP arm: 7.2 h/night versus 6.6 h/night, P = .989; APAP arm: 7.2 h/night versus 6.9 h/night, P = .836). During the APAP trial arm there were no differences in median pressure (9.8 cm H2O versus 9.8 cm H2O for nasal mask versus full face mask, respectively, P = .581), 95th centile pressure (11.8 cm H2O versus 12.1 cm H2O for nasal mask versus full face mask, respectively, P = .968), or 95th centile leak (13.2 L/ min versus 8.4 L/min for nasal mask versus full face mask, respectively, P = .059). The 95th centile leak was also similar during the CPAP trial arm (25.2 L/min versus 17.4 L/min for nasal mask versus full face mask, respectively, P = .126). No differences were observed in residual AHI for both the CPAP (4.6 events/h versus 3.6 events/h for nasal mask versus full face mask, respectively, P = .516) and APAP (5.0 events/h versus 5.0 events/h for nasal mask versus full face mask, respectively, P = .563) trial arms. Symptoms of aerophagia were similar at baseline (29.4 versus 35.6 events/h for nasal mask versus full face mask, respectively, P = .091), and after the APAP trial arm (18.1 versus 13.0 for nasal mask versus full face mask, respectively, P = .328). However, the symptoms of aerophagia were reduced with nasal mask use during the CPAP trial arm (19.1 versus 28.8 for nasal mask versus full face mask, respectively, P = .019, effect size r = 0.311).
DISCUSSION
In this study, we investigated the ability of APAP to alleviate the effects of aerophagia in OSA patients who complain of this side effect in comparison with CPAP. Aerophagia is a side effect of CPAP therapy whereby the pressurized air is swallowed and results in abdominal pain, bloating, belching, and flatulence. Our results demonstrate that, in patients who experience symptoms of aerophagia, APAP reduces these aerophagia symptoms while still effectively treating their OSA. The use of APAP reduces the median and 95th centile therapeutic pressures and reduces the leak compared to CPAP therapy. This reduction in aerophagia symptoms does not, however, result in any improvement in therapy compliance or sleep-related outcome measures.
This is the first study that we are aware of that tries to formally address this side effect of CPAP therapy. The use of APAP, in addition to reductions in therapeutic pressure, has been a common intervention for aerophagia in the clinical sleep laboratory. These interventions are initiated on the belief that they will improve therapy acceptance and thus lead to improved compliance with therapy. Furthermore, anecdotal evidence in our laboratory suggested that APAP therapy was associated with increased compliance. It was therefore somewhat surprising that APAP did not improve therapy compliance compared with CPAP for patients with aerophagia; patients often cite this complication as a reason for discontinuing CPAP. There may be a couple of factors that could explain the lack of difference in compliance between CPAP and APAP. First, a recent meta-analysis comparing CPAP with APAP for the treatment of OSA18 demonstrated a slight increase in compliance for APAP compared to CPAP. This increase in compliance with APAP therapy was in the magnitude of approximately 10 minutes. Because our study was powered on the basis of a 30-minute difference in compliance, considered by our sleep physicians to be clinically significant, it is unlikely to find any difference. Furthermore, according to the same meta-analysis, groups with an AHI of greater than 20 events/h did not display any difference in compliance overall. The patient group recruited for this trial had mostly moderate to severe OSA and would appear to complement this subgroup analysis. Second, CPAP compliance has been demonstrated to be unaffected by side effects experienced by users.7 There has yet to be any studies that demonstrate that the incidence and severity of side-effects reliably account for variances in therapeutic compliance. Last, our baseline compliance at 5.5 h/night could be considered quite good in comparison with various CPAP trials in the literature. An argument could be made that this relatively high CPAP compliance at commencement of the study could make it less likely to demonstrate an appreciable change in compliance between the two therapy modes.
Despite the lack of improvement in therapy compliance, we did see improvements in the subjective symptoms associated with aerophagia. These improvements were statistically significant for bloating, flatulence, belching, and overall symptoms of aerophagia. Although they were statistically significant, most of these were small in effect. APAP was only able to reduce the total symptom score and belching with a moderate effect. It is interesting to note that even with two weeks of CPAP, subjective symptoms of aerophagia decreased from baseline. Correspondingly, there was also an increase in compliance during the CPAP arm when compared to baseline. We believe that these differences in symptoms and compliance between baseline and the CPAP trial arm could be explained by a combination of recall bias of symptoms, the motivations of the patient, and the extra attention given to the patients. Evidence has shown that active coping can explain some of the variance associated with CPAP compliance.19 The motivation to enroll and actively participate in the study could be viewed as an active coping mechanism and lead to improved compliance overall and a reduction in the perception of symptoms.
A feature of this study that deserves comment is the high proportion of full face-mask interfaces used by subjects in this trial. In retrospective and experimental studies, full face masks decrease the retroglossal or retropalatal area20,21 leading to higher CPAP requirements22,23 although this has not been demonstrated by prospective studies conducted with small sample sizes.24,25 Still, the possibility that full face masks could pre-dispose to aerophagia in CPAP patients cannot be discounted. With that in mind, we conducted post hoc subgroup analysis to assess this possibility. Our study could not demonstrate any difference in APAP pressure requirements (median and 95th centile pressures), leak, residual AHI, or compliance between subjects using a full face mask and subjects using a nasal mask. The use of a full face mask was, however, associated with greater aerophagia symptoms in comparison with a nasal mask during the CPAP trial arm but not the APAP trial arm. These results suggest that a full face mask could predispose to aerophagia but this is more likely due to the route of air delivery rather than elevated therapeutic pressure requirements in these subjects. Nonetheless, APAP therapy has shown to be beneficial in reducing the symptoms of aerophagia with both mask types.
There are a number of limitations to the study. Firstly, we have no objective measures of aerophagia in these enrolled subjects; therefore, it could not be confirmed that the symptoms were directly due to CPAP and the study could not be controlled for the severity of the aerophagia. It has been reported that other concerns such as pressure intolerance, mask interface problems, and even gastric cancer can mimic the subjective symptoms of aerophagia.26 However, this limitation is somewhat mitigated by the fact that, in a usual clinical situation, a physician will take these patient symptoms at face value and instigate interventions to alleviate these symptoms immediately. Thus, our recruitment could be considered a strength due to its generalizability. There was an attempt to blind patients to the therapy mode; however, a limitation of the study is the potential for patients to break their blinding and determine the mode by removing the concealment of the device display. We are not aware of any patient in this study attempting to break the blinding, but it should not be discounted as a possibility. Patients were not provided with any information on how to interrogate the study device. A possible limitation could be the lack of a formal washout period in the study design. The rationale was that aerophagia is likely a direct mechanical effect of the PAP and these effects are not likely to carry over from one mode to the next. Therefore, the lack of a washout period should not influence average treatment compliance over a two-week period. Our data proved our rationale to be correct with no discernible carryover or order effect observed. Another limitation of this study is that it is probably underpowered to detect any difference in therapy compliance. Recent meta-analysis has shown compliance to increase by approximately 10 minutes with APAP compared to CPAP. However, we determined 30 minutes to be the minimum clinical difference and powered the study accordingly. Again, we believe that this aspect enhances the applicability to your usual sleep clinic.
CONCLUSIONS
This study has demonstrated that APAP therapy is effective for the relief of aerophagia symptoms in OSA patients. Furthermore, APAP was able to reduce the effect of aerophagia without compromising effective treatment of OSA. More patients believed that APAP was more effective in relieving these symptoms and hence described their preference for this therapy.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors declare no conflicts of interest in relation to this study. The authors received partial funding from the 2009–2010 Queensland Health - Health Practitioner Research Scheme to carry out this study.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- APAP
autotitrating positive airway pressure
- ArI
arousal index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- FOSQ
Functional Outcomes of Sleep Questionnaire
- GERD
gastroesophageal reflux disease
- NREM
non-rapid eye movement
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- REM
rapid eye movement
- TST
total sleep time
- VAS
visual analogue scale
- WE
worst episode
REFERENCES
- 1.Gottlieb DJ, Whitney CW, Bonekat WH, et al. Relation of sleepiness to respiratory disturbance index: the sleep heart health study. Am J Respir Crit Care Med. 1999;159(2):502–507. doi: 10.1164/ajrccm.159.2.9804051. [DOI] [PubMed] [Google Scholar]
- 2.Moyer CA, Sonnad SS, Garetz SL, Helman JI, Chervin RD. Quality of life in obstructive sleep apnea: a systematic review of the literature. Sleep Med. 2016;2(6):477–491. doi: 10.1016/s1389-9457(01)00072-7. [DOI] [PubMed] [Google Scholar]
- 3.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5(6):573–581. [PMC free article] [PubMed] [Google Scholar]
- 4.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 5.Heinzer RC, Stanchina ML, Malhotra A, et al. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172(1):114–117. doi: 10.1164/rccm.200404-552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giles TL, Lasserson TJ, Smith BJ, White J, Wright J, Cates CJ. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 1996. [Google Scholar]
- 7.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147(4):887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 9.Pepin JL, Leger P, Veale D, Langevin B, Robert D, Levy P. Side effects of nasal continuous positive airway pressure in sleep apnea syndrome: study of 193 patients in two French sleep centers. Chest. 1995;107(2):375–381. doi: 10.1378/chest.107.2.375. [DOI] [PubMed] [Google Scholar]
- 10.Chai CL, Pathinathan A, Smith B. Continuous positive airway pressure delivery interfaces for obstructive sleep apnoea. Cochrane Database Syst Rev. 2006;(4):CD005308. doi: 10.1002/14651858.CD005308.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaile JC, Levin T, Wung JT, Abramson SJ, Ruzal-Shapiro C, Berdon WE. Benign gaseous distension of the bowel in premature infants treated with nasal continuous airway pressure: a study of contributing factors. Am J Roentgenol. 1992;158(1):125–127. doi: 10.2214/ajr.158.1.1727337. [DOI] [PubMed] [Google Scholar]
- 12.Watson NF, Mystkowski SK. Aerophagia and gastroesophageal reflux disease in patients using continuous positive airway pressure: a preliminary observation. J Clin Sleep Med. 2008;4(5):434–438. [PMC free article] [PubMed] [Google Scholar]
- 13.Hukins C. Comparative study of autotitrating and fixed-pressure CPAP in the home: a randomized, single-blind crossover trial. Sleep. 2004;27(8):1512–1517. doi: 10.1093/sleep/27.8.1512. [DOI] [PubMed] [Google Scholar]
- 14.Senn S. Cross-Over Trials in Clinical Research. 2nd ed. Vol 156. London, UK: John Wiley & Sons, Ltd; 2012. [Google Scholar]
- 15.D'Agostino R, Pearson ES. Tests for departure from results for the normality of b2 and √b1. Biometrika. 1973;60(3):613–622. [Google Scholar]
- 16.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82(4):591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- 17.Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141(1):2–18. doi: 10.1037/a0024338. [DOI] [PubMed] [Google Scholar]
- 18.Ip S, D'Ambrosio C, Patel K, et al. Auto-titrating versus fixed continuous positive airway pressure for the treatment of obstructive sleep apnea: a systematic review with meta-analyses. Syst Rev. 2012;1(1):20. doi: 10.1186/2046-4053-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stepnowsky CJ, Jr, Bardwell WA, Moore PJ, Ancoli-Israel S, Dimsdale JE. Psychologic correlates of compliance with continuous positive airway pressure. Sleep. 2002;25(7):758–762. doi: 10.1093/sleep/25.7.758. [DOI] [PubMed] [Google Scholar]
- 20.Andrade RG, Madeiro F, Piccin VS, et al. Impact of acute changes in CPAP flow route in sleep apnea treatment. Chest. 2016;150(6):1194–1201. doi: 10.1016/j.chest.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Schorr F, Genta PR, Gregório MG, Danzi-Soares NJ, Lorenzi-Filho G. Continuous positive airway pressure delivered by oronasal mask may not be effective for obstructive sleep apnoea. Eur Respir J. 2012;40(2):503–505. doi: 10.1183/09031936.00145111. [DOI] [PubMed] [Google Scholar]
- 22.Borel JC, Tamisier R, Dias-Domingos S, et al. Type of mask may impact on continuous positive airway pressure adherence in apneic patients. PLoS One. 2013;8(5):e64382. doi: 10.1371/journal.pone.0064382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deshpande S, Joosten S, Turton A, et al. Oronasal masks require a higher pressure than nasal and nasal pillow masks for the treatment of obstructive sleep apnea. J Clin Sleep Med. 2016;12(9):1263–1268. doi: 10.5664/jcsm.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakker JP, Neill AM, Campbell AJ. Nasal versus oronasal continuous positive airway pressure masks for obstructive sleep apnea: a pilot investigation of pressure requirement, residual disease, and leak. Sleep Breath. 2012;16(3):709–716. doi: 10.1007/s11325-011-0564-3. [DOI] [PubMed] [Google Scholar]
- 25.Teo M, Amis T, Lee S, Falland K, Lambert S, Wheatley J. Equivalence of nasal and oronasal masks during initial CPAP titration for obstructive sleep apnea syndrome. Sleep. 2011;34(7):951–955. doi: 10.5665/SLEEP.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayoralas Alises S, Gómez Mendieta M, Díaz Lobato S. Aerophagia due to noninvasive mechanical ventilation: a first manifestation of silent gastric carcinoma. Arch Bronconeumol. 2003;39(7):321–323. doi: 10.1016/s0300-2896(03)75393-9. [DOI] [PubMed] [Google Scholar]